化工学报 ›› 2023, Vol. 74 ›› Issue (9): 3731-3741.DOI: 10.11949/0438-1157.20230517
王俐智1( ), 杭钱程1, 郑叶玲2, 丁延1, 陈家继1, 叶青1, 李进龙1(
), 杭钱程1, 郑叶玲2, 丁延1, 陈家继1, 叶青1, 李进龙1( )
)
收稿日期:2023-05-29
修回日期:2023-08-09
出版日期:2023-09-25
发布日期:2023-11-20
通讯作者:
李进龙
作者简介:王俐智(1999—),男,硕士研究生,m18761020178@163.com
基金资助:
Lizhi WANG1( ), Qiancheng HANG1, Yeling ZHENG2, Yan DING1, Jiaji CHEN1, Qing YE1, Jinlong LI1(
), Qiancheng HANG1, Yeling ZHENG2, Yan DING1, Jiaji CHEN1, Qing YE1, Jinlong LI1( )
)
Received:2023-05-29
Revised:2023-08-09
Online:2023-09-25
Published:2023-11-20
Contact:
Jinlong LI
摘要:
以离子液体(ILs)[BMIM][NTF2]和[HMIM][NTF2]为萃取剂,萃取精馏分离丙酸甲酯+甲醇共沸物,通过分子模拟分析了ILs促进目标共沸物的分离机理;基于汽液平衡实验数据,获得新的NRTL热力学模型参数;选用常规的二组分双塔分离流程,实现了目标共沸体系的分离。作为对比,同时构建了以苯酚为萃取剂的萃取精馏和变压精馏流程。基于Aspen Plus软件平台,分析了上述各流程分离单元主要操作参数对分离过程性能的影响,考察并对比了各流程能耗、年总成本(TAC)和碳排放。结果表明:离子液体工艺可实现丙酸甲酯+甲醇共沸物的有效分离,产品纯度达到99.9%(质量分数),[HMIM][NTF2]工艺与[BMIM][NTF2]、苯酚及变压精馏工艺相比,TAC降低11.68%~43.68%、CO2 排放减少32.11%~68.46%。结果可为共沸物丙酸甲酯+甲醇分离新工艺设计及优化提供理论支撑和实际指导。
中图分类号:
王俐智, 杭钱程, 郑叶玲, 丁延, 陈家继, 叶青, 李进龙. 离子液体萃取剂萃取精馏分离丙酸甲酯+甲醇共沸物[J]. 化工学报, 2023, 74(9): 3731-3741.
Lizhi WANG, Qiancheng HANG, Yeling ZHENG, Yan DING, Jiaji CHEN, Qing YE, Jinlong LI. Separation of methyl propionate + methanol azeotrope using ionic liquid entrainers[J]. CIESC Journal, 2023, 74(9): 3731-3741.
