化工学报 ›› 2021, Vol. 72 ›› Issue (6): 2972-3001.DOI: 10.11949/0438-1157.20210108
收稿日期:2021-01-18
修回日期:2021-04-06
出版日期:2021-06-05
发布日期:2021-06-05
通讯作者:
杨宇森
作者简介:周石杰(1998—),女,硕士研究生,基金资助:
ZHOU Shijie( ),REN Zhen,YANG Yusen(
),REN Zhen,YANG Yusen( ),WEI Min
),WEI Min
Received:2021-01-18
Revised:2021-04-06
Online:2021-06-05
Published:2021-06-05
Contact:
YANG Yusen
摘要:
金属氧化物作为一类重要工业催化剂,广泛应用于合成氨工业、能源化工、精细化工等重要的工业生产过程。金属氧化物的形貌对其性能有重要的影响,具有特定形貌的金属氧化物催化剂因其结构上的优势,使其在许多方面表现出不同于常规块体材料的独特性能,成为当前材料科学领域的研究热点。本文总结了不同形貌的金属氧化物的制备方法、生长机制及其结构特性,聚焦于金属氧化物在氧化反应、加氢反应以及蒸汽重整反应中的最新研究进展。最后,进一步讨论了金属氧化物催化剂未来的发展趋势以及面临的挑战,并提出了解决这些问题的有效方案。
中图分类号:
周石杰, 任祯, 杨宇森, 卫敏. 不同形貌金属氧化物的制备及其在工业催化反应中的应用[J]. 化工学报, 2021, 72(6): 2972-3001.
ZHOU Shijie, REN Zhen, YANG Yusen, WEI Min. Preparation and application of metal oxides with various morphology for industrial catalysis[J]. CIESC Journal, 2021, 72(6): 2972-3001.
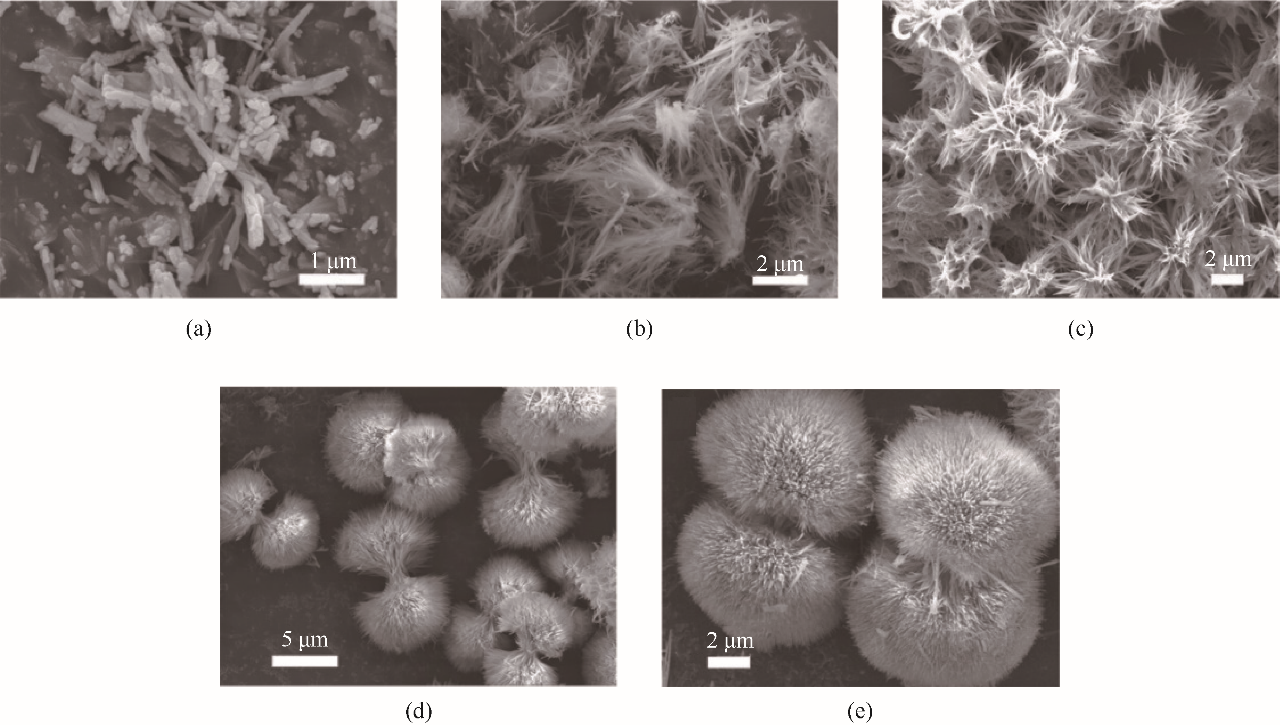
图1 在160℃下前体氧化物合成的FE-SEM图:2 h (a)、3 h (b)、4 h (c)、5 h (d)、6 h (e) [48]
Fig.1 FE-SEM images of the precursors synthesized at 160℃ for 2 h (a), 3 h (b), 4 h (c), 5 h (d), 6 h (e)[48]

图2 多壳ZnO空心微球的演化过程。碳微球在加热之前(室温)和在不同温度(400℃、400℃下30 min、420℃、440℃、460℃、480℃和500℃)下加热后浸入硝酸锌溶液中的透射电子显微镜图像。3 mol/L和5 mol/L硝酸锌溶液分别用于样品Ⅰ、Ⅱ、Ⅲ和样品Ⅳ、Ⅴ、Ⅵ、Ⅶ、Ⅷ、Ⅸ。样品Ⅰ、Ⅱ、Ⅲ、Ⅳ、Ⅴ和Ⅵ中使用的碳质微球的直径为3 μm, 样品Ⅶ、Ⅷ和Ⅸ中使用的碳质微球的直径为4 μm。快速加热模式和中速加热模式下的温度分别在2和1℃/min下直接升高到500℃而慢速加热模式下的温度在1℃/min下直接升高到500℃并且在400℃保持30 min。在第一列中,样品Ⅰ~Ⅵ比例尺为1 μm,样品Ⅶ~Ⅸ比例尺为1.3 μm。第二列中的所有样品比例尺均为0.5 μm, 而第三列及更高列中样品比例尺均为0.3 μm(a)。通过不同的加热过程形成多壳ZnO中空微球的图示(b) [57]
Fig.2 Evolution process of the family of multishelled ZnO hollow microspheres. Transmission electron microscopy images of carbonaceous microspheres after immersion in zinc nitrate solutions before heating (room temperature) and after heating at different temperatures (400℃, 400℃ for 30 min, 420℃, 440℃, 460℃, 480℃ and 500℃). 3 and 5 mol/L zinc nitrate solutions were used in samples Ⅰ, Ⅱ, Ⅲ and samples Ⅳ, Ⅴ, Ⅵ, Ⅶ, Ⅷ, Ⅸ, respectively. The diameters of carbonaceous microspheres used in samples Ⅰ, Ⅱ, Ⅲ, Ⅳ, Ⅴ and Ⅵ are 3 μm, and those used in samples Ⅶ, Ⅷ, and Ⅸ are 4 μm. The temperature in the fast and medium heating modes is directly increased to 500℃ at 2 and 1℃/min respectively, while the temperature in the slow heating mode is increased to 500℃ at 1℃/minwith 30 min holding at 400℃. The scale bars are 1 μm for the samples from Ⅰ to Ⅵ and 1.3 μm for samples from Ⅶ to Ⅸ in the first column. All the scale bars in the second column are 0.5 μm, while the scale bars in the third and higher columns are all 0.3 μm(a). Illustration of the formation of multishelled ZnO hollow microspheres through different heating processes(b) [57]
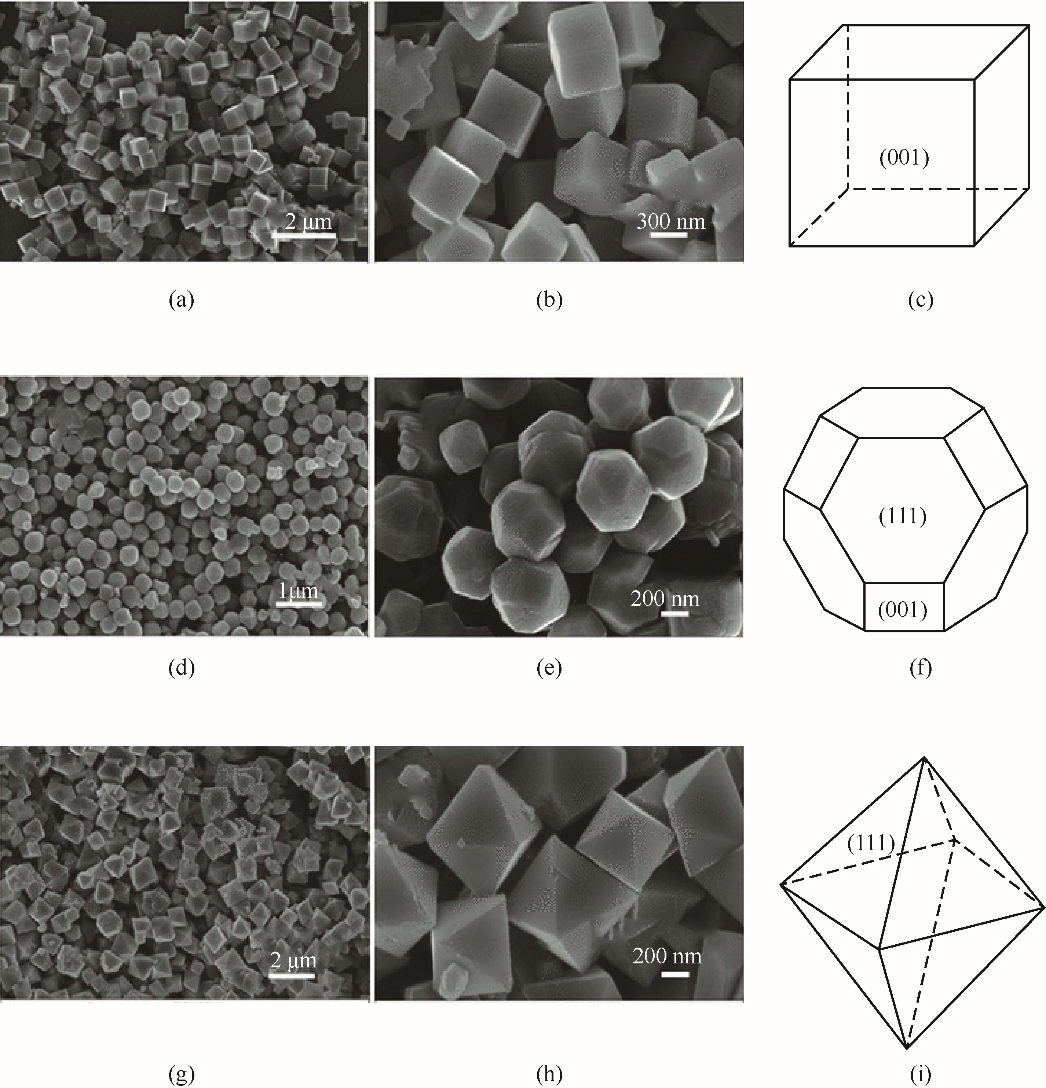
图3 三种形貌的焙烧后的Co3O4的SEM图像及其结构模型: Co3O4立方体[(a)~(c)], Co3O4截短的八面体[(d)~(f)]和Co3O4八面体[(g)~(i)][58]
Fig.3 SEM images of the three types of calcined Co3O4 and their structure models: Co3O4 cubes[(a)~(c)], Co3O4 truncated octahedra[(d)—(f)], and Co3O4 octahedra[(g)—(i)] [58]

图5 ZnO纳米晶体随时间演变为具有双蛋黄结构的ZnO空心球: 1 h (a), 12 h (b), 24 h (c), 相应演变过程的示意图(d) [64]
Fig.5 Time-dependent evolution of ZnO nanocrystals to ZnO hollow spheres with double-yolk egg structure: 1 h (a), 12 h (b), 24 h (c). The corresponding schematic graphs of the evolution process (d) [64]

图6 制备BHC-TiO2的总流程图[(a)~(d)]: 相应的BHC-TiO2制备程序的FESEM[(e)~(h)], BSE-SEM[(i)~(l)]和TEM[(m)~(p)]图。白色箭头表示破裂的空心立方结构。(e)~(h) 比例尺为1 μm, (i)~(p) 比例尺为500 nm[66]
