化工学报 ›› 2020, Vol. 71 ›› Issue (7): 3009-3017.DOI: 10.11949/0438-1157.20191001
杨冲1,2( ),林旭枫1,2,张金锋1,2,陈宏1,2,肖业鹏1,2,王慧1,2,程丽华1,2(
),林旭枫1,2,张金锋1,2,陈宏1,2,肖业鹏1,2,王慧1,2,程丽华1,2( ),欧阳新平3
),欧阳新平3
收稿日期:2019-09-06
修回日期:2020-04-27
出版日期:2020-07-05
发布日期:2020-07-05
通讯作者:
程丽华
作者简介:杨冲(1988—),男,博士,讲师,基金资助:
Chong YANG1,2( ),Xufeng LIN1,2,Jinfeng ZHANG1,2,Hong CHEN1,2,Yepeng XIAO1,2,Hui WANG1,2,Lihua CHENG1,2(
),Xufeng LIN1,2,Jinfeng ZHANG1,2,Hong CHEN1,2,Yepeng XIAO1,2,Hui WANG1,2,Lihua CHENG1,2( ),Xinping OUYANG3
),Xinping OUYANG3
Received:2019-09-06
Revised:2020-04-27
Online:2020-07-05
Published:2020-07-05
Contact:
Lihua CHENG
摘要:
为了给溶剂萃取分离正己烷–异丙醇混合物的过程设计和流程模拟计算提供基础数据,以二甲亚砜、1,4-丁二醇、1,2-丙二醇、乙腈、糠醛和N,N-二甲基甲酰胺为萃取剂,测定了30℃下萃取分离正己烷–异丙醇混合物的液液相平衡数据,并利用分离因子评价不同萃取剂的萃取分离性能。根据Hand方程对实验数据进行一致性和可靠性检验,拟合方程的相关系数都在0.98以上。随后,采用Aspen Plus软件选取NRTL活度系数模型对相平衡数据进行关联,得到相应的二元交互作用参数,并以此计算相应的相平衡组成。结果显示,计算值和实验值的均方根偏差小于1.0%,表明NRTL模型能够准确描述三元体系的液液相平衡。
中图分类号:
杨冲, 林旭枫, 张金锋, 陈宏, 肖业鹏, 王慧, 程丽华, 欧阳新平. 正己烷–异丙醇共沸体系液液相平衡数据测定及关联[J]. 化工学报, 2020, 71(7): 3009-3017.
Chong YANG, Xufeng LIN, Jinfeng ZHANG, Hong CHEN, Yepeng XIAO, Hui WANG, Lihua CHENG, Xinping OUYANG. Measurement and correlation of liquid-liquid equilibrium data for n-hexane- isopropanol azeotropic system[J]. CIESC Journal, 2020, 71(7): 3009-3017.
| 试剂名称 | CAS① | 纯度/%(mass) | 生产厂家 |
|---|---|---|---|
| 正己烷 | 110-54-3 | >99 | 上海阿拉丁生化科技股份有限公司 |
| 异丙醇 | 67-63-0 | ≥99.7 | 国药集团化学试剂有限公司 |
| 二甲亚砜 | 67-68-5 | ≥99.0 | 国药集团化学试剂有限公司 |
| 1,4-丁二醇 | 110-63-4 | ≥99.0 | 国药集团化学试剂有限公司 |
| 1,2-丙二醇 | 57-55-6 | ≥99.0 | 国药集团化学试剂有限公司 |
| 乙腈 | 75-05-8 | ≥99.0 | 国药集团化学试剂有限公司 |
| 糠醛 | 98-01-1 | ≥99.0 | 国药集团化学试剂有限公司 |
| N,N-二甲基甲酰胺 | 68-12-2 | ≥99.5 | 国药集团化学试剂有限公司 |
| 1,4-二氧六环 | 123-91-1 | ≥99.0 | 上海阿拉丁生化科技股份有限公司 |
| 无水乙醇 | 64-17-5 | ≥99.7 | 广州市东红实业发展有限公司 |
| 氢气 | —— | 99.999 | 茂名市民兴气体有限公司 |
表1 实验试剂
Table 1 Experimental reagents
| 试剂名称 | CAS① | 纯度/%(mass) | 生产厂家 |
|---|---|---|---|
| 正己烷 | 110-54-3 | >99 | 上海阿拉丁生化科技股份有限公司 |
| 异丙醇 | 67-63-0 | ≥99.7 | 国药集团化学试剂有限公司 |
| 二甲亚砜 | 67-68-5 | ≥99.0 | 国药集团化学试剂有限公司 |
| 1,4-丁二醇 | 110-63-4 | ≥99.0 | 国药集团化学试剂有限公司 |
| 1,2-丙二醇 | 57-55-6 | ≥99.0 | 国药集团化学试剂有限公司 |
| 乙腈 | 75-05-8 | ≥99.0 | 国药集团化学试剂有限公司 |
| 糠醛 | 98-01-1 | ≥99.0 | 国药集团化学试剂有限公司 |
| N,N-二甲基甲酰胺 | 68-12-2 | ≥99.5 | 国药集团化学试剂有限公司 |
| 1,4-二氧六环 | 123-91-1 | ≥99.0 | 上海阿拉丁生化科技股份有限公司 |
| 无水乙醇 | 64-17-5 | ≥99.7 | 广州市东红实业发展有限公司 |
| 氢气 | —— | 99.999 | 茂名市民兴气体有限公司 |
| 仪器名称 | 型号 | 生产厂家 |
|---|---|---|
| 气相色谱仪 | GC9790 | 浙江福立分析仪器有限公司 |
| 电子天平 | CP512 | 广州泸瑞明仪器有限公司 |
| 低温恒温水槽 | DC-3010 | 上海比朗仪器制造有限公司 |
| 烘箱 | DHG-9076A | 上海精宏实验设备有限公司 |
| 磁力搅拌器 | FK-2A | 腾科科学仪器有限公司 |
| 微量进样器 | 1 μl | 上海高鸽工贸有限公司 |
表2 实验仪器
Table 2 Experimental equipments
| 仪器名称 | 型号 | 生产厂家 |
|---|---|---|
| 气相色谱仪 | GC9790 | 浙江福立分析仪器有限公司 |
| 电子天平 | CP512 | 广州泸瑞明仪器有限公司 |
| 低温恒温水槽 | DC-3010 | 上海比朗仪器制造有限公司 |
| 烘箱 | DHG-9076A | 上海精宏实验设备有限公司 |
| 磁力搅拌器 | FK-2A | 腾科科学仪器有限公司 |
| 微量进样器 | 1 μl | 上海高鸽工贸有限公司 |
| Extraction phase | Raffinate phase | S | ||||
|---|---|---|---|---|---|---|
| w1 | w2 | w3 | w1 | w2 | w3 | |
| 0.0182 | 0 | 0.9818 | 0.9984 | 0 | 0.0016 | — |
| 0.0246 | 0.0518 | 0.9236 | 0.9818 | 0.0026 | 0.0156 | 795.14 |
| 0.0429 | 0.1040 | 0.8531 | 0.9757 | 0.0064 | 0.0179 | 369.58 |
| 0.0630 | 0.1566 | 0.7804 | 0.9644 | 0.0118 | 0.0238 | 203.15 |
| 0.0888 | 0.1998 | 0.7114 | 0.9505 | 0.0187 | 0.0308 | 114.36 |
| 0.1104 | 0.2409 | 0.6487 | 0.9410 | 0.0271 | 0.0319 | 75.77 |
| 0.1675 | 0.2691 | 0.5634 | 0.9086 | 0.0414 | 0.0500 | 35.26 |
| 0.2272 | 0.2953 | 0.4775 | 0.8825 | 0.0592 | 0.0583 | 19.38 |
表3 在30℃和101325 Pa下,正己烷(1)-异丙醇(2)-二甲基亚砜(3)三元体系的液液相平衡数据
Table 3 LLE data for n-hexane (1) - isopropanol (2) - dimethyl sulfoxide (3) ternary system at 30℃ and 101325 Pa
| Extraction phase | Raffinate phase | S | ||||
|---|---|---|---|---|---|---|
| w1 | w2 | w3 | w1 | w2 | w3 | |
