化工学报 ›› 2020, Vol. 71 ›› Issue (6): 2660-2677.DOI: 10.11949/0438-1157.20200508
王晓波1( ),赵青山1(
),赵青山1( ),程智年1,张浩然3,胡涵1,王路海3,吴明铂1,2(
),程智年1,张浩然3,胡涵1,王路海3,吴明铂1,2( )
)
收稿日期:2020-05-07
修回日期:2020-05-18
出版日期:2020-06-05
发布日期:2020-06-05
通讯作者:
吴明铂
作者简介:王晓波(1988—),男,博士研究生, 基金资助:
Xiaobo WANG1( ),Qingshan ZHAO1(
),Qingshan ZHAO1( ),Zhinian CHENG1,Haoran ZHANG3,Han HU1,Luhai WANG3,Mingbo WU1,2(
),Zhinian CHENG1,Haoran ZHANG3,Han HU1,Luhai WANG3,Mingbo WU1,2( )
)
Received:2020-05-07
Revised:2020-05-18
Online:2020-06-05
Published:2020-06-05
Contact:
Mingbo WU
摘要:
电化学储能器件的性能很大程度上决定于其电极材料。碳材料具有来源广泛、化学稳定性好、易于调控、环境友好等优点,被广泛应用于各类能量存储系统,但仍存在能量密度低、倍率性能差等问题。本文从碳材料孔结构调控、杂原子掺杂、与金属氧化物复合三个角度,综述了构建高性能碳基储能材料的设计合成策略,介绍了其在锂/钠离子二次电池、超级电容器等领域的研究进展,对几种方法策略的优缺点进行了总结,并对未来的研究方向进行了展望。本文对高性能碳基储能电极材料的设计开发具有积极意义。
中图分类号:
王晓波,赵青山,程智年,张浩然,胡涵,王路海,吴明铂. 高性能碳基储能材料的设计、合成与应用[J]. 化工学报, 2020, 71(6): 2660-2677.
Xiaobo WANG,Qingshan ZHAO,Zhinian CHENG,Haoran ZHANG,Han HU,Luhai WANG,Mingbo WU. Design, synthesis and application of high-performance carbon-based energy storage materials[J]. CIESC Journal, 2020, 71(6): 2660-2677.
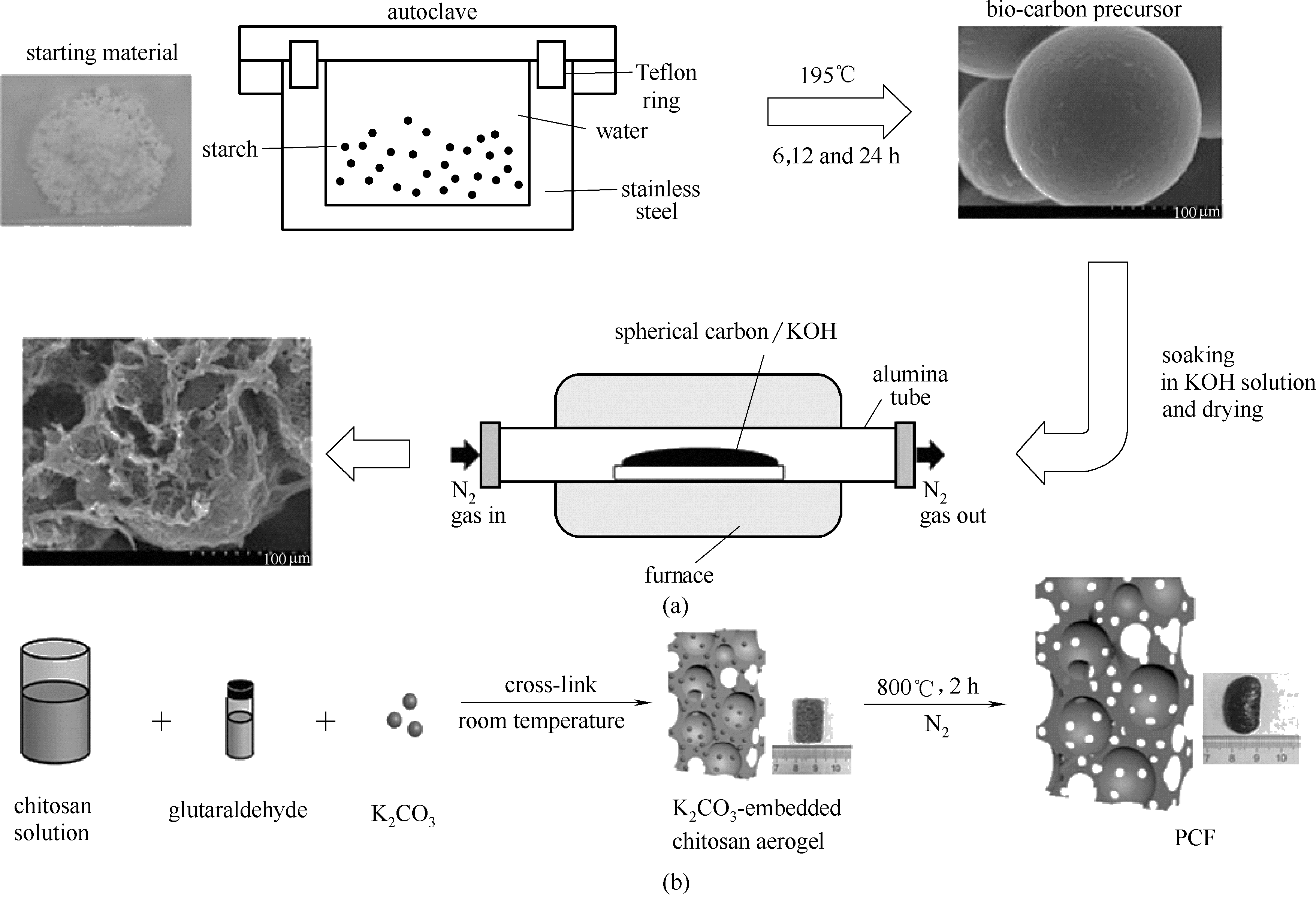
图2 HPC制备流程示意图(a) [10]; PCF制备流程与结构示意图(b) [12]
Fig.2 Schematic illustration for the preparation of HPC(a) [10]; schematic illustration for the preparation and structure of PCF(b) [12]

图3 介孔碳材料(a)[20]、HPCs(b)[21]和三维蜂窝状多孔碳材料(c)[22]制备流程示意图;三维蜂窝状多孔碳材料扫描电镜图[(d)、(e)][22]
Fig.3 Schematic illustration for the preparation of mesoporous carbon material (a) [20], HPCs(b) [21] and 3D honeycomb-like porous carbon material (c) [22]; SEM images of 3D honeycomb-like porous carbon material[(d),(e)][22]
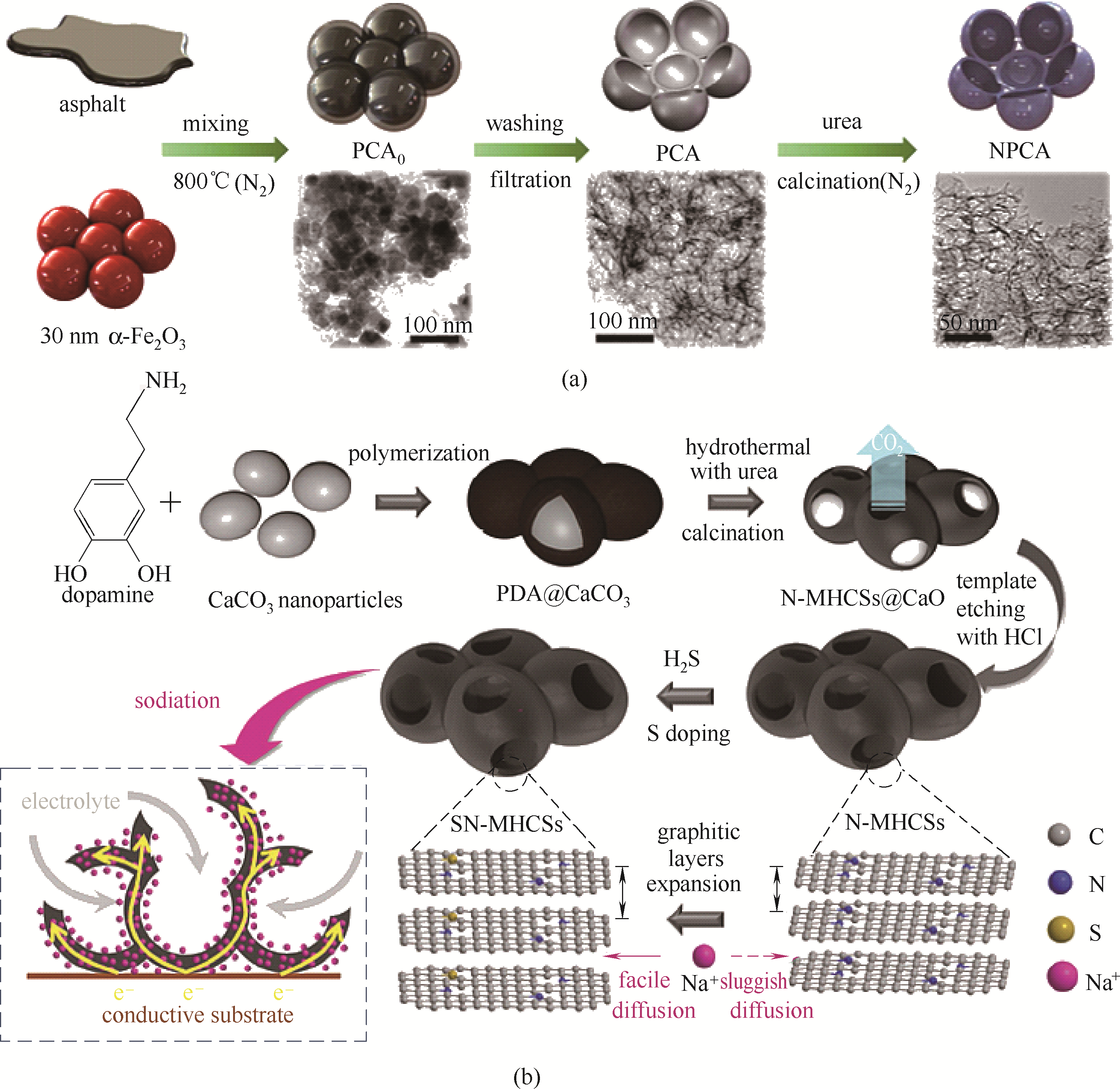
图5 NPCA制备流程及形貌示意图(a) [36]; SNMHCSs制备流程及元素组成示意图(b) [37]
Fig.5 Schematic illustration for the preparation and morphology of NPCA(a) [36]; schematic illustration for the preparation and element composition of SNMHCSs(b)[37]
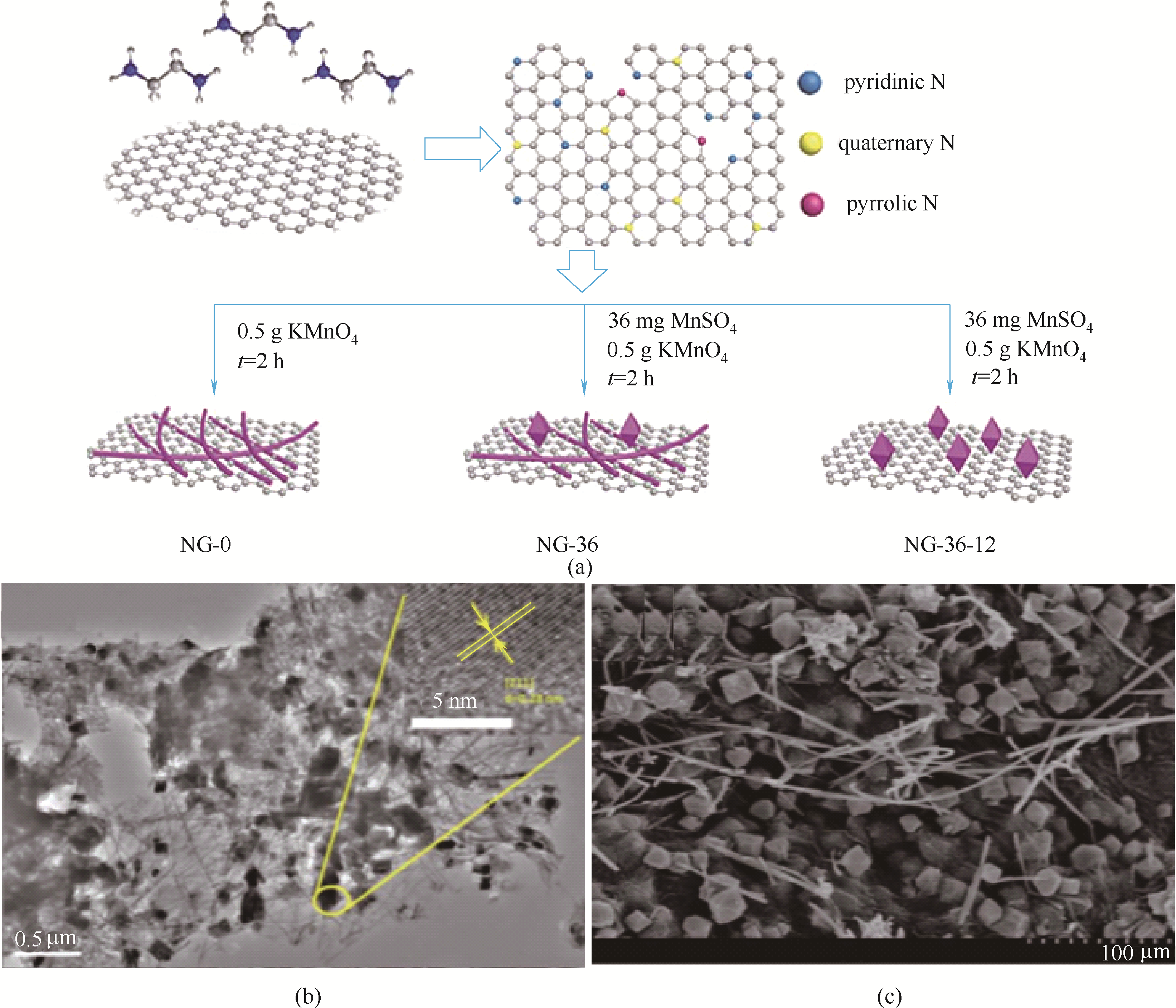
图6 N-Graphene/MnOOH/Mn3O4制备流程与结构示意图(a)、透射电镜图(b)和扫描电镜图(c) [38]
Fig.6 Schematic illustration for the preparation and structure of N-Graphene/MnOOH/Mn3O4 (a); TEM (b) and SEM (c) images of N-Graphene/MnOOH/Mn3O4[38]
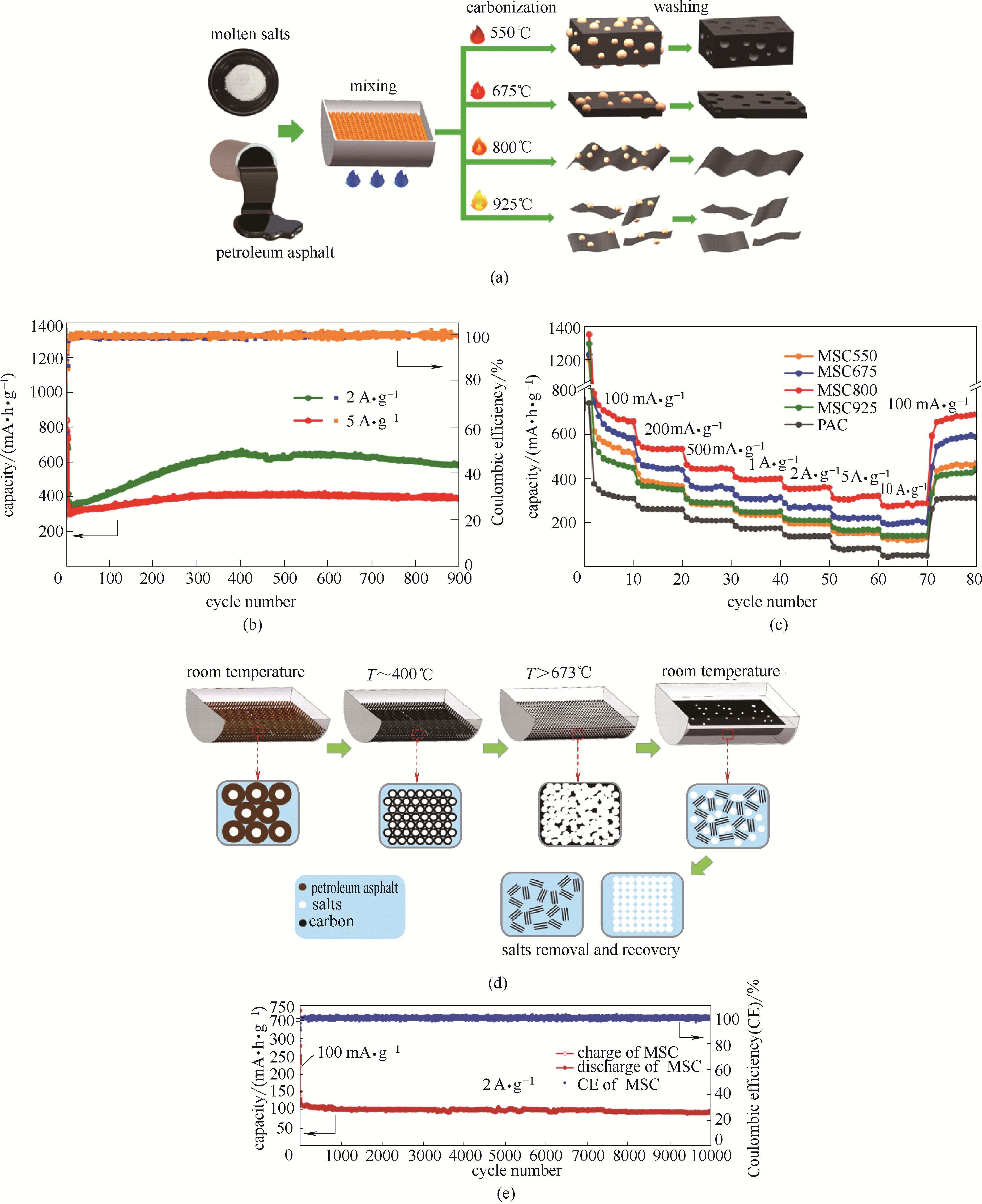
图9 MSC制备流程示意图(a)及储锂循环(b)和倍率性能图(c)[57];碳纳米片制备流程示意图(d)及储钠循环性能图(e) [58]
Fig.9 Schematic illustration for the preparation (a), long cycling stability(b) and rate performance of MSC for lithium storage(c) [57]; schematic illustration for the preparation(d) and long cycling stability performance (e) of carbon nanosheets for sodium storage at 2 A·g-1 [58]

图10 NP-CNS扫描电镜(a)和高倍透射电镜图(b);1.2 mV·s-1扫速下储锂(c)和储钠(d)赝电容贡献图;1 A·g-1电流密度下储锂(e)和储钠(f)的循环性能图[59]
