化工学报 ›› 2020, Vol. 71 ›› Issue (6): 2492-2509.DOI: 10.11949/0438-1157.20200106
收稿日期:2020-02-03
修回日期:2020-03-31
出版日期:2020-06-05
发布日期:2020-06-05
通讯作者:
郝广平
作者简介:董灵玉(1997—),女,硕士研究生,基金资助:
Lingyu DONG( ),Rui GE,Yafei YUAN,Songyuan TANG,Guangping HAO(
),Rui GE,Yafei YUAN,Songyuan TANG,Guangping HAO( ),Anhui LU
),Anhui LU
Received:2020-02-03
Revised:2020-03-31
Online:2020-06-05
Published:2020-06-05
Contact:
Guangping HAO
摘要:
二氧化碳(CO2)电催化转化引起广泛关注,其中非贵金属多孔炭基催化剂是研究热点。重点介绍了近年来多孔炭基CO2电催化材料的孔结构、表面化学、形貌调控策略,归纳了增强多孔炭基CO2电催化还原效率的方法,探讨了多孔炭基催化材料的活性中心类型与分布,分析了提高催化活性位密度的手段。在总结近年来取得研究进展的基础上,展望了多孔炭基催化剂在电催化CO2转化方面的发展趋势和面临的挑战。
中图分类号:
董灵玉, 葛睿, 原亚飞, 唐宋元, 郝广平, 陆安慧. 多孔炭基二氧化碳电催化材料研究进展[J]. 化工学报, 2020, 71(6): 2492-2509.
Lingyu DONG, Rui GE, Yafei YUAN, Songyuan TANG, Guangping HAO, Anhui LU. Recent advances in porous carbon-based carbon dioxide electrocatalytic materials[J]. CIESC Journal, 2020, 71(6): 2492-2509.
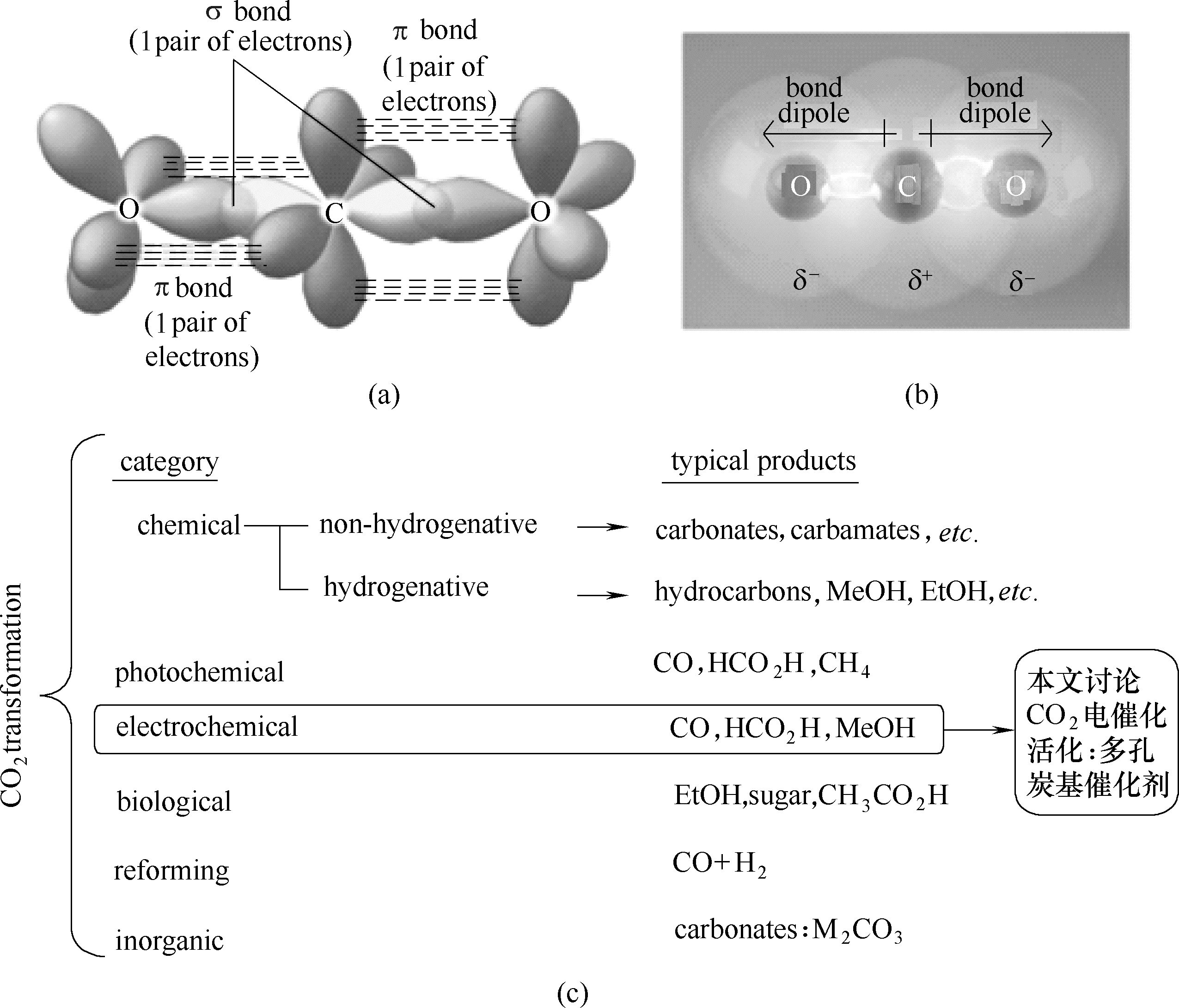
图1 CO2分子杂化轨道及域电子分配(a), CO2原子电负性(b)和CO2活化方式(c)
Fig.1 Hybrid orbital of CO2and delocalized electrons (a), C and O element’s electronegativity in CO2(b) and the methods to activate CO2(c)
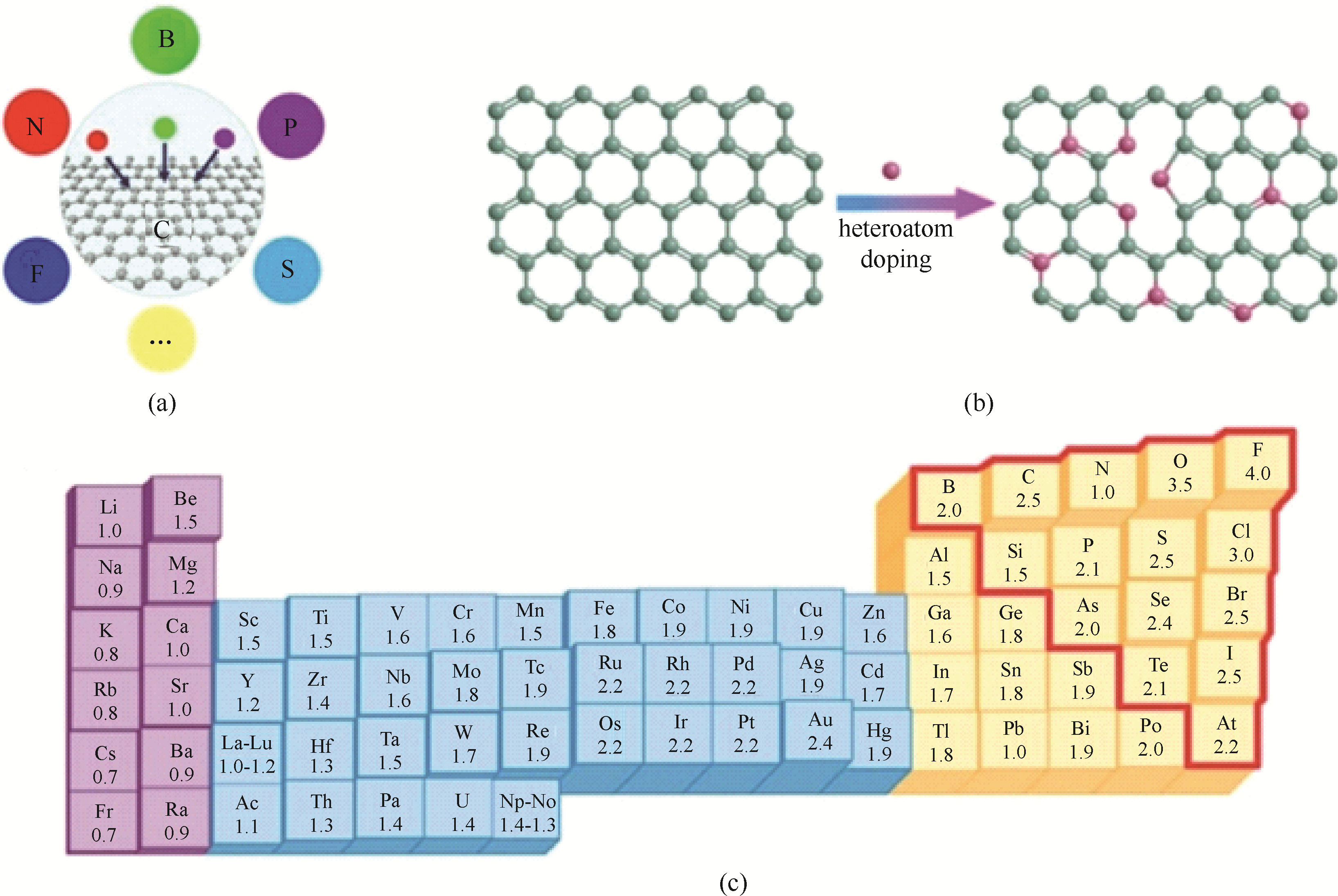
图4 杂原子掺杂炭结构(a)及掺杂方法(b), 杂原子电负性(c)
Fig.4 Doping(a) and doping method(b) of graphitic carbon structure with heteroatoms, and the corresponding electronegativity of elements(c)

图5 石墨烯负载型氮掺杂石墨烯量子点形貌以及CO2还原电化学测试
Fig.5 Morphology of graphene-loaded nitrogen-doped graphene quantum dots and electrochemical measurement of CO2 reduction

图7 M-Nx材料模型与局部结构示意图(a), 与Au基催化剂相比, M-N-C催化剂的质量归一化CO部分电流(质量活性) (b)和实验与模拟的相关性(c)
Fig.7 Model and a schematic local structure(a), and catalyst mass-normalized CO partial currents(mass activity) vs applied potential compared to state-of-art Au catalysts(b) and experimental correlation to simulations(c) for M–N–C catalysts

图9 Cu–N4结构上*CO转化为CH3OH的自由能(橙色、灰色、红色和浅蓝色球体分别代表Cu、C、O和H原子)
Fig.9 Free energies for conversion of *CO to CH3OH on Cu-N4 structure(orange, gray, red and light blue spheres stand for Cu, C, O, and H atoms, respectively)
| 1 | Roncancio R, Ulcay M S, Arango J E, et al. Experimental study of CO2 corn stover char gasification using iron nitrate as a catalyst under a high-pressure environment[J]. Fuel, 2020, 267: 117237. |
| 2 | Zhu D D, Liu J L, Qiao S Z. Recent advances in inorganic heterogeneous electrocatalysts for reduction of carbon dioxide[J]. Advanced Materials, 2016, 28(18): 3423-3452. |
| 3 | 杨东明, 梁相程. CO2绿色利用技术[J]. 当代化工, 2019, 48(8): 1838-1841. |
| Yang D M, Liang X C. Green utilization technology of CO2[J]. Contemporary Chemical Industry, 2019, 48(8): 1838-1841. | |
| 4 | Hossain M N, Wen J, Chen A. Unique copper and reduced graphene oxide nanocomposite toward the efficient electrochemical reduction of carbon dioxide[J]. Scientific Reports, 2017, 7(1): 3184. |
| 5 | Mariano R G, McKelvey K, White H S, et al. Selective increase in CO2 electroreduction activity at grain-boundary surface terminations[J]. Science, 2017, 358(6367): 1187-1192. |
| 6 | Gao D, Zhou H, Wang J, et al. Size-dependent electrocatalytic reduction of CO2 over Pd nanoparticles[J]. Journal of the American Chemical Society, 2015, 137(13): 4288-4291. |
| 7 | Wang W, Ning H, Yang Z, et al. Interface-induced controllable synthesis of Cu2O nanocubes for electroreduction CO2 to C2H4[J]. Electrochimica Acta, 2019, 306: 360-365. |
| 8 | Gao D, Zhang Y, Zhou Z, et al. Enhancing CO2 electroreduction with the metal-oxide interface[J]. Journal of the American Chemical Society, 2017, 139(16): 5652-5655. |
