化工学报 ›› 2023, Vol. 74 ›› Issue (8): 3256-3265.DOI: 10.11949/0438-1157.20230470
收稿日期:2023-05-12
修回日期:2023-06-28
出版日期:2023-08-25
发布日期:2023-10-18
通讯作者:
曾英
作者简介:于旭东(1985—),男,博士,副教授,xwdlyxd@126.com
基金资助:
Xudong YU( ), Qi LI, Niancu CHEN, Li DU, Siying REN, Ying ZENG(
), Qi LI, Niancu CHEN, Li DU, Siying REN, Ying ZENG( )
)
Received:2023-05-12
Revised:2023-06-28
Online:2023-08-25
Published:2023-10-18
Contact:
Ying ZENG
摘要:
采用等温溶解平衡法开展了298.2、323.2、348.2 K三元体系KCl + CaCl2 + H2O相平衡研究,测定了平衡液相组成及密度,采用Schreinemakers湿渣法和X射线粉晶衍射法确定了平衡固相组成。研究发现:298.2 K时,该体系为简单三元体系,无复盐形成,相图由1个三元共饱点、2条单变量曲线和2个结晶相区组成;323.2和348.2 K时,该体系为复杂三元体系,有复盐氯钾钙石(KCl·CaCl2)形成,相图均由2个三元共饱点、3条单变量曲线和3个结晶相区组成。对比该三元体系278.2、298.2、308.2、323.2、348.2 K相图可知:随温度升高,CaCl2结晶形式发生变化,CaCl2·6H2O(278.2、298.2 K)→CaCl2·4H2O(308.2 K)→CaCl2·2H2O(323.2、348.2 K);KCl结晶面积在323.2 K时最大,氯钾钙石结晶面积随温度升高而增大。采用Pitzer-Simonson-Clegg模型进行了三元体系KCl + CaCl2 + H2O(298.2、323.2、348.2 K)热力学计算,理论计算值与实验值吻合较好。
中图分类号:
于旭东, 李琪, 陈念粗, 杜理, 任思颖, 曾英. 三元体系KCl + CaCl2 + H2O 298.2、323.2及348.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(8): 3256-3265.
Xudong YU, Qi LI, Niancu CHEN, Li DU, Siying REN, Ying ZENG. Phase equilibria and calculation of aqueous ternary system KCl + CaCl2 + H2O at 298.2, 323.2, and 348.2 K[J]. CIESC Journal, 2023, 74(8): 3256-3265.
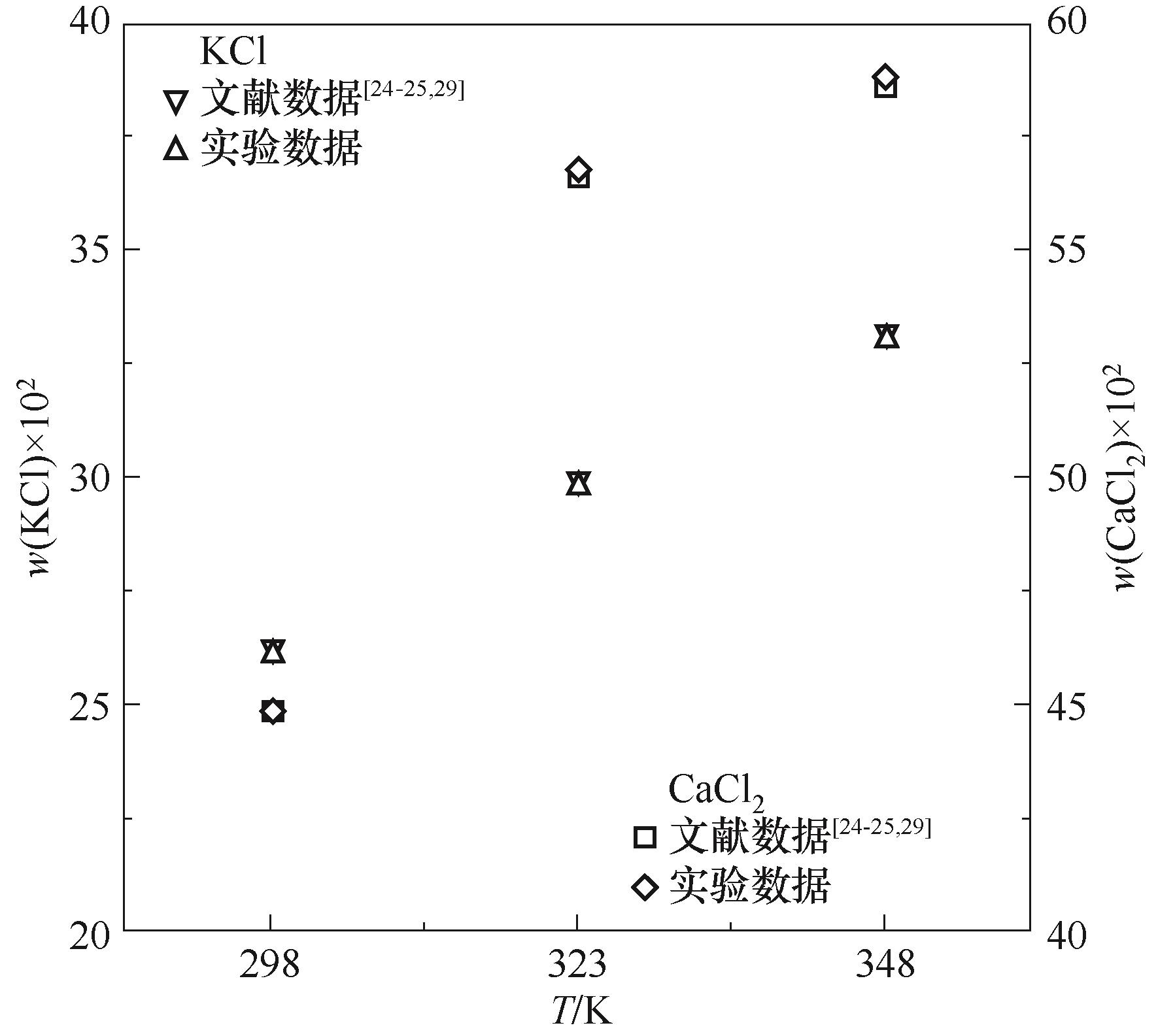
图1 二元体系KCl + H2O、CaCl2 + H2O 298.2、323.2、348.2 K溶解度数据对比
Fig.1 Comparison of solubility data of the binary systems KCl + H2O, CaCl2+H2O at 298.2, 323.2, and 348.2 K
| 编号 | 密度 ρ/(g/cm3) | 液相组成 w(B)×102 | 湿固相组成 w(B)×102 | 平衡固相 | ||
|---|---|---|---|---|---|---|
| w(KCl) | w(CaCl2) | w(KCl) | w(CaCl2) | |||
| T = 298.2 K | ||||||
| 1, A | 1.1837 | 26.18 | 0.00 | — | — | KCl |
| 2 | 1.1919 | 23.23 | 2.77 | 59.29 | 1.54 | KCl |
| 3 | 1.1953 | 21.07 | 5.16 | 59.94 | 2.63 | KCl |
| 4 | 1.2130 | 18.31 | 8.14 | 53.82 | 4.57 | KCl |
| 5 | 1.2217 | 15.55 | 11.26 | 72.01 | 3.67 | KCl |
| 6 | 1.2459 | 12.07 | 15.57 | 51.16 | 8.68 | KCl |
| 7 | 1.2605 | 9.80 | 19.19 | 62.52 | 8.00 | KCl |
| 8 | 1.2847 | 7.58 | 22.71 | 80.77 | 4.75 | KCl |
| 9 | 1.3010 | 5.58 | 26.41 | 71.16 | 7.82 | KCl |
| 10 | 1.3383 | 4.91 | 28.74 | 53.74 | 14.18 | KCl |
| 11 | 1.3490 | 4.07 | 31.84 | 67.50 | 10.44 | KCl |
| 12 | 1.3873 | 3.58 | 34.72 | 66.73 | 11.79 | KCl |
| 13 | 1.3961 | 3.34 | 36.81 | 63.62 | 13.41 | KCl |
| 14 | 1.4252 | 3.18 | 40.47 | 63.92 | 14.24 | KCl |
| 15 | 1.4503 | 3.52 | 43.08 | 77.75 | 9.21 | KCl |
| 16 | 1.4615 | 3.31 | 44.41 | 50.94 | 21.71 | KCl |
| 17, S1 | 1.4664 | 2.81 | 45.62 | 1.10 | 48.59 | KCl + CaCl2·6H2O |
| 18, B | 1.4570 | 0.00 | 44.83 | — | — | CaCl2·6H2O |
| T = 323.2 K | ||||||
| 1, C | 1.2032 | 29.87 | 0.00 | — | — | KCl |
| 2 | 1.2119 | 26.52 | 3.28 | 65.74 | 1.57 | KCl |