Fig.10 SEM(a) and HRTEM(b) images of NP-CNS; pseudocapacitance contribution of lithium storage (c) and sodium storage(d) at scanning rate of 1.2 mV·s-1; cycle performance of lithium storage (e) and sodium storage (f) at 1 A·g-1[59]

图11 SnO2-QDs/N-GNs的TEM图(a)和100 mA·g-1电流下的循环性能图(b) [60]; H-MoO2/C的TEM图(c)和1 A·g-1电流下的循环性能图(d)[61];Fe2O3/rGO/CNFs的锂离子嵌入/脱出示意图(e)、分级微结构示意图(f)及2 A·g-1电流下的循环性能图(g)[62]
Fig.11 TEM image (a) and cycle performance image(b) at 100 mA·g-1 of SnO2-QDs/N-GNs[60]; TEM image (c) and cycle performance image (d) at 1 A·g-1 of H-MoO2/C[61]; schematic illustration of lithium ion intercalation/de-intercalation (e), hierarchical microstructure(f), and cycle performance image at 2 A·g-1 of Fe2O3/rGO/CNFs(g)[62]

图12 HPC扫描电镜图(a)和不同电流密度下的比电容性能(b)[65];NHCA扫描电镜图(c)和5 A·g-1电流下循环稳定性能(d)[66]
Fig.12 SEM image(a) and specific capacitance performance at different current densities(b) of HPC[65]; SEM image(c) and cycling stability performance at 5 A·g-1(d) of NHCA[66]

图13 HC/KOH和HC/N/KOH在不同电流密度下的功率和能量密度(a) [67];PCNs在不同电流密度下的比电容(b) [68]; CSAAs在8 A·g-1电流密度下的循环性能(c) [69];NSCF的循环性能和库仑效率(插图为10 mV·s-1扫速下的循环伏安曲线) (d) [72]
Fig.13 Power density of HC/KOH and HC/N/KOH samples vs average energy density at different current density(a)[67]; specific capacitance of PCNs at different current densities(b) [68]; cycle stability and Coulombic efficiency of CSAAs at the current density of 8 A·g-1(c) [69]; cycling performance and Coulombic efficiency of NSCF (inset shows the corresponding CV curves during the cycling at scanning rate of 10 mV·s-1) (d)[72]

图14 Fe2O3@ACC不同放大倍数下的扫描电镜图(a)和不同电流下的面电容和比电容(b) [76]; NaOH刻蚀后MoO3/C中夹层石墨烯透射电镜图(c) [77];MoO3/C的电荷/电子迁移示意图(d)及倍率性能(e) [77]
Fig.14 SEM images at different magnification (a), areal and specific capacitances vs current density(b) of the Fe2O3@ACC electrode [76]; TEM image of pure interlayered graphene in MoO3/C after NaOH etching(c) [77]; schematic illustration showing the bicontinuous paths for H+ ions and fast electron migration(d) and rate performance (e) of MoO3/C[77]
| 设计策略 | 优点 | 缺点 |
|---|---|---|
| 孔结构调控 | 提高了材料的比表面积及活性接触位点数;丰富了离子传输通道;提高了离子迁移速率 | 微、介、大孔相对含量的可控化设计难度较大;制备过程比较耗时;废酸洗液难以处理 |
| 杂原子掺杂 | 增加了材料的缺陷活性位点数;提高了材料的电负性;增大了材料的层间距;促进电子传输速率和离子储存效率 | 难以保证掺杂的均匀性;难以精确调控杂原子掺杂位置和类型 |
| 与金属氧化物复合 | 显著提高了材料的能量密度 | 材料循环稳定性仍较差 |
表1 碳基储能材料设计策略的优缺点
Table 1 Advantages and disadvantages of the design strategy for carbon-based energy storage materials
| 设计策略 | 优点 | 缺点 |
|---|---|---|
| 孔结构调控 | 提高了材料的比表面积及活性接触位点数;丰富了离子传输通道;提高了离子迁移速率 | 微、介、大孔相对含量的可控化设计难度较大;制备过程比较耗时;废酸洗液难以处理 |
| 杂原子掺杂 | 增加了材料的缺陷活性位点数;提高了材料的电负性;增大了材料的层间距;促进电子传输速率和离子储存效率 | 难以保证掺杂的均匀性;难以精确调控杂原子掺杂位置和类型 |
| 与金属氧化物复合 | 显著提高了材料的能量密度 | 材料循环稳定性仍较差 |
| 1 | Simon P, Gogoysi Y. Materials for electrochemical capacitors[J]. Nature Materials, 2008, 7(11): 845-854. |