| 9 | Lin S, Diercks C S, Zhang Y B, et al. Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water[J]. Science, 2015, 349(6253): 1208-1213. |
| 10 | Diercks C S, Lin S, Kornienko N, et al. Reticular electronic tuning of porphyrin active sites in covalent organic frameworks for electrocatalytic carbon dioxide reduction[J]. Journal of the American Chemical Society, 2018, 140(3): 1116-1122. |
| 11 | Kornienko N, Zhao Y, Kley C S, et al. Metal-organic frameworks for electrocatalytic reduction of carbon dioxide[J]. Journal of the American Chemical Society, 2015, 137(44): 14129-14135. |
| 12 | Angamuthu R, Byers P, Lutz M, et al. Electrocatalytic CO2 conversion to oxalate by a copper complex[J]. Science, 2010, 327(5963): 313-315. |
| 13 | Costentin C, Drouet S, Robert M, et al. A local proton source enhances CO2 electroreduction to CO by a molecular Fe catalyst[J]. Science, 2012, 338(6103): 90-94. |
| 14 | Ye Y, Cai F, Li H, et al. Surface functionalization of ZIF-8 with ammonium ferric citrate toward high exposure of Fe-N active sites for efficient oxygen and carbon dioxide electroreduction[J]. Nano Energy, 2017, 38: 281-289. |
| 15 | Pan F, Zhao H, Deng W, et al. A novel N, Fe-decorated carbon nanotube/carbon nanosheet architecture for efficient CO2 reduction[J]. Electrochimica Acta, 2018, 273: 154-161. |
| 16 | Ning H, Wang X, Wang W, et al. Cubic Cu2O on nitrogen-doped carbon shell for electrocatalytic CO2 reduction to C2H4[J]. Carbon, 2019, 146: 218-223. |
| 17 | Zhang F, Zhang H, Liu Z. Recent advances on electrochemical reduction of CO2[J]. Current Opinion in Green and Sustainable Chemistry, 2019, 16: 77-84. |
| 18 | Zhang J, Terrones M, Park C R, et al. Carbon science in 2016: status, challenges and perspectives[J]. Carbon, 2016, 98(70): 708-732. |
| 19 | 王同洲, 王鸿. 多孔碳材料的研究进展[J]. 中国科学: 化学, 2019, 49(5): 729-740. |
| Wang T Z, Wang H. Research progress of porous carbon materials[J]. Scientia Sinica Chimica, 2019, 49(5): 729-740. | |
| 20 | Zhang H, Liu Q, Fang Y, et al. Boosting Zn-ion energy storage capability of hierarchically porous carbon by promoting chemical adsorption[J]. Advanced Materials, 2019, 31(44): 1904948. |
| 21 | Zhu Q, Wang X, Chen D, et al. Highly porous carbon xerogels doped with cuprous chloride for effective CO adsorption[J]. ACS Omega, 2019, 4(4): 6138-6143. |
| 22 | 郝广平, 李文翠, 陆安慧. 纳米结构多孔固体在二氧化碳吸附分离中的应用[J]. 化工进展, 2012, 31(11): 2493-2510. |
| Hao G P, Li W C, Lu A H. Application of nanostructured porous solids in carbon dioxide adsorption and separation[J]. Chemical Industry and Engineering Progress, 2012, 31(11): 2493-2510. | |
| 23 | Wang R T, Lang J W, Yan X B. Effect of surface area and heteroatom of porous carbon materials on electrochemical capacitance in aqueous and organic electrolytes[J]. Science China Chemistry, 2014, 57(11): 1570-1578. |
| 24 | Chen Y, Li J, Yue G, et al. Novel Ag@nitrogen-doped porous carbon composite with high electrochemical performance as anode materials for lithium-ion batteries[J]. Nano-Micro Letters, 2017, 9(3): 82-92. |
| 25 | Matos I, Bernardo M, Fonseca I. Porous carbon: a versatile material for catalysis[J]. Catalysis Today, 2017, 285: 194-203. |
| 26 | Novoselov K S, Geim A K, Morozov S V, et al. Electric field effect in atomically thin carbon films[J]. Science, 2004, 306(5696): 666-669. |