| 3 | 1.2149 | 23.39 | 6.46 | 73.40 | 2.19 | KCl |
| 4 | 1.2327 | 20.21 | 10.18 | 67.70 | 4.27 | KCl |
| 5 | 1.2474 | 17.00 | 13.43 | 66.42 | 5.55 | KCl |
| 6 | 1.2600 | 13.40 | 17.67 | 67.87 | 6.64 | KCl |
| 7 | 1.2792 | 10.97 | 21.21 | 65.99 | 8.06 | KCl |
| 8 | 1.2954 | 8.55 | 25.18 | 65.82 | 9.29 | KCl |
| 9 | 1.3149 | 6.40 | 29.11 | 67.27 | 10.41 | KCl |
| 10 | 1.3532 | 6.09 | 31.26 | 65.90 | 11.46 | KCl |
| 11 | 1.3908 | 5.12 | 36.74 | 71.33 | 11.29 | KCl |
| 12 | 1.4188 | 4.87 | 40.10 | 72.83 | 11.56 | KCl |
| 13 | 1.4559 | 5.01 | 42.97 | 76.87 | 12.51 | KCl |
| 14 | 1.4843 | 5.28 | 44.98 | 75.73 | 13.47 | KCl |
| 15 | 1.5001 | 5.63 | 47.10 | 74.76 | 13.45 | KCl |
| 16 | 1.5131 | 5.84 | 48.83 | 73.58 | 14.40 | KCl |
| 17 | 1.5526 | 7.23 | 50.86 | 33.33 | 36.61 | KCl |
| 18, S2 | 1.5571 | 7.40 | 51.88 | 40.08 | 54.27 | KCl + KCl·CaCl2 |
| 19 | 1.5556 | 7.24 | 52.74 | 10.14 | 52.97 | KCl·CaCl2 |
| 20 | 1.5508 | 5.01 | 55.02 | 26.21 | 57.97 | KCl·CaCl2 |
| 21, S3 | 1.5511 | 4.24 | 55.07 | 7.48 | 61.15 | KCl·CaCl2 + CaCl2·2H2O |
| 22 | 1.5496 | 4.10 | 55.18 | 2.57 | 62.16 | CaCl2·2H2O |
| 23 | 1.5384 | 2.18 | 55.58 | 2.05 | 60.68 | CaCl2·2H2O |
| 24, D | 1.5366 | 0.00 | 56.74 | — | — | CaCl2·2H2O |
| T = 348.2 K | ||||||
| 1, E | 1.2992 | 33.08 | 0.00 | — | — | KCl |
| 2 | 1.3057 | 29.78 | 3.64 | 77.93 | 1.56 | KCl |
| 3 | 1.3153 | 26.22 | 7.07 | 75.07 | 2.30 | KCl |
| 4 | 1.3236 | 22.94 | 10.43 | 70.94 | 4.52 | KCl |
| 5 | 1.3442 | 19.10 | 14.59 | 61.73 | 7.51 | KCl |
| 6 | 1.3490 | 15.64 | 19.79 | 65.71 | 9.51 | KCl |
| 7 | 1.3889 | 12.42 | 24.19 | 77.24 | 7.93 | KCl |
| 8 | 1.4435 | 10.21 | 28.66 | 68.01 | 10.59 | KCl |
| 9 | 1.4597 | 7.70 | 33.05 | 69.57 | 12.08 | KCl |
| 10 | 1.5251 | 7.16 | 38.38 | 71.84 | 12.05 | KCl |
| 11 | 1.5890 | 6.50 | 41.34 | 65.19 | 15.48 | KCl |
| 12 | 1.6241 | 6.42 | 43.68 | 67.34 | 15.57 | KCl |
| 13 | 1.6385 | 7.09 | 46.21 | 66.84 | 16.23 | KCl |
| 14, S4 | 1.6511 | 9.36 | 48.36 | 19.73 | 44.10 | KCl + KCl·CaCl2 |
| 15 | 1.6612 | 7.99 | 51.52 | 19.08 | 53.98 | KCl·CaCl2 |
| 16 | 1.6708 | 5.85 | 53.98 | 17.23 | 55.68 | KCl·CaCl2 |
| 17 | 1.6765 | 4.66 | 55.38 | 10.18 | 56.31 | KCl·CaCl2 |
| 18, S5 | 1.6820 | 3.69 | 56.56 | 8.79 | 60.30 | KCl·CaCl2 + CaCl2·2H2O |
| 19 | 1.7013 | 2.43 | 56.82 | 1.34 | 63.88 | CaCl2·2H2O |
| 20 | 1.7214 | 1.95 | 57.12 | 1.16 | 63.62 | CaCl2·2H2O |
| 21, F | 1.7432 | 0.00 | 58.84 | — | — | CaCl2·2H2O |
表1 三元体系KCl + CaCl2 + H2O 在298.2、323.2和348.2 K的固液相平衡数据
Table 1 Solid-liquid phase equilibria data of the ternary system KCl + CaCl2 + H2O at 298.2, 323.2, and 348.2 K
| 编号 | 密度 ρ/(g/cm3) | 液相组成 w(B)×102 | 湿固相组成 w(B)×102 | 平衡固相 | ||
|---|---|---|---|---|---|---|
| w(KCl) | w(CaCl2) | w(KCl) | w(CaCl2) | |||