| 2 | Liu C, Li F, Ma L P, et al. Advanced materials for energy storage[J]. Advanced Materials, 2010, 22(8): 28-62. |
| 3 | Arico A S, Bruce P G, Scrosti B, et al. Nanostructured materials for advanced energy conversion and storage devices[J]. Nature Materials, 2005, 4(5): 366-377. |
| 4 | Bruce P G, Freunberger S A, Hardwick L J, et al. Li-O2 and Li-S batteries with high energy storage[J]. Nature Materials, 2011, 11(1): 19-29. |
| 5 | Wen L, Li F, Luo H Z, et al. Graphene for Flexible Lithium-ion Batteries: Development and Prospects[M]. John, Wiley & Sons, Ltd., 2015. |
| 6 | Li P, Liu J Y, Wang Y W, et al. Synthesis of ultrathin hollow carbon shell from petroleum asphalt for high-performance anode material in lithium-ion batteries[J]. Chemical Engineering Journal, 2016, 286: 632-639. |
| 7 | Wang Y W, Xiao N, Wang Z Y, et al. Rational design of high-performance sodium-ion battery anode by molecular engineering of coal tar pitch[J]. Chemical Engineering Journal, 2018, 342: 52-60. |
| 8 | Zhu C Y, Akiyama T. Cotton derived porous carbon via an MgO template method for high performance lithium ion battery anodes[J]. Green Chemistry, 2016, 18(7): 2106-2114. |
| 9 | Stein A, Wang Z Y, Fierke M A, et al. Functionalization of porous carbon materials with designed pore architecture[J]. Advanced Materials, 2009, 21(3): 265-293. |
| 10 | Kim Y I, Lee Y J, Yoo J, et al. High-capacitance activated bio-carbons with controlled pore size distribution for sustainable energy storage[J]. Journal of Power Sources, 2019, 438: 226969- 226978. |
| 11 | He X J, Wang J X, Xu G H, et al. Synthesis of microporous carbon/graphene composites for high-performance supercapacitors[J]. Diamond and Related Materials, 2016, 66: 119-125. |
| 12 | Zhang F, Liu T Y, Hou G H, et al. Hierarchically porous carbon foams for electric double layer capacitors[J]. Nano Research, 2016, 9(10): 2875-2888. |
| 13 | He X J, Ling P H, Yu M X, et al. Rice husk-derived porous carbons with high capacitance by ZnCl2 activation for supercapacitors[J]. Electrochimica Acta, 2013, 105(105): 635-641. |
| 14 | Olivaresmarin M, Fernandezgonzalez C, Maciasgarcia A, et al. Preparation of activated carbon from cherry stones by chemical activation with ZnCl2[J]. Applied Surface Science, 2006, 252(17): 5967-5971. |
| 15 | Kim S, Lee J, Jaewook L. Hierarchical porous carbon from algae biomass by peroxide-assisted microwave activation for dye adsorption[J]. Journal of Advanced Engineering and Technology, 2019, 12(3): 143-148. |
| 16 | Guo Y P, Qi J R, Jiang Y Q, et al. Performance of electrical double layer capacitors with porous carbons derived from rice husk[J]. Materials Chemistry and Physics, 2003, 80(3): 704-709. |
