化工学报 ›› 2021, Vol. 72 ›› Issue (10): 5002-5015.DOI: 10.11949/0438-1157.20210327
收稿日期:2021-03-04
修回日期:2021-04-21
出版日期:2021-10-05
发布日期:2021-10-05
通讯作者:
汪靖伦
作者简介:唐子龙(1967—),男,博士,教授,基金资助:
Zilong TANG( ),Fanfan XIAO,Yuhua YIN,Senyu LI,Jinglun WANG(
),Fanfan XIAO,Yuhua YIN,Senyu LI,Jinglun WANG( )
)
Received:2021-03-04
Revised:2021-04-21
Online:2021-10-05
Published:2021-10-05
Contact:
Jinglun WANG
摘要:
有机-无机复合固态电解质不仅具有聚合物电解质的柔韧性和界面相容性,还能显著提高离子传导性和力学性能。然而,构建良好的填料/聚合物分散体系是制备此类复合电解质的难点,设计新型有强相互作用的功能化填料以调控界面渗流结构也面临巨大挑战。通过功能硅烷对无机填料进行化学键联改性或原位合成是解决无机填料与聚合物间分散性和界面相容性问题的有效策略。本文综述了在复合固态电解质中利用功能硅烷对无机填料进行表面改性和原位合成、功能硅烷作为复合固态电解质的交联中心和制备离子胶类复合固态电解质四方面的研究进展,重点阐述了硅烷功能化填料与固态电解质结构和性能之间的关系。最后对功能硅烷在有机-无机复合固态电解质中的应用研究进行了总结和展望。
中图分类号:
唐子龙,肖凡凡,尹玉华,李森雨,汪靖伦. 功能硅烷在有机-无机复合固态电解质中的应用研究进展[J]. 化工学报, 2021, 72(10): 5002-5015.
Zilong TANG,Fanfan XIAO,Yuhua YIN,Senyu LI,Jinglun WANG. Recent advances in application of functional organosilane for organic-inorganic composite solid electrolyte[J]. CIESC Journal, 2021, 72(10): 5002-5015.
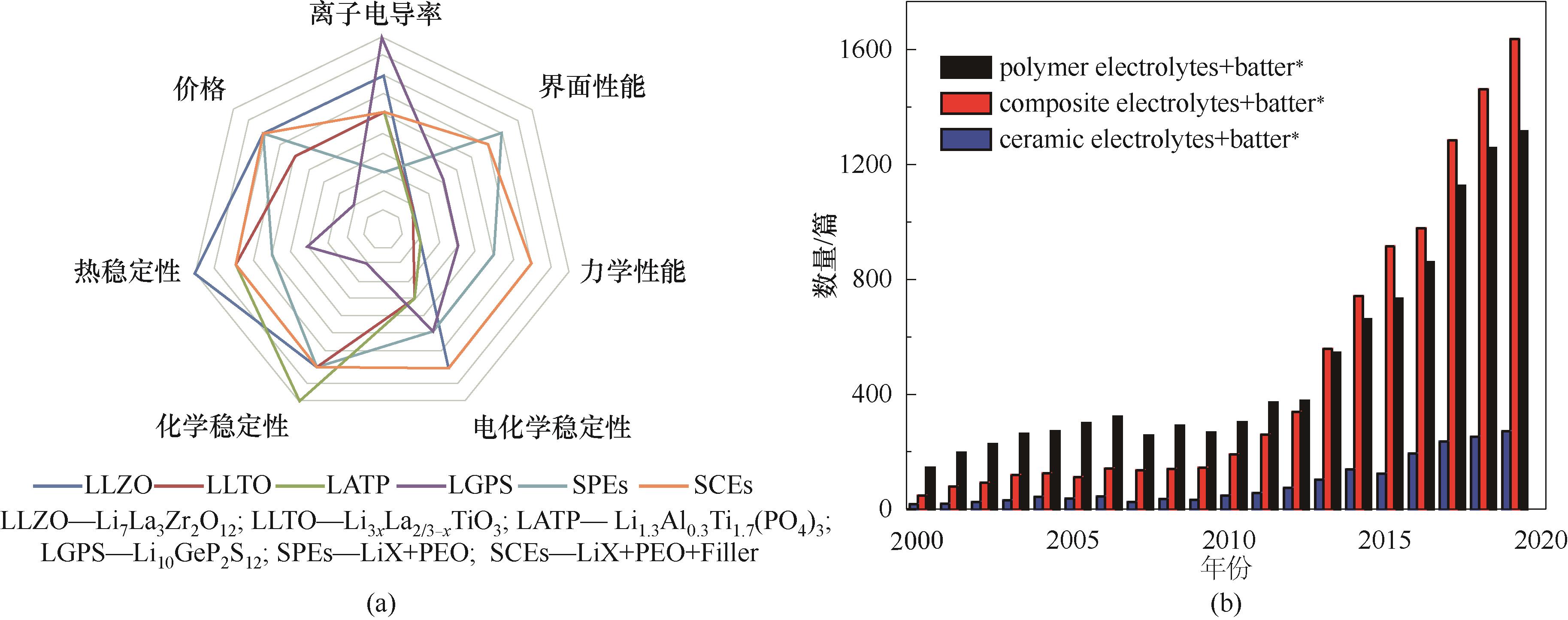
图1 各类固态电解质性能对比雷达图(a)[5]和相关研究论文增长趋势图(b)
Fig.1 The radar chart of various solid electrolytes (a) [5] and the research growth trend of reported articles(b)
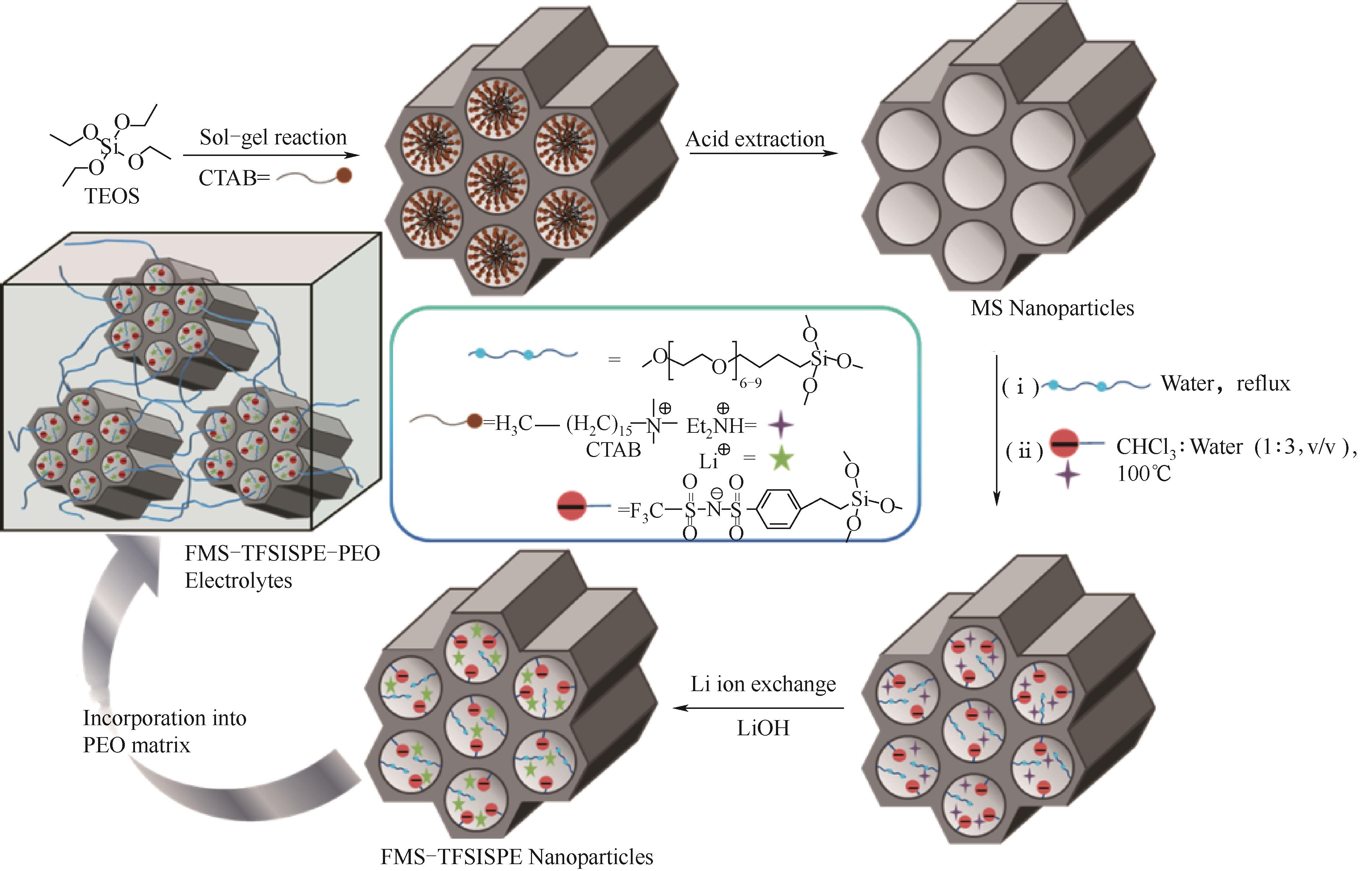
图3 PEO/FMS-TFSISPE复合固态电解质合成示意图[42]
Fig.3 Schematic synthetic route of a single-ion conducting nanohybrid solid polymer electrolyte (PEO/FMS-TFSISPE) [42]

图6 原位水解制备复合固态电解质和分子间相互作用机理示意图[30]
Fig.6 In-situ preparation of PEO-SiO2 composite electrolyte and schematic representation of molecular interaction[30]
| 复合固态电解质 | 离子电导率/(S/cm) | 功能硅烷化学式 | 功能硅烷作用 | 文献 |
|---|---|---|---|---|
| 原位制备SiO2复合PEO基固态电解质 | 4.4×10-5@30℃ | (C2H5O)4Si | 高分散性 | [ |
| 聚乙二醇基三维交联复合电解质 | 8.0×10-5@room temp. | (C2H5O)3Si(CH2)3OCH2(CHCH2O) | 交联中心 | [ |
| 丙烯酸酯交联SiO2复合丁二腈基固态电解质 | 7.0×10-4@25℃ | (C2H5O)3SiCH | 交联中心 | [ |
| 接枝改性SiO2复合聚乙烯醇基复合电解质 | 1.5×10-4@25℃ | (C2H5O)3SiCH2CH2CH2NH2 | 化学键联 | [ |
| 接枝改性Al2O3复合PEO基复合电解质 | 1.9×10-4@70℃ | (CH3O)3Si(CH2)3O(CH2CH2O)nCH3 (n=6~9) | 化学键联 | [ |
| 离子键修饰SiO2复合PEO基固态电解质 | 3.6×10-5@30℃ | (C2H5O)3SiCH2CH2CH2SH | 化学键联 | [ |
| 离子胶类固态电解质 | 3.2×10-3@30℃ | (C2H5O)4Si | 离子胶 | [ |
| SiO2复合PEO基复合电解质 | 2.0×10-6@30℃ | 物理混合,无硅烷功能化 | — | [ |