| 27 | Sing K S W, Everett D H, Haul R A W, et al. Reporting physisorption data for gas/solid systems-with special reference to the determination of surface area and porosity[J]. Pure and Applied Chemistry, 1985, 57(4): 603-619. |
| 28 | Thommes M, Kaneko K, Neimark A V, et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report)[J]. Pure and Applied Chemistry, 2015, 87(9/10): 1051-1069. |
| 29 | Ma Z, Kyotani T, Tomita A. Preparation of a high surface area microporous carbon having the structural regularity of Y zeolite[J]. Chemical Communications, 2000, 23: 2365-2366. |
| 30 | Braun E, Lee Y, Moosavi S M, et al. Generating carbon schwarzites via zeolite-templating[J]. Proceedings of the National Academy of Sciences, 2018, 115(35): E8116-E8124. |
| 31 | Presser V, Heon M, Gogotsi Y. Carbide-derived carbons-from porous networks to nanotubes and graphene[J]. Adv. Funct. Mater., 2011, 21: 810-833. |
| 32 | Kim K, Lee T, Kwon Y, et al. Lanthanum-catalysed synthesis of microporous 3D graphene-like carbons in a zeolite template[J]. Nature, 2016, 535: 131-135. |
| 33 | Oschatz M, Boukhalfa S, Nickel W, et al. Carbide-derived carbon aerogels with tunable pore structure as versatile electrode material in high power supercapacitors[J]. Carbon, 2017, 113: 283-291. |
| 34 | Shao L, Sang Y, Huang J, et al. Triazine-based hyper-cross-linked polymers with inorganic-organic hybrid framework derived porous carbons for CO2 capture[J]. Chemical Engineering Journal, 2018, 353: 1-14. |
| 35 | Lu A H, Schüth F. Nanocasting: a versatile strategy for creating nanostructured porous materials[J]. Advanced Materials, 2006, 18: 1793. |
| 36 | Li W, Liu J, Zhao D. Mesoporous materials for energy conversion and storage devices[J]. Nature Reviews Materials, 2016, 1(6): 1-17. |
| 37 | Hursan D, Samu A A, Janovak L, et al. Morphological attributes govern carbon dioxide reduction on N-doped carbon electrodes[J]. Joule, 2019, 3: 1719-1733. |
| 38 | Deng Y, Liu C, Yu T, et al. Facile synthesis of hierarchically porous carbons from dual colloidal crystal/block copolymer template approach[J]. Chemistry of Materials, 2007, 19(13): 3271-3277. |
| 39 | Wang H, Jia J, Song P, et al. Efficient electrocatalytic reduction of CO2 by nitrogen-doped nanoporous carbon/carbon nanotube membranes: a step towards the electrochemical CO2 refinery[J]. Angewandte Chemie International Edition, 2017, 56: 7847-7852. |
| 40 | Hao G P, Mondin G, Zheng Z, et al. Unusual ultra-hydrophilic, porous carbon cuboids for atmospheric-water capture[J]. Angewandte Chemie International Edition, 2015, 54: 1941-1945. |
| 41 | Hao G P, Sahraie N R, Zhang Q, et al. Hydrophilic non-precious metal nitrogen-doped carbon electrocatalysts for enhanced efficiency in oxygen reduction reaction[J]. Chemical Communications, 2015, 51: 17285-17288. |
| 42 | Hao G P, Zhang Q, Sin M, et al. Design of hierarchically porous carbons with interlinked hydrophilic and hydrophobic surface and their capacitive behavior[J]. Chemistry of Materials, 2016, 28: 8715-8725. |
| 43 | Zhang G R, Munoz M, Etzold B J M. Accelerating oxygen-reduction catalysts through preventing poisoning with non-reactive species by using hydrophobic ionic liquids[J]. Angewandte Chemie International Edition, 2016, 55: 2257-2261. |
| 44 | Xu W, Lu Z, Sun X, et al. Superwetting electrodes for gas-involving electrocatalysis[J]. Accounts of Chemical Research, 2018, 51: 1590-1598. |
| 45 | Li H, Xiao N, Wang Y, et al. Promoted electroreduction of CO2 with oxygen vacancies on plasma-activated SnOx/carbon foam monolithic electrode[J]. Journal of Materials Chemistry A, 2020, 8(4): 1779-1786. |