| 物质 | 分子结构优化 | 相互作用能/(kJ/mol) |
|---|---|---|
| [BMIM]+ +丙酸甲酯 |  | -83.53 |
| [BMIM]+ +甲醇 |  | -11.72 |
| [HMIM]+ +丙酸甲酯 |  | -104.59 |
| [HMIM]+ +甲醇 |  | -48.95 |
| [NTF2]-+丙酸甲酯 |  | -58.42 |
| [NTF2]-+甲醇 |  | -17.97 |
| 苯酚+丙酸甲酯 |  | -12.80 |
| 苯酚+甲醇 |  | -4.47 |
| 甲醇+丙酸甲酯 |  | -4.36 |
表1 分子/离子间的相互作用能
Table 1 The interaction energy between molecules and ions
| 物质 | 分子结构优化 | 相互作用能/(kJ/mol) |
|---|---|---|
| [BMIM]+ +丙酸甲酯 |  | -83.53 |
| [BMIM]+ +甲醇 |  | -11.72 |
| [HMIM]+ +丙酸甲酯 |  | -104.59 |
| [HMIM]+ +甲醇 |  | -48.95 |
| [NTF2]-+丙酸甲酯 |  | -58.42 |
| [NTF2]-+甲醇 |  | -17.97 |
| 苯酚+丙酸甲酯 |  | -12.80 |
| 苯酚+甲醇 |  | -4.47 |
| 甲醇+丙酸甲酯 |  | -4.36 |
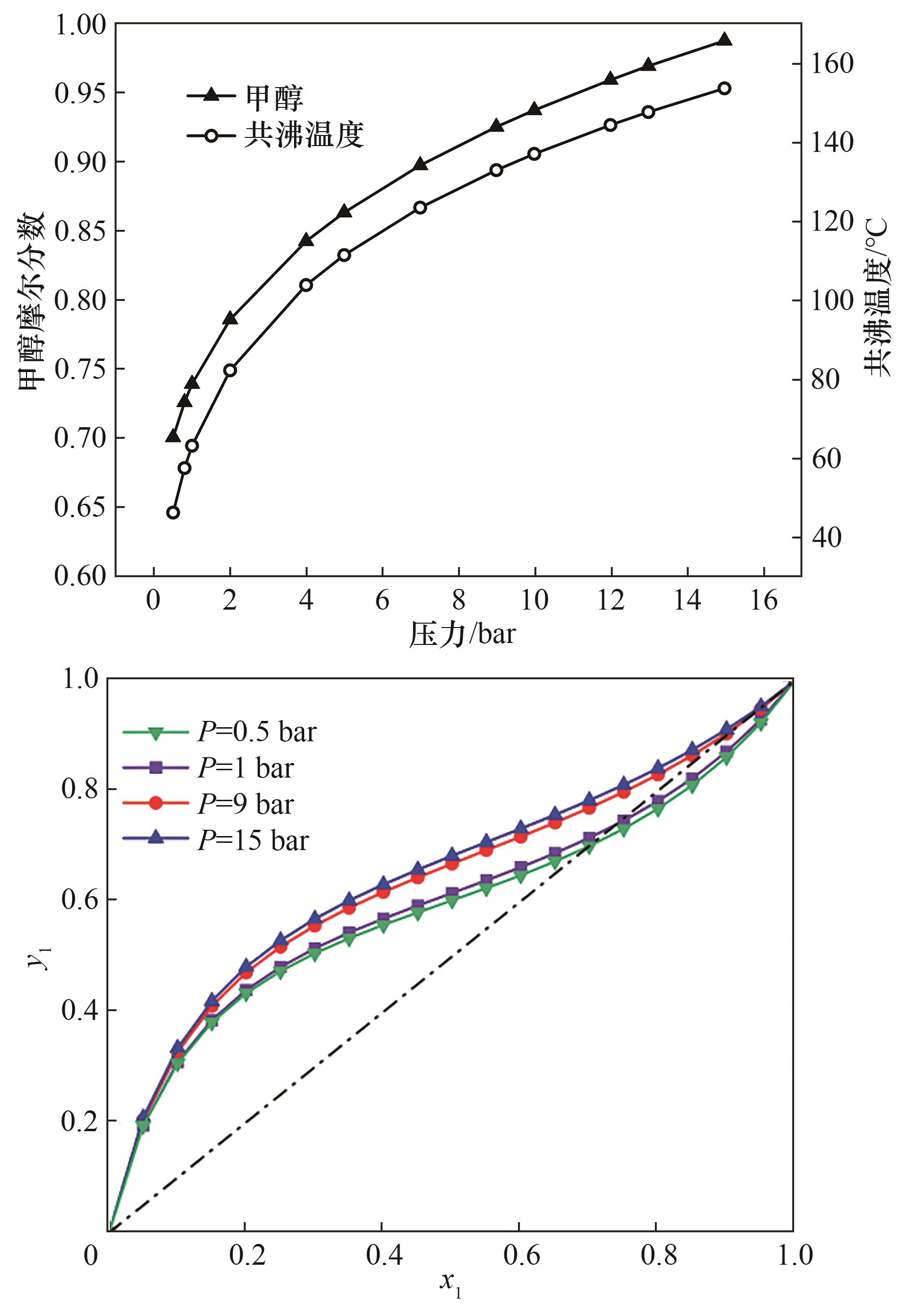
图3 (a)压力对共沸温度及组成的影响; (b)不同压力下MEOH(1)+MP(2)x-y相图
Fig.3 (a) Effect of pressure on the azeotropic temperature and composition; (b) x-y phase diagram of methand (1)+ methly propionate (2) at different pressures
| 组分 i | 组分 j | Δgij | Δgji | ΔT/K | Δy1 |
|---|---|---|---|---|---|
| MEOH | MP | 291.225 | 102.277 | 0.08 | 0.0017 |
| MEOH | [BMIM][NTF2] | -88.141 | 2744.110 | 0.3035 | 0.0062 |
| MP | [BMIM][NTF2] | 792.627 | -319.243 | ||
| MEOH | [HMIM][NTF2] | 353.792 | -433.952 | 0.4517 | 0.0071 |
| MP | [HMIM][NTF2] | -636.198 | 347.593 | ||
| MEOH | BF① | -159.758 | -68.302 | 2.12 | 0.052 |
| MP | BF① | -123.557 | -317.395 |
表 2 NRTL二元交互参数
Table 2 NRTL binary interaction parameters for the investigated mixtures
| 组分 i | 组分 j | Δgij | Δgji | ΔT/K | Δy1 |
|---|---|---|---|---|---|
| MEOH | MP | 291.225 | 102.277 | 0.08 | 0.0017 |
| MEOH | [BMIM][NTF2] | -88.141 | 2744.110 | 0.3035 | 0.0062 |
| MP | [BMIM][NTF2] | 792.627 | -319.243 | ||
| MEOH | [HMIM][NTF2] | 353.792 | -433.952 | 0.4517 | 0.0071 |
| MP | [HMIM][NTF2] | -636.198 | 347.593 | ||
| MEOH | BF① | -159.758 | -68.302 | 2.12 | 0.052 |
| MP | BF① | -123.557 | -317.395 |
| 参数 | 萃取精馏 | 变压精馏 | |||||
|---|---|---|---|---|---|---|---|
| [BMIM][NTF2] | [HMIM][NTF2] | 苯酚 | 常规流程 | 热集成流程 | |||
| 萃取塔(高压塔) | |||||||
| 理论级 | 34 | 34 | 34 | 34 | 34 | ||
| 摩尔溶剂比 | 1.60 | 0.80 | 2.10 | — | — | ||
| 进料塔板数 | 26 | 20 | 26 | 25 | 25 | ||
| 质量回流比 | 0.25 | 0.22 | 1.20 | 1.65 | 1.67 | ||
| 压力/bar | 1 | 1 | 1 | 9 | |||
| 萃取剂进料位置 | 5 | 2 | 4 | — | — | ||
| 萃取剂流量/(kg/h) | 67099.60 | 35793.90 | 19763.40 | — | — | ||