| Al2O3复合PEO基复合电解质 | 1.0×10-6@30℃ | 物理混合,无硅烷功能化 | — | [ |
表1 功能硅烷化学结构及在复合固态电解质中应用
Table 1 Chemical structure of functional silane and its application in composite solid electrolyte
| 复合固态电解质 | 离子电导率/(S/cm) | 功能硅烷化学式 | 功能硅烷作用 | 文献 |
|---|---|---|---|---|
| 原位制备SiO2复合PEO基固态电解质 | 4.4×10-5@30℃ | (C2H5O)4Si | 高分散性 | [ |
| 聚乙二醇基三维交联复合电解质 | 8.0×10-5@room temp. | (C2H5O)3Si(CH2)3OCH2(CHCH2O) | 交联中心 | [ |
| 丙烯酸酯交联SiO2复合丁二腈基固态电解质 | 7.0×10-4@25℃ | (C2H5O)3SiCH | 交联中心 | [ |
| 接枝改性SiO2复合聚乙烯醇基复合电解质 | 1.5×10-4@25℃ | (C2H5O)3SiCH2CH2CH2NH2 | 化学键联 | [ |
| 接枝改性Al2O3复合PEO基复合电解质 | 1.9×10-4@70℃ | (CH3O)3Si(CH2)3O(CH2CH2O)nCH3 (n=6~9) | 化学键联 | [ |
| 离子键修饰SiO2复合PEO基固态电解质 | 3.6×10-5@30℃ | (C2H5O)3SiCH2CH2CH2SH | 化学键联 | [ |
| 离子胶类固态电解质 | 3.2×10-3@30℃ | (C2H5O)4Si | 离子胶 | [ |
| SiO2复合PEO基复合电解质 | 2.0×10-6@30℃ | 物理混合,无硅烷功能化 | — | [ |
| Al2O3复合PEO基复合电解质 | 1.0×10-6@30℃ | 物理混合,无硅烷功能化 | — | [ |
| 1 | Xu K. Electrolytes and interphases in Li-ion batteries and beyond[J]. Chemical Reviews, 2014, 114(23): 11503-11618. |
| 2 | Li M, Lu J, Chen Z W, et al. 30 years of lithium-ion batteries[J]. Advanced Materials, 2018, 30(33): 1800561. |
| 3 | Chen R, Li Q, Yu X, et al. Approaching practically accessible solid-state batteries: stability issues related to solid electrolytes and interfaces[J]. Chemical Reviews, 2020, 120(14): 6820-6877. |
| 4 | Zheng Y, Yao Y Z, Ou J H, et al. A review of composite solid-state electrolytes for lithium batteries: fundamentals, key materials and advanced structures[J]. Chemical Society Reviews, 2020, 49(23): 8790-8839. |
| 5 | Manthiram A, Yu X W, Wang S F. Lithium battery chemistries enabled by solid-state electrolytes[J]. Nature Reviews Materials, 2017, 2: 16103. |
| 6 | Mauger A, Julien C M, Paolella A, et al. Building better batteries in the solid state: a review[J]. Materials, 2019, 12(23): 3892. |
| 7 | Keller M, Varzi A, Passerini S. Hybrid electrolytes for lithium metal batteries[J]. Journal of Power Sources, 2018, 392: 206-225. |
| 8 | Liang J N, Luo J, Sun Q, et al. Recent progress on solid-state hybrid electrolytes for solid-state lithium batteries[J]. Energy Storage Materials, 2019, 21: 308-334. |
