化工学报 ›› 2023, Vol. 74 ›› Issue (1): 408-415.DOI: 10.11949/0438-1157.20221178
收稿日期:2022-08-29
修回日期:2022-11-24
出版日期:2023-01-05
发布日期:2023-03-20
通讯作者:
王晓辉,陈光进
作者简介:蔡进(1998—),男,博士研究生,cj716788@163.com
基金资助:
Jin CAI( ), Xiaohui WANG(
), Xiaohui WANG( ), Han TANG, Guangjin CHEN(
), Han TANG, Guangjin CHEN( ), Changyu SUN
), Changyu SUN
Received:2022-08-29
Revised:2022-11-24
Online:2023-01-05
Published:2023-03-20
Contact:
Xiaohui WANG, Guangjin CHEN
摘要:
四丁基溴化铵(TBAB)是一种广泛使用的水合物促进剂,可以显著降低水合反应的形成条件。针对TBAB水溶液中离子与溶剂间较强的相互作用力,采用电解质NRTL方程确定液相组分的活度系数,结合含TBAB水合物的晶体结构特征和Chen-Guo水合物模型,建立了TBAB水溶液体系中水合物形成热力学条件的预测模型。与TBAB质量分数为5%~60%的221个实验数据点进行对比分析,结果显示温度平均相对误差为0.112%,预测值与实验数据具有较好的一致性。通过关联混合电解质体系下盐-溶剂间的交互作用参数,该模型还可进一步扩展到TBAB+NaCl复合盐体系下水合物相平衡条件的预测。在TBAB质量分数范围为5%~20%、NaCl质量分数为3.5%~10%的复合盐溶液体系中,CO2形成半笼型水合物的相平衡条件的实验值与预测值的温度偏差范围为0.01~1.17 K,二者比较吻合。本模型可以为水合物法气体分离、气体储运等技术的实际应用和工艺包开发提供理论基础。
中图分类号:
蔡进, 王晓辉, 汤涵, 陈光进, 孙长宇. TBAB水溶液体系中半笼型水合物的相平衡预测模型[J]. 化工学报, 2023, 74(1): 408-415.
Jin CAI, Xiaohui WANG, Han TANG, Guangjin CHEN, Changyu SUN. Prediction of the phase equilibrium of semi-clathrate hydrate in TBAB aqueous solution[J]. CIESC Journal, 2023, 74(1): 408-415.
| 组分 | Structure A | Structure B | ||
|---|---|---|---|---|
| A1 | A2 | A1 | A2 | |
| H2 | -1006.46 | 34269.46 | 270.95 | 2185.29 |
| CH4 | 7.27 | -2833.25 | 44.87 | -1015.54 |
| CO2 | -0.185 | -311.54 | 7.15 | -121.91 |
| N2 | -3.91 | -672.69 | 18.27 | -747.76 |
表1 式(12)中关联参数值
Table 1 Correlated parameters in Eq. (12)
| 组分 | Structure A | Structure B | ||
|---|---|---|---|---|
| A1 | A2 | A1 | A2 | |
| H2 | -1006.46 | 34269.46 | 270.95 | 2185.29 |
| CH4 | 7.27 | -2833.25 | 44.87 | -1015.54 |
| CO2 | -0.185 | -311.54 | 7.15 | -121.91 |
| N2 | -3.91 | -672.69 | 18.27 | -747.76 |
| 水合物 | λ1 | λ2 | β/(K·MPa-1) |
|---|---|---|---|
| A型水合物 | 3/26 | 1/26 | 9.381 |
| B型水合物 | 3/38 | 1/38 | 13.711 |
表2 TBAB水合物模型结构参数[11]
Table 2 Structural parameters of TBAB semi-clathrate hydrate[11]
| 水合物 | λ1 | λ2 | β/(K·MPa-1) |
|---|---|---|---|
| A型水合物 | 3/26 | 1/26 | 9.381 |
| B型水合物 | 3/38 | 1/38 | 13.711 |
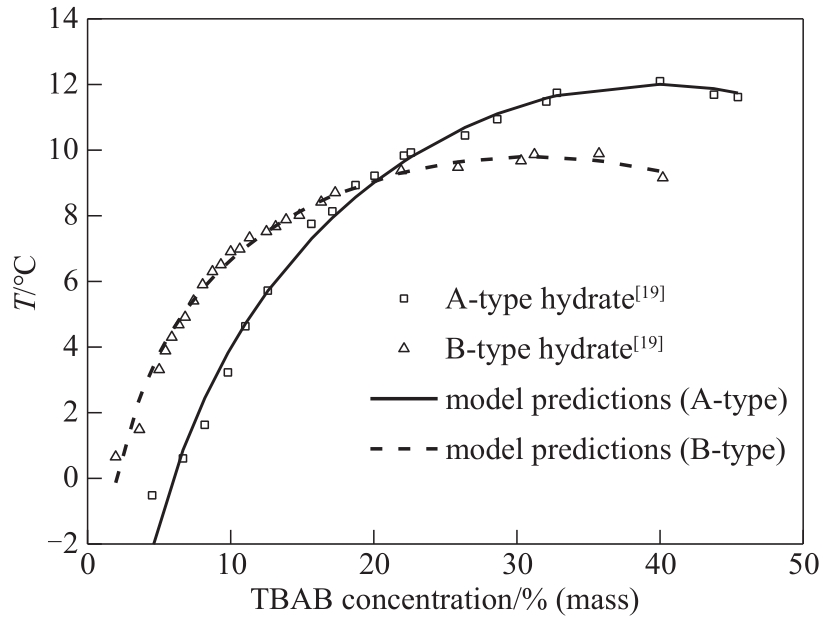
图1 (TBAB+水)体系中水合物相平衡温度-TBAB质量分数图(P=0.1 MPa)
Fig.1 Phase diagram of TBAB hydrate in terms of temperature and mass fraction in (TBAB+water) system (P=0.1 MPa)
| System | Np | Conc./%(mass) | T/K | P/MPa | AADT/% | Ref. |
|---|---|---|---|---|---|---|
| CH4 | 6 | 5 | 287.15—296.75 | 4.688—41.369 | 0.182 | [ |
| 7 | 10 | 284.4—292.2 | 1.112—10.158 | 0.208 | [ | |