| 17 | Zhang W F, Huang Z H, Guo Z, et al. Porous carbons prepared from deoiled asphalt and their electrochemical properties for supercapacitors[J]. Materials Letters, 2010, 64(17): 1868-1870. |
| 18 | Lupul I, Yperman J, Carleer R, et al. Tailoring of porous texture of hemp stem-based activated carbon produced by phosphoric acid activation in steam atmosphere[J]. Journal of Porous Materials, 2015, 22(1): 283-289. |
| 19 | Liu S L, Wang Y N, Lu K, et al. Preparation and pore characterization of activated carbon from Ma bamboo (Dendrocalamus latiflorus) by H3PO4 chemical activation[J]. Journal of Porous Materials, 2014, 21(4): 459-466. |
| 20 | He X J, Li R C, Qiu J S, et al. Synthesis of mesoporous carbons for supercapacitors from coal tar pitch by coupling microwave-assisted KOH activation with a MgO template[J]. Carbon, 2012, 50(13): 4911-4921. |
| 21 | He X J, Zhao N, Qiu J S, et al. Synthesis of hierarchical porous carbons for supercapacitors from coal tar pitch with nano-Fe2O3 as template and activation agent coupled with KOH activation[J]. Journal of Materials Chemistry, 2013, 1(33): 9440-9448. |
| 22 | Pan L, Li X X, Wang Y X, et al. 3D interconnected honeycomb-like and high rate performance porous carbons from petroleum asphalt for supercapacitors[J]. Applied Surface Science, 2018, 444 (30): 739-746. |
| 23 | Sun Q Y. Porous carbon material based on biomass prepared by MgO template method and ZnCl2 activation method as electrode for high performance supercapacitor[J]. International Journal of Electrochemical Science, 2019, 14: 1-14. |
| 24 | Paraknowitsch J P, Thomas A. Doping carbons beyond nitrogen: an overview of advanced heteroatom doped carbons with boron, sulphur and phosphorus for energy applications[J]. Energy and Environmental Science, 2013, 6(10): 2839-2855. |
| 25 | Wood K N, Ohayre R, Pylypenko S, et al. Recent progress on nitrogen/carbon structures designed for use in energy and sustainability applications[J]. Energy and Environmental Science, 2014, 7(4): 1212-1249. |
| 26 | Zhu Y P, Guo C X, Zheng Y, et al. Surface and interface engineering of noble-metal-free electrocatalysts for efficient energy conversion processes[J]. Accounts of Chemical Research, 2017, 50(4): 915-923. |
| 27 | Tian W, Liu J L, Wang Y X, et al. Substrate-assisted in situ confinement pyrolysis of zeolitic imidazolate frameworks to nitrogen-doped hierarchical porous carbon nanoframes with superior lithium storage[J]. ACS Applied Materials & Interfaces, 2017, 9(49): 42845-42855. |
| 28 | Qie L, Chen W M, Xiong X Q, et al. Sulfur-doped carbon with enlarged interlayer distance as a high-performance anode material for sodium-ion batteries[J]. Advanced Science, 2015, 2(12): 1500195- 1500200. |