| 9 | Chen R J, Qu W J, Guo X, et al. The pursuit of solid-state electrolytes for lithium batteries: from comprehensive insight to emerging horizons[J]. Materials Horizons, 2016, 3(6): 487-516. |
| 10 | Croce F, Appetecchi G B, Persi L, et al. Nanocomposite polymer electrolytes for lithium batteries[J]. Nature, 1998, 394(6692): 456-458. |
| 11 | Li S, Zhang S Q, Shen L, et al. Progress and perspective of ceramic/polymer composite solid electrolytes for lithium batteries[J]. Advanced Science, 2020, 7(5): 1903088. |
| 12 | Wu N, Chien P H, Li Y T, et al. Fast Li+ conduction mechanism and interfacial chemistry of a NASICON/polymer composite electrolyte[J]. Journal of the American Chemical Society, 2020, 142(5): 2497-2505. |
| 13 | Wu N, Chien P H, Qian Y M, et al. Enhanced surface interactions enable fast Li+ conduction in oxide/polymer composite electrolyte[J]. Angewandte Chemie, 2020, 132(10): 4160-4166. |
| 14 | Li Z, Huang H M, Zhu J K, et al. Ionic conduction in composite polymer electrolytes: case of PEO: Ga-LLZO composites[J]. ACS Applied Materials & Interfaces, 2019, 11(1): 784-791. |
| 15 | 虞鑫润, 马君, 牟春博, 等. 高锂离子电导的有机-无机复合电解质的渗流结构设计[J]. 物理化学学报, 2020, 36: 1912061-1912070. |
| Yu X R, Ma J, Mou C B, et al. Percolation structure design of organic-inorganic composite electrolyte with high lithium ion-conductivity[J]. Acta Phys.-Chim. Sin., 2020, 36: 1912061-1912070. | |
| 16 | Tan S J, Zeng X X, Ma Q, et al. Recent advancements in polymer-based composite electrolytes for rechargeable lithium batteries[J]. Electrochemical Energy Reviews, 2018, 1(2): 113-138. |
| 17 | 孙宗杰, 丁书江. PEO基聚合物电解质在锂离子电池中的研究进展[J]. 科学通报, 2018, 63(22): 2280-2295. |
| Sun Z J, Ding S J. PEO-based polymer electrolytes in lithium ion batteries[J]. Chinese Science Bulletin, 2018, 63(22): 2280-2295. | |
| 18 | Lim H D, Park J H, Shin H J, et al. A review of challenges and issues concerning interfaces for all-solid-state batteries[J]. Energy Storage Materials, 2020, 25: 224-250. |
| 19 | Pan K C, Zhang L, Qian W W, et al. A flexible ceramic/polymer hybrid solid electrolyte for solid-state lithium metal batteries[J]. Advanced Materials, 2020, 32(17): 2000399. |
| 20 | Zhang L Z, Zhang Z C, Harring S, et al. Highly conductive trimethylsilyl oligo(ethylene oxide) electrolytes for energy storage applications[J]. Journal of Materials Chemistry, 2008, 18(31): 3713. |