| 5 | 10 | 286.35—291.45 | 2.23—8.87 | 0.198 | [ | |
| N2 | 5 | 5 | 281.3—284.3 | 4.12—9.18 | 0.008 | [ |
| 6 | 10 | 284.3—287.6 | 3.31—9.79 | 0.097 | [ | |
| 6 | 10 | 288.15—292.95 | 10.945—33.503 | 0.087 | [ | |
| CO2 | 6 | 5 | 280.2—286.5 | 0.40—3.42 | 0.113 | [ |
| 13 | 10 | 281.81—289.14 | 0.349—4.075 | 0.065 | [ | |
| CO2+N2 | 9 | 5 | 282.8—289.2 | 1.47—19.07 | 0.068 | [ |
| 16 | 10 | 284.57—288.81 | 2.108—5.851 | 0.022 | [ | |
| 10 | 15 | 284.3—291.6 | 1.42—15.94 | 0.242 | [ | |
| CH4+CO2 | 10 | 5 | 286.26—289.46 | 3.619—6.493 | 0.143 | [ |
| 8 | 10 | 288.51—291.41 | 2.828—6.540 | 0.094 | [ | |
| CO2+H2 | 10 | 5 | 277.35—284.55 | 0.25—6.23 | 0.129 | [ |
| 10 | 8.30 | 279.75—286.55 | 0.27—5.91 | 0.095 | [ | |
| 8 | 15.3 | 283.05—287.75 | 0.64—5.18 | 0.076 | [ | |
| 5 | 15.3 | 281.75—285.45 | 0.51—7.04 | 0.252 | [ | |
| overall | 140 | 0.113 |
表3 TBAB水溶液体系中B型半笼型水合物生成条件的模型计算结果
Table 3 Predictions of phase equilibrium for B-type semi-clathrate hydrate in aqueous solutions containing TBAB
| System | Np | Conc./%(mass) | T/K | P/MPa | AADT/% | Ref. |
|---|---|---|---|---|---|---|
| CH4 | 6 | 5 | 287.15—296.75 | 4.688—41.369 | 0.182 | [ |
| 7 | 10 | 284.4—292.2 | 1.112—10.158 | 0.208 | [ | |
| 5 | 10 | 286.35—291.45 | 2.23—8.87 | 0.198 | [ | |
| N2 | 5 | 5 | 281.3—284.3 | 4.12—9.18 | 0.008 | [ |
| 6 | 10 | 284.3—287.6 | 3.31—9.79 | 0.097 | [ | |
| 6 | 10 | 288.15—292.95 | 10.945—33.503 | 0.087 | [ | |
| CO2 | 6 | 5 | 280.2—286.5 | 0.40—3.42 | 0.113 | [ |
| 13 | 10 | 281.81—289.14 | 0.349—4.075 | 0.065 | [ | |
| CO2+N2 | 9 | 5 | 282.8—289.2 | 1.47—19.07 | 0.068 | [ |
| 16 | 10 | 284.57—288.81 | 2.108—5.851 | 0.022 | [ | |
| 10 | 15 | 284.3—291.6 | 1.42—15.94 | 0.242 | [ | |
| CH4+CO2 | 10 | 5 | 286.26—289.46 | 3.619—6.493 | 0.143 | [ |
| 8 | 10 | 288.51—291.41 | 2.828—6.540 | 0.094 | [ | |
| CO2+H2 | 10 | 5 | 277.35—284.55 | 0.25—6.23 | 0.129 | [ |
| 10 | 8.30 | 279.75—286.55 | 0.27—5.91 | 0.095 | [ | |
| 8 | 15.3 | 283.05—287.75 | 0.64—5.18 | 0.076 | [ | |
| 5 | 15.3 | 281.75—285.45 | 0.51—7.04 | 0.252 | [ | |
| overall | 140 | 0.113 |
| System | Np | Conc./%(mass) | T/K | P/MPa | AADT/% | Ref. |
|---|---|---|---|---|---|---|
| N2 | 5 | 20 | 286.6—289.2 | 4.24—9.49 | 0.133 | [ |
| 8 | 32 | 285.51—290.6 | 2.01—11.48 | 0.120 | [ | |
| 5 | 40 | 286.8—289.4 | 4.04—8.97 | 0.074 | [ | |
| CO2 | 10 | 19 | 283.55—290.46 | 0.42—4.36 | 0.095 | [ |
| 3 | 32 | 288.77—291.3 | 1.60—3.78 | 0.082 | [ | |