| 46 | Tang C, Zhang Q. Nanocarbon for oxygen reduction electrocatalysis: dopants, edges, and defects[J]. Advanced Materials, 2017, 29(13): 1604103. |
| 47 | Liu X, Dai L. Carbon-based metal-free catalysts[J]. Nature Reviews Materials, 2016, 1: 16064. |
| 48 | Yan D, Li Y, Huo J, et al. Defect chemistry of nonprecious-metal electrocatalysts for oxygen reactions[J]. Advanced Materials, 2017, 29(48): 1606459. |
| 49 | Hao G P, Tang C, Zhang E, et al. Thermal exfoliation of layered metal-organic frameworks into ultrahydrophilic graphene stacks and their applications in Li-S batteries[J]. Advanced Materials, 2017, 29(37): 1702829. |
| 50 | Zhong G, Wang H, Yu H, et al. Chemically drilling carbon nanotubes for electrocatalytic oxygen reduction reaction[J]. Electrochimica Acta, 2016, 190: 49-56. |
| 51 | Zhong G, Wang H, Yu H, et al. The effect of edge carbon of carbon nanotubes on the electrocatalytic performance of oxygen reduction reaction[J]. Electrochemistry Communications, 2014, 40: 5-8. |
| 52 | Huang Y, Liang J, Chen Y. An overview of the applications of graphene-based materials in supercapacitors[J]. Small, 2012, 8(12): 1805-1834. |
| 53 | Zhang L, Xu Q, Niu J, et al. Role of lattice defects in catalytic activities of graphene clusters for fuel cells[J]. Physical Chemistry Chemical Physics, 2015, 17(26): 16733-16743. |
| 54 | Sa Y J, Kim J H, Joo S H. Active edge-site-rich carbon nanocatalysts with enhanced electron transfer for efficient electrochemical hydrogen peroxide production[J]. Angewandte Chemie International Edition, 2019, 58: 1100-1105. |
| 55 | Hou Y, Wen Z, Cui S, et al. Strongly coupled ternary hybrid aerogels of N-deficient porous graphitic-C3N4 nanosheets/N-doped graphene/NiFe-layered double hydroxide for solar-driven photoelectrochemical water oxidation[J]. Nano Letters, 2016, 16(4): 2268-2277. |
| 56 | Jiang Y, Yang L, Sun T, et al. Significant contribution of intrinsic carbon defects to oxygen reduction activity[J]. ACS Catalysis, 2015, 5: 6707-6712. |
| 57 | Roman D S, Krishnamurthy D, Garg R, et al. Engineering three-dimensional (3D) out-of-plane graphene edge sites for highly selective two-electron oxygen reduction electrocatalysis[J]. ACS Catalysis, 2020, 10(3): 1993-2008. |
| 58 | Florian B, Jani K, Arkady V K. Structural defects in graphene[J]. ACS Nano, 2011, 5(1): 26-41. |
| 59 | 黄秋玲. 掺杂/缺陷石墨烯的制备及其电催化性能研究[D]. 广州: 华南理工大学, 2019. |
| Huang Q L. Preparation and electrocatalytic properties of doped/defective graphene[D]. Guangzhou: South China University of Technology, 2019. | |
| 60 | Antonietti M, Oschatz M. The concept of “noble, heteroatom-doped carbons” their directed synthesis by electronic band control of carbonization, and applications in catalysis and energy materials[J]. Advanced Materials, 2018, 30(21): 1706836. |
| 61 | Li X X, Zhao Q S, Feng X, et al. Pyridinic nitrogen-doped graphene nanoshells boost the catalytic efficiency of palladium nanoparticles for the N-allylation reaction[J]. Chem. Sus. Chem., 2019, 12(4): 1-9. |
| 62 | Li S, Pasc A, Fierro V, et al. Hollow carbon spheres, synthesis and applications-a review[J]. Journal of Materials Chemistry, 2016, 4(33): 12686-12713. |
| 63 | Jesica C Q, Esther B G, Francisco C M, et al. Mesoporous carbon nanospheres with improved conductivity for electro-catalytic reduction of O2 and CO2[J]. Carbon, 2019, 155: 88-99. |