| 21 | Zhang L Z, Lyons L, Newhouse J, et al. Synthesis and characterization of alkylsilane ethers with oligo(ethylene oxide) substituents for safe electrolytes in lithium-ion batteries[J]. Journal of Materials Chemistry, 2010, 20(38): 8224. |
| 22 | Wang J L, Mai Y J, Luo H, et al. Fluorosilane compounds with oligo(ethylene oxide) substituent as safe electrolyte solvents for high-voltage lithium-ion batteries[J]. Journal of Power Sources, 2016, 334: 58-64. |
| 23 | Dong J, Zhang Z C, Kusachi Y, et al. A study of tri(ethylene glycol)-substituted trimethylsilane (1NM3)/LiBOB as lithium battery electrolyte[J]. Journal of Power Sources, 2011, 196(4): 2255-2259. |
| 24 | Rossi N A, West R. Silicon-containing liquid polymer electrolytes for application in lithium ion batteries[J]. Polymer International, 2009, 58(3): 267-272. |
| 25 | 崔孟忠, 李竹云, 张洁, 等. 硅氧烷基聚合物电解质[J]. 化学进展, 2008, 20(12): 1987-1997. |
| Cui M Z, Li Z Y, Zhang J, et al. Siloxane-based polymer electrolytes[J]. Progress in Chemistry, 2008, 20(12): 1987-1997. | |
| 26 | Wang Q L, Zhang H R, Cui Z L, et al. Siloxane-based polymer electrolytes for solid-state lithium batteries[J]. Energy Storage Materials, 2019, 23: 466-490. |
| 27 | Chinnam P R, Wunder S L. Engineered interfaces in hybrid ceramic-polymer electrolytes for use in all-solid-state Li batteries[J]. ACS Energy Letters, 2017, 2(1): 134-138. |
| 28 | Kango S, Kalia S, Celli A, et al. Surface modification of inorganic nanoparticles for development of organic-inorganic nanocomposites—a review[J]. Progress in Polymer Science, 2013, 38(8): 1232-1261. |
| 29 | Ji K S, Moon H S, Kim J W, et al. Role of functional nano-sized inorganic fillers in poly(ethylene) oxide-based polymer electrolytes[J]. Journal of Power Sources, 2003, 117(1/2): 124-130. |
| 30 | Lin D C, Liu W, Liu Y Y, et al. High ionic conductivity of composite solid polymer electrolyte viain situ synthesis of monodispersed SiO2 nanospheres in poly(ethylene oxide)[J]. Nano Letters, 2016, 16(1): 459-465. |
| 31 | Zhu Y H, Cao J, Chen H, et al. High electrochemical stability of a 3D cross-linked network PEO@nano-SiO2 composite polymer electrolyte for lithium metal batteries[J]. Journal of Materials Chemistry A, 2019, 7(12): 6832-6839. |