| T = 298.2 K | ||||||
| 1, A | 1.1837 | 26.18 | 0.00 | — | — | KCl |
| 2 | 1.1919 | 23.23 | 2.77 | 59.29 | 1.54 | KCl |
| 3 | 1.1953 | 21.07 | 5.16 | 59.94 | 2.63 | KCl |
| 4 | 1.2130 | 18.31 | 8.14 | 53.82 | 4.57 | KCl |
| 5 | 1.2217 | 15.55 | 11.26 | 72.01 | 3.67 | KCl |
| 6 | 1.2459 | 12.07 | 15.57 | 51.16 | 8.68 | KCl |
| 7 | 1.2605 | 9.80 | 19.19 | 62.52 | 8.00 | KCl |
| 8 | 1.2847 | 7.58 | 22.71 | 80.77 | 4.75 | KCl |
| 9 | 1.3010 | 5.58 | 26.41 | 71.16 | 7.82 | KCl |
| 10 | 1.3383 | 4.91 | 28.74 | 53.74 | 14.18 | KCl |
| 11 | 1.3490 | 4.07 | 31.84 | 67.50 | 10.44 | KCl |
| 12 | 1.3873 | 3.58 | 34.72 | 66.73 | 11.79 | KCl |
| 13 | 1.3961 | 3.34 | 36.81 | 63.62 | 13.41 | KCl |
| 14 | 1.4252 | 3.18 | 40.47 | 63.92 | 14.24 | KCl |
| 15 | 1.4503 | 3.52 | 43.08 | 77.75 | 9.21 | KCl |
| 16 | 1.4615 | 3.31 | 44.41 | 50.94 | 21.71 | KCl |
| 17, S1 | 1.4664 | 2.81 | 45.62 | 1.10 | 48.59 | KCl + CaCl2·6H2O |
| 18, B | 1.4570 | 0.00 | 44.83 | — | — | CaCl2·6H2O |
| T = 323.2 K | ||||||
| 1, C | 1.2032 | 29.87 | 0.00 | — | — | KCl |
| 2 | 1.2119 | 26.52 | 3.28 | 65.74 | 1.57 | KCl |
| 3 | 1.2149 | 23.39 | 6.46 | 73.40 | 2.19 | KCl |
| 4 | 1.2327 | 20.21 | 10.18 | 67.70 | 4.27 | KCl |
| 5 | 1.2474 | 17.00 | 13.43 | 66.42 | 5.55 | KCl |
| 6 | 1.2600 | 13.40 | 17.67 | 67.87 | 6.64 | KCl |
| 7 | 1.2792 | 10.97 | 21.21 | 65.99 | 8.06 | KCl |
| 8 | 1.2954 | 8.55 | 25.18 | 65.82 | 9.29 | KCl |
| 9 | 1.3149 | 6.40 | 29.11 | 67.27 | 10.41 | KCl |
| 10 | 1.3532 | 6.09 | 31.26 | 65.90 | 11.46 | KCl |
| 11 | 1.3908 | 5.12 | 36.74 | 71.33 | 11.29 | KCl |
| 12 | 1.4188 | 4.87 | 40.10 | 72.83 | 11.56 | KCl |
| 13 | 1.4559 | 5.01 | 42.97 | 76.87 | 12.51 | KCl |
| 14 | 1.4843 | 5.28 | 44.98 | 75.73 | 13.47 | KCl |
| 15 | 1.5001 | 5.63 | 47.10 | 74.76 | 13.45 | KCl |
| 16 | 1.5131 | 5.84 | 48.83 | 73.58 | 14.40 | KCl |
| 17 | 1.5526 | 7.23 | 50.86 | 33.33 | 36.61 | KCl |
| 18, S2 | 1.5571 | 7.40 | 51.88 | 40.08 | 54.27 | KCl + KCl·CaCl2 |
| 19 | 1.5556 | 7.24 | 52.74 | 10.14 | 52.97 | KCl·CaCl2 |
| 20 | 1.5508 | 5.01 | 55.02 | 26.21 | 57.97 | KCl·CaCl2 |
| 21, S3 | 1.5511 | 4.24 | 55.07 | 7.48 | 61.15 | KCl·CaCl2 + CaCl2·2H2O |
| 22 | 1.5496 | 4.10 | 55.18 | 2.57 | 62.16 | CaCl2·2H2O |
| 23 | 1.5384 | 2.18 | 55.58 | 2.05 | 60.68 | CaCl2·2H2O |
| 24, D | 1.5366 | 0.00 | 56.74 | — | — | CaCl2·2H2O |
| T = 348.2 K | ||||||
| 1, E | 1.2992 | 33.08 | 0.00 | — | — | KCl |
| 2 | 1.3057 | 29.78 | 3.64 | 77.93 | 1.56 | KCl |
| 3 | 1.3153 | 26.22 | 7.07 | 75.07 | 2.30 | KCl |
| 4 | 1.3236 | 22.94 | 10.43 | 70.94 | 4.52 | KCl |
| 5 | 1.3442 | 19.10 | 14.59 | 61.73 | 7.51 | KCl |
| 6 | 1.3490 | 15.64 | 19.79 | 65.71 | 9.51 | KCl |
| 7 | 1.3889 | 12.42 | 24.19 | 77.24 | 7.93 | KCl |