| 0.0182 | 0 | 0.9818 | 0.9984 | 0 | 0.0016 | — |
| 0.0246 | 0.0518 | 0.9236 | 0.9818 | 0.0026 | 0.0156 | 795.14 |
| 0.0429 | 0.1040 | 0.8531 | 0.9757 | 0.0064 | 0.0179 | 369.58 |
| 0.0630 | 0.1566 | 0.7804 | 0.9644 | 0.0118 | 0.0238 | 203.15 |
| 0.0888 | 0.1998 | 0.7114 | 0.9505 | 0.0187 | 0.0308 | 114.36 |
| 0.1104 | 0.2409 | 0.6487 | 0.9410 | 0.0271 | 0.0319 | 75.77 |
| 0.1675 | 0.2691 | 0.5634 | 0.9086 | 0.0414 | 0.0500 | 35.26 |
| 0.2272 | 0.2953 | 0.4775 | 0.8825 | 0.0592 | 0.0583 | 19.38 |
| Extraction phase | Raffinate phase | S | ||||
|---|---|---|---|---|---|---|
| w1 | w2 | w3 | w1 | w2 | w3 | |
| 0.0171 | 0 | 0.9829 | 0.9998 | 0 | 0.0002 | — |
| 0.0220 | 0.0572 | 0.9208 | 0.9949 | 0.0037 | 0.0014 | 699.12 |
| 0.0297 | 0.1149 | 0.8554 | 0.9925 | 0.0068 | 0.0007 | 564.66 |
| 0.0350 | 0.1673 | 0.7977 | 0.9860 | 0.0124 | 0.0016 | 380.09 |
| 0.0589 | 0.2721 | 0.6690 | 0.9698 | 0.0253 | 0.0049 | 177.08 |
| 0.0763 | 0.3185 | 0.6052 | 0.9449 | 0.0436 | 0.0115 | 90.47 |
| 0.1007 | 0.3665 | 0.5328 | 0.9324 | 0.0542 | 0.0134 | 62.61 |
| 0.1281 | 0.4035 | 0.4684 | 0.9108 | 0.0739 | 0.0153 | 38.82 |
| 0.1678 | 0.4353 | 0.3969 | 0.8530 | 0.1196 | 0.0274 | 18.50 |
表4 在30℃和101325 Pa下,正己烷(1)-异丙醇(2)-1,4-丁二醇(3)三元体系的液液相平衡数据
Table 4 LLE data for n-hexane (1) – isopropanol (2) - 1,4-butanediol (3) ternary system at 30℃ and 101325 Pa
| Extraction phase | Raffinate phase | S | ||||
|---|---|---|---|---|---|---|
| w1 | w2 | w3 | w1 | w2 | w3 | |
| 0.0171 | 0 | 0.9829 | 0.9998 | 0 | 0.0002 | — |
| 0.0220 | 0.0572 | 0.9208 | 0.9949 | 0.0037 | 0.0014 | 699.12 |
| 0.0297 | 0.1149 | 0.8554 | 0.9925 | 0.0068 | 0.0007 | 564.66 |
| 0.0350 | 0.1673 | 0.7977 | 0.9860 | 0.0124 | 0.0016 | 380.09 |
| 0.0589 | 0.2721 | 0.6690 | 0.9698 | 0.0253 | 0.0049 | 177.08 |
| 0.0763 | 0.3185 | 0.6052 | 0.9449 | 0.0436 | 0.0115 | 90.47 |
| 0.1007 | 0.3665 | 0.5328 | 0.9324 | 0.0542 | 0.0134 | 62.61 |
| 0.1281 | 0.4035 | 0.4684 | 0.9108 | 0.0739 | 0.0153 | 38.82 |
| 0.1678 | 0.4353 | 0.3969 | 0.8530 | 0.1196 | 0.0274 | 18.50 |
| Extraction phase | Raffinate phase | S | ||||
|---|---|---|---|---|---|---|
| w1 | w2 | w3 | w1 | w2 | w3 | |
| 0.0208 | 0 | 0.9792 | 1 | 0 | < 1.0-4 | — |
| 0.0316 | 0.0726 | 0.8958 | 0.9953 | 0.0034 | 0.0013 | 672.55 |
| 0.0467 | 0.1358 | 0.8175 | 0.9894 | 0.0083 | 0.0023 | 346.64 |
| 0.0677 | 0.1962 | 0.7361 | 0.9794 | 0.0157 | 0.0049 | 180.79 |
| 0.0947 | 0.2462 | 0.6591 | 0.9614 | 0.0277 | 0.0109 | 90.23 |
| 0.1185 | 0.2920 | 0.5895 | 0.9356 | 0.0452 | 0.0192 | 51.01 |
| 0.1654 | 0.3265 | 0.5081 | 0.8984 | 0.0696 | 0.0320 | 25.48 |
| 0.2669 | 0.3711 | 0.3620 | 0.7995 | 0.1364 | 0.0641 | 8.15 |
表5 在30℃和101325 Pa下,正己烷(1)-异丙醇(2)-1,2-丙二醇(3)三元体系的液液相平衡数据
Table 5 LLE data for n-hexane (1) – isopropanol (2) - 1,2-propanediol (3) ternary system at 30℃ and 101325 Pa
| Extraction phase | Raffinate phase | S | ||||
|---|---|---|---|---|---|---|
| w1 | w2 | w3 | w1 | w2 | w3 | |
| 0.0208 | 0 | 0.9792 | 1 | 0 | < 1.0-4 | — |
| 0.0316 | 0.0726 | 0.8958 | 0.9953 | 0.0034 | 0.0013 | 672.55 |
| 0.0467 | 0.1358 | 0.8175 | 0.9894 | 0.0083 | 0.0023 | 346.64 |
| 0.0677 | 0.1962 | 0.7361 | 0.9794 | 0.0157 | 0.0049 | 180.79 |
| 0.0947 | 0.2462 | 0.6591 | 0.9614 | 0.0277 | 0.0109 | 90.23 |
| 0.1185 | 0.2920 | 0.5895 | 0.9356 | 0.0452 | 0.0192 | 51.01 |
| 0.1654 | 0.3265 | 0.5081 | 0.8984 | 0.0696 | 0.0320 | 25.48 |
| 0.2669 | 0.3711 | 0.3620 | 0.7995 | 0.1364 | 0.0641 | 8.15 |
| Extraction phase | Raffinate phase | S | ||||
|---|---|---|---|---|---|---|
| w1 | w2 | w3 | w1 | w2 | w3 | |
| 0.1452 | 0 | 0.8548 | 0.9628 | 0 | 0.0372 | — |
| 0.1912 | 0.0477 | 0.7611 | 0.9545 | 0.0068 | 0.0387 | 35.02 |
| 0.2226 | 0.0891 | 0.6883 | 0.9347 | 0.0160 | 0.0493 | 23.38 |
| 0.2311 | 0.1321 | 0.6368 | 0.9286 | 0.0259 | 0.0455 | 20.49 |