Fig.6 Overall flowchart for fabrication of BHC-TiO2[(a)—(d)]; Corresponding FESEM[(e)—(h)], BSE-SEM [(i)—(l)] and TEM images[(m)—(p)] of the BHC-TiO2 fabrication procedure. The white arrows indicate the cracked hollow cubic structures. The scale bars are 1 μm [(e)—(h)] and 500 nm[(i)—(p)][66]
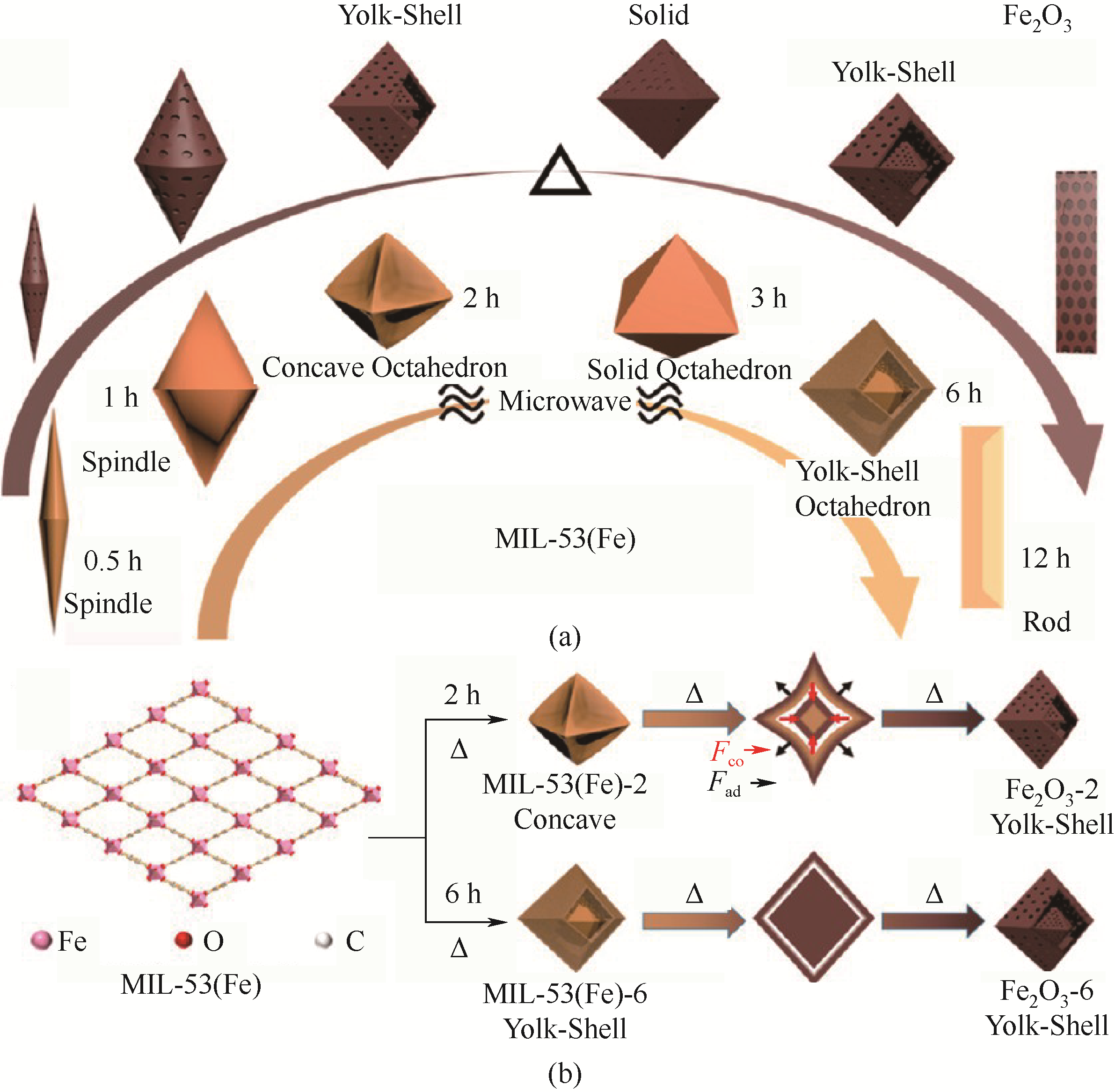
图7 MIL-53(Fe)的生长过程及其相应的铁氧化物的形貌(a)以及Fe2O3-2和Fe2O3-6的形成过程的示意图(b) [67]
Fig.7 Schematic illustration of the process of MIL-53(Fe) growth and morphology of their corresponding iron oxides (a) and the formation process of Fe2O3-2 and Fe2O3-6 (b) [67]

图8 在9.55 kW/cm2(a), 15.92 kW/cm2 (b), 22.29 kW/cm2 (c), 28.66 kW/cm2 (d)和200 kHz的激光功率密度下生长5 min的ZnO晶体的SEM图像。动力学控制(左侧)和热力学控制(右侧)过程中晶体生长的示意图(e)。所有比例尺代表长度均为500 nm[68]
Fig.8 SEM pictures of ZnO crystals grown under laser power density of 9.55 kW/cm2 (a), 15.92 kW/cm2 (b), 22.29 kW/cm2 (c), 28.66 kW/cm2 (d) in 200 kHz for 5 min. Schematic illustration of crystal growth in kinetically controlled (left direction) and thermodynamically controlled (right direction) processes (e). All scale bars represent 500 nm in length[68]

图9 Tdep对Co3O4薄膜的TC(220)、(311)、(111)、(400)和(511)的影响(a), (111)面的生长机理(b), 放大的FESEM图像(c)和在773 K下制备的Co3O4薄膜的纳米墙结构示意图(d) [69]
Fig.9 Effect of Tdep on TC(220), (311), (111), (400) and (511) of Co3O4 films (a), growth mechanism of (111) planes (b), enlarged FESEM image (c) and schematic of nanowall structure of Co3O4 film prepared at 773 K(d) [69]

图10 在不同温度下制备的样品的SEM和TEM图像: S-600[(a),(b)], S-750[(c),(d)], S-850[(e),(f)]和S-950[(g),(h)][70]
Fig.10 SEM and TEM images of samples prepared at different temperatures: S-600[(a),(b)], S-750[(c),(d)], S-850[(e),(f)] and S-950[(g),(h)][70]
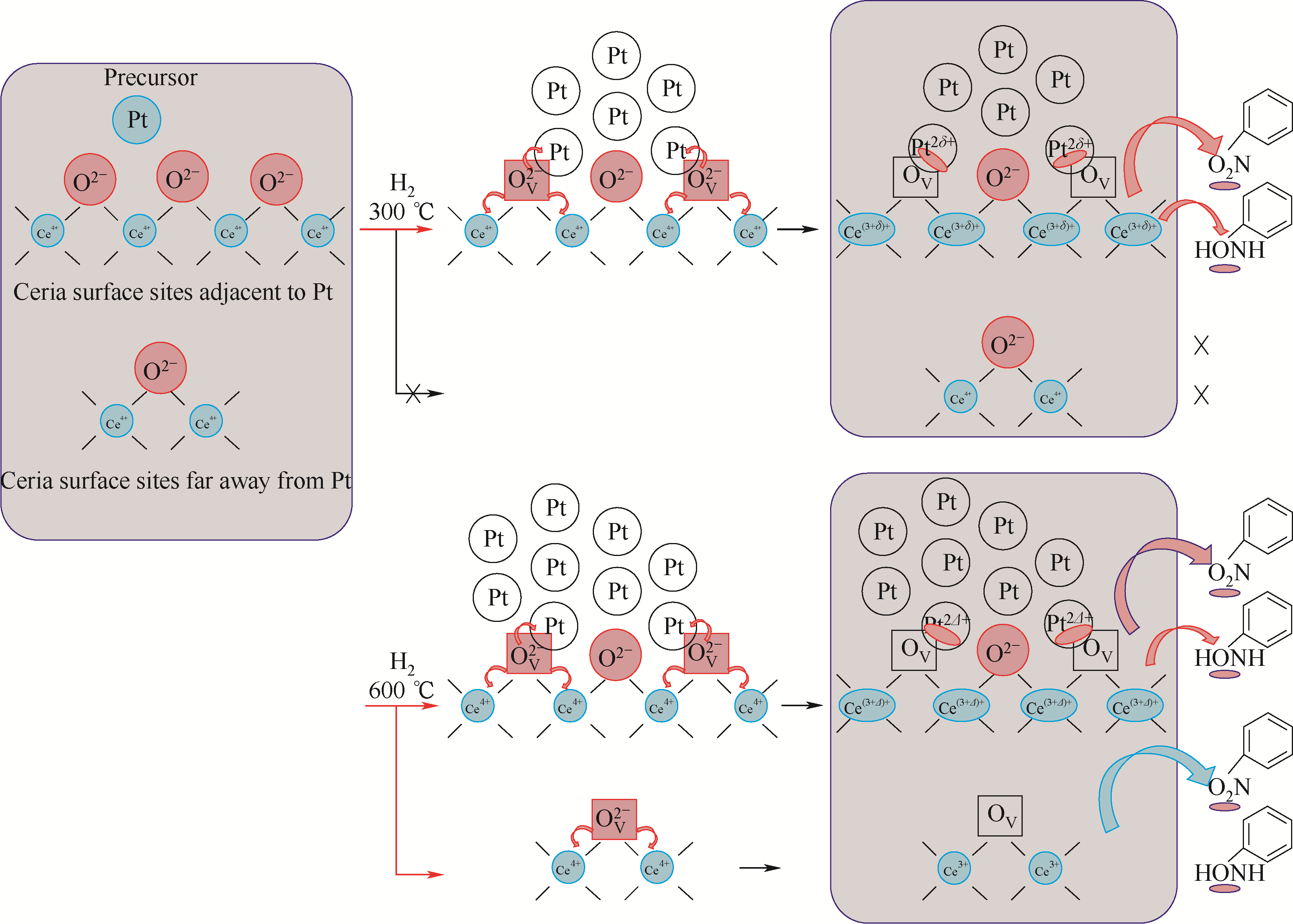
图11 高温还原产生的额外的Ce3+位点有利于反应物和中间体的吸附的示意图[75]
Fig.11 Additional Ce3+ sites derived from high-temperature reduction favoring adsorption of the reactant and intermediate[75]

图12 CeO2-R[(a),(b)], CeO2-P[(c),(d)], CeO2-C[(e),(f)]的TEM和高分辨率TEM (HRTEM) 图像, CeO2-O的SEM(g)和HRTEM(h)图像, 插图为CeO2-R, CeO2-P, CeO2-C和CeO2-O的暴露晶面[78]
Fig.12 TEM and high-resolution TEM (HRTEM) images of CeO2-R[(a),(b)], CeO2-P[(c),(d)], CeO2-C[(e),(f)], SEM(g) and HRTEM(h) images of CeO2-O. The insets schematically illustrate the crystal planes exposed on the CeO2-R, CeO2-P, CeO2-C and CeO2-O[78]
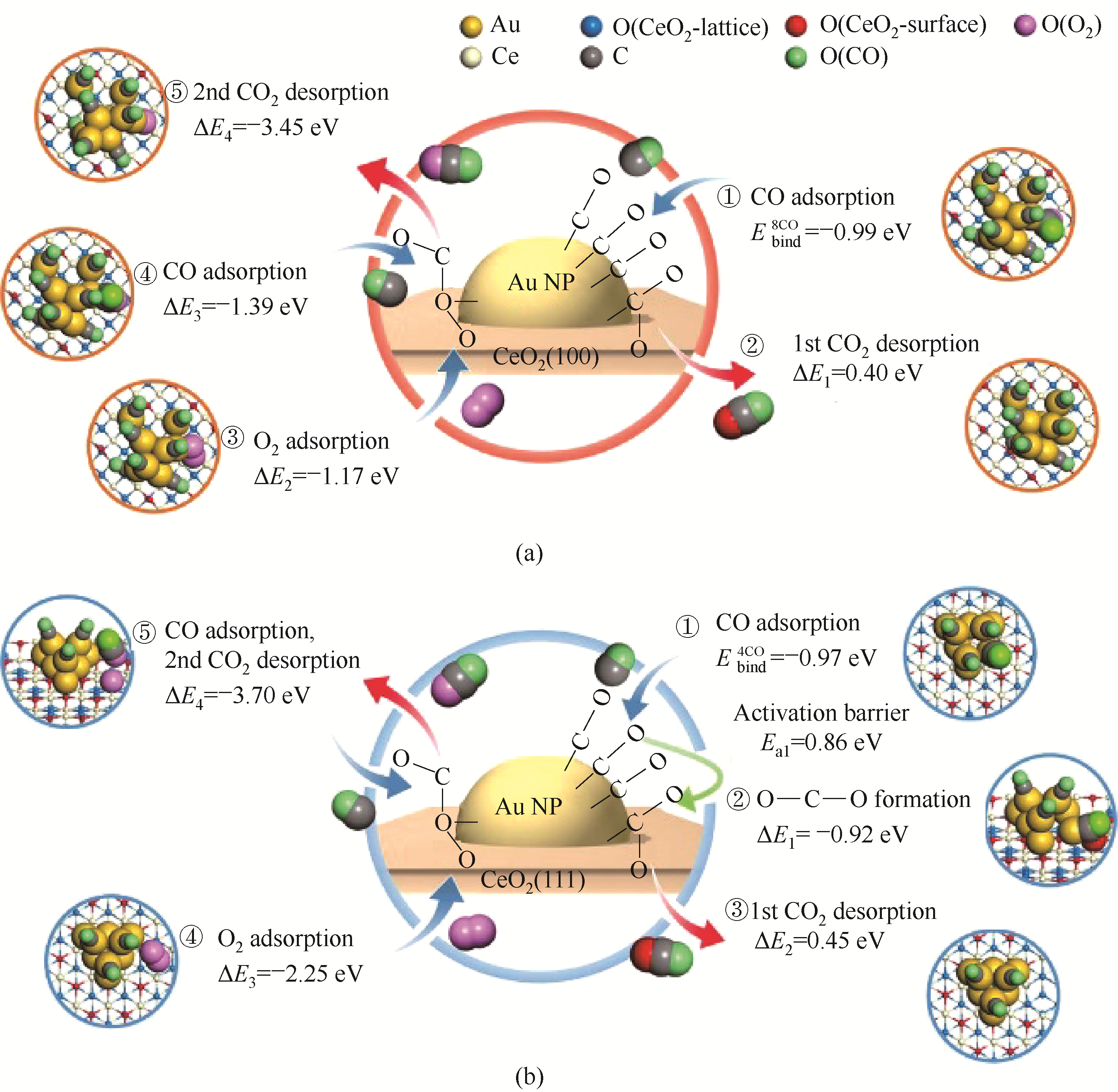
图13 具有8或4个吸附的CO分子的Au/CeO2(100) (a)和Au/CeO2(111) (b)催化的示意性CO氧化途径表明尽管Au/CeO2(111)对于附加的O—C—O形成步骤是必须的[(b)图,步骤②], 但CO氧化发生在Au-CeO2界面[86]
Fig.13 Schematic CO oxidation pathways catalyzed by Au/CeO2(100) (a) and Au/CeO2(111) (b) with 8 or 4 adsorbed CO molecules show that CO oxidation occurs at the Au-CeO2 interface, although an additional O—C—O formation step [(b), step②] is required for Au/CeO2(111) [86]

图14 α-Fe2O3-THB样品的TEM图[(ⅰ),(ⅱ)], HRTEM图(ⅲ), IFFT图像(ⅳ)(a). α-Fe2O3-QC样品的TEM图[(ⅰ),(ⅱ)], HRTEM图(ⅲ), IFFT图(ⅳ)(b). α-Fe2O3-HS样品的 TEM图(ⅰ), SAED模式(ⅱ)和示意图(ⅲ)(c)[87]
Fig.14 TEM images [(ⅰ),(ⅱ)], HRTEM image (ⅲ), IFFT image (ⅳ) of the α-Fe2O3-THB sample(a). TEM images [(ⅰ),(ⅱ)], HRTEM image (ⅲ), IFFT image (ⅳ) of α-Fe2O3-QC sample (b). TEM image (ⅰ), SAED pattern (ⅱ), and schematic illustration (ⅲ) of α-Fe2O3-HS sample(c)[87]

图15 在甲醇转化率为30%~40%时, Pd/ZnO-N (a)和Pd/ZnO-P(b)的CO选择性(反应条件: 100~200 mg催化剂, 甲醇浓度 6.4%(mol), 催化剂质量/进料气流速=0.037~0.123 g?s/ml, T = 250℃)[88]
Fig.15 CO selectivity of Pd/ZnO-N (a) and Pd/ZnO-P (b) with Pd loading amount under methanol conversion of 30%—40% [88]