| 冷凝负荷/kW | -882.38 | -885.06 | -1547.80 | -3267.86 | -2018.78 | ||
| 加热负荷/kW | 3455.03 | 2484.78 | 3524.34 | 4083.18 | 4067.28 | ||
| 闪蒸罐(溶剂回收塔/低压塔) | |||||||
| 温度/℃ | 150 | 150 | — | — | |||
| 压力 | 30 Pa | 30 Pa | 0.4 bar | 1 bar | |||
| 加热负荷/kW | 514.95 | 210.38 | 1145.39 | 4463.04 | 3534.31 | ||
| 冷凝负荷/kW | — | — | -1501.61 | -4875.94 | -5415.36 | ||
| 理论级 | — | — | 20 | 34 | 34 | ||
| 进料位置 | — | — | 5 | 15 | 13 | ||
| 质量回流比 | — | — | 4.48 | 5.36 | 6.06 | ||
| 换热器 | |||||||
| 热负荷/kW | -2612.68 | -1334.53 | -1503.00 | -101.80 | -101.84 | ||
| 甲醇产品流股 | |||||||
| 温度/℃ | 64.20 | 64.20 | 64.20 | 64.20 | 64.20 | ||
| 甲醇纯度 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | ||
| 产品流量/(kg/h) | 2300.65 | 2298.75 | 2300.84 | 2298.41 | 2298.41 | ||
| 丙酸甲酯产品流股 | |||||||
| 温度/℃ | 150.00 | 150.00 | 53.57 | 166.02 | 25 | ||
| 丙酸甲酯纯度 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | ||
| 产品流量/(kg/h) | 2488.11 | 2490.01 | 2490.19 | 2490.36 | 2490.35 | ||
| 溶剂回收流股 | |||||||
| 温度/℃ | 150.00 | 150.00 | 151.05 | — | |||
| 甲醇质量分数 | 0 | 0 | 0 | — | |||
| 丙酸甲酯含量/ppm | 0.3 | 43 | 25 | — | |||
| 溶剂质量分数 | 0.999997 | 0.999957 | 0.999975 | — | |||
| 工艺总加热负荷/kW | 3970.0 | 2695.2 | 4669.7 | 8546.2 | 7601.6 | ||
| 工艺总冷凝负荷/kW | -3495.1 | -2219.6 | -4552.4 | -8245.6 | -7536.0 | ||
表 3 流程模拟优化工艺条件
Table 3 Optimization parameters for flowsheet simulation
| 参数 | 萃取精馏 | 变压精馏 | |||||
|---|---|---|---|---|---|---|---|
| [BMIM][NTF2] | [HMIM][NTF2] | 苯酚 | 常规流程 | 热集成流程 | |||
| 萃取塔(高压塔) | |||||||
| 理论级 | 34 | 34 | 34 | 34 | 34 | ||
| 摩尔溶剂比 | 1.60 | 0.80 | 2.10 | — | — | ||
| 进料塔板数 | 26 | 20 | 26 | 25 | 25 | ||
| 质量回流比 | 0.25 | 0.22 | 1.20 | 1.65 | 1.67 | ||
| 压力/bar | 1 | 1 | 1 | 9 | |||
| 萃取剂进料位置 | 5 | 2 | 4 | — | — | ||
| 萃取剂流量/(kg/h) | 67099.60 | 35793.90 | 19763.40 | — | — | ||
| 冷凝负荷/kW | -882.38 | -885.06 | -1547.80 | -3267.86 | -2018.78 | ||
| 加热负荷/kW | 3455.03 | 2484.78 | 3524.34 | 4083.18 | 4067.28 | ||
| 闪蒸罐(溶剂回收塔/低压塔) | |||||||
| 温度/℃ | 150 | 150 | — | — | |||
| 压力 | 30 Pa | 30 Pa | 0.4 bar | 1 bar | |||
| 加热负荷/kW | 514.95 | 210.38 | 1145.39 | 4463.04 | 3534.31 | ||
| 冷凝负荷/kW | — | — | -1501.61 | -4875.94 | -5415.36 | ||
| 理论级 | — | — | 20 | 34 | 34 | ||
| 进料位置 | — | — | 5 | 15 | 13 | ||
| 质量回流比 | — | — | 4.48 | 5.36 | 6.06 | ||
| 换热器 | |||||||
| 热负荷/kW | -2612.68 | -1334.53 | -1503.00 | -101.80 | -101.84 | ||