| 29 | Yi J N, Qing Y, Wu C T, et al. Lignocellulose-derived porous phosphorus-doped carbon as advanced electrode for supercapacitors[J]. Journal of Power Sources, 2017, 351: 130-137. |
| 30 | Geng Z, Li B, Liu H Z, et al. Oxygen-doped carbon host with enhanced bonding and electron attraction abilities for efficient and stable SnO2/carbon composite battery anode[J]. Science China-Materials, 2018, 61(8): 1067-1077. |
| 31 | Rodriguez E, Camean I, Garcia R, et al. Graphitized boron-doped carbon foams: performance as anodes in lithium-ion batteries[J]. Electrochimica Acta, 2011, 56(14): 5090-5094. |
| 32 | Peng Y Y, Zhang Y Y, Huang J X, et al. Nitrogen and oxygen dual-doped hollow carbon nanospheres derived from catechol/polyamine as sulfur hosts for advanced lithium sulfur batteries[J]. Carbon, 2017, 124: 23-33. |
| 33 | Zhou J, Shen H L, Li Z H, et al. Porous carbon materials with dual N, S-doping and uniform ultra-microporosity for high performance supercapacitors[J]. Electrochimica Acta, 2016, 209: 557-564. |
| 34 | Jin C B, Zhang W K, Zhuang Z Z, et al. Enhanced sulfide chemisorption using boron and oxygen dually doped multi-walled carbon nanotubes for advanced lithium-sulfur batteries[J]. Journal of Materials Chemistry, 2017, 5(2): 632-640. |
| 35 | Wang L, Wang Y Q, Wu M G, et al. Nitrogen, fluorine, and boron ternary doped carbon fibers as cathode electrocatalysts for zinc–air batteries[J]. Small, 2018, 14(20): 1800737- 1800744. |
| 36 | Liu J Y, Liu Y, Li P, et al. Fe-N-doped porous carbon from petroleum asphalt for highly efficient oxygen reduction reaction[J]. Carbon, 2018, 126: 1-8. |
| 37 | Ni D, Sun W, Wang Z H, et al. Heteroatom‐doped mesoporous hollow carbon spheres for fast sodium storage with an ultralong cycle life[J]. Advanced Energy Materials, 2019, 9(19): 1900036-1900046. |
| 38 | Li Z T, Wang Y K, Chen Y, et al. Controllable growth of MnOx dual-nanocrystals on N-doped graphene as lithium-ion battery anode[J]. RSC Advances, 2017, 7(11): 6396-6402. |
| 39 | Li Z H, Cui Y H, Chen J, et al. Fabrication of (Co, Mn)3O4/rGO composite for lithium ion battery anode by a one-step hydrothermal process with H2O2 as additive[J]. Plos One, 2016, 11(10): 164657- 164668. |
| 40 | Feng J Z, Dong Y F, Yan Y C, et al. Extended lattice space of TiO2 hollow nanocubes for improved sodium storage[J]. Chemical Engineering Journal, 2019, 373: 565-571. |
| 41 | Wang D, Zhou W W, Zhang R, et al. MOF-derived Zn-Mn mixed oxides@carbon hollow disks with robust hierarchical structure for high-performance lithium-ion batteries[J]. Journal of Materials Chemistry A, 2018, 6(7): 2974-2983. |