| 4 | 40.74 | 287.35—291.75 | 1.12—4.55 | 0.143 | [ | |
| 11 | 55 | 285.33—290.69 | 0.778—4.249 | 0.065 | [ | |
| 4 | 60 | 287.05—290.20 | 1.72—4.37 | 0.046 | [ | |
| CO2+H2 | 4 | 30 | 289.1—290.0 | 1.98—3.45 | 0.214 | [ |
| 5 | 32.9 | 285.95—288.55 | 0.50—4.20 | 0.205 | [ | |
| 8 | 35.6 | 286.05—288.56 | 0.54—4.12 | 0.123 | [ | |
| CO2+N2 | 5 | 30 | 286.6—291.0 | 1.06—3.70 | 0.075 | [ |
| 5 | 30 | 285.7—293.2 | 1.57—16.21 | 0.194 | [ | |
| 4 | 40.7 | 286.15—287.55 | 2.04—3.85 | 0.050 | [ | |
| overall | 81 | 0.112 |
表4 TBAB水溶液体系中A型半笼型水合物生成条件的模型计算结果
Table 4 Predictions of phase equilibrium for A-type semi-clathrate hydrate in aqueous solutions containing TBAB
| System | Np | Conc./%(mass) | T/K | P/MPa | AADT/% | Ref. |
|---|---|---|---|---|---|---|
| N2 | 5 | 20 | 286.6—289.2 | 4.24—9.49 | 0.133 | [ |
| 8 | 32 | 285.51—290.6 | 2.01—11.48 | 0.120 | [ | |
| 5 | 40 | 286.8—289.4 | 4.04—8.97 | 0.074 | [ | |
| CO2 | 10 | 19 | 283.55—290.46 | 0.42—4.36 | 0.095 | [ |
| 3 | 32 | 288.77—291.3 | 1.60—3.78 | 0.082 | [ | |
| 4 | 40.74 | 287.35—291.75 | 1.12—4.55 | 0.143 | [ | |
| 11 | 55 | 285.33—290.69 | 0.778—4.249 | 0.065 | [ | |
| 4 | 60 | 287.05—290.20 | 1.72—4.37 | 0.046 | [ | |
| CO2+H2 | 4 | 30 | 289.1—290.0 | 1.98—3.45 | 0.214 | [ |
| 5 | 32.9 | 285.95—288.55 | 0.50—4.20 | 0.205 | [ | |
| 8 | 35.6 | 286.05—288.56 | 0.54—4.12 | 0.123 | [ | |
| CO2+N2 | 5 | 30 | 286.6—291.0 | 1.06—3.70 | 0.075 | [ |
| 5 | 30 | 285.7—293.2 | 1.57—16.21 | 0.194 | [ | |
| 4 | 40.7 | 286.15—287.55 | 2.04—3.85 | 0.050 | [ | |
| overall | 81 | 0.112 |

图2 CO2+TBAB水合物相平衡条件计算结果points—experimental data[34]; lines—predicted equilibrium temperature
Fig.2 Predictions of phase equilibrium of CO2+TBAB semi-clathrate hydrate

图3 N2+TBAB水合物相平衡条件计算结果points—experimental data[22]; lines—predicted equilibrium temperature
Fig.3 Predictions of phase equilibrium of N2+TBAB semi-clathrate hydrate

图4 H2+TBAB水合物相平衡条件计算结果points—experimental data[19]; lines—predicted equilibrium temperature
Fig.4 Predictions of phase equilibrium of H2+TBAB semi-clathrate hydrate

图5 CO2+N2+TBAB水合物相平衡条件计算结果points—experimental data[26]; lines—predicted equilibrium temperature
Fig.5 Predictions of phase equilibrium of CO2+N2+TBAB semi-clathrate hydrate

图6 CO2+H2+TBAB水合物相平衡条件计算结果points—experimental data[35]; lines—predicted equilibrium temperature
Fig.6 Predictions of phase equilibrium of CO2+H2+TBAB semi-clathrate hydrate

图7 CH4(1)+N2(2)+TBAB水合物相平衡条件计算结果points—experimental data[36]; lines—predicted equilibrium temperature