| 甲醇产品流股 | |||||||
| 温度/℃ | 64.20 | 64.20 | 64.20 | 64.20 | 64.20 | ||
| 甲醇纯度 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | ||
| 产品流量/(kg/h) | 2300.65 | 2298.75 | 2300.84 | 2298.41 | 2298.41 | ||
| 丙酸甲酯产品流股 | |||||||
| 温度/℃ | 150.00 | 150.00 | 53.57 | 166.02 | 25 | ||
| 丙酸甲酯纯度 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | ||
| 产品流量/(kg/h) | 2488.11 | 2490.01 | 2490.19 | 2490.36 | 2490.35 | ||
| 溶剂回收流股 | |||||||
| 温度/℃ | 150.00 | 150.00 | 151.05 | — | |||
| 甲醇质量分数 | 0 | 0 | 0 | — | |||
| 丙酸甲酯含量/ppm | 0.3 | 43 | 25 | — | |||
| 溶剂质量分数 | 0.999997 | 0.999957 | 0.999975 | — | |||
| 工艺总加热负荷/kW | 3970.0 | 2695.2 | 4669.7 | 8546.2 | 7601.6 | ||
| 工艺总冷凝负荷/kW | -3495.1 | -2219.6 | -4552.4 | -8245.6 | -7536.0 | ||
| 项目 | 费用/(105 USD/a) | ||||
|---|---|---|---|---|---|
| [BMIM][NTF2] | [HMIM][NTF2] | 苯酚 | 常规 流程 | 热集成流程 | |
| 投资成本 | 9.01 | 6.48 | 13.15 | 15.39 | 15.31 |
| 能源成本 | 9.53 | 6.68 | 11.46 | 20.11 | 17.99 |
| 溶剂成本 | 6.71 | 5.37 | 0.24 | — | — |
| 运行成本 | 16.24 | 12.05 | 11.70 | 20.11 | 17.99 |
| TAC | 19.25 | 14.21 | 16.09 | 25.23 | 23.10 |
表4 各个流程的经济评价
Table 4 Economic evaluation of each case
| 项目 | 费用/(105 USD/a) | ||||
|---|---|---|---|---|---|
| [BMIM][NTF2] | [HMIM][NTF2] | 苯酚 | 常规 流程 | 热集成流程 | |
| 投资成本 | 9.01 | 6.48 | 13.15 | 15.39 | 15.31 |
| 能源成本 | 9.53 | 6.68 | 11.46 | 20.11 | 17.99 |
| 溶剂成本 | 6.71 | 5.37 | 0.24 | — | — |
| 运行成本 | 16.24 | 12.05 | 11.70 | 20.11 | 17.99 |
| TAC | 19.25 | 14.21 | 16.09 | 25.23 | 23.10 |
| 1 | de Maron J, Eberle M, Cavani F, et al. Continuous-flow methyl methacrylate synthesis over gallium-based bifunctional catalysts[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(4): 1790-1803. |
| 2 | Ai M. Formation of methyl methacrylate by condensation of methyl propionate with formaldehyde over silica-supported cesium hydroxide catalysts[J]. Applied Catalysis A: General, 2005, 288(1/2): 211-215. |
| 3 | 李柏春, 张静雅, 王凤竹, 等. 酯化法合成丙酸甲酯的动力学研究[J]. 石油化工, 2017, 46(12): 1468-1472. |
| Li B C, Zhang J Y, Wang F Z, et al. Reaction kinetics of synthesized methyl propionate by esterification[J]. Petrochemical Technology, 2017, 46(12): 1468-1472. | |
| 4 | 赵彦玲, 赵艳平, 赵丽云. 利用离子液体催化剂合成丙酸甲酯[J]. 煤炭与化工, 2013, 36(11): 39-41, 79. |