| 0.2556 | 0.1695 | 0.5749 | 0.9100 | 0.0414 | 0.0486 | 14.58 |
| 0.3049 | 0.1982 | 0.4969 | 0.8628 | 0.0668 | 0.0704 | 8.40 |
| 0.2941 | 0.2472 | 0.4587 | 0.8164 | 0.0998 | 0.0838 | 6.88 |
表6 在30℃和101325 Pa下,正己烷(1)-异丙醇(2)-乙腈(3)三元体系的液液相平衡数据
Table 6 LLE data for n-hexane (1) - isopropanol (2) - acetonitrile (3) ternary system at 30℃ and 101325 Pa
| Extraction phase | Raffinate phase | S | ||||
|---|---|---|---|---|---|---|
| w1 | w2 | w3 | w1 | w2 | w3 | |
| 0.1452 | 0 | 0.8548 | 0.9628 | 0 | 0.0372 | — |
| 0.1912 | 0.0477 | 0.7611 | 0.9545 | 0.0068 | 0.0387 | 35.02 |
| 0.2226 | 0.0891 | 0.6883 | 0.9347 | 0.0160 | 0.0493 | 23.38 |
| 0.2311 | 0.1321 | 0.6368 | 0.9286 | 0.0259 | 0.0455 | 20.49 |
| 0.2556 | 0.1695 | 0.5749 | 0.9100 | 0.0414 | 0.0486 | 14.58 |
| 0.3049 | 0.1982 | 0.4969 | 0.8628 | 0.0668 | 0.0704 | 8.40 |
| 0.2941 | 0.2472 | 0.4587 | 0.8164 | 0.0998 | 0.0838 | 6.88 |
| Extraction phase | Raffinate phase | S | ||||
|---|---|---|---|---|---|---|
| w1 | w2 | w3 | w1 | w2 | w3 | |
| 0.0767 | 0 | 0.9233 | 0.9307 | 0 | 0.0693 | — |
| 0.0960 | 0.0374 | 0.8666 | 0.9130 | 0.0113 | 0.0757 | 31.48 |
| 0.0981 | 0.0769 | 0.8250 | 0.8843 | 0.0254 | 0.0903 | 27.29 |
| 0.1335 | 0.1089 | 0.7576 | 0.8465 | 0.0469 | 0.1066 | 14.72 |
| 0.1339 | 0.1488 | 0.7173 | 0.7911 | 0.0785 | 0.1304 | 11.20 |
| 0.1997 | 0.1804 | 0.6199 | 0.7249 | 0.1141 | 0.1610 | 5.74 |
| 0.2456 | 0.2098 | 0.5446 | 0.6430 | 0.1574 | 0.1996 | 3.49 |
表7 在30℃和101325 Pa下,正己烷(1)-异丙醇(2)-糠醛(3)三元体系的液液相平衡数据
Table 7 LLE data for n-hexane (1) - isopropanol (2) - furfural (3) ternary system at 30℃ and 101325 Pa
| Extraction phase | Raffinate phase | S | ||||
|---|---|---|---|---|---|---|
| w1 | w2 | w3 | w1 | w2 | w3 | |
| 0.0767 | 0 | 0.9233 | 0.9307 | 0 | 0.0693 | — |
| 0.0960 | 0.0374 | 0.8666 | 0.9130 | 0.0113 | 0.0757 | 31.48 |
| 0.0981 | 0.0769 | 0.8250 | 0.8843 | 0.0254 | 0.0903 | 27.29 |
| 0.1335 | 0.1089 | 0.7576 | 0.8465 | 0.0469 | 0.1066 | 14.72 |
| 0.1339 | 0.1488 | 0.7173 | 0.7911 | 0.0785 | 0.1304 | 11.20 |
| 0.1997 | 0.1804 | 0.6199 | 0.7249 | 0.1141 | 0.1610 | 5.74 |
| 0.2456 | 0.2098 | 0.5446 | 0.6430 | 0.1574 | 0.1996 | 3.49 |
| Extraction phase | Raffinate phase | S | ||||
|---|---|---|---|---|---|---|
| w1 | w2 | w3 | w1 | w2 | w3 | |
| 0.1448 | 0 | 0.8552 | 0.9064 | 0 | 0.0936 | — |
| 0.1627 | 0.0543 | 0.7830 | 0.8920 | 0.0098 | 0.0982 | 30.38 |
| 0.2368 | 0.1012 | 0.6620 | 0.8569 | 0.0261 | 0.1170 | 14.03 |
| 0.3506 | 0.1272 | 0.5222 | 0.7996 | 0.0476 | 0.1528 | 6.09 |
表8 在30℃和101325 Pa下,正己烷(1)-异丙醇(2)-N,N-二甲基甲酰胺(3)三元体系的液液相平衡数据
Table 8 LLE data for n-hexane (1) - isopropanol (2) -N,N-dimethylformamide (3) ternary system at 30℃ and 101325 Pa
| Extraction phase | Raffinate phase | S | ||||
|---|---|---|---|---|---|---|
| w1 | w2 | w3 | w1 | w2 | w3 | |
| 0.1448 | 0 | 0.8552 | 0.9064 | 0 | 0.0936 | — |
| 0.1627 | 0.0543 | 0.7830 | 0.8920 | 0.0098 | 0.0982 | 30.38 |
| 0.2368 | 0.1012 | 0.6620 | 0.8569 | 0.0261 | 0.1170 | 14.03 |
| 0.3506 | 0.1272 | 0.5222 | 0.7996 | 0.0476 | 0.1528 | 6.09 |
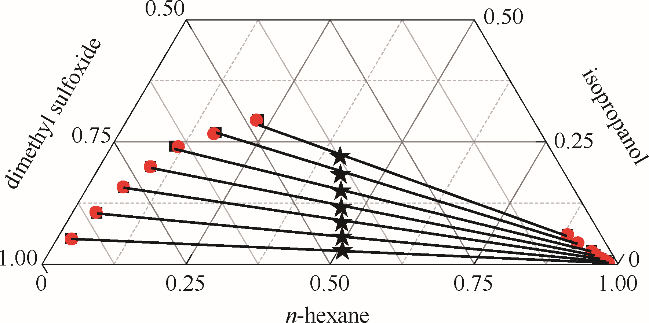
图1 正己烷-异丙醇-二甲亚砜三元体系的液液平衡相图
Fig.1 LLE phase diagram of n-hexane - isopropanol - dimethyl sulfoxide ternary system★ feed compositions; ■ experimental data; ● calculated values using NRTL model

图2 正己烷-异丙醇-1,4-丁二醇三元体系的液液平衡相图
Fig.2 LLE phase diagram of n-hexane - isopropanol - 1,4-butanediol ternary system★ feed compositions; ■ experimental data; ● calculated values using NRTL model
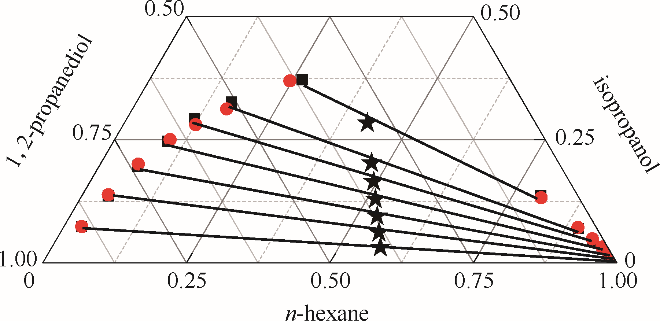
图3 正己烷-异丙醇-1,2-丙二醇三元体系的液液平衡相图
Fig.3 LLE phase diagram of n-hexane - isopropanol - 1,2-propanediol ternary system★ feed compositions; ■ experimental data; ● calculated values using NRTL model
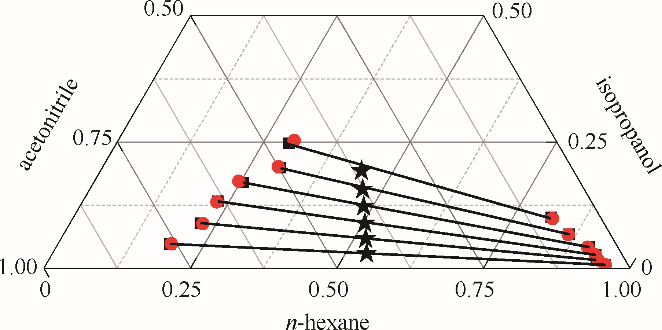
图4 正己烷-异丙醇-乙腈三元体系的液液平衡相图