| 1 | Polo-Garzon F, Bao Z H, Zhang X, et al. Surface reconstructions of metal oxides and the consequences on catalytic chemistry[J]. ACS Catalysis, 2019, 9(6): 5692-5707. |
| 2 | Jia J, Qian C, Dong Y, et al. Heterogeneous catalytic hydrogenation of CO2 by metal oxides: defect engineering-perfecting imperfection[J]. Chemical Society Reviews, 2017, 46(15): 4631-4644. |
| 3 | Patzke G R, Zhou Y, Kontic R, et al. Oxide nanomaterials: synthetic developments, mechanistic studies, and technological innovations[J]. Angewandte Chemie International Edition, 2011, 50(4): 826-859. |
| 4 | Ren Y, Ma Z, Bruce P G. Ordered mesoporous metal oxides: synthesis and applications[J]. Chemical Society Reviews, 2012, 41(14): 4909-4927. |
| 5 | Song I, Lee H, Jeon S W, et al. Time-resolved observation of V2O5/TiO2 in NH3-SCR reveals the equivalence of Brønsted and Lewis acid sites[J]. Chemical Communications, 2020, 56(98): 15450-15453. |
| 6 | Salavati-Fard T, Vasiliadou E S, Jenness G R, et al. Lewis acid site and hydrogen-bond-mediated polarization synergy in the catalysis of Diels-Alder cycloaddition by band-gap transition-metal oxides[J]. ACS Catalysis, 2019, 9(1): 701-715. |
| 7 | Tamura M, Tomishige K. Redox properties of CeO2 at low temperature: the direct synthesis of imines from alcohol and amine[J]. Angewandte Chemie International Edition, 2015, 54(3): 864-867. |
| 8 | Zhang H P, Wu C, Wang W B, et al. Effect of ceria on redox-catalytic property in mild condition: a solvent-free route for imine synthesis at low temperature[J]. Applied Catalysis B: Environmental, 2018, 227: 209-217. |
| 9 | Ren Q M, Mo S P, Peng R S, et al. Controllable synthesis of 3D hierarchical Co3O4 nanocatalysts with various morphologies for the catalytic oxidation of toluene[J]. Journal of Materials Chemistry A, 2018, 6(2): 498-509. |
| 10 | Mo S P, Li S D, Ren Q M, et al. Vertically-aligned Co3O4 arrays on Ni foam as monolithic structured catalysts for CO oxidation: effects of morphological transformation[J]. Nanoscale, 2018, 10(16): 7746-7758. |
| 11 | González-Prior J, López-Fonseca R, Gutiérrez-Ortiz J I, et al. Oxidation of 1, 2-dichloroethane over nanocube-shaped Co3O4 catalysts[J]. Applied Catalysis B: Environmental, 2016, 199: 384-393. |
| 12 | Zhou X C, Liu Z, Wang Y F, et al. Facet effect of Co3O4 nanocrystals on visible-light driven water oxidation[J]. Applied Catalysis B: Environmental, 2018, 237: 74-84. |
| 13 | Zhang P, Yu L, Lou X W. Construction of heterostructured Fe2O3-TiO2 microdumbbells for photoelectrochemical water oxidation[J]. Angewandte Chemie International Edition, 2018, 57(46): 15076-15080. |
| 14 | Chen W Y, Han B, Tian C, et al. MOFs-derived ultrathin holey Co3O4 nanosheets for enhanced visible light CO2 reduction[J]. Applied Catalysis B: Environmental, 2019, 244: 996-1003. |
| 15 | Zhang R, Fang Y Y, Chen T, et al. Enhanced photoelectrochemical water oxidation performance of Fe2O3 nanorods array by S doping[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(9): 7502-7506. |
| 16 | Wang Z, Lang X J. Visible light photocatalysis of dye-sensitized TiO2: the selective aerobic oxidation of amines to imines[J]. Applied Catalysis B: Environmental, 2018, 224: 404-409. |
| 17 | Hao X D, Chen C L, Saito M, et al. Direct imaging for single molecular chain of surfactant on CeO2 nanocrystals[J]. Small, 2018, 14(31): 1801093. |