| Zhao Y L, Zhao Y P, Zhao L Y. Synthesis of methyl propionate using ionic liquid as catalyst[J]. Coal and Chemical Industry, 2013, 36(11): 39-41, 79. | |
| 5 | Shariati A, Florusse L J, Kroon M C, et al. Bubble point pressures of binary system of methanol and methyl propionate[J]. Fluid Phase Equilibria, 2016, 417: 166-170. |
| 6 | 张伟静, 张雷. 变压精馏分离丙酸甲酯-甲醇的节能设计[J]. 天然气化工—C1化学与化工, 2022, 47(1): 152-158. |
| Zhang W J, Zhang L. Energy saving design for separation of methyl propionate-methanol by pressure swing distillation[J]. Natural Gas Chemical Industry, 2022, 47(1): 152-158. | |
| 7 | 李顺民, 张亲亲, 辛华, 等. 不同阳离子醋酸类离子液体作萃取剂分离丙酸甲酯-甲醇共沸体系[J]. 石油化工, 2022, 51(3): 302-309. |
| Li S M, Zhang Q Q, Xin H, et al. Separation of methyl propionate-methanol azeotropic system using acetic acid ionic liquids with different cations as entrainers[J]. Petrochemical Technology, 2022, 51(3): 302-309. | |
| 8 | 韩淑萃, 杨金杯. 丙酸甲酯和甲醇共沸物萃取精馏分离工艺的研究[J]. 现代化工, 2018, 38(7): 214-218. |
| Han S C, Yang J B. Separation of methyl propionate-methanol azeotrope by extractive distillation[J]. Modern Chemical Industry, 2018, 38(7): 214-218. | |
| 9 | 郑明石, 宋泽, 李群生, 等. 耦合变压精馏分离甲醇-丙酸甲酯共沸体系的工艺模拟和优化[J]. 北京化工大学学报(自然科学版), 2019, 46(3): 28-34. |
| Zheng M S, Song Z, Li Q S, et al. Process simulation and optimization of the separation of a methanol-methyl propionate azeotropic system by coupled pressure swing distillation[J]. Journal of Beijing University of Chemical Technology(Natural Science Edition), 2019, 46(3): 28-34. | |
| 10 | Akinlua A, Jochmann M A, Schmidt T C. Ionic liquid as green solvent for leaching of polycyclic aromatic hydrocarbons from petroleum source rock[J]. Industrial & Engineering Chemistry Research, 2015, 54(51): 12960-12965. |
| 11 | Zhang Y L, Su Z H, Xue K, et al. Efficient separation of methyl tert-butyl ether using ionic liquids from computational thermodynamics to process intensification[J]. Industrial & Engineering Chemistry Research, 2022, 61(48): 17631-17643. |
| 12 | Broderick Erin M, Manuela S, Beckay M, et al. Scientific approach for a cleaner environment using ionic liquids[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(5): 3681-3684. |
| 13 | Ding Y, Guo Y C, Sun Y H, et al. Mixed ionic liquids as entrainers for aromatic extraction processes: energy, economic, and environmental evaluations[J]. Industrial & Engineering Chemistry Research, 2022, 61(43): 16193-16208. |