Fig. 4 LLE phase diagram of n-hexane - isopropanol - acetonitrile ternary system★ feed compositions; ■ experimental data; ● calculated values using NRTL model
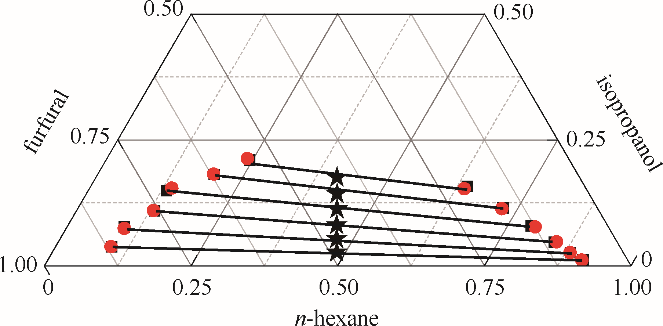
图5 正己烷-异丙醇-糠醛三元体系的液液平衡相图
Fig.5 LLE phase diagram of n-hexane - isopropanol - furfural ternary system★ feed compositions; ■ experimental data; ● calculated values using NRTL model
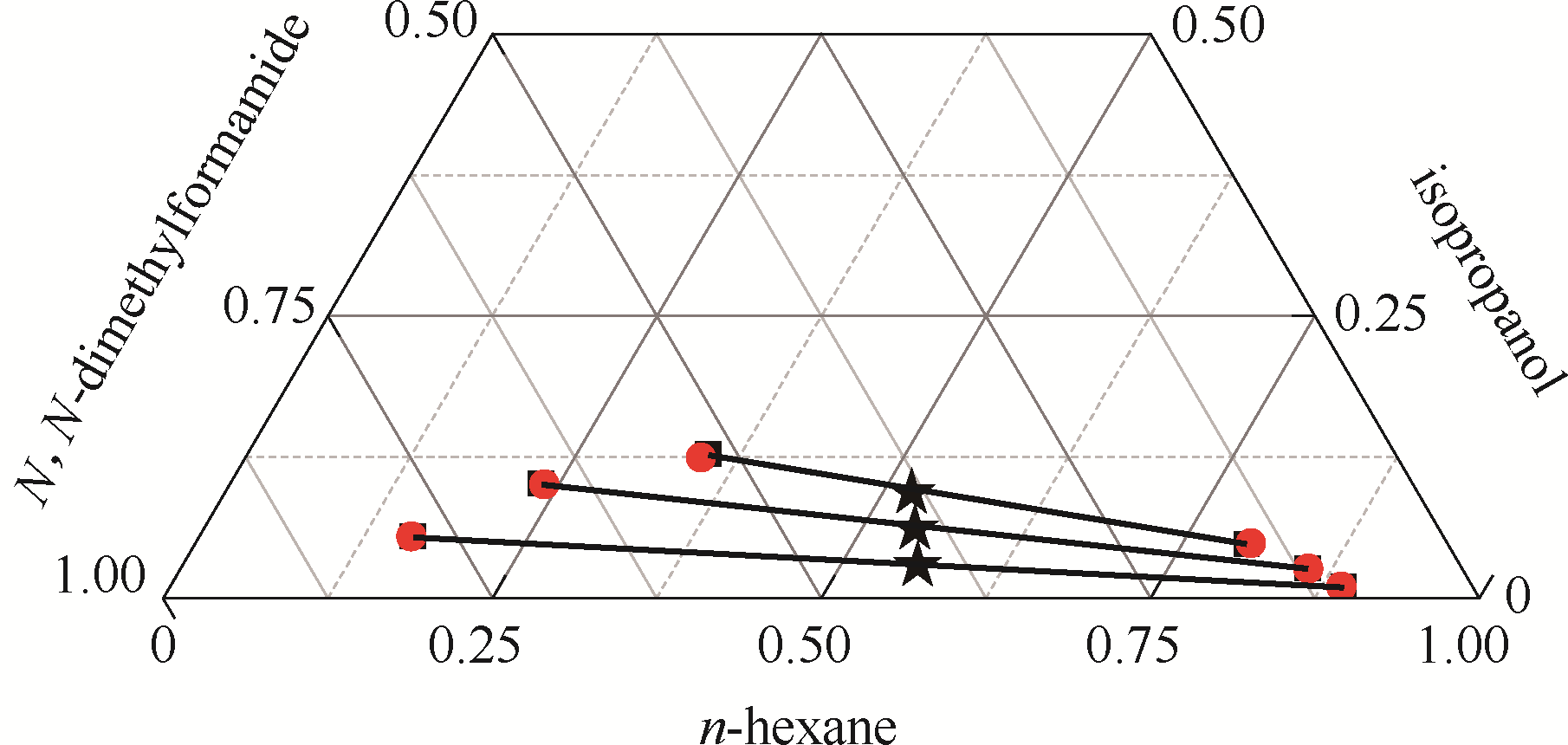
图6 正己烷–异丙醇-N,N-二甲基甲酰胺三元体系的液液平衡相图
Fig.6 LLE phase diagram of n-hexane - isopropanol - N,N-dimethylformamide ternary system★ feed compositions; ■ experimental data; ● calculated values using NRTL model
| System | a | b | R2 |
|---|---|---|---|
| n-hexane-isopropanol-dimethyl sulfoxide | 1.5843 | 0.7391 | 0.9947 |
| n-hexane-isopropanol-1,4-butanediol | 1.7891 | 0.7805 | 0.9842 |
| n-hexane-isopropanol-1,2-propanediol | 1.2155 | 0.6371 | 0.9929 |
| n-hexane-isopropanol-acetonitrile | 1.0470 | 0.7584 | 0.9920 |
| n-hexane-isopropanol-furfural | 0.0981 | 0.7197 | 0.9947 |
| n-hexane-isopropanol-N,N-dimethylformamide | 0.7054 | 0.7461 | 0.9991 |
表9 Hand方程拟合参数
Table 9 Fitting parameters in Hand equations
| System | a | b | R2 |
|---|---|---|---|
| n-hexane-isopropanol-dimethyl sulfoxide | 1.5843 | 0.7391 | 0.9947 |
| n-hexane-isopropanol-1,4-butanediol | 1.7891 | 0.7805 | 0.9842 |
| n-hexane-isopropanol-1,2-propanediol | 1.2155 | 0.6371 | 0.9929 |
| n-hexane-isopropanol-acetonitrile | 1.0470 | 0.7584 | 0.9920 |
| n-hexane-isopropanol-furfural | 0.0981 | 0.7197 | 0.9947 |
| n-hexane-isopropanol-N,N-dimethylformamide | 0.7054 | 0.7461 | 0.9991 |
| Extraction phase | Raffinate phase | ||||
|---|---|---|---|---|---|
| w1 | w2 | w3 | w1 | w2 | w3 |
| 0.0256 | 0.0510 | 0.9234 | 0.9820 | 0.0026 | 0.0154 |
| 0.0405 | 0.1063 | 0.8532 | 0.9748 | 0.0065 | 0.0187 |
| 0.0623 | 0.1578 | 0.7799 | 0.9644 | 0.0119 | 0.0237 |
| 0.0890 | 0.1988 | 0.7122 | 0.9510 | 0.0190 | 0.0300 |
| 0.1163 | 0.2399 | 0.6438 | 0.9417 | 0.0255 | 0.0328 |
| 0.1650 | 0.2667 | 0.5683 | 0.9088 | 0.0429 | 0.0483 |
| 0.2252 | 0.2943 | 0.4805 | 0.8822 | 0.0597 | 0.0581 |
表10 在30℃和101325 Pa下,利用NRTL方程计算的正己烷(1)-异丙醇(2)-二甲基亚砜(3)三元体系的液液相平衡数据
Table 10 Calculated LLE data using NRTL equation for n-hexane (1) - isopropanol (2) - dimethyl sulfoxide (3) ternary system at 30℃ and 101325 Pa
| Extraction phase | Raffinate phase | ||||
|---|---|---|---|---|---|
| w1 | w2 | w3 | w1 | w2 | w3 |
| 0.0256 | 0.0510 | 0.9234 | 0.9820 | 0.0026 | 0.0154 |
| 0.0405 | 0.1063 | 0.8532 | 0.9748 | 0.0065 | 0.0187 |
| 0.0623 | 0.1578 | 0.7799 | 0.9644 | 0.0119 | 0.0237 |
| 0.0890 | 0.1988 | 0.7122 | 0.9510 | 0.0190 | 0.0300 |
| 0.1163 | 0.2399 | 0.6438 | 0.9417 | 0.0255 | 0.0328 |
| 0.1650 | 0.2667 | 0.5683 | 0.9088 | 0.0429 | 0.0483 |
| 0.2252 | 0.2943 | 0.4805 | 0.8822 | 0.0597 | 0.0581 |
| Extraction phase | Raffinate phase | ||||
|---|---|---|---|---|---|
| w1 | w2 | w3 | w1 | w2 | w3 |
| 0.0216 | 0.0585 | 0.9199 | 0.9950 | 0.0038 | 0.0012 |
| 0.0258 | 0.1207 | 0.8535 | 0.9929 | 0.0062 | 0.0009 |
| 0.0380 | 0.1613 | 0.8007 | 0.9863 | 0.0121 | 0.0016 |
| 0.0661 | 0.2631 | 0.6708 | 0.9655 | 0.0303 | 0.0042 |
| 0.0836 | 0.3211 | 0.5953 | 0.9381 | 0.0515 | 0.0104 |
| 0.0999 | 0.3457 | 0.5544 | 0.9272 | 0.0600 | 0.0128 |
| 0.1199 | 0.3775 | 0.5026 | 0.9130 | 0.0711 | 0.0159 |
| 0.1510 | 0.4524 | 0.3966 | 0.8615 | 0.1094 | 0.0291 |
表11 在30℃和101325 Pa下,利用NRTL方程计算的正己烷(1)-异丙醇(2)-1,4-丁二醇(3)三元体系的液液相平衡数据
Table 11 Calculated LLE data using NRTL equation for n-hexane (1) - isopropanol (2) - 1,4-butanediol (3) ternary system at 30℃ and 101325 Pa
| Extraction phase | Raffinate phase | ||||
|---|---|---|---|---|---|
| w1 | w2 | w3 | w1 | w2 | w3 |
| 0.0216 | 0.0585 | 0.9199 | 0.9950 | 0.0038 | 0.0012 |
| 0.0258 | 0.1207 | 0.8535 | 0.9929 | 0.0062 | 0.0009 |
| 0.0380 | 0.1613 | 0.8007 | 0.9863 | 0.0121 | 0.0016 |