| 18 | Wei J F, Wen X J, Zhu F. Influence of surfactant on the morphology and photocatalytic activity of anatase TiO2 by solvothermal synthesis[J]. Journal of Nanomaterials, 2018, 2018: 3086269. |
| 19 | Saravanakumar B, Radhakrishnan C, Ramasamy M, et al. Surfactant determines the morphology, structure and energy storage features of CuO nanostructures[J]. Results in Physics, 2019, 13: 102185. |
| 20 | Zhu Z Y, Han C, Li T T, et al. MOF-templated syntheses of porous Co3O4 hollow spheres and micro-flowers for enhanced performance in supercapacitors[J]. CrystEngComm, 2018, 20(27): 3812-3816. |
| 21 | Memar A, Phan C M, Tade M O. Influence of surfactants on Fe2O3 nanostructure photoanode[J]. International Journal of Hydrogen Energy, 2012, 37(22): 16835-16843. |
| 22 | Wang H X, Li X X, Tang L. Effects of surfactants on the morphology and properties of TiO2[J]. Applied Physics A, 2020, 126(6): 1-7. |
| 23 | Hong B D, Lee C L. Specific activities of rhombic dodecahedral, octahedral, and cubic Cu2O nanocrystals as glucose oxidation catalysts[J]. Chemical Engineering Journal, 2020, 382: 122994. |
| 24 | Zhu C Z, Wei X Q, Li W Q, et al. Crystal-plane effects of CeO2{110} and CeO2{100} on photocatalytic CO2 reduction: synergistic interactions of oxygen defects and hydroxyl groups[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(38): 14397-14406. |
| 25 | Hua Q, Cao T, Gu X K, et al. Crystal-plane-controlled selectivity of Cu2O catalysts in propylene oxidation with molecular oxygen[J]. Angewandte Chemie International Edition, 2014, 53(19): 4856-4861. |
| 26 | Yang C W, Yu X J, Heißler S, et al. Surface faceting and reconstruction of ceria nanoparticles[J]. Angewandte Chemie International Edition, 2017, 56(1): 375-379. |
| 27 | Vilé G, Colussi S, Krumeich F, et al. Opposite face sensitivity of CeO2 in hydrogenation and oxidation catalysis[J]. Angewandte Chemie International Edition, 2014, 53(45): 12069-12072. |
| 28 | Wu Z L, Mann A K P, Li M J, et al. Spectroscopic investigation of surface-dependent acid-base property of ceria nanoshapes[J]. The Journal of Physical Chemistry C, 2015, 119(13): 7340-7350. |
| 29 | Chen S L, Cao T, Gao Y X, et al. Probing surface structures of CeO2, TiO2, and Cu2O nanocrystals with CO and CO2 chemisorption[J]. The Journal of Physical Chemistry C, 2016, 120(38): 21472-21485. |
| 30 | Tan Z C, Li G C, Chou H L, et al. Differentiating surface Ce species among CeO2 facets by solid-state NMR for catalytic correlation[J]. ACS Catalysis, 2020, 10(7): 4003-4011. |
| 31 | Wang F, Li C M, Zhang X Y, et al. Catalytic behavior of supported Ru nanoparticles on the {1 0 0}, {1 1 0}, and {1 1 1} facet of CeO2[J]. Journal of Catalysis, 2015, 329: 177-186. |
| 32 | Sakpal T, Lefferts L. Structure-dependent activity of CeO2 supported Ru catalysts for CO2 methanation[J]. Journal of Catalysis, 2018, 367: 171-180. |
| 33 | Hu Z, Liu X F, Meng D M, et al. Effect of ceria crystal plane on the physicochemical and catalytic properties of Pd/ceria for CO and propane oxidation[J]. ACS Catalysis, 2016, 6(4): 2265-2279. |
| 34 | Lin L L, Yao S Y, Liu Z Y, et al. In situ characterization of Cu/CeO2 nanocatalysts for CO2 hydrogenation: morphological effects of nanostructured ceria on the catalytic activity[J]. The Journal of Physical Chemistry C, 2018, 122(24): 12934-12943. |