| 64 | Chen Y, Zou L, Liu H, et al. Fe and N co-doped porous carbon nanospheres with high density of active sites for efficient CO2 electroreduction[J]. The Journal of Physical Chemistry C, 2019, 123(27): 16651-16659. |
| 65 | Wang S, Li W, Hao G, et al. Temperature-programmed precise control over the sizes of carbon nanospheres based on benzoxazine chemistry[J]. Journal of the American Chemical Society, 2011, 133(39): 15304-15307. |
| 66 | Wu J, Yadav R M, Liu M, et al. Achieving highly efficient, selective, and stable CO2 reduction on nitrogen-doped carbon nanotubes[J]. ACS Nano, 2015, 9(5): 5364-5371. |
| 67 | Yang H, Wu Y, Li G, et al. Scalable production of efficient single-atom copper decorated carbon membranes for CO2 electroreduction to methanol[J]. Journal of the American Chemical Society, 2019, 141(32): 12717-12723. |
| 68 | Zhang W, Zeng J, Liu H, et al. CoxNi1-x nanoalloys on N-doped carbon nanofibers: electronic regulation toward efficient electrochemical CO2 reduction[J]. Journal of Catalysis, 2019, 372: 277-286. |
| 69 | Zhou W, Shen H, Wang Q, et al. N-doped peanut-shaped carbon nanotubes for efficient CO2 electrocatalytic reduction[J]. Carbon, 2019, 152: 241-246. |
| 70 | Modi A, Bhaduri B, Verma N, et al. Facile one-step synthesis of nitrogen-doped carbon nanofibers for the removal of potentially toxic metals from water[J]. Industrial & Engineering Chemistry Research, 2015, 54(18): 5172-5178. |
| 71 | Duan J, Chen S, Jaroniec M, et al. Heteroatom-doped graphene-based materials for energy-relevant electrocatalytic processes[J]. ACS Catalysis, 2015, 5(9): 5207-5234. |
| 72 | Mao X, Kour G, Zhang L, et al. Silicon-doped graphene edges: an efficient metal-free catalyst for the reduction of CO2 into methanol and ethanol[J]. Catalysis Science & Technology, 2019, 9(23): 6800-6807. |
| 73 | Zhang H, Li J, Xi S, et al. A graphene-supported single-atom FeN5 catalytic site for efficient electrochemical CO2 reduction[J]. Angewandte Chemie International Edition, 2019, 131(42): 15013-15018. |
| 74 | Jiang K, Siahrostami S, Zheng T, et al. Isolated Ni single atoms in graphene nanosheets for high-performance CO2 reduction[J]. Energy & Environmental Science, 2018, 11(4): 893-903. |
| 75 | Gierszal K P, Jaroniec M. Carbons with extremely large volume of uniform mesopores synthesized by carbonization of phenolic resin film formed on colloidal silica template[J]. Journal of the American Chemical Society, 2006, 128(31): 10026-10027. |
| 76 | Lu A H, Hao G P, Sun Q, et al. Chemical synthesis of carbon materials with intriguing nanostructure and morphology[J]. Macromolecular Chemistry and Physics, 2012, 213: 1107-1131. |
| 77 | Hao G P, Li W C, Qian D, et al. Structurally designed synthesis of mechanically stable poly (benzoxazine-co-resol)-based porous carbon monoliths and their application as high-performance CO2 capture sorbents[J]. Journal of the American Chemical Society, 2011, 133(29): 11378-11388. |
| 78 | Sharma P P, Wu J, Yadav R M, et al. Nitrogen-doped carbon nanotube arrays for high-efficiency electrochemical reduction of CO2: on the understanding of defects, defect density, and selectivity[J]. Angewandte Chemie International Edition, 2015, 54(46): 13701-13705. |
| 79 | Varela A S, Sahraie N R, Steinberg J, et al. Metal-doped nitrogenated carbon as an efficient catalyst for direct CO2 electroreduction to CO and hydrocarbons[J]. Angewandte Chemie International Edition, 2015, 54(37): 10758-10762. |