Fig.7 Predictions of phase equilibrium of CH4(1)+N2(2)+TBAB semi-clathrate hydrate (x1=0.3)

图8 CO2+TBAB+NaCl水合物相平衡条件计算结果points—experimental data[37]; lines—predicted equilibrium temperature; Na3TBA5 denotes the mass composition of aqueous system is 3.35% (mass) NaCl and 5% (mass) TBAB
Fig.8 Predictions of phase equilibrium of CO2+TBAB+NaCl semi-clathrate hydrate
| 1 | Sloan E D, Koh C A. Clathrate Hydrates of Natural Gases[M]. 3rd ed. Boca Raton: CRC Press, 2008. |
| 2 | 孙长宇, 黄强, 陈光进. 气体水合物形成的热力学与动力学研究进展[J]. 化工学报, 2006, 57(5): 1031-1039. |
| Sun C Y, Huang Q, Chen G J. Progress of thermodynamics and kinetics of gas hydrate formation[J]. Journal of Chemical Industry and Engineering (China), 2006, 57(5): 1031-1039. | |
| 3 | 王晓辉, 许强, 郑华星, 等. 天然气水合物置换开采的能源效率研究[J]. 化工学报, 2020, 71(12): 5754-5762. |
| Wang X H, Xu Q, Zheng H X, et al. Energy efficiency analysis of natural gas hydrates production method[J]. CIESC Journal, 2020, 71(12): 5754-5762. | |
| 4 | Chen J, Yan K L, Chen G J, et al. Insights into the formation mechanism of hydrate plugging in pipelines[J]. Chemical Engineering Science, 2015, 122: 284-290. |
| 5 | 樊栓狮, 程宏远, 陈光进, 等. 水合物法分离技术研究[J]. 现代化工, 1999, 19(2): 11-14. |
| Fan S S, Cheng H Y, Chen G J, et al. Separation technique based on gas hydrate formation[J]. Modern Chemical Industry, 1999, 19(2): 11-14. | |
| 6 | Fukumoto A, Paricaud P, Dalmazzone D, et al. Modeling the dissociation conditions of carbon dioxide+TBAB, TBAC, TBAF, and TBPB semiclathrate hydrates[J]. Journal of Chemical & Engineering Data, 2014, 59(10): 3193-3204. |
| 7 | Avula V R, Gupta P, Gardas R L, et al. Thermodynamic modeling of phase equilibrium of carbon dioxide clathrate hydrate in aqueous solutions of promoters and inhibitors suitable for gas separation[J]. Asia-Pacific Journal of Chemical Engineering, 2017, 12(5): 709-722. |
| 8 | Eslamimanesh A, Mohammadi A H, Richon D. Thermodynamic modeling of phase equilibria of semi-clathrate hydrates of CO2, CH4, or N2+tetra-n-butylammonium bromide aqueous solution[J]. Chemical Engineering Science, 2012, 81: 319-328. |
| 9 | Chen G J, Guo T M. Thermodynamic modeling of hydrate formation based on new concepts[J]. Fluid Phase Equilibria, 1996, 122(1/2): 43-65. |
| 10 | Wang L B, Cui J L, Sun C Y, et al. Review on the applications and modifications of the Chen-Guo model for hydrate formation and dissociation[J]. Energy & Fuels, 2021, 35(4): 2936-2964. |