| 35 | Lei Y Y, Li W Z, Liu Q C, et al. Typical crystal face effects of different morphology ceria on the activity of Pd/CeO2 catalysts for lean methane combustion[J]. Fuel, 2018, 233: 10-20. |
| 36 | Xie X, Li Y, Liu Z Q, et al. Low-temperature oxidation of CO catalysed by Co3O4 nanorods[J]. Nature, 2009, 458(7239): 746-749. |
| 37 | Jian Y F, Tian M J, He C, et al. Efficient propane low-temperature destruction by Co3O4 crystal facets engineering: unveiling the decisive role of lattice and oxygen defects and surface acid-base pairs[J]. Applied Catalysis B: Environmental, 2021, 283: 119657. |
| 38 | Tumuluri U, Howe J D, Mounfield W P, et al. Effect of surface structure of TiO2 nanoparticles on CO2 adsorption and SO2 resistance[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(10): 9295-9306. |
| 39 | Tawfilas M, Mauri M, De Trizio L, et al. Surface characterization of TiO2 polymorphic nanocrystals through 1H-TD-NMR[J]. Langmuir, 2018, 34(32): 9460-9469. |
| 40 | Hua Q, Cao T, Bao H Z, et al. Crystal-plane-controlled surface chemistry and catalytic performance of surfactant-free Cu2O nanocrystals[J]. ChemSusChem, 2013, 6(10): 1966-1972. |
| 41 | Bao H Z, Zhang W H, Hua Q, et al. Crystal-plane-controlled surface restructuring and catalytic performance of oxide nanocrystals[J]. Angewandte Chemie International Edition, 2011, 50(51): 12294-12298. |
| 42 | Wu Z L, Li M J, Mullins D R, et al. Probing the surface sites of CeO2 nanocrystals with well-defined surface planes via methanol adsorption and desorption[J]. ACS Catalysis, 2012, 2(11): 2224-2234. |
| 43 | Zhang W, Ma X L, Xiao H, et al. Mechanistic investigations on thermal hydrogenation of CO2 to methanol by nanostructured CeO2(100): the crystal-plane effect on catalytic reactivity[J]. The Journal of Physical Chemistry C, 2019, 123(18): 11763-11771. |
| 44 | Wang Y G, Yang X F, Li J. Theoretical studies of CO oxidation with lattice oxygen on Co3O4 surfaces[J]. Chinese Journal of Catalysis, 2016, 37(1): 193-198. |
| 45 | Chen S Q, Zhao Y F, Sun B, et al. Microwave-assisted synthesis of mesoporous Co3O4 nanoflakes for applications in lithium ion batteries and oxygen evolution reactions[J]. ACS Applied Materials & Interfaces, 2015, 7(5): 3306-3313. |
| 46 | Ryu W H, Yoon T H, Song S H, et al. Bifunctional composite catalysts using Co3O4 nanofibers immobilized on nonoxidized graphene nanoflakes for high-capacity and long-cycle Li-O2 batteries[J]. Nano Letters, 2013, 13(9): 4190-4197. |
| 47 | Bahlawane N, Ngamou P H, Vannier V, et al. Tailoring the properties and the reactivity of the spinel cobalt oxide[J]. Physical Chemistry Chemical Physics, 2009, 11(40): 9224-9232. |
| 48 | Xiao Y H, Liu S J, Li F, et al. Hierarchical nanoarchitectures: 3D hierarchical Co3O4 twin-spheres with an urchin-like structure: large-scale synthesis, multistep-splitting growth, and electrochemical pseudocapacitors [J]. Advanced Functional Materials, 2012, 22(19): 4051. |
| 49 | Wang B, Zhu T, Wu H B, et al. Porous Co3O4 nanowires derived from long Co(CO3)0.5OH·0.11H2O nanowires with improved supercapacitive properties[J]. Nanoscale, 2012, 4(6): 2145-2149. |
| 50 | Wang L, Wan J W, Zhao Y S, et al. Hollow multi-shelled structures of Co3O4 dodecahedron with unique crystal orientation for enhanced photocatalytic CO2 reduction[J]. Journal of the American Chemical Society, 2019, 141(6): 2238-2241. |