| 8 | 1.4435 | 10.21 | 28.66 | 68.01 | 10.59 | KCl |
| 9 | 1.4597 | 7.70 | 33.05 | 69.57 | 12.08 | KCl |
| 10 | 1.5251 | 7.16 | 38.38 | 71.84 | 12.05 | KCl |
| 11 | 1.5890 | 6.50 | 41.34 | 65.19 | 15.48 | KCl |
| 12 | 1.6241 | 6.42 | 43.68 | 67.34 | 15.57 | KCl |
| 13 | 1.6385 | 7.09 | 46.21 | 66.84 | 16.23 | KCl |
| 14, S4 | 1.6511 | 9.36 | 48.36 | 19.73 | 44.10 | KCl + KCl·CaCl2 |
| 15 | 1.6612 | 7.99 | 51.52 | 19.08 | 53.98 | KCl·CaCl2 |
| 16 | 1.6708 | 5.85 | 53.98 | 17.23 | 55.68 | KCl·CaCl2 |
| 17 | 1.6765 | 4.66 | 55.38 | 10.18 | 56.31 | KCl·CaCl2 |
| 18, S5 | 1.6820 | 3.69 | 56.56 | 8.79 | 60.30 | KCl·CaCl2 + CaCl2·2H2O |
| 19 | 1.7013 | 2.43 | 56.82 | 1.34 | 63.88 | CaCl2·2H2O |
| 20 | 1.7214 | 1.95 | 57.12 | 1.16 | 63.62 | CaCl2·2H2O |
| 21, F | 1.7432 | 0.00 | 58.84 | — | — | CaCl2·2H2O |
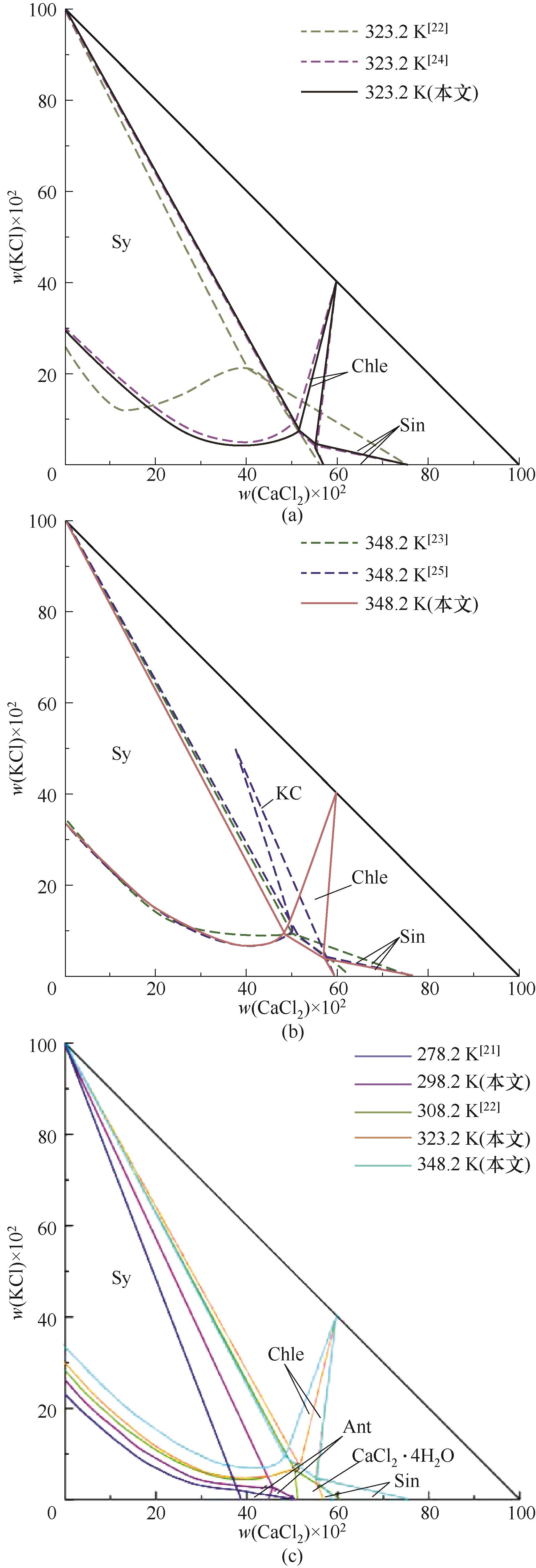
图10 三元体系KCl + CaCl2 + H2O对比相图Sy—KCl; Chle—KCl·CaCl2; KC—2KCl·CaCl2·2H2O; Ant—CaCl2·6H2O; Sin—CaCl2·2H2O
Fig.10 Comparison of phase diagrams of the ternary system KCl + CaCl2 + H2O
| 体系 | αMX | BMX | W1,MX | U1,MX | V1,MX | 文献 | ||
|---|---|---|---|---|---|---|---|---|
| T = 298.2 K | [ | |||||||
| KCl-H2O | 13 | 0 | 9.1029 | 0 | -3.7326 | -3.1452 | 0.3974 | |
| CaCl2-H2O | 13 | 2 | 57.5105 | 163.5972 | 23.2003 | 71.0962 | -79.9384 | |
| T = 323.2 K | ||||||||
| KCl-H2O | 13 | 0 | 10.4427 | 0 | -3.2804 | -2.3879 | 0.4077 | |
| CaCl2-H2O | 13 | 2 | 90.4708 | 88.6000 | 15.0772 | 57.7981 | -60.2934 | |
| T = 348.2 K | ||||||||
| KCl-H2O | 13 | 0 | 15.4538 | 0 | 0.2626 | 6.1647 | -4.7684 | |
| CaCl2-H2O | 13 | 2 | 119.6073 | 14.5708 | 4.9119 | 39.4562 | -37.3883 | |
表2 三元体系KCl + CaCl2 + H2O 298.2、323.2和348.2 K单盐参数
Table 2 Single salt parameters of the ternary system KCl + CaCl2 + H2O at 298.2, 323.2, and 348.2 K
| 体系 | αMX | BMX | W1,MX | U1,MX | V1,MX | 文献 | ||
|---|---|---|---|---|---|---|---|---|
| T = 298.2 K | [ | |||||||
| KCl-H2O | 13 | 0 | 9.1029 | 0 | -3.7326 | -3.1452 | 0.3974 | |
| CaCl2-H2O | 13 | 2 | 57.5105 | 163.5972 | 23.2003 | 71.0962 | -79.9384 | |
| T = 323.2 K | ||||||||
| KCl-H2O | 13 | 0 | 10.4427 | 0 | -3.2804 | -2.3879 | 0.4077 | |
| CaCl2-H2O | 13 | 2 | 90.4708 | 88.6000 | 15.0772 | 57.7981 | -60.2934 | |
| T = 348.2 K | ||||||||
| KCl-H2O | 13 | 0 | 15.4538 | 0 | 0.2626 | 6.1647 | -4.7684 | |
| CaCl2-H2O | 13 | 2 | 119.6073 | 14.5708 | 4.9119 | 39.4562 | -37.3883 | |
| T/K | WMNX | QMNX | UMNX | 文献 |
|---|---|---|---|---|
| 298.2 | 216.2946 | -333.0332 | 254.5483 | [ |
| 323.2 | 201.1790 | -322.7688 | 255.6148 | |
| 348.2 | 157.8346 | -267.3083 | 217.4444 |
表3 三元体系KCl + CaCl2 + H2O 298.2、323.2和348.2 K混合参数
Table 3 Mixing parameters of the ternary system KCl + CaCl2 + H2O at 298.2, 323.2, and 348.2 K