| 14 | Zhang Z S, Zhao X X, Wang Y, et al. Eco-efficient heat-integrated extractive distillation process using ionic liquid as entrainer for ethyl acetate-isopropyl alcohol-water mixture[J]. Separation and Purification Technology, 2022, 287: 120491. |
| 15 | Guo J, Hu B F, Li Z X, et al. Vapor-liquid equilibrium experiment and extractive distillation process design for the azeotrope ethyl propionate n-propanol using ionic liquid[J]. Journal of Molecular Liquids, 2022, 350: 118492. |
| 16 | Dong Y C, Yang Q C, Li Z W, et al. Extractive distillation of the benzene and acetonitrile mixture using an ionic liquid as the entrainer[J]. Green & Energy Environment, 2021, 6(3): 444-451. |
| 17 | Chen Z R, Zhang Y L, Zhou M J, et al. Mechanism analysis and process optimization of acetone-methanol azeotrope separation using 1-ethyl-3-methylimidazolium acetate based mixed extractants[J]. Journal of Cleaner Production, 2022, 379:134687. |
| 18 | Larriba M, Navarro P, García J, et al. Liquid-liquid extraction of toluene from heptane using [emim][DCA], [bmim][DCA], and [emim][TCM] ionic liquids[J]. Industrial & Engineering Chemistry Research, 2013, 52(7): 2714-2720. |
| 19 | Li J L, Li T T, Peng C J, et al. Extractive distillation with ionic liquid entrainers for the separation of acetonitrile and water[J]. Industrial & Engineering Chemistry Research, 2019, 58(14): 5602-5612. |
| 20 | Li J L, Li J S, Zheng Y L, et al. Measurement and correlation of isobaric vapour-liquid equilibrium for the (acetonitrile+water) system containing different ionic liquids at atmospheric pressure[J]. The Journal of Chemical Thermodynamics, 2019, 138: 366-373. |
| 21 | 李进龙, 李佳书, 杨青, 等. 乙腈+水+离子液体等压汽液平衡测定与计算[J]. 化工学报, 2019, 70(6): 2110-2116. |
| Li J L, Li J S, Yang Q, et al. Determination and calculation of isobaric vapor equilibrium for acetonitrile+water+ionic liquid[J]. CIESC Journal, 2019, 70(6): 2110-2116. | |
| 22 | Lu Q L, Li J L, Peng C J, et al. Experimental determination of vapor liquid equilibrium for methanol+methyl propionate+1-butyl-3-methylimidazo-lium bis(trifluoromethylsulfonyl)imide at atmospheric pressure[J]. The Journal of Chemical Thermodynamics, 2019, 132: 289- 294. |