| 51 | Yin J Z, Zhang Y, Lu Q Y, et al. Tunable Co3O4 hollow structures (from yolk-shell to multi-shell) and their Li storage properties[J]. Journal of Materials Chemistry A, 2017, 5(25): 12757-12761. |
| 52 | Jiang X, Huang X C, Zeng W, et al. Facile morphology control of 3D porous CeO2 for CO oxidation[J]. RSC Advances, 2018, 8(38): 21658-21663. |
| 53 | Xu B, Zhang Q T, Yuan S S, et al. Morphology control and characterization of broom-like porous CeO2[J]. Chemical Engineering Journal, 2015, 260: 126-132. |
| 54 | Li J, Wang C B, Zhu X J, et al. Synthesis of hierarchical CeO2 octahedrons with tunable size and the catalytic properties[J]. Materials Letters, 2019, 240: 73-76. |
| 55 | Que Y P, Weng J, Hu L H, et al. High open voltage and superior light-harvesting dye-sensitized solar cells fabricated by flower-like hierarchical TiO2 composed with highly crystalline nanosheets[J]. Journal of Power Sources, 2016, 307: 138-145. |
| 56 | Pan J H, Wang X Z, Huang Q Z, et al. Large-scale synthesis of urchin-like mesoporous TiO2 hollow spheres by targeted etching and their photoelectrochemical properties[J]. Advanced Functional Materials, 2014, 24(1): 95-104. |
| 57 | Dong Z H, Lai X Y, Halpert J E, et al. Accurate control of multishelled ZnO hollow microspheres for dye-sensitized solar cells with high efficiency[J]. Advanced Materials, 2012, 24(8): 1046-1049. |
| 58 | Xiao X L, Liu X F, Zhao H, et al. Facile shape control of Co3O4 and the effect of the crystal plane on electrochemical performance[J]. Advanced Materials, 2012, 24(42): 5762-5766. |
| 59 | Liao F, Han X R, Zhang Y F, et al. Template-free hydrothermal synthesis of 3D hierarchical Co3O4 microflowers constructed by mesoporous nanoneedles[J]. Materials Letters, 2018, 215: 179-182. |
| 60 | Qiao X R, Ma C, Chang X, et al. 3D radial Co3O4 nanorod cluster derived from cobalt-based layered hydroxide metal salt for enhanced trace acetone detection[J]. Sensors and Actuators B: Chemical, 2021, 327: 128926. |
| 61 | Sun P, Wang B Q, Zhao L P, et al. Enhanced gas sensing by amorphous double-shell Fe2O3 hollow nanospheres functionalized with PdO nanoparticles[J]. Sensors and Actuators B: Chemical, 2017, 252: 322-329. |
| 62 | Cao K Z, Jiao L F, Liu H Q, et al. 3D hierarchical porous α-Fe2O3 nanosheets for high-performance lithium-ion batteries [J]. Advanced Energy Materials, 2015, 5(4): 1401-1421. |
| 63 | Zhao Y, Pan K M, Wei S Z, et al. Template-free hydrothermal synthesis of 3D hollow aggregate spherical structure WO3 nano-plates and photocatalytic properties[J]. Materials Research Bulletin, 2018, 101: 280-286. |
| 64 | Wang X, Liao M Y, Zhong Y T, et al. ZnO hollow spheres with double-yolk egg structure for high-performance photocatalysts and photodetectors[J]. Advanced Materials, 2012, 24(25): 3421-3425. |
| 65 | Liao J Y, Lei B X, Kuang D B, et al. Tri-functional hierarchical TiO2 spheres consisting of anatase nanorods and nanoparticles for high efficiency dye-sensitized solar cells[J]. Energy & Environmental Science, 2011, 4(10): 4079. |
| 66 | Ziarati A, Badiei A, Luque R. Black hollow TiO2 nanocubes: advanced nanoarchitectures for efficient visible light photocatalytic applications[J]. Applied Catalysis B: Environmental, 2018, 238: 177-183. |
| 67 | Guo W X, Sun W W, Lv L P, et al. Microwave-assisted morphology evolution of Fe-based metal-organic frameworks and their derived Fe2O3 nanostructures for Li-ion storage[J]. ACS Nano, 2017, 11(4): 4198-4205. |