| 11 | Ma Q L, Qi J L, Chen G J, et al. Modeling study on phase equilibria of semiclathrate hydrates of pure gases and gas mixtures in aqueous solutions of TBAB and TBAF[J]. Fluid Phase Equilibria, 2016, 430: 178-187. |
| 12 | 门文欣, 彭庆收, 桂霞. 不同季铵盐作用下的CO2水合物相平衡[J]. 化工学报, 2022, 73(4): 1472-1482. |
| Men W X, Peng Q S, Gui X. Phase equilibrium of CO2 hydrate in the presence of four different quaternary ammonium salts[J]. CIESC Journal, 2022, 73(4): 1472-1482. | |
| 13 | Chen C C, Song Y H. Generalized electrolyte-NRTL model for mixed-solvent electrolyte systems[J]. AIChE Journal, 2004, 50(8): 1928-1941. |
| 14 | Chen C C, Evans L B. A local composition model for the excess Gibbs energy of aqueous electrolyte systems[J]. AIChE Journal, 1986, 32(3): 444-454. |
| 15 | Renon H, Prausnitz J M. Local compositions in thermodynamic excess functions for liquid mixtures[J]. AIChE Journal, 1968, 14(1): 135-144. |
| 16 | Chen G J, Guo T M. A new approach to gas hydrate modelling[J]. Chemical Engineering Journal, 1998, 71(2): 145-151. |
| 17 | Lindenbaum S, Boyd G E. Osmotic and activity coefficients for the symmetrical tetraalkyl ammonium halides in aqueous solution at 25℃[J]. The Journal of Physical Chemistry, 1964, 68(4): 911-917. |
| 18 | Oyama H, Shimada W, Ebinuma T, et al. Phase diagram, latent heat, and specific heat of TBAB semiclathrate hydrate crystals[J]. Fluid Phase Equilibria, 2005, 234(1/2): 131-135. |
| 19 | Arjmandi M, Chapoy A, Tohidi B. Equilibrium data of hydrogen, methane, nitrogen, carbon dioxide, and natural gas in semi-clathrate hydrates of tetrabutyl ammonium bromide[J]. Journal of Chemical & Engineering Data, 2007, 52(6): 2153-2158. |
| 20 | Mohammadi A H, Eslamimanesh A, Belandria V, et al. Phase equilibria of semiclathrate hydrates of CO2, N2, CH4, or H2+tetra-n-butylammonium bromide aqueous solution[J]. Journal of Chemical & Engineering Data, 2011, 56(10): 3855-3865. |
| 21 | Lee S, Park S, Lee Yet al. Guest gas enclathration in semiclathrates of tetra-n-butylammonium bromide: stability condition and spectroscopic analysis[J]. Langmuir, 2011, 27(17): 10597-10603. |
| 22 | Lee S, Lee Y, Park Set al. Phase equilibria of semiclathrate hydrate for nitrogen in the presence of tetra-n-butylammonium bromide and fluoride[J]. Journal of Chemical & Engineering Data, 2010, 55(12): 5883-5886. |