| T/K | WMNX | QMNX | UMNX | 文献 |
|---|---|---|---|---|
| 298.2 | 216.2946 | -333.0332 | 254.5483 | [ |
| 323.2 | 201.1790 | -322.7688 | 255.6148 | |
| 348.2 | 157.8346 | -267.3083 | 217.4444 |
| 固相 | T/K | Ksp | 文献 |
|---|---|---|---|
| KCl | 298.2 | 8.1347 | [ |
| 323.2 | 12.7311 | ||
| 348.2 | 16.9827 | ||
| CaCl2·6H2O | 298.2 | 7.4367×103 | |
| CaCl2·2H2O | 323.3 | 2.5202×106 | |
| 348.2 | 6.3200×105 | ||
| KCl·CaCl2 | 323.2 | 2.9535×108 | |
| 348.2 | 7.8489×107 |
表4 三元体系KCl + CaCl2 + H2O 298.2、323.2和348.2 K各盐溶度积常数
Table 4 Ksp of solid phase in the ternary system KCl + CaCl2 + H2O at 298.2, 323.2, and 348.2 K
| 固相 | T/K | Ksp | 文献 |
|---|---|---|---|
| KCl | 298.2 | 8.1347 | [ |
| 323.2 | 12.7311 | ||
| 348.2 | 16.9827 | ||
| CaCl2·6H2O | 298.2 | 7.4367×103 | |
| CaCl2·2H2O | 323.3 | 2.5202×106 | |
| 348.2 | 6.3200×105 | ||
| KCl·CaCl2 | 323.2 | 2.9535×108 | |
| 348.2 | 7.8489×107 |

图11 三元体系KCl + CaCl2 + H2O 298.2(a)、323.2(b)、348.2 K(c)实验与计算相图
Fig.11 Experimental and calculated phase diagrams of the ternary system KCl + CaCl2 + H2O at 298.2(a), 323.2(b), and 348.2 K(c)
| 1 | 侯殿保, 贺茂勇, 陈育刚, 等. 资源优化配置与循环经济在钾资源开发利用中的应用分析[J]. 化工进展, 2023, 42(6): 3197-3208. |
| Hou D B, He M Y, Chen Y G, et al. Application analysis of resource allocation optimization and circular economy in development and utilization of potassium resources[J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3197-3208. | |
| 2 | 郑绵平, 侯献华, 于常青, 等. 成盐理论引领我国找钾取得重要进展[J]. 地球学报, 2015, 36(2): 129-139. |
| Zheng M P, Hou X H, Yu C Q, et al. The leading role of salt formation theory in the breakthrough and important progress in potash deposit prospecting[J]. Acta Geoscientica Sinica, 2015, 36(2): 129-139. | |
| 3 | 郑绵平, 张震, 张永生, 等. 我国钾盐找矿规律新认识和进展[J]. 地球学报, 2012, 33(3): 280-294. |
| Zheng M P, Zhang Z, Zhang Y S, et al. Potash exploration characteristics in China: new understanding and research progress[J]. Acta Geoscientica Sinica, 2012, 33(3): 280-294. | |
| 4 | 郑绵平, 张永生, 商雯君, 等. 川东北普光地区发现新型杂卤石钾盐矿[J]. 中国地质, 2018, 45(5): 1074-1075. |
| Zheng M P, Zhang Y S, Shang W J, et al. Discovery of a new type of polyhalite potassium ore in Puguang region, northeastern Sichuan[J]. Geology in China, 2018, 45(5): 1074-1075. | |
| 5 | 牛雪, 焦鹏程, 曹养同, 等. 青海察尔汗盐湖别勒滩区段杂卤石成因及其成钾指示意义[J]. 地质学报, 2015, 89(11): 2087-2095. |
| Niu X, Jiao P C, Cao Y T, et al. The origin of polyhalite and its indicating significance for the potash formation in the bieletan area of the Qarhan salt lake, Qinghai[J]. Acta Geologica Sinica, 2015, 89(11): 2087-2095. | |
| 6 | Yu X D, Zeng Y, Yao H X, et al. Metastable phase equilibria in the aqueous ternary systems KCl + MgCl2+H2O and KCl + RbCl + H2O at 298.15 K[J]. Journal of Chemical & Engineering Data, 2011, 56(8): 3384-3391. |