| 80 | Zheng Y, Jiao Y, Jaroniec M, et al. Nanostructured metal-free electrochemical catalysts for highly efficient oxygen reduction[J]. Small, 2012, 8(23): 3550-3566. |
| 81 | Su D S, Zhang J, Frank B, et al. Metal-free heterogeneous catalysis for sustainable chemistry[J]. Chem. Sus. Chem., 2010, 3(2): 169-180. |
| 82 | Li Q, Cao R, Cho J, et al. Nanostructured carbon-based cathode catalysts for nonaqueous lithium-oxygen batteries[J]. Physical Chemistry Chemical Physics, 2014, 16(27): 13568-13582. |
| 83 | Wu J, Sharifi T, Gao Y, et al. Emerging carbon-based heterogeneous catalysts for electrochemical reduction of carbon dioxide into value-added chemicals[J]. Advanced Materials, 2019, 31(13): 1804257. |
| 84 | Kong X K, Chen C L, Chen Q W. Doped graphene for metal-free catalysis[J]. Chemical Society Reviews, 2014, 43(8): 2841-2857. |
| 85 | Dai L, Xue Y, Qu L, et al. Metal-free catalysts for oxygen reduction reaction[J]. Chemical Reviews, 2015, 115(11): 4823-4892. |
| 86 | Zhao L, He R, Rim K T, et al. Visualizing individual nitrogen dopants in monolayer graphene[J]. Science, 2011, 333(6045): 999-1003. |
| 87 | Wei D, Liu Y, Wang Y, et al. Synthesis of N-doped graphene by chemical vapor deposition and its electrical properties[J]. Nano Letters, 2009, 9(5): 1752-1758. |
| 88 | Duan X, Xu J, Wei Z, et al. Metal-free carbon materials for CO2 electrochemical reduction[J]. Advanced Materials, 2017, 29(41): 1701784. |
| 89 | Kumar B, Asadi M, Pisasale D, et al. Renewable and metal-free carbon nanofibre catalysts for carbon dioxide reduction[J]. Nature Communications, 2013, 4: 2819. |
| 90 | Zhang S, Kang P, Ubnoske S, et al. Polyethylenimine-enhanced electrocatalytic reduction of CO2 to formate at nitrogen-doped carbon nanomaterials[J]. Journal of the American Chemical Society, 2014, 136(22): 7845-7848. |
| 91 | Wu J, Yadav R M, Liu M, et al. Achieving highly efficient, selective, and stable CO2 reduction on nitrogen-doped carbon nanotubes[J]. ACS Nano, 2015, 9(5): 5364-5371. |
| 92 | Liu Y, Chen S, Quan X, et al. Efficient electrochemical reduction of carbon dioxide to acetate on nitrogen-doped nanodiamond[J]. Journal of the American Chemical Society, 2015, 137(36): 11631-11636. |
| 93 | Wu J, Ma S, Sun J, et al. A metal-free electrocatalyst for carbon dioxide reduction to multi-carbon hydrocarbons and oxygenates[J]. Nature Communications, 2016, 7: 13869. |
| 94 | Jhong H R M, Tornow C E, Smid B, et al. A nitrogen-doped carbon catalyst for electrochemical CO2 conversion to CO with high selectivity and current density[J]. Chem. Sus. Chem., 2017, 10(6): 1094-1099. |
| 95 | Song Y, Chen W, Zhao C, et al. Metal-free nitrogen-doped mesoporous carbon for electroreduction of CO2 to ethanol[J]. Angewandte Chemie International Edition, 2017, 56(36): 10840-10844. |
| 96 | Zhang L, Niu J, Li M, et al. Catalytic mechanisms of sulfur-doped graphene as efficient oxygen reduction reaction catalysts for fuel cells[J]. The Journal of Physical Chemistry C, 2014, 118(7): 3545-3553. |
| 97 | Pan F, Li B, Deng W, et al. Promoting electrocatalytic CO2 reduction on nitrogen-doped carbon with sulfur addition[J]. Applied Catalysis B: Environmental, 2019, 252: 240-249. |
| 98 | Sun Q, Li Z, Searles D J, et al. Charge-controlled switchable CO2 capture on boron nitride nanomaterials[J]. Journal of the American Chemical Society, 2013, 135(22): 8246-8253. |