| 0.0661 | 0.2631 | 0.6708 | 0.9655 | 0.0303 | 0.0042 |
| 0.0836 | 0.3211 | 0.5953 | 0.9381 | 0.0515 | 0.0104 |
| 0.0999 | 0.3457 | 0.5544 | 0.9272 | 0.0600 | 0.0128 |
| 0.1199 | 0.3775 | 0.5026 | 0.9130 | 0.0711 | 0.0159 |
| 0.1510 | 0.4524 | 0.3966 | 0.8615 | 0.1094 | 0.0291 |
| Extraction phase | Raffinate phase | ||||
|---|---|---|---|---|---|
| w1 | w2 | w3 | w1 | w2 | w3 |
| 0.0313 | 0.0736 | 0.8951 | 0.9952 | 0.0033 | 0.0015 |
| 0.0451 | 0.1382 | 0.8167 | 0.9893 | 0.0084 | 0.0023 |
| 0.0663 | 0.2004 | 0.7333 | 0.9782 | 0.0174 | 0.0044 |
| 0.0971 | 0.2498 | 0.6531 | 0.9582 | 0.0320 | 0.0098 |
| 0.1266 | 0.2799 | 0.5935 | 0.9331 | 0.0486 | 0.0183 |
| 0.1645 | 0.3119 | 0.5236 | 0.8966 | 0.0718 | 0.0316 |
| 0.2464 | 0.3692 | 0.3844 | 0.8023 | 0.1319 | 0.0658 |
表12 在30℃和101325 Pa下,利用NRTL方程计算的正己烷(1)-异丙醇(2)-1,2-丙二醇(3)三元体系的液液相平衡数据
Table 12 Calculated LLE data using NRTL equation for n-hexane (1) - isopropanol (2) - 1,2-propanediol (3) ternary system at 30℃ and 101325 Pa
| Extraction phase | Raffinate phase | ||||
|---|---|---|---|---|---|
| w1 | w2 | w3 | w1 | w2 | w3 |
| 0.0313 | 0.0736 | 0.8951 | 0.9952 | 0.0033 | 0.0015 |
| 0.0451 | 0.1382 | 0.8167 | 0.9893 | 0.0084 | 0.0023 |
| 0.0663 | 0.2004 | 0.7333 | 0.9782 | 0.0174 | 0.0044 |
| 0.0971 | 0.2498 | 0.6531 | 0.9582 | 0.0320 | 0.0098 |
| 0.1266 | 0.2799 | 0.5935 | 0.9331 | 0.0486 | 0.0183 |
| 0.1645 | 0.3119 | 0.5236 | 0.8966 | 0.0718 | 0.0316 |
| 0.2464 | 0.3692 | 0.3844 | 0.8023 | 0.1319 | 0.0658 |
| Extraction phase | Raffinate phase | ||||
|---|---|---|---|---|---|
| w1 | w2 | w3 | w1 | w2 | w3 |
| 0.1933 | 0.0481 | 0.7586 | 0.9547 | 0.0067 | 0.0386 |
| 0.2273 | 0.0877 | 0.6850 | 0.9374 | 0.0162 | 0.0464 |
| 0.2301 | 0.1307 | 0.6392 | 0.9282 | 0.0261 | 0.0457 |
| 0.2469 | 0.1717 | 0.5814 | 0.9076 | 0.0409 | 0.0515 |
| 0.2995 | 0.2009 | 0.4996 | 0.8619 | 0.0663 | 0.0718 |
| 0.3012 | 0.2522 | 0.4466 | 0.8192 | 0.0975 | 0.0833 |
表13 在30℃和101325 Pa下,利用NRTL方程计算的正己烷(1)-异丙醇(2)-乙腈(3)三元体系的液液相平衡数据
Table 13 Calculated LLE data using NRTL equation for n-hexane (1) - isopropanol (2) - acetonitrile (3) ternary system at 30℃ and 101325 Pa
| Extraction phase | Raffinate phase | ||||
|---|---|---|---|---|---|
| w1 | w2 | w3 | w1 | w2 | w3 |
| 0.1933 | 0.0481 | 0.7586 | 0.9547 | 0.0067 | 0.0386 |
| 0.2273 | 0.0877 | 0.6850 | 0.9374 | 0.0162 | 0.0464 |
| 0.2301 | 0.1307 | 0.6392 | 0.9282 | 0.0261 | 0.0457 |
| 0.2469 | 0.1717 | 0.5814 | 0.9076 | 0.0409 | 0.0515 |
| 0.2995 | 0.2009 | 0.4996 | 0.8619 | 0.0663 | 0.0718 |
| 0.3012 | 0.2522 | 0.4466 | 0.8192 | 0.0975 | 0.0833 |
| Extraction phase | Raffinate phase | ||||
|---|---|---|---|---|---|
| w1 | w2 | w3 | w1 | w2 | w3 |
| 0.0949 | 0.0377 | 0.8674 | 0.9107 | 0.0112 | 0.0781 |
| 0.0995 | 0.0739 | 0.8266 | 0.8835 | 0.0260 | 0.0905 |
| 0.1312 | 0.1095 | 0.7593 | 0.8495 | 0.0476 | 0.1029 |
| 0.1404 | 0.1540 | 0.7056 | 0.7981 | 0.0773 | 0.1246 |
| 0.1975 | 0.1820 | 0.6205 | 0.7235 | 0.1130 | 0.1635 |
| 0.2401 | 0.2126 | 0.5473 | 0.6395 | 0.1528 | 0.2077 |
表14 在30℃和101325 Pa下,利用NRTL方程计算的正己烷(1)-异丙醇(2)-糠醛(3)三元体系的液液相平衡数据
Table 14 Calculated LLE data using NRTL equation for n-hexane (1) - isopropanol (2) - furfural (3) ternary system at 30℃ and 101325 Pa
| Extraction phase | Raffinate phase | ||||
|---|---|---|---|---|---|
| w1 | w2 | w3 | w1 | w2 | w3 |
| 0.0949 | 0.0377 | 0.8674 | 0.9107 | 0.0112 | 0.0781 |
| 0.0995 | 0.0739 | 0.8266 | 0.8835 | 0.0260 | 0.0905 |
| 0.1312 | 0.1095 | 0.7593 | 0.8495 | 0.0476 | 0.1029 |
| 0.1404 | 0.1540 | 0.7056 | 0.7981 | 0.0773 | 0.1246 |
| 0.1975 | 0.1820 | 0.6205 | 0.7235 | 0.1130 | 0.1635 |
| 0.2401 | 0.2126 | 0.5473 | 0.6395 | 0.1528 | 0.2077 |
| Extraction phase | Raffinate phase | ||||
|---|---|---|---|---|---|
| w1 | w2 | w3 | w1 | w2 | w3 |
| 0.1616 | 0.0546 | 0.7838 | 0.8905 | 0.0098 | 0.0997 |