| 23 | Li S F, Fan S S, Wang J Q, et al. Semiclathrate hydrate phase equilibria for CO2 in the presence of tetra-n-butylammonium halide (bromide, chloride, or fluoride)[J]. Journal of Chemical & Engineering Data, 2010, 55(9): 3212-3215. |
| 24 | Ye N, Zhang P. Equilibrium data and morphology of tetra-n-butyl ammonium bromide semiclathrate hydrate with carbon dioxide[J]. Journal of Chemical & Engineering Data, 2012, 57(5): 1557-1562. |
| 25 | Belandria V, Mohammadi A H, Eslamimanesh A, et al. Phase equilibrium measurements for semi-clathrate hydrates of the (CO2+N2+tetra-n-butylammonium bromide) aqueous solution systems: Part 2[J]. Fluid Phase Equilibria, 2012, 322: 105-112. |
| 26 | Meysel P, Oellrich L, Bishnoi P R, et al. Experimental investigation of incipient equilibrium conditions for the formation of semi-clathrate hydrates from quaternary mixtures of (CO2+N2+TBAB+H2O)[J]. The Journal of Chemical Thermodynamics, 2011, 43(10): 1475-1479. |
| 27 | Mohammadi A H, Eslamimanesh A, Belandria V, et al. Phase equilibrium measurements for semi-clathrate hydrates of the (CO2+N2+tetra-n-butylammonium bromide) aqueous solution system[J]. The Journal of Chemical Thermodynamics, 2012, 46: 57-61. |
| 28 | Acosta H Y, Bishnoi P R, Clarke M A. Experimental measurements of the thermodynamic equilibrium conditions of tetra-n-butylammonium bromide semiclathrates formed from synthetic landfill gases[J]. Journal of Chemical & Engineering Data, 2011, 56(1): 69-73. |
| 29 | Li X S, Xia Z M, Chen Z Y, et al. Equilibrium hydrate formation conditions for the mixtures of CO2+H2+tetrabutyl ammonium bromide[J]. Journal of Chemical & Engineering Data, 2010, 55(6): 2180-2184. |
| 30 | Kim S M, Lee J D, Lee H J, et al. Gas hydrate formation method to capture the carbon dioxide for pre-combustion process in IGCC plant[J]. International Journal of Hydrogen Energy, 2011, 36(1): 1115-1121. |
| 31 | Muromachi S, Hashimoto H, Maekawa T, et al. Phase equilibrium and characterization of ionic clathrate hydrates formed with tetra-n-butylammonium bromide and nitrogen gas[J]. Fluid Phase Equilibria, 2016, 413: 249-253. |
| 32 | Mohammadi A H, Eslamimanesh A, Richon D. Semi-clathrate hydrate phase equilibrium measurements for the CO2+H2/CH4+tetra-n-butylammonium bromide aqueous solution system[J]. Chemical Engineering Science, 2013, 94: 284-290. |
| 33 | 鲁涛, 张郁, 李小森, 等. CO2-N2-TBAB和CO2-N2-THF体系的水合物平衡生成条件[J]. 过程工程学报, 2009, 9(3): 541-544. |
| Lu T, Zhang Y, Li X S, et al. Equilibrium conditions of hydrate formation in the systems of CO2-N2-TBAB and CO2-N2-THF[J]. The Chinese Journal of Process Engineering, 2009, 9(3): 541-544. | |