| 32 | 秦雪英, 汪靖伦, 张灵志. 锂离子电池有机硅电解液[J]. 化学进展, 2012, 24(5): 810-822. |
| Qin X Y, Wang J L, Zhang L Z. Organosilicon based electrolytes for lithium-ion batteries[J]. Progress in Chemistry, 2012, 24(5): 810-822. | |
| 33 | 汪靖伦, 冉琴, 韩冲宇, 等. 锂离子电池有机硅功能电解液[J]. 化学进展, 2020, 32(4): 467-480. |
| Wang J L, Ran Q, Han C Y, et al. Organosilicon functionalized electrolytes for lithium-ion batteries[J]. Progress in Chemistry, 2020, 32(4): 467-480. | |
| 34 | Yao P H, Yu H B, Ding Z Y, et al. Review on polymer-based composite electrolytes for lithium batteries[J]. Frontiers in Chemistry, 2019, 7: 522. |
| 35 | 陈嘉苗, 熊靖雯, 籍少敏, 等. 锂电池用全固态聚合物电解质[J]. 化学进展, 2020, 32(4): 481-496. |
| Chen J M, Xiong J W, Ji S M, et al. All solid polymer electrolytes for lithium batteries[J]. Progress in Chemistry, 2020, 32(4): 481-496. | |
| 36 | 陈龙, 池上森, 董源, 等. 全固态锂电池关键材料——固态电解质研究进展[J]. 硅酸盐学报, 2018, 46(1): 21-34. |
| Chen L, Chi S S, Dong Y, et al. Research progress of key materials for all-solid-state lithium batteries[J]. Journal of the Chinese Ceramic Society, 2018, 46(1): 21-34. | |
| 37 | Boaretto N, Meabe L, Martinez-Ibañez M, et al. Review—polymer electrolytes for rechargeable batteries: from nanocomposite to nanohybrid[J]. Journal of the Electrochemical Society, 2020, 167(7): 070524. |
| 38 | Croce F, Persi L, Scrosati B, et al. Role of the ceramic fillers in enhancing the transport properties of composite polymer electrolytes[J]. Electrochimica Acta, 2001, 46(16): 2457-2461. |
| 39 | Liu Y, Lee J Y, Hong L. Functionalized SiO2 in poly(ethylene oxide)-based polymer electrolytes[J]. Journal of Power Sources, 2002, 109(2): 507-514. |
| 40 | Fan L Z, Nan C W, Zhao S J. Effect of modified SiO2 on the properties of PEO-based polymer electrolytes[J]. Solid State Ionics, 2003, 164(1/2): 81-86. |
| 41 | Lago N, Garcia-Calvo O, Lopez del Amo J M, et al. All-solid-state lithium-ion batteries with grafted ceramic nanoparticles dispersed in solid polymer electrolytes[J]. ChemSusChem, 2015, 8(18): 3039-3043. |