| 7 | Yu X D, Zeng Y. Metastable phase equilibria in the aqueous ternary systems KCl + MgCl2 + H2O and KCl + RbCl + H2O at 323.15 K[J]. Journal of Chemical & Engineering Data, 2010, 55(12): 5771-5776. |
| 8 | Yu X D, Zeng Y, Yin Q H, et al. Solubilities, densities, and refractive indices of the ternary systems KCl + RbCl + H2O and KCl + MgCl2 + H2O at 348.15 K[J]. Journal of Chemical & Engineering Data, 2012, 57(12): 3658-3663. |
| 9 | Yu X D, Wang L, Chen J, et al. Salt-water phase equilibria in ternary systems K+(Mg2+), N H 4 + // Cl- - H2O at T = 273 K[J]. Journal of Chemical & Engineering Data, 2017, 62(4): 1427-1432. |
| 10 | Yu X D, Zeng Y, Zhang Z X. Solid-liquid metastable phase equilibria in the ternary systems KCl + NH4Cl+ H2O and NH4Cl + MgCl2 + H2O at 298.15 K[J]. Journal of Chemical & Engineering Data, 2012, 57(6): 1759-1765. |
| 11 | 任思颖, 于旭东, 罗军, 等. 298.2 K四元体系Li+, K+, N H 4 + //Cl-- H2O相平衡研究[J]. 化工学报, 2022, 73(10): 4335-4344. |
| Ren S Y, Yu X D, Luo J, et al. Phase equilibria of aqueous quaternary system Li+, K+, N H 4 + // Cl- - H2O at 298.2 K[J]. CIESC Journal, 2022, 73(10): 4335-4344. | |
| 12 | Dong O Y, Li D D, Zeng D W. A novel eutectic phase-change material: CaCl2·6H2O + NH4Cl + KCl[J]. Calphad, 2018, 63: 92-99. |
| 13 | Wang X, Zhao K Y, Guo Y F, et al. Experimental determination and thermodynamics model of solid-liquid equilibria in the ternary system (LiCl + CaCl2 + H2O) at 273.15 K[J]. Journal of Chemical & Engineering Data, 2019, 64(1): 249-254. |
| 14 | Li L, Yuan F, Guo Y F, et al. Experimental and predictive equilibrium thermodynamics of the aqueous ternary system (LiCl+ CaCl2 + H2O) at T = 288.15 K[J]. Journal of Chemical & Engineering Data, 2020, 65(9): 4369-4377. |
| 15 | Zeng D W, Xu W F, Voigt W, et al. Thermodynamic study of the system (LiCl + CaCl2 + H2O)[J]. The Journal of Chemical Thermodynamics, 2008, 40(7): 1157-1165. |
| 16 | 冯霞, 于雪峰, 姚智豪, 等.三元体系Na+(Mg2+), Ca2+ //Cl- - H2O 278.2 K稳定相平衡[J]. 无机盐工业, 2023, DOI: 10.19964/j.issn.1006-4990.2023-0074 . |
| Feng X, Yu X F, Yao Z H, et al. Phase equilibria of aqueous ternary system Na+(Mg2+), Ca2+ //Cl- - H2O at 278.2 K[J]. Inorganic Chinese Industry, 2023, DOI: 10.19964/j.issn.1006-4990.2023-0074 . | |
| 17 | 崔瑞芝, 李武, 董亚萍, 等. 298 K四元体系LiCl-MgCl2-CaCl2-H2O相平衡实验及溶解度计算[J]. 化工学报, 2018, 69(10): 4148-4155. |
| Cui R Z, Li W, Dong Y P, et al. Measurements and calculations of solid-liquid equilibria in quaternary system LiCl-MgCl2-CaCl2-H2O at 298 K[J]. CIESC Journal, 2018, 69(10): 4148-4155. | |
| 18 | 陈科, 杜理, 任思颖, 等. 四元体系LiCl + MgCl2 + CaCl2 + H2O 323.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(5): 1896-1903. |