| 42 | Ning H, Xie H, Zhao Q S, et al. Electrospinning ZnO/carbon nanofiber as binder-free and self-supported anode for Li-ion batteries[J]. Journal of Alloys and Compounds, 2017, 722: 716-720. |
| 43 | Zhang C Y, Su L, Huang L N, et al. A facile synthesis of Cu-MnO/CNF by electrospinning method and its electrochemical performance for supercapacitor application[J]. International Journal of Electrochemical Science, 2019, 14(12): 11358-11366. |
| 44 | Nayak P K, Yang L T, Brehm W, et al. From lithium‐ion to sodium-ion batteries: advantages, challenges, and surprises[J]. Angewandte Chemie, 2018, 57(1): 102-120. |
| 45 | Nitta N, Wu F X, Lee J, et al. Li-ion battery materials: present and future[J]. Materials Today, 2015, 18(5): 252-264. |
| 46 | Visstrom H, Ddvidsson S, Hook M. Lithium availability and future production outlooks[J]. Applied Energy, 2013, 110(10): 252-266. |
| 47 | 张伶俐. 石墨烯及过渡金属氧化物在锂/钠离子电池负极材料中的应用[D]. 南宁: 广西大学, 2018. |
| Zhang L L. Application of graphene and transition metal oxides for anode materials of lithium/sodium ion batteries[D]. Nanning: Guangxi University, 2018. | |
| 48 | Tarascon J M, Armand M. Issues and challenges facing rechargeable lithium batteries[J]. Nature, 2001, 414(6861): 359-367. |
| 49 | Armand M, Tarascon J M. Building better batteries[J]. Nature, 2008, 451(7179): 652-657. |
| 50 | Scrosati B, Garche J. Lithium batteries: status, prospects and future[J]. Journal of Power Sources, 2010, 195(9): 2419-2430. |
| 51 | Hwang J Y, Myung S, Sun Y. Sodium-ion batteries: present and future[J]. Chemical Society Reviews, 2017, 46(12): 3529-3614. |
| 52 | Peters J, Buchholz D, Passerini S, et al. Life cycle assessment of sodium-ion batteries[J]. Energy and Environmental Science, 2016, 9(5): 1744-1751. |
| 53 | Yabuuchi N, Kubota K, Dahbi M, et al. Research development on sodium-ion batteries[J]. Chemical Reviews, 2014, 114(23): 11636-11682. |
| 54 | Hong S Y, Kim Y J, Park Y, et al. Charge carriers in rechargeable batteries: Na ions vs. Li ions[J]. Energy and Environmental Science, 2013, 6(7): 2067-2081. |
| 55 | Luo W, Shen F, Bommier C, et al. Na-ion battery anodes: materials and electrochemistry[J]. Accounts of Chemical Research, 2016, 49(2): 231-240. |
| 56 | Bresser D, Passerini S, Scrosati B, et al. Leveraging valuable synergies by combining alloying and conversion for lithium-ion anodes[J]. Energy and Environmental Science, 2016, 9(11): 3348-3367. |
| 57 | Wang Y X, Tian W, Wang L H, et al. A tunable molten-salt route for scalable synthesis of ultrathin amorphous carbon nanosheets as high-performance anode materials for lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2018, 10(6): 5577-5585. |