| 42 | Kim Y, Kwon S J, Jang H K, et al. High ion conducting nanohybrid solid polymer electrolytes via single-ion conducting mesoporous organosilica in poly(ethylene oxide)[J]. Chemistry of Materials, 2017, 29(10): 4401-4410. |
| 43 | Hu J, Wang W H, Yu R H, et al. Solid polymer electrolyte based on ionic bond or covalent bond functionalized silica nanoparticles[J]. RSC Advances, 2017, 7(87): 54986-54994. |
| 44 | Hu J, Wang W H, Zhou B H, et al. Poly(ethylene oxide)-based composite polymer electrolytes embedding with ionic bond modified nanoparticles for all-solid-state lithium-ion battery[J]. Journal of Membrane Science, 2019, 575: 200-208. |
| 45 | Wang J L, Yong T Q, Yang J W, et al. Organosilicon functionalized glycerol carbonates as electrolytes for lithium-ion batteries[J]. RSC Advances, 2015, 5(23): 17660-17666. |
| 46 | 汪靖伦, 秦雪英, 赵欣悦, 等. 高安全性三甲基硅功能化碳酸丙烯酯电解液的合成及其在锂离子电池中的应用[J]. 化工学报, 2013, 64(9): 3454-3459. |
| Wang J L, Qin X Y, Zhao X Y, et al. Synthesis of novel safe organosilicon functionalized carbonate as electrolyte and its application in lithium-ion batteries[J]. CIESC Journal, 2013, 64(9): 3454-3459. | |
| 47 | 汪靖伦, 孙天霷, 韩冲宇, 等. 一种复合固态聚合物电解质及其制备方法: 111416147A[P]. 2020-07-14. |
| Wang J L, Sun T Y, Han C Y, et al. Composite solid polymer electrolyte and preparation method thereof: 111416147A[P]. 2020-07-14. | |
| 48 | Liu Y, Lee J Y, Hong L. In situ preparation of poly(ethylene oxide)-SiO2 composite polymer electrolytes[J]. Journal of Power Sources, 2004, 129(2): 303-311. |
| 49 | Pan C Y, Gao J H, Zhang Q, et al. Preparation and properties of PEO/LiClO4/KH560-SiO2 composite polymer electrolyte by sol-gel composite-in-situ method[J]. Journal of Central South University of Technology, 2008, 15(3): 295-300. |
| 50 | 潘春跃, 巢猛, 王小花, 等. 偶联剂原位改性SiO2提高PEO/LiClO4/SiO2电导率[J]. 应用化学, 2006, 23(6): 663-667. |
| Pan C Y, Chao M, Wang X H, et al. Conductivity enhancement of (PEO)8LiClO4-SiO2 by in situ SiO2 modification[J]. Chinese Journal of Applied Chemistry, 2006, 23(6): 663-667. | |
| 51 | Pan C Y, Zhang Q, Feng Q, et al. Effect of catalyst on structure of (PEO)8LiClO4-SiO2 composite polymer electrolyte films[J]. Journal of Central South University of Technology, 2008, 15(4): 438-442. |