| 34 | Zhou X B, Long Z, He Y, et al. Phase equilibria and the crystallographic properties of TBAB-CO2 semiclathrate hydrates[J]. Journal of Chemical & Engineering Data, 2018, 63(5): 1249-1255. |
| 35 | Wang S, Danner M, Kuchling T, et al. Measurement of the three-phase (vapour+liquid+solid) equilibrium conditions of semi-clathrates formed from mixtures of CO2, CO and H2 [J]. The Journal of Chemical Thermodynamics, 2013, 56: 149-152. |
| 36 | Zhong D L, Englezos P. Methane separation from coal mine methane gas by tetra-n-butyl ammonium bromide semiclathrate hydrate formation[J]. Energy & Fuels, 2012, 26(4): 2098-2106. |
| 37 | Godishala K K, Sangwai J S, Sami N A, et al. Phase stability of semiclathrate hydrates of carbon dioxide in synthetic sea water[J]. Journal of Chemical & Engineering Data, 2013, 58(4): 1062-1067. |
| [1] | 于旭东, 李琪, 陈念粗, 杜理, 任思颖, 曾英. 三元体系KCl + CaCl2 + H2O 298.2、323.2及348.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(8): 3256-3265. |
| [2] | 汪尔奇, 彭书舟, 杨震, 段远源. 含HFO混合体系气液相平衡的理论模型评价[J]. 化工学报, 2023, 74(8): 3216-3225. |
| [3] | 陈科, 杜理, 曾英, 任思颖, 于旭东. 四元体系LiCl+MgCl2+CaCl2+H2O 323.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(5): 1896-1903. |
| [4] | 高靖博, 孙强, 李青, 王逸伟, 郭绪强. 考虑水合物结构转变的含氢气体水合物相平衡模型[J]. 化工学报, 2023, 74(2): 666-673. |
| [5] | 程文婷, 李杰, 徐丽, 程芳琴, 刘国际. AlCl3·6H2O在FeCl3、CaCl2、KCl及KCl–FeCl3溶液中溶解度的实验及预测[J]. 化工学报, 2023, 74(2): 642-652. |
| [6] | 周桓, 张梦丽, 郝晴, 吴思, 李杰, 徐存兵. 硫酸镁型光卤石转化钾盐镁矾的过程机制与动态规律[J]. 化工学报, 2022, 73(9): 3841-3850. |
| [7] | 刘潜, 张香兰, 李志平, 李玉龙, 韩梦醒. 油酚分离过程低共熔溶剂的筛选及萃取性能研究[J]. 化工学报, 2022, 73(9): 3915-3928. |
| [8] | 张家仁, 刘海超. 大豆油与甲醇酯交换反应体系的相平衡研究[J]. 化工学报, 2022, 73(5): 1920-1929. |
| [9] | 姜焱龙, 张妮, 李淡然, 朱冰冰, 蒋怡晨, 陈海军, 朱跃钊. 基于COSMO-RS方法筛选离子液体用于焦油脱除[J]. 化工学报, 2022, 73(4): 1704-1713. |
| [10] | 门文欣, 彭庆收, 桂霞. 不同季铵盐作用下的CO2水合物相平衡[J]. 化工学报, 2022, 73(4): 1472-1482. |
| [11] | 吴子睿, 孙瑞, 石凌峰, 田华, 王轩, 舒歌群. CO2混合工质的气液相平衡的混合规则对比与预测研究[J]. 化工学报, 2022, 73(4): 1483-1492. |
| [12] | 孙裕坤, 杨焘, 吴江涛. R32+R1234yf+R1234ze(E)混合制冷剂气液相平衡实验研究[J]. 化工学报, 2022, 73(3): 1063-1071. |
| [13] | 许昊, 陈伟, 李邹路. 以[Li(TX-7)]SCN/H2O为工质对的第二类热泵特性研究[J]. 化工学报, 2022, 73(2): 577-586. |
| [14] | 高腾飞, 李国选, 雷志刚. 从催化裂化柴油中分离联苯的溶剂筛选:实验和计算热力学[J]. 化工学报, 2022, 73(12): 5314-5323. |
| [15] | 彭昌炜, 桑世华, 崔瑞芝, 任红保. 五元体系NaBr-KBr-MgBr2-CaBr2-H2O在298.15 K下的空间立体相图研究[J]. 化工学报, 2022, 73(11): 4850-4858. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号