| 58 | Wang Y X, Wang Y W, Liu J Y, et al. Preparation of carbon nanosheets from petroleum asphalt via recyclable molten-salt method for superior lithium and sodium storage[J]. Carbon, 2017, 122: 344-351. |
| 59 | Gao C, Feng J Z, Dai J R, et al. Manipulation of interlayer spacing and surface charge of carbon nanosheets for robust lithium/sodium storage[J]. Carbon, 2019, 153: 372-380. |
| 60 | Li Z T, Wu G L, Deng S Z, et al. Combination of uniform SnO2 nanocrystals with nitrogen doped graphene for high-performance lithium-ion batteries anode[J]. Chemical Engineering Journal, 2016, 283: 1435-1442. |
| 61 | Tian W, Hu H, Wang Y X, et al. Metal–organic frameworks mediated synthesis of one-dimensional molybdenum-based/carbon composites for enhanced lithium storage[J]. ACS Nano, 2018, 12(2): 1990-2000. |
| 62 | Zhao Q S, Liu J L, Li X X, et al. Graphene oxide-induced synthesis of button-shaped amorphous Fe2O3/rGO/CNFs films as flexible anode for high-performance lithium-ion batteries[J]. Chemical Engineering Journal, 2019, 369: 215-222. |
| 63 | Kou T Y, Yao B, Liu T Y, et al. Recent advances in chemical methods for activating carbon and metal oxide based electrodes for supercapacitors[J]. Journal of Materials Chemistry, 2017, 5(33): 17151-17173. |
| 64 | Bonaccorso F, Colombo L, Yu G H, et al. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage[J]. Science, 2015, 347: 1246501- 1246510. |
| 65 | Pan L, Wang Y X, Hu H, et al. 3D self-assembly synthesis of hierarchical porous carbon from petroleum asphalt for supercapacitors[J]. Carbon, 2018, 134: 345-353. |
| 66 | Guan L, Pan L, Peng T Y, et al. Green and scalable synthesis of porous carbon nanosheet-assembled hierarchical architectures for robust capacitive energy harvesting[J]. Carbon, 2019, 152: 537-544. |
| 67 | Gao F, Shao G H, Qu J Y, et al. Tailoring of porous and nitrogen-rich carbons derived from hydrochar for high-performance supercapacitor electrodes[J]. Electrochimica Acta, 2015, 155: 201-208. |
| 68 | Guan L, Pan L, Peng T Y, et al. Synthesis of biomass-derived nitrogen-doped porous carbon nanosheests for high-performance supercapacitors[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(9): 8405-8412. |
| 69 | Xia L C, Huang H, Fan Z, et al. Hierarchical macro-/meso-/microporous oxygen-doped carbon derived from sodium alginate: A cost-effective biomass material for binder-free supercapacitors[J]. Materials & Design, 2019, 182: 108048- 108056. |
| 70 | Glenis S, Nelson A J, Labes M M, et al. Sulfur doped graphite prepared via arc discharge of carbon rods in the presence of thiophenes[J]. Journal of Applied Physics, 1999, 86(8): 4464-4466. |