| 52 | Xu Z, Yang T, Chu X, et al. Strong lewis acid-base and weak hydrogen bond synergistically enhancing ionic conductivity of poly(ethylene oxide)@SiO2 electrolytes for a high rate capability Li-metal battery[J]. ACS Applied Materials & Interfaces, 2020, 12(9): 10341-10349. |
| 53 | Popall M, Andrei M, Kappel J, et al. ORMOCERs as inorganic-organic electrolytes for new solid state lithium batteries and supercapacitors[J]. Electrochimica Acta, 1998, 43(10/11): 1155-1161. |
| 54 | Popall M, Buestrich R, Semrau G, et al. New polymer lithium secondary batteries based on ORMOCER® electrolytes-inorganic-organic polymers[J]. Electrochimica Acta, 2001, 46(10/11): 1499-1508. |
| 55 | Boaretto N, Bittner A, Brinkmann C, et al. Highly conducting 3D-hybrid polymer electrolytes for lithium batteries based on siloxane networks and cross-linked organic polar interphases[J]. Chemistry of Materials, 2014, 26(22): 6339-6350. |
| 56 | Hu X L, Hou G M, Zhang M Q, et al. A new nanocomposite polymer electrolyte based on poly(vinyl alcohol) incorporating hypergrafted nano-silica[J]. Journal of Materials Chemistry, 2012, 22(36): 18961. |
| 57 | Han P F, Zhu Y W, Liu J. An all-solid-state lithium ion battery electrolyte membrane fabricated by hot-pressing method[J]. Journal of Power Sources, 2015, 284: 459-465. |
| 58 | Pan Q W, Smith D M, Qi H, et al. Hybrid electrolytes with controlled network structures for lithium metal batteries[J]. Advanced Materials, 2015, 27(39): 5995-6001. |
| 59 | Liu K, Ding F, Liu J, et al. A cross-linking succinonitrile-based composite polymer electrolyte with uniformly dispersed vinyl-functionalized SiO2 particles for Li-ion batteries[J]. ACS Applied Materials & Interfaces, 2016, 8(36): 23668-23675. |
| 60 | 王琛璐, 王艳磊, 赵秋, 等. 低维纳米受限离子液体的研究进展[J]. 化工学报, 2021, 72(1): 366-383. |
| Wang C L, Wang Y L, Zhao Q, et al. Research progress of low-dimensional nanoconfined ionic liquids[J]. CIESC Journal, 2021, 72(1): 366-383. | |
| 61 | Zhang S, Zhang J, Zhang Y, et al. Nanoconfined ionic liquids[J]. Chemical Reviews, 2017, 117(10): 6755-6833. |
| 62 | Hapiot P, Lagrost C. Electrochemical reactivity in room-temperature ionic liquids[J]. Chemical Reviews, 2008, 108(7): 2238-2264. |
| 63 | 张文林, 霍宇, 李功伟, 等. 离子液体作为电解液添加剂用于高压锂离子电池[J]. 化工学报, 2019, 70(6): 2334-2342. |