| 23 | 陆钱磊. 促进甲醇-丙酸甲酯分离的离子液体筛选、实验验证与过程模拟[D]. 上海: 华东理工大学, 2019. |
| Lu Q L. Screening of ionic liquid, experimental verification and process simulation for methanol-methyl propionate separation[D]. Shanghai: East China University of Science and Technology, 2019. | |
| 24 | Valderrama J O, Rojas R E. Critical properties of ionic liquids. Revisited[J]. Industrial & Engineering Chemistry Research, 2009, 48(14): 6890-6900. |
| 25 | Valderrama J O, Robles P A. Critical properties, normal boiling temperatures, and acentric factors of fifty ionic liquids[J]. Industrial & Engineering Chemistry Research, 2007, 46(4): 1338-1344. |
| 26 | Valderrama J O, Robles P A. Reply to "comment on 'critical properties, normal boiling temperature, and acentric factor of fifty ionic liquids'"[J]. Industrial & Engineering Chemistry Research, 2007, 46(18): 6063-6064. |
| 27 | Valderrama J O, Zarricueta K. A simple and generalized model for predicting the density of ionic liquids[J]. Fluid Phase Equilibria, 2009, 275(2): 145-151. |
| 28 | Valderrama J O, Rojas R E. Mass connectivity index, a new molecular parameter for the estimation of ionic liquid properties[J]. Fluid Phase Equilibria, 2010, 297(1): 107-112. |
| 29 | Joback K G. Knowledge bases for computerized physical property estimation[J]. Fluid Phase Equilibria, 2001, 185(1/2): 45-52. |
| 30 | Renon H, Prausnitz J M. Local compositions in thermodynamic excess functions for liquid mixtures[J]. AIChE Journal, 1968, 14(1): 135-144. |
| 31 | 王菊, 谭平华, 宋吉英. 甲醇-丙酸甲酯二元体系汽液平衡数据的测定与关联[J]. 天然气化工—C1化学与化工, 2017, 42(2): 65-69. |
| Wang J, Tan P H, Song J Y. Vapor-liquid equilibrium measurements and correlation of the methanol-methy propionate binary system[J]. Natural Gas Chemical Industry, 2017, 42(2): 65-69. | |
| 32 | Luyben W L. Comparison of extractive distillation and pressure-swing distillation for acetone-methanol separation[J]. Industrial & Engineering Chemistry Research, 2008, 47(8): 2696-2707. |
| 33 | Chen Y C, Li K L, Chen C L, et al. Design and control of a hybrid extraction-distillation system for the separation of pyridine and water[J]. Industrial & Engineering Chemistry Research, 2015, 54(31): 7715-7727. |
| 34 | Cheng J K L, Lee C L, Jhuang Y T, et al. Design and control of the glycerol tertiary butyl ethers process for the utilization of a renewable resource[J]. Industrial & Engineering Chemistry Research, 2011, 50(22): 12706-12716. |