| 68 | Liu S Y, Liu C R. Morphology control by pulsed laser in chemical deposition illustrated in ZnO crystal growth[J]. Crystal Growth & Design, 2019, 19(5): 2912-2918. |
| 69 | Chen J C, Du H W, Zhang J H, et al. Influence of deposition temperature on crystalline structure and morphologies of Co3O4 films prepared by a direct liquid injection chemical vapor deposition[J]. Surface and Coatings Technology, 2017, 319: 110-116. |
| 70 | Li T, Li X H, Wang Z X, et al. Synthesis of nanoparticles-assembled Co3O4 microspheres as anodes for Li-ion batteries by spray pyrolysis of CoCl2 solution[J]. Electrochimica Acta, 2016, 209: 456-463. |
| 71 | Yang S L, Sha S M, Lu H, et al. Electrodeposition of hierarchical zinc oxide nanostructures on metal meshes as photoanodes for flexible dye-sensitized solar cells[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2020, 594: 124665. |
| 72 | Fei S X, Xia K S, Tian X L, et al. Fabrication of ordered mesoporous MoO3 for olefin catalytic hydrogenation[J]. International Journal of Hydrogen Energy, 2016, 41(13): 5652-5660. |
| 73 | Nguyen-Huy C, Lee J, Seo J H, et al. Structure-dependent catalytic properties of mesoporous cobalt oxides in furfural hydrogenation[J]. Applied Catalysis A: General, 2019, 583: 117125. |
| 74 | Masunga N, Tito G S, Meijboom R. Catalytic evaluation of mesoporous metal oxides for liquid phase oxidation of styrene[J]. Applied Catalysis A: General, 2018, 552: 154-167. |
| 75 | Zhang Q S, Bu J H, Wang J D, et al. Highly efficient hydrogenation of nitrobenzene to aniline over Pt/CeO2 catalysts: the shape effect of the support and key role of additional Ce3+ sites[J]. ACS Catalysis, 2020, 10(18): 10350-10363. |
| 76 | Li T, Xia D P, Zhou G L, et al. Effect of the morphology on the vapor phase benzene catalytic hydrogenation over Pd/CeO2 catalyst[J]. Catalysis Communications, 2018, 112: 35-38. |
| 77 | Ouyang B, Xiong S H, Zhang Y H, et al. The study of morphology effect of Pt/Co3O4 catalysts for higher alcohol synthesis from CO2 hydrogenation[J]. Applied Catalysis A: General, 2017, 543: 189-195. |
| 78 | Jiang F, Wang S S, Liu B, et al. Insights into the influence of CeO2 crystal facet on CO2 hydrogenation to methanol over Pd/CeO2 catalysts[J]. ACS Catalysis, 2020, 10(19): 11493-11509. |
| 79 | Chai M Q, Tan Y, Pei G X, et al. Crystal plane effect of ZnO on the catalytic activity of gold nanoparticles for the acetylene hydrogenation reaction[J]. The Journal of Physical Chemistry C, 2017, 121(36): 19727-19734. |
| 80 | Jia A P, Zhang Y S, Song T Y, et al. Crystal-plane effects of anatase TiO2 on the selective hydrogenation of crotonaldehyde over Ir/TiO2 catalysts[J]. Journal of Catalysis, 2021, 395: 10-22. |
| 81 | Liu X J, Liu J F, Chang Z, et al. Crystal plane effect of Fe2O3 with various morphologies on CO catalytic oxidation[J]. Catalysis Communications, 2011, 12(6): 530-534. |
| 82 | May Y A, Wang W W, Yan H, et al. Insights into facet-dependent reactivity of CuO-CeO2 nanocubes and nanorods as catalysts for CO oxidation reaction[J]. Chinese Journal of Catalysis, 2020, 41(6): 1017-1027. |
| 83 | Zheng Y E, Li K Z, Wang H, et al. Structure dependence and reaction mechanism of CO oxidation: a model study on macroporous CeO2 and CeO2-ZrO2 catalysts[J]. Journal of Catalysis, 2016, 344: 365-377. |
| 84 | Chen Z P, Wang S, Ding Y, et al. Pd catalysts supported on Co3O4 with the specified morphologies in CO and CH4 oxidation[J]. Applied Catalysis A: General, 2017, 532: 95-104. |