| Chen K, Du L, Ren S Y, et al. Phase equilibria and calculation of quaternary system LiCl + MgCl2 + CaCl2 + H2O at 323.2 K[J]. CIESC Journal, 2023, 74(5): 1896-1903. | |
| 19 | Guo L J, Zeng D W, Yao Y, et al. Isopiestic measurement and solubility evaluation of the ternary system (CaCl2 + SrCl2 + H2O) at T = 298.15 K[J]. The Journal of Chemical Thermodynamics, 2013, 63: 60-66. |
| 20 | 曹大群, 金艳, 陈杭, 等. 338.15 K时四元体系CaCl2-SrCl2-BaCl2-H2O相平衡测定及溶解度计算[J]. 化工学报, 2021, 72(10): 5028-5039. |
| Cao D Q, Jin Y, Chen H, et al. Phase equilibria determination and solubility calculation of the quaternary system CaCl2-SrCl2-BaCl2-H2O at 338.15 K[J]. CIESC Journal, 2021, 72(10): 5028-5039. | |
| 21 | 姚智豪, 孟浩, 于旭东, 等. 三元体系KCl + CaCl2 + H2O在278.2 K及308.2 K下的稳定相平衡研究[J]. 矿产保护与利用, 2021, 41(6): 112-116. |
| Yao Z H, Meng H, Yu X D, et al. Stable phase equilibria of ternary system KCl + CaCl2 + H2O at 278.2 K and 308.2 K[J]. Conservation and Utilization of Mineral Resources, 2021, 41(6): 112-116. | |
| 22 | Yang J M, Hou G Y, Ding T R, et al. Measurement of solubilities in the ternary system NaCl + CaCl2 + H2O and KCl + CaCl2 + H2O at 50℃[J]. Journal of the Korean Chemical Society, 2010, 54(3): 269-274. |
| 23 | Yang J M, Liu X L, Liang P P. Solubilities of salts in the ternary systems NaCl + CaCl2 + H2O and KCl + CaCl2 + H2O at 75℃[J]. Russian Journal of Physical Chemistry A, 2011, 85(7): 1149-1154. |
| 24 | Assarsson G O. Equilibria in aqueous systems containing K+, Na+, Ca2+, Mg2+ and Cl-(Ⅰ): The ternary system CaCl2-KCl-H2O[J]. Journal of the American Chemical Society, 1950, 72(4): 1433-1436. |
| 25 | Lightfoot W J, Prutton C F. Equilibria in saturated salt solutions(Ⅱ): The ternary systems CaCl2-MgCl2-H2O, CaCl2-KCl-H2O and MgCl2-KCl-H2O at 75℃[J]. Journal of the American Chemical Society, 1947, 69(9): 2098-2100. |
| 26 | 宋彭生. 湿渣法在水盐体系相平衡研究中的应用[J]. 盐湖研究, 1991(1): 15-23, 7. |
| Song P S. Application of wet residue method in the study of phase equilibria of water-salt system[J]. Journal of Salt Lake Research, 1991(1): 15-23, 7. | |
| 27 | 中国科学院青海盐湖研究所分析室. 卤水和盐的分析方法[M]. 2版. 北京: 科学出版社, 1988: 56-59, 69-72. |
| Institute of Qinghai Salt-Lake of Chinese Academy of Sciences. Analysis Method of Brine and Salt[M]. 2nd ed. Beijing: Science Press, 1988: 56-59, 69-72. | |
| 28 | 国家市场监督管理总局, 国家标准化管理委员会. 铝及铝合金化学分析方法第33部分: 钾含量的测定火焰原子吸收光谱法: [S]. 北京: 中国标准出版社, 2020. |
| Standardization Administration of the People's Republic of China. Methods for chemical analysis of aluminium and aluminium alloys—Part 33: Determination of potassium content—Flame atomic absorption spectrometric method: [S]. Beijing: Standards Press of China, 2020. | |
| 29 | Haynes W M, Lide D R, Bruno T J. CRC Handbook of Chemistry and Physics[M]. 97thed. Boca Raton: CRC Press, 2016. |