| 71 | Kuper C A, Labes M M. A new method of doping pyrolytic graphite utilizing laser heating in the presence of organic heteroatomic vapors[J]. Chemistry of Materials, 1999, 11(2): 408-411. |
| 72 | Lei W, Zhang H J, Liu D Z, et al. Fabrication of nitrogen and sulfur co-doped carbon nanofibers with three-dimensional architecture for high performance supercapacitors[J]. Applied Surface Science, 2019, 495: 143572- 143580. |
| 73 | 肖谧, 宿玉鹏, 杜伯学. 超级电容器研究进展[J]. 电子元件与材料, 2019, (9): 1-12. |
| Xiao M, Su Y P, Du B X. Research advance of supercapacitors[J]. Electronic Components and Materials, 2019, (9): 1-12. | |
| 74 | Pandolfo A, Hollenkamp A F. Carbon properties and their role in supercapacitors[J]. Journal of Power Sources, 2006, 157(1): 11-27. |
| 75 | 杨波, 伍俊霖, 刘文宝, 等. 商业化石墨烯材料在超级电容器中的应用研究[J]. 化工新型材料, 2019, 47(8): 220-223. |
| Yang B, Wu J L, Liu W B, et al. Application of commercialized graphene material for supercapacitor[J]. New Chemical Materials, 2019, 47(8): 220-223. | |
| 76 | Li J E, Wang Y W, Xu W N, et al. Porous Fe2O3 nanospheres anchored on activated carbon cloth for high-performance symmetric supercapacitors[J]. Nano Energy, 2019, 57: 379-387. |
| 77 | Ji H M, Liu X L, Liu Z J, et al. In situ preparation of sandwich MoO3/C hybrid nanostructures for high‐rate and ultralong‐life supercapacitors[J]. Advanced Functional Materials, 2015, 25(12): 1886-1894. |
| [1] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| [2] | 郭旭, 张永政, 夏厚兵, 杨娜, 朱真珍, 齐晶瑶. 碳基材料电氧化去除水体污染物的研究进展[J]. 化工学报, 2023, 74(5): 1862-1874. |
| [3] | 张永泉, 玄伟伟. 碱金属/(FeO+CaO+MgO)对硅酸盐灰熔渣结构和黏度的影响机理[J]. 化工学报, 2023, 74(4): 1764-1771. |
| [4] | 黄心童, 耿宇昊, 刘恒源, 陈卓, 徐建鸿. 微流控制备新型功能纳米粒子研究进展[J]. 化工学报, 2023, 74(1): 355-364. |
| [5] | 周石杰, 任祯, 杨宇森, 卫敏. 不同形貌金属氧化物的制备及其在工业催化反应中的应用[J]. 化工学报, 2021, 72(6): 2972-3001. |
| [6] | 汪艳秋,仲兆祥,邢卫红. 三维金属氧化物纳米材料的研究进展[J]. 化工学报, 2021, 72(5): 2339-2353. |
| [7] | 杨耀钧, 刁瑞, 王储, 朱锡锋. 不同金属氧化物对重质生物油再裂解的比较性研究[J]. 化工学报, 2021, 72(11): 5820-5830. |
| [8] | 卞维柏, 潘建明. 电吸附技术及吸附电极材料研究进展[J]. 化工学报, 2021, 72(1): 304-319. |
| [9] | 张伶, 陈红梅, 魏子栋. 过渡金属氧化物催化析氧反应研究进展[J]. 化工学报, 2020, 71(9): 3876-3904. |
| [10] | 张亚婷, 张博超, 张建兰, 李可可, 党永强, 段瑛峰. “自下而上”化学合成纳米石墨烯的研究进展[J]. 化工学报, 2020, 71(6): 2628-2642. |
| [11] | 董灵玉, 葛睿, 原亚飞, 唐宋元, 郝广平, 陆安慧. 多孔炭基二氧化碳电催化材料研究进展[J]. 化工学报, 2020, 71(6): 2492-2509. |
| [12] | 周宇, 王宇新. 杂原子掺杂碳基氧还原反应电催化剂研究进展[J]. 化工学报, 2017, 68(2): 519-534. |
| [13] | 王泽, 史婉君, 宋文立, 李松庚. 金属氧化物对油页岩热解产物收率及组成分布的影响[J]. 化工学报, 2017, 68(10): 3884-3891. |
| [14] | 顾欧昀, 廖永涛, 陈锐杰, 贾璐, 龟山秀雄, 林益, 周吕, 马华, 郭燏. 铜锰复合氧化物催化剂上甲苯的催化燃烧[J]. 化工学报, 2016, 67(7): 2832-2840. |
| [15] | 黄兴, 张筱娴, 帅永, 袁远, 李炳熙. 铁基氧化物微颗粒的光谱辐射特性[J]. CIESC Journal, 2015, 66(S1): 308-313. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号