| 0.2391 | 0.1008 | 0.6601 | 0.8573 | 0.0260 | 0.1167 |
| 0.3467 | 0.1242 | 0.5291 | 0.8021 | 0.0487 | 0.1492 |
表15 在30℃和101325 Pa下,利用NRTL方程计算的正己烷(1)-异丙醇(2)-N,N-二甲基甲酰胺(3)三元体系的液液相平衡数据
Table 15 Calculated LLE data using NRTL equation for n-hexane (1) - isopropanol (2) -N,N-dimethylformamide (3) ternary system at 30℃ and 101325 Pa
| Extraction phase | Raffinate phase | ||||
|---|---|---|---|---|---|
| w1 | w2 | w3 | w1 | w2 | w3 |
| 0.1616 | 0.0546 | 0.7838 | 0.8905 | 0.0098 | 0.0997 |
| 0.2391 | 0.1008 | 0.6601 | 0.8573 | 0.0260 | 0.1167 |
| 0.3467 | 0.1242 | 0.5291 | 0.8021 | 0.0487 | 0.1492 |
| Solvent | i | j | Δgij/ (J·mol-1) | Δgji/ (J·mol-1) | αij | RMSD/% |
|---|---|---|---|---|---|---|
| dimethyl sulfoxide | 1 | 2 | -56284.05 | 968.83 | 0.20 | 0.18 |
| 1 | 3 | 6854.25 | 6761.20 | 0.20 | ||
| 2 | 3 | -1112.04 | -63742.32 | 0.30 | ||
| 1,4-butanediol | 1 | 2 | 1068.26 | 5991.22 | 0.20 | 0.94 |
| 1 | 3 | 15068.36 | 9058.82 | 0.29 | ||
| 2 | 3 | -5312.66 | 4087.68 | 0.20 | ||
| 1,2-propanediol | 1 | 2 | 1381.50 | 4668.83 | 0.20 | 0.65 |
| 1 | 3 | 13203.53 | 9005.39 | 0.30 | ||
| 2 | 3 | -4250.53 | 3503.82 | 0.40 | ||
| acetonitrile | 1 | 2 | -2156.02 | 11393.89 | 0.20 | 0.37 |
| 1 | 3 | 5242.92 | 4668.25 | 0.30 | ||
| 2 | 3 | -3200.37 | 2489.72 | 0.40 | ||
| furfural | 1 | 2 | -3222.48 | 5342.67 | 0.20 | 0.38 |
| 1 | 3 | 5330.74 | 4750.76 | 0.30 | ||
| 2 | 3 | -2225.34 | -1745.79 | 0.30 | ||
| N,N-dimethylformamide | 1 | 2 | -946.20 | 13600.58 | 0.20 | 0.25 |
| 1 | 3 | 4290.41 | 5929.85 | 0.30 | ||
| 2 | 3 | -5841.67 | 10715.00 | 0.30 |
表16 正己烷(1)-异丙醇(2)-溶剂(3)三元体系的NRTL模型参数和RMSD值
Table 16 Parameters of NRTL model and RMSD values for n-hexane (1) - isopropanol (2) - solvent (3) ternary system
| Solvent | i | j | Δgij/ (J·mol-1) | Δgji/ (J·mol-1) | αij | RMSD/% |
|---|---|---|---|---|---|---|
| dimethyl sulfoxide | 1 | 2 | -56284.05 | 968.83 | 0.20 | 0.18 |
| 1 | 3 | 6854.25 | 6761.20 | 0.20 | ||
| 2 | 3 | -1112.04 | -63742.32 | 0.30 | ||
| 1,4-butanediol | 1 | 2 | 1068.26 | 5991.22 | 0.20 | 0.94 |
| 1 | 3 | 15068.36 | 9058.82 | 0.29 | ||
| 2 | 3 | -5312.66 | 4087.68 | 0.20 | ||
| 1,2-propanediol | 1 | 2 | 1381.50 | 4668.83 | 0.20 | 0.65 |
| 1 | 3 | 13203.53 | 9005.39 | 0.30 | ||
| 2 | 3 | -4250.53 | 3503.82 | 0.40 | ||
| acetonitrile | 1 | 2 | -2156.02 | 11393.89 | 0.20 | 0.37 |
| 1 | 3 | 5242.92 | 4668.25 | 0.30 | ||
| 2 | 3 | -3200.37 | 2489.72 | 0.40 | ||
| furfural | 1 | 2 | -3222.48 | 5342.67 | 0.20 | 0.38 |
| 1 | 3 | 5330.74 | 4750.76 | 0.30 | ||
| 2 | 3 | -2225.34 | -1745.79 | 0.30 | ||
| N,N-dimethylformamide | 1 | 2 | -946.20 | 13600.58 | 0.20 | 0.25 |
| 1 | 3 | 4290.41 | 5929.85 | 0.30 | ||
| 2 | 3 | -5841.67 | 10715.00 | 0.30 |
| 1 | Zhu Z Y, Bai W T, Xu Y, et al. Liquid-liquid extraction of methanol from its mixtures with hexane using three imidazolium-based ionic liquids[J]. J. Chem. Thermodyn., 2019, 138: 189-195. |
| 2 | 赵利霞, 马永, 孙利, 等. 正己烷生产纯化工艺的理论及设备研究进展[J]. 现代化工, 2009, 29(9): 15-20. |
| Zhao L X, Ma Y, Sun L, et al. Research progress in theory and equipment for hexane production and purification[J]. Modern Chemical Industry, 2009, 29(9): 15-20. | |
| 3 | 张飞, 薛晓金. 异丙醇浸出棉籽油的研究[J]. 中国油脂, 2008, 33(7): 13-16. |
| Zhang F, Xue X J. Isopropanol extraction of cotton seed oil[J]. China Oils and Fats, 2008, 33(7): 13-16. | |
| 4 | 程能林. 溶剂手册[M]. 第五版. 北京: 化学工业出版社, 2015: 106. |
| Cheng N L. Solvents Handbook[M]. 5thed. Beijing: Chemical Industry Press, 2015: 106. | |
| 5 | 孙毅, 谢清若, 韦藤幼, 等. 水-乙酸-糠醛三元体系相平衡数据关联与共沸精馏过程模拟[J]. 化工学报, 2011, 62(7): 1800-1807. |
| Sun Y, Xie Q R, Wei T Y, et al. VLE correlation and azeotropic distillation simulation for water-acetic acid-furfural ternary system[J]. CIESC Journal, 2011, 62(7): 1800-1807. | |