| 35 | Seider W D, Seader J D, Lewin D R. Product and Process Design Principles: Synthesis, Analysis, and Evaluation [M]. 2nd ed. New York: Wiley, 2004. |
| 36 | Gadalla M A, Olujic Z, Jansens P J, et al. Reducing CO2 emissions and energy consumption of heat-integrated distillation systems[J]. Environmental Science & Technology, 2005, 39(17): 6860-6870. |
| 37 | 张桂华, 孙洪举. 苯酚国内市场分析、预测[J]. 化学工业, 2014, 32(10): 33-35. |
| Zhang G H, Sun H J. Production and market analysis of phenol in domestic[J]. Chemical Industry, 2014, 32(10): 33-35. | |
| 38 | Davis J H, Gordon C M, Hilgers C, et al. Ionic Liquids in Synthesis[M]. New York: Wiley-VCH Verlag Gmbh & Co., 2003: 7-40. |
| 39 | Lian Y, Li H X, Renyang Q Q, et al. Mapping the net ecosystem exchange of CO2 of global terrestrial systems[J]. International Journal of Applied Earth Observation and Geoinformation, 2023, 116: 103176. |
| [1] | 王琪, 张斌, 张晓昕, 武虎建, 战海涛, 王涛. 氯铝酸-三乙胺离子液体/P2O5催化合成伊索克酸和2-乙基蒽醌[J]. 化工学报, 2023, 74(S1): 245-249. |
| [2] | 车睿敏, 郑文秋, 王小宇, 李鑫, 许凤. 基于离子液体的纤维素均相加工研究进展[J]. 化工学报, 2023, 74(9): 3615-3627. |
| [3] | 陆俊凤, 孙怀宇, 王艳磊, 何宏艳. 离子液体界面极化及其调控氢键性质的分子机理[J]. 化工学报, 2023, 74(9): 3665-3680. |
| [4] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [5] | 宋明昊, 赵霏, 刘淑晴, 李国选, 杨声, 雷志刚. 离子液体脱除模拟油中挥发酚的多尺度模拟与研究[J]. 化工学报, 2023, 74(9): 3654-3664. |
| [6] | 杨绍旗, 赵淑蘅, 陈伦刚, 王晨光, 胡建军, 周清, 马隆龙. Raney镍-质子型离子液体体系催化木质素平台分子加氢脱氧制备烷烃[J]. 化工学报, 2023, 74(9): 3697-3707. |
| [7] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [8] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [9] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [10] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [11] | 张缘良, 栾昕奇, 苏伟格, 李畅浩, 赵钟兴, 周利琴, 陈健民, 黄艳, 赵祯霞. 离子液体复合萃取剂选择性萃取尼古丁的研究及DFT计算[J]. 化工学报, 2023, 74(7): 2947-2956. |
| [12] | 毛磊, 刘冠章, 袁航, 张光亚. 可捕集CO2的纳米碳酸酐酶粒子的高效制备及性能研究[J]. 化工学报, 2023, 74(6): 2589-2598. |
| [13] | 龙臻, 王谨航, 任俊杰, 何勇, 周雪冰, 梁德青. 离子液体协同PVCap抑制天然气水合物生成实验研究[J]. 化工学报, 2023, 74(6): 2639-2646. |
| [14] | 李木金, 胡松, 施德磐, 赵鹏, 高瑞, 李进龙. 环氧丁烷尾气溶剂吸收及精制工艺[J]. 化工学报, 2023, 74(4): 1607-1618. |
| [15] | 龙臻, 王谨航, 何勇, 梁德青. 离子液体与动力学抑制剂作用下混合气体水合物生成特性研究[J]. 化工学报, 2023, 74(4): 1703-1711. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号