| 30 | 成怀刚, 程芳琴. 水盐体系相分离[M]. 北京: 冶金工业出版社, 2022. |
| Cheng H G, Cheng F Q. Phase Separation of Salt-water System[M]. Beijing: Metallurgical Industry Press, 2022. | |
| 31 | 张杰, 史学伟, 赵双良, 等. 水盐体系相平衡研究进展[J]. 化工学报, 2016, 67(2): 379-389. |
| Zhang J, Shi X W, Zhao S L, et al. Progress in study on phase equilibria of salt-water systems[J]. CIESC Journal, 2016, 67(2): 379-389. | |
| 32 | Li D D, Zeng D W, Yin X, et al. Phase diagrams and thermochemical modeling of salt lake brine systems ( Ⅳ ) : Thermodynamic framework and program implementation for multicomponent systems[J]. Calphad, 2020, 71: 101806. |
| 33 | Pitzer K S, Simonson J M. Thermodynamics of multicomponent, miscible, ionic systems: theory and equations[J]. The Journal of Physical Chemistry, 1986, 90(13): 3005-3009. |
| 34 | Clegg S L, Pitzer K S. Thermodynamics of multicomponent, miscible, ionic solutions: generalized equations for symmetrical electrolytes[J]. The Journal of Physical Chemistry, 1992, 96(8): 3513-3520. |
| 35 | Clegg S L, Pitzer K S, Brimblecombe P. Thermodynamics of multicomponent, miscible, ionic solutions(2): Mixtures including unsymmetrical electrolytes[J]. The Journal of Physical Chemistry, 1992, 96(23): 9470-9479. |
| [1] | 杨百玉, 寇悦, 姜峻韬, 詹亚力, 王庆宏, 陈春茂. 炼化碱渣湿式氧化预处理过程DOM的化学转化特征[J]. 化工学报, 2023, 74(9): 3912-3920. |
| [2] | 龙臻, 王谨航, 任俊杰, 何勇, 周雪冰, 梁德青. 离子液体协同PVCap抑制天然气水合物生成实验研究[J]. 化工学报, 2023, 74(6): 2639-2646. |
| [3] | 徐文超, 孙志高, 李翠敏, 李娟, 黄海峰. 静态条件下表面活性剂E-1310对HCFC-141b水合物生成的影响[J]. 化工学报, 2023, 74(5): 2179-2185. |
| [4] | 陈科, 杜理, 曾英, 任思颖, 于旭东. 四元体系LiCl+MgCl2+CaCl2+H2O 323.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(5): 1896-1903. |
| [5] | 李明川, 樊栓狮, 徐赋海, 卢惠东, 李晓军. 水合物热分解Stefan相变模型解的存在性及Laplace变换求解[J]. 化工学报, 2023, 74(4): 1746-1754. |
| [6] | 龙臻, 王谨航, 何勇, 梁德青. 离子液体与动力学抑制剂作用下混合气体水合物生成特性研究[J]. 化工学报, 2023, 74(4): 1703-1711. |
| [7] | 胡晗, 杨亮, 李春晓, 刘道平. 天然烟浸滤液水合物法储甲烷动力学研究[J]. 化工学报, 2023, 74(3): 1313-1321. |
| [8] | 高靖博, 孙强, 李青, 王逸伟, 郭绪强. 考虑水合物结构转变的含氢气体水合物相平衡模型[J]. 化工学报, 2023, 74(2): 666-673. |
| [9] | 程文婷, 李杰, 徐丽, 程芳琴, 刘国际. AlCl3·6H2O在FeCl3、CaCl2、KCl及KCl–FeCl3溶液中溶解度的实验及预测[J]. 化工学报, 2023, 74(2): 642-652. |
| [10] | 蔡进, 王晓辉, 汤涵, 陈光进, 孙长宇. TBAB水溶液体系中半笼型水合物的相平衡预测模型[J]. 化工学报, 2023, 74(1): 408-415. |
| [11] | 周桓, 张梦丽, 郝晴, 吴思, 李杰, 徐存兵. 硫酸镁型光卤石转化钾盐镁矾的过程机制与动态规律[J]. 化工学报, 2022, 73(9): 3841-3850. |
| [12] | 刘潜, 张香兰, 李志平, 李玉龙, 韩梦醒. 油酚分离过程低共熔溶剂的筛选及萃取性能研究[J]. 化工学报, 2022, 73(9): 3915-3928. |
| [13] | 张炜, 李昊阳, 徐纯刚, 李小森. 气体水合物生成微观机理及分析方法研究进展[J]. 化工学报, 2022, 73(9): 3815-3827. |
| [14] | 郎雪梅, 姚柳眉, 樊栓狮, 李刚, 王燕鸿. 多孔材料中甲烷水合物生成的传热数值模拟研究[J]. 化工学报, 2022, 73(9): 3851-3860. |
| [15] | 张家仁, 刘海超. 大豆油与甲醇酯交换反应体系的相平衡研究[J]. 化工学报, 2022, 73(5): 1920-1929. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号