| 85 | Gao Y N, Chiang F K, Li S J, et al. Influence of hematite morphology on the CO oxidation performance of Au/α-Fe2O3[J]. Chinese Journal of Catalysis, 2021, 42(4): 658-665. |
| 86 | Ha H, Yoon S, An K, et al. Catalytic CO oxidation over Au nanoparticles supported on CeO2 nanocrystals: effect of the Au-CeO2 interface[J]. ACS Catalysis, 2018, 8(12): 11491-11501. |
| 87 | Gu L L, Su Q, Jiang W, et al. How do the unique Au/α-Fe2O3 interfacial structures determine activity in CO oxidation?[J]. Catalysis Science & Technology, 2018, 8(22): 5782-5793. |
| 88 | Zhang H, Sun J M, Dagle V L, et al. Influence of ZnO facets on Pd/ZnO catalysts for methanol steam reforming[J]. ACS Catalysis, 2014, 4(7): 2379-2386. |
| 89 | Kourtelesis M, Moraes T S, Mattos L V, et al. The effects of support morphology on the performance of Pt/CeO2 catalysts for the low temperature steam reforming of ethanol[J]. Applied Catalysis B: Environmental, 2021, 284: 119757. |
| 90 | Wang F G, Zhang L J, Zhu J Y, et al. Study on different CeO2 structure stability during ethanol steam reforming reaction over Ir/CeO2 nanocatalysts[J]. Applied Catalysis A: General, 2018, 564: 226-233. |
| 91 | Zhao K, Zheng A Q, Li H B, et al. Exploration of the mechanism of chemical looping steam methane reforming using double perovskite-type oxides La1.6Sr0.4FeCoO6[J]. Applied Catalysis B: Environmental, 2017, 219: 672-682. |
| 92 | Padmakar D, Surendar M, Chandrashekar P, et al. A highly stable and efficient Co-Mg-Sr mixed oxide catalysts for hydrogen production from glycerol steam reforming[J]. Catalysis Letters, 2020, 150(9): 2734-2743. |
| 93 | Xi H J, Hou X N, Liu Y J, et al. Cu-Al spinel oxide as an efficient catalyst for methanol steam reforming[J]. Angewandte Chemie, 2014, 126(44): 12080-12083. |
| 94 | Yang L, Bukhovko M P, Brezicki G, et al. Steam reforming of ethylene over manganese-chromium spinel oxides[J]. Journal of Catalysis, 2019, 380: 224-235. |
| [1] | 吴雷, 刘姣, 李长聪, 周军, 叶干, 刘田田, 朱瑞玉, 张秋利, 宋永辉. 低阶粉煤催化微波热解制备含碳纳米管的高附加值改性兰炭末[J]. 化工学报, 2023, 74(9): 3956-3967. |
| [2] | 王绍宇, 马翰泽, 吴洪, 梁旭, 王洪建, 朱姿亭, 姜忠义. 有机框架膜在气体分离中的研究进展[J]. 化工学报, 2021, 72(7): 3488-3510. |
| [3] | 张眉佳, 吴登峰, 许昊翔, 程道建. 氢氧直接合成过氧化氢用钯基催化剂研究进展[J]. 化工学报, 2021, 72(1): 292-303. |
| [4] | 田隆, 刘婷, 孙克宁. 用于水质净化的氧化石墨烯膜研究进展[J]. 化工学报, 2020, 71(9): 4112-4130. |
| [5] | 张双正, 陈国, 苏鹏飞. 磁响应交联糖化酶聚集体的制备及催化特性[J]. 化工学报, 2017, 68(7): 2763-2770. |
| [6] | 李振花, 张晓珊, 曲江磊, 王玮涵, 王保伟, 马新宾. 制备方法对钼基耐硫甲烷化催化剂性能的影响[J]. 化工学报, 2017, 68(1): 129-135. |
| [7] | 耿春香,柴倩倩,王陈珑. Mn-Fe-Ce/TiO2低温脱硝催化剂的制备条件优化及其表征[J]. 化工进展, 2014, 33(04): 921-924. |
| [8] | 唐海燕1,2,3,4,肖清贵1,2,3,徐红彬1,2,3,张 懿1,2,3. 新型铬化学品——有机铬研究进展[J]. 化工进展, 2013, 32(09): 2205-2215. |
| [9] | 成 欢,朱光明,宋 蕊. 时间温度指示剂研究进展[J]. 化工进展, 2013, 32(04): 885-890. |
| [10] | 郭晓明,毛东森,卢冠忠,王 嵩. CO2加氢合成甲醇催化剂的研究进展[J]. 化工进展, 2012, 31(03): 477-488. |
| [11] | 毛 伟,吕 剑,张 伟,杜咏梅,王 伟. 六氟丁二烯的制备技术进展 [J]. CIESC Journal, 2011, 30(3): 627-. |
| [12] | 顾汉念1,3,王 宁1,杨永琼2,3,田元江1. 不溶性含钾岩石制备钾肥研究现状与评述[J]. CIESC Journal, 2011, 30(11): 2450-. |
| [13] | 刘利军,彭金辉,郭胜惠,李东波. 超细碳酸钡粉体制备方法的研究进展及展望[J]. CIESC Journal, 2011, 30(10): 2247-. |
| [14] | 马明亮,张秋禹,刘燕燕,王为强,陆树新. 稀土纳米材料制备方法的研究进展 [J]. CIESC Journal, 2009, 28(5): 822-. |
| [15] | 李敏娜,罗青枝,安 静,王德松,段彦栋. 纳米银粒子制备及应用研究进展 [J]. CIESC Journal, 2008, 27(11): 1765-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号