| 99 | Sreekanth N, Nazrulla M A, Vineesh T V, et al. Metal-free boron-doped graphene for selective electroreduction of carbon dioxide to formic acid/formate[J]. Chemical Communications, 2015, 51(89): 16061-16064. |
| 100 | Nakata K, Ozaki T, Terashima C, et al. High-yield electrochemical production of formaldehyde from CO2 and seawater[J]. Angewandte Chemie International Edition, 2014, 53(3): 871-874. |
| 101 | 张宇晶. 硼氮共掺杂纳米金刚石的制备及其电还原CO2性能研究[D]. 大连: 大连理工大学, 2016. |
| Zhang Y J. Preparation of boron-nitrogen co-doped nanodiamonds and its electroreduction of CO2[D]. Dalian: Dalian University of Technology, 2016. | |
| 102 | Liu Y, Zhang Y, Cheng K, et al. Selective electrochemical reduction of carbon dioxide to ethanol on a boron- and nitrogen-co-doped nanodiamond[J]. Angewandte Chemie International Edition, 2017, 56(49): 15607-15611. |
| 103 | Liu T F, Ali S, Lian Z, et al. Phosphorus-doped onion-like carbon for CO2 electrochemical reduction: the decisive role of the bonding configuration of phosphorus[J]. Journal of Materials Chemistry A, 2018, 6(41): 19998-20004. |
| 104 | Varela A S, Ju W, Bagger A, et al. Electrochemical reduction of CO2 on metal-nitrogen-doped carbon catalysts[J]. ACS Catalysis, 2019, 9(8): 7270-7284. |
| 105 | Yan C, Lin L, Wang G, et al. Transition metal-nitrogen sites for electrochemical carbon dioxide reduction reaction[J]. Chinese Journal of Catalysis, 2019, 40(1): 23-37. |
| 106 | Hu X M, Hval H H, Bjerglund E T, et al. Selective CO2 reduction to CO in water using earth-abundant metal and nitrogen-doped carbon electrocatalysts[J]. ACS Catalysis, 2018, 8(7): 6255-6264. |
| 107 | Pan F, Deng W, Justiniano C, et al. Identification of champion transition metals centers in metal and nitrogen-co-doped carbon catalysts for CO2 reduction[J]. Applied Catalysis B: Environmental, 2018, 226: 463-472. |
| 108 | Jiang K, Siahrostami S, Akey A J, et al. Transition-metal single atoms in a graphene shell as active centers for highly efficient artificial photosynthesis[J]. Chem, 2017, 3(6): 950-960. |
| 109 | Wang X, Zhao Q, Yang B, et al. Emerging nanostructured carbon-based non-precious metal electrocatalysts for selective electrochemical CO2 reduction to CO[J]. Journal of Materials Chemistry A, 2019, 7(44): 25191-25202. |
| 110 | Lei C, Wang Y, Hou Y, et al. Efficient alkaline hydrogen evolution on atomically dispersed Ni-Nx species anchored porous carbon with embedded Ni nanoparticles by accelerating water dissociation kinetics[J]. Energy & Environmental Science, 2019, 12(1): 149-156. |
| 111 | Pérez-Rodríguez S, Pastor E, Lázaro M J. Noble metal-free catalysts supported on carbon for CO2 electrochemical reduction[J]. Journal of CO2 Utilization, 2017, 18: 41-52. |
| 112 | Ju W, Bagger A, Hao G P, et al. Understanding activity and selectivity of metal-nitrogen-doped carbon catalysts for electrochemical reduction of CO2[J]. Nature Communications, 2017, 8(1): 944. |
| 113 | Zhang B, Zhang J, Shi J, et al. Manganese acting as a high-performance heterogeneous electrocatalyst in carbon dioxide reduction[J]. Nature Communications, 2019, 10(1): 1-8. |
| 114 | Genovese C, Schuster M E, Gibson E K, et al. Operando spectroscopy study of the carbon dioxide electro-reduction by iron species on nitrogen-doped carbon[J]. Nature Communications, 2018, 9(1): 935. |
| 115 | Pan F, Zhang H, Liu K, et al. Unveiling active sites of CO2 reduction on nitrogen-coordinated and atomically dispersed iron and cobalt catalysts[J]. ACS Catalysis, 2018, 8(4): 3116-3122. |