| 6 | Chen M Q, Yu N, Cong L, et al. Design and control of a heat pump-assisted azeotropic dividing wall column for EDA/water separation[J]. Ind. Eng. Chem. Res., 2017, 56(34): 9770-9777. |
| 7 | Shen W F, Dong L C, Wei S A, et al. Systematic design of an extractive distillation for maximum-boiling azeotropes with heavy entrainers[J]. AIChE J., 2015, 61(11): 3898-3910. |
| 8 | Shang X Y, Ma S T, Pan Q, et al. Process analysis of extractive distillation for the separation of ethanol-water[J]. Chem. Eng. Res. Des., 2019, 148: 298-311. |
| 9 | 胡松, 李进龙, 李木金, 等. 萃取精馏生产高纯度环氧丙烷的工艺研究[J]. 化工学报, 2019, 70(2): 670-677. |
| Hu S, Li J L, Li M J, et al. Extractive refining process for production of propylene oxide with high purification[J]. CIESC Journal, 2019, 70(2): 670-677. | |
| 10 | Modla G, Lang P. Removal and recovery of organic solvents from aqueous waste mixtures by extractive and pressure swing distillation[J]. Ind. Eng. Chem. Res., 2012, 51: 11473-11481. |
| 11 | 张海永, 刘潜, 刘兴坤, 等. 低温煤焦油中正十二烷-甲苯-苯酚的相平衡及分离[J]. 化工学报, 2018, 69(8): 3479-3487. |
| Zhang H Y, Liu Q, Liu X K, et al. Phase equilibrium and separation of n-dodecane-toluene-phenol in low temperature coal tar[J]. CIESC Journal, 2018, 69(8): 3479-3487. | |
| 12 | 陈赟, 吕冉, 熊康宁, 等. 甲基异丙基甲酮-苯酚-对苯二酚-水的液液相平衡数据测定、模型关联及萃取过程模拟[J]. 化工学报, 2018, 69(4): 1299-1306. |
| Chen Y, Lü R, Xiong K N, et al. Experimental determination, thermodynamic modeling and process simulation of methyl isopropyl ketone-phenol-hydroquinone-water quaternary systems[J]. CIESC Journal, 2018, 69(4): 1299-1306. | |
| 13 | Ma S T, Li J F, Li L M, et al. Liquid–liquid extraction of benzene and cyclohexane using sulfolane-based low transition temperature mixtures as solvents: experiments and simulation[J]. Energ. Fuel., 2018, 32(7): 8006-8015. |
| 14 | Gomes R S, Mattedi S, Santos G R. Liquid-liquid equilibria data of protic ionic liquid (ethyl-2-hydroxyethylammonium propionate, diethylammonium propionate, or butylammonium propionate) with 1-butanol + water at 298.15 K[J]. J. Chem. Eng. Data, 2019, 64(7): 2915-2922. |
| 15 | Zhang Y H, Gao H R, Wang M X, et al. Research on the effect of the solvent structure and group on separation of 1-hexene, benzene, and thiophene[J]. Energ. Fuel., 2019, 33(6): 5162-5172. |
| 16 | 苏佳伟. 液液萃取分离正己烷-异丙醇混合体系的研究[D]. 南京: 南京师范大学, 2017. |
| Su J W. Research of the separation of n-hexane–isopropanol mixed system by liquid-liquid extraction[D]. Nanjing: Nanjing Normal University, 2017. | |
| 17 | Hand D B. Dineric distribution[J]. J. Phys. Chem., 1930, 34(9): 1961-2000. |
| 18 | Renon H, Prausnitz J M. Local compositions in thermodynamic excess functions for liquid mixtures[J]. AIChE J., 1968, 14(1): 135-144. |
| 19 | Dai F F, Xin K, Song Y H, et al. Liquid-liquid equilibria for the ternary system containing 1-butanol + methoxy(methoxymethoxy)methane + water at temperatures of 303.15, 323.15 and 343.15 K[J]. Fluid Phase Equilib., 2016, 409: 466-471. |
| 20 | Dai F F, Xin K, Song Y H, et al. Liquid–liquid equilibria for the extraction of phenols from alkane using ethylene glycol[J]. Fluid Phase Equilib., 2016, 419: 50-56. |
| 21 | Liu H, Cui P, Xin K, et al. Experimental determination and correlation of liquid-liquid equilibria for water + cyclohexanone + solvents (toluene or p-xylene) ternary systems at 303.15 and 323.15 K under 101.3 kPa[J]. J. Chem. Eng. Data, 2017, 62: 2367-2373. |
| 22 | Mokhtarani B, Musavi J, Parvini M, et al. Ternary (liquid–liquid) equilibria of nitrate based ionic liquid+alkane+benzene at 298.15 K: experiments and correlation[J]. Fluid Phase Equilib., 2013, 341: 35-41. |
| 23 | Marciniak A, Królikowski M. Ternary (liquid+liquid) equilibria of {trifluorotris (perfluoroethyl) phosphate based ionic liquids+thiophene+heptane}[J]. J. Chem. Thermodyn., 2012, 49: 154-158. |
| 24 | 汪勤, 张冰剑, 何畅, 等. 环丁砜萃取精馏过程模拟分析及工艺参数优化[J]. 化工学报, 2017, 68(5): 1969-1976. |
| Wang Q, Zhang B J, He C, et al. Process simulation and optimization of sulfolane extractive distillation[J]. CIESC Journal, 2017, 68(5): 1969-1976. | |
| 25 | 庄志海, 张建强, 刘殿华. 聚甲氧基二甲醚+水+正己烷三元体系的液液相平衡[J]. 化工学报, 2016, 67(9): 3545-3551. |
| Zhuang Z H, Zhang J Q, Liu D H. Liquid-liquid equilibria for ternary systems polyoxymethylene dimethyl ethers + water + n-hexane[J]. CIESC Journal, 2016, 67(9): 3545-3551. | |
| 26 | Toledo I E P, Ferreira-Pinto L, Voll F A P, et al. Liquid–liquid equilibrium of the system {peanut biodiesel + glycerol + ethanol} at atmospheric pressure[J]. J. Chem. Eng. Data, 2019, 64(5): 2207-2212. |
| 27 | 时米东, 王利平, 何高银, 等. 低浓度甲缩醛水溶液–萃取剂液液相平衡数据的测定与关联[J]. 天然气化工(C1化学与化工), 2018, 43(1): 34-39+46. |
| Shi M D, Wang L P, He G Y, et al. Measurement and correlation of liquid-liquid equilibria data for low concentrated methylal aqueous solution and extractants[J]. Natural Gas Chemical Industry, 2018, 43(1): 34-39+46. | |
| 28 | 崔鹏, 刘海, 于雪敏, 等. 水-环己酮-甲基异丁基酮液液萃取相平衡数据的测定与关联[J]. 物理化学学报, 2018, 34(1): 65-72. |
| Cui P, Liu H, Yu X M, et al. Measurement and correlation of liquid-liquid equilibrium data for the water + cyclohexanone + methyl isobutyl ketone ternary system[J]. Acta Physico-Chimica Sinica, 2018, 34(1): 65-72. | |
| 29 | Yang C, Yu X M, Wang L P, et al. Experimental measurement and thermodynamic modelling of liquid-liquid equilibria for the separation of 1,2-dichloroethane from cyclohexane using various extractants[J]. J. Mol. Liq., 2018, 252: 263-270. |
| 30 | Naik P K, Paul S, Banerjee T. Liquid-liquid equilibria measurements for the extraction of poly aromatic nitrogen hydrocarbons with a low cost deep eutectic solvent: experimental and theoretical insights[J]. J. Mol. Liq., 2017, 243: 542-552. |
| 31 | Al-Tuwaim M S, Alkhaldi K H A E, Fandary M S, et al. Extraction of propylbenzene from its mixtures with heptadecane using 4-methyl-N-butylpyridinium tetrafluoroborate[J]. Fluid Phase Equilib., 2012, 315: 21-28. |
| 32 | 冯艺荣, 盖恒军, 郭凯, 等. 甲基戊烯酮-水-邻苯二酚三元物系液液相平衡数据的测定与关联[J]. 化工学报, 2017, 68(3): 848-853. |
| Feng Y R, Gai H J, Guo K, et al. Measurement and correlation of liquid-liquid equilibrium data for mesityl oxide-water-catechol ternary system[J]. CIESC Journal, 2017, 68(3): 848-853. |
| [1] | 王俐智, 杭钱程, 郑叶玲, 丁延, 陈家继, 叶青, 李进龙. 离子液体萃取剂萃取精馏分离丙酸甲酯+甲醇共沸物[J]. 化工学报, 2023, 74(9): 3731-3741. |
| [2] | 于旭东, 李琪, 陈念粗, 杜理, 任思颖, 曾英. 三元体系KCl + CaCl2 + H2O 298.2、323.2及348.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(8): 3256-3265. |
| [3] | 陈科, 杜理, 曾英, 任思颖, 于旭东. 四元体系LiCl+MgCl2+CaCl2+H2O 323.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(5): 1896-1903. |
| [4] | 吕阳光, 左培培, 杨正金, 徐铜文. 三嗪框架聚合物膜用于有机纳滤甲醇/正己烷分离[J]. 化工学报, 2023, 74(4): 1598-1606. |
| [5] | 程文婷, 李杰, 徐丽, 程芳琴, 刘国际. AlCl3·6H2O在FeCl3、CaCl2、KCl及KCl–FeCl3溶液中溶解度的实验及预测[J]. 化工学报, 2023, 74(2): 642-652. |
| [6] | 高靖博, 孙强, 李青, 王逸伟, 郭绪强. 考虑水合物结构转变的含氢气体水合物相平衡模型[J]. 化工学报, 2023, 74(2): 666-673. |
| [7] | 蔡进, 王晓辉, 汤涵, 陈光进, 孙长宇. TBAB水溶液体系中半笼型水合物的相平衡预测模型[J]. 化工学报, 2023, 74(1): 408-415. |
| [8] | 周桓, 张梦丽, 郝晴, 吴思, 李杰, 徐存兵. 硫酸镁型光卤石转化钾盐镁矾的过程机制与动态规律[J]. 化工学报, 2022, 73(9): 3841-3850. |
| [9] | 刘潜, 张香兰, 李志平, 李玉龙, 韩梦醒. 油酚分离过程低共熔溶剂的筛选及萃取性能研究[J]. 化工学报, 2022, 73(9): 3915-3928. |
| [10] | 张家仁, 刘海超. 大豆油与甲醇酯交换反应体系的相平衡研究[J]. 化工学报, 2022, 73(5): 1920-1929. |
| [11] | 门文欣, 彭庆收, 桂霞. 不同季铵盐作用下的CO2水合物相平衡[J]. 化工学报, 2022, 73(4): 1472-1482. |
| [12] | 吴子睿, 孙瑞, 石凌峰, 田华, 王轩, 舒歌群. CO2混合工质的气液相平衡的混合规则对比与预测研究[J]. 化工学报, 2022, 73(4): 1483-1492. |
| [13] | 李春晖, 何辉, 何明键, 张萌, 高杨, 矫彩山. 离子液体萃取硝酸中Ce(Ⅳ)的动力学研究[J]. 化工学报, 2022, 73(4): 1606-1614. |
| [14] | 孙裕坤, 杨焘, 吴江涛. R32+R1234yf+R1234ze(E)混合制冷剂气液相平衡实验研究[J]. 化工学报, 2022, 73(3): 1063-1071. |
| [15] | 贝鹏志, 李文英. 能量分解前提下萃取剂的选择策略[J]. 化工学报, 2022, 73(2): 739-746. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号