| 116 | Qu Y, Chen B, Li Z, et al. Thermal emitting strategy to synthesize atomically dispersed Pt metal sites from bulk Pt metal[J]. Journal of the American Chemical Society, 2019, 141(11): 4505-4509. |
| 117 | Liu J. Catalysis by supported single metal atoms[J]. ACS Catalysis, 2016, 7(1): 34-59. |
| 118 | Jiao J, Lin R, Liu S, et al. Copper atom-pair catalyst anchored on alloy nanowires for selective and efficient electrochemical reduction of CO2[J]. Nature Chemistry, 2019, 11(3): 222-228. |
| 119 | Yang H B, Hung S F, Liu S, et al. Atomically dispersed Ni (I) as the active site for electrochemical CO2 reduction[J]. Nature Energy, 2018, 3(2): 140. |
| 120 | Yang H, Wu Y, Li G, et al. Scalable production of efficient single-atom copper decorated carbon membranes for CO2 electroreduction to methanol[J]. Journal of the American Chemical Society, 2019, 141(32): 12717-12723. |
| 121 | Wang M, Chen L, Lau T C, et al. A hybrid Co quaterpyridine complex/carbon nanotube catalytic material for CO2 reduction in water[J]. Angewandte Chemie International Edition, 2018, 57(26): 7769-7773. |
| 122 | Francke R, Schille B, Roemelt M. Homogeneously catalyzed electroreduction of carbon dioxide-methods, mechanisms, and catalysts[J]. Chemical Reviews, 2018, 118(9): 4631-4701. |
| 123 | Wu Y, Jiang Z, Lu X, et al. Domino electroreduction of CO2 to methanol on a molecular catalyst[J]. Nature, 2019, 575(7784): 639-642. |
| 124 | Li F, Thevenon A, Rosas-Hernández A, et al. Molecular tuning of CO2-to-ethylene conversion[J]. Nature, 2020, 577(7791): 509-513. |
| 125 | Wang J, Kattel S, Hawxhurst C J, et al. Enhancing activity and reducing cost for electrochemical reduction of CO2 by supporting palladium on metal carbides[J]. Angewandte Chemie International Edition, 2019, 58(19): 6271-6275. |
| 126 | Liu J, Jiao M, Mei B, et al. Carbon-supported divacancy-anchored platinum single-atom electrocatalysts with superhigh Pt utilization for the oxygen reduction reaction[J]. Angewandte Chemie International Edition, 2019, 58(4): 1163-1167. |
| [1] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| [2] | 王峰, 张顺鑫, 余方博, 刘亚, 郭烈锦. 光催化CO2还原制碳氢燃料系统优化策略研究[J]. 化工学报, 2023, 74(1): 29-44. |
| [3] | 陈健鑫, 朱瑞杰, 盛楠, 朱春宇, 饶中浩. 纤维素基生物质多孔炭的制备及其超级电容器性能研究[J]. 化工学报, 2022, 73(9): 4194-4206. |
| [4] | 王淋, 付乾, 肖帅, 李卓, 李俊, 张亮, 朱恂, 廖强. 高效可见光响应微生物/光电化学耦合人工光合作用系统[J]. 化工学报, 2022, 73(2): 887-893. |
| [5] | 温怡静, 张博, 陈晓霏, 赵思洋, 周欣, 黄艳, 李忠. 多孔炭吸附剂的乙烯-乙烷选择性反转机制[J]. 化工学报, 2021, 72(9): 4768-4774. |
| [6] | 赵林洲, 郑燕娥, 李孔斋, 王亚明, 蒋丽红, 范浩熙, 王雅静, 祝星, 魏永刚. Ce1-xNixOy氧载体在化学链甲烷重整耦合CO2还原中的应用[J]. 化工学报, 2021, 72(8): 4371-4380. |
| [7] | 杨金曼, 朱兴旺, 周固礼, 许晖, 李华明. MOFs诱导中空Co3O4/CdIn2S4合成及光催化CO2还原性能研究[J]. 化工学报, 2020, 71(6): 2780-2787. |
| [8] | 张亚婷, 张博超, 张建兰, 李可可, 党永强, 段瑛峰. “自下而上”化学合成纳米石墨烯的研究进展[J]. 化工学报, 2020, 71(6): 2628-2642. |
| [9] | 王晓波,赵青山,程智年,张浩然,胡涵,王路海,吴明铂. 高性能碳基储能材料的设计、合成与应用[J]. 化工学报, 2020, 71(6): 2660-2677. |
| [10] | 周宇, 王宇新. 杂原子掺杂碳基氧还原反应电催化剂研究进展[J]. 化工学报, 2017, 68(2): 519-534. |
| [11] | 张志盼, 陈承镇, 王倩倩, 钟菊花, 张波, 程振民. 卤族元素对Ag电极电催化还原CO2的影响[J]. 化工学报, 2017, 68(2): 687-693. |
| [12] | 王恩民, 李文翠, 雷成, 陆安慧. 碱式碳酸镁催化酚醛聚合制备多孔炭及其CO2吸附性能[J]. 化工学报, 2015, 66(7): 2565-2572. |
| [13] | 吴美容, 张瑞, 周俊, 谢欣欣, 雍晓雨, 闫志英, 葛明民, 郑涛. 温度对产甲烷菌代谢途径和优势菌群结构的影响[J]. 化工学报, 2014, 65(5): 1602-1606. |
| [14] | 杨杰, 浦群, 包永忠. 基于偏氯乙烯嵌段共聚物的多级多孔炭的制备[J]. 化工学报, 2014, 65(1): 358-364. |
| [15] | 吴启强, 包永忠. 偏氯乙烯共聚物/纳米水滑石复合材料及多孔炭的制备与表征 [J]. 化工学报, 2011, 62(4): 1130-1135. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号