| Zhang W L, Huo Y, Li G W, et al. Ionic liquids as electrolyte additives for high-voltage lithium-ion batteries[J]. CIESC Journal, 2019, 70(6): 2334-2342. | |
| 64 | Moganty S S, Jayaprakash N, Nugent J L, et al. Ionic-liquid-tethered nanoparticles: hybrid electrolytes[J]. Angewandte Chemie International Edition, 2010, 49(48): 9158-9161. |
| 65 | Lu Y Y, Moganty S S, Schaefer J L, et al. Ionic liquid-nanoparticle hybrid electrolytes[J]. Journal of Materials Chemistry, 2012, 22(9): 4066. |
| 66 | Lu Y Y, Korf K, Kambe Y, et al. Ionic-liquid-nanoparticle hybrid electrolytes: applications in lithium metal batteries[J]. Angewandte Chemie, 2014, 126(2): 498-502. |
| 67 | Tripathi A K. Ionic liquid-based solid electrolytes (ionogels) for application in rechargeable lithium battery[J]. Materials Today Energy, 2021, 20: 100643. |
| 68 | Echelmeyer T, Meyer H W, van Wüllen L. Novel ternary composite electrolytes: Li ion conducting ionic liquids in silica glass[J]. Chemistry of Materials, 2009, 21(11): 2280-2285. |
| 69 | Noor S A M, Bayley P M, Forsyth M, et al. Ionogels based on ionic liquids as potential highly conductive solid state electrolytes[J]. Electrochimica Acta, 2013, 91: 219-226. |
| 70 | Wu F, Chen N, Chen R J, et al. Self-regulative nanogelator solid electrolyte: a new option to improve the safety of lithium battery[J]. Advanced Science, 2016, 3(1): 1500306. |
| 71 | 谭国强. 新型固态化锂二次电池及相关材料的制备与性能研究[D]. 北京: 北京理工大学, 2014. |
| Tan G Q. Preparation and performance of the novel solid-state rechargeable lithium batteries and relative materials[D]. Beijing: Beijing Institute of Technology, 2014. | |
| 72 | Sagara A, Chen X B, Gandrud K B, et al. High-rate performance solid-state lithium batteries with silica-gel solid nanocomposite electrolytes using bis(fluorosulfonyl)imide-based ionic liquid[J]. Journal of the Electrochemical Society, 2020, 167(7): 070549. |
| 73 | 南皓雄, 赵辰孜, 袁洪, 等. 固态金属锂电池研究进展:外部压力和内部应力的影响[J]. 化工学报, 2021, 72(1): 61-70. |
| Nan H X, Zhao C Z, Yuan H, et al. Recent advances in solid-state lithium metal batteries: the role of external pressure and internal stress[J]. CIESC Journal, 2021, 72(1): 61-70. | |
| 74 | 冉琴, 孙天霷, 韩冲宇, 等. 多酚类化合物: 丹宁酸用作锂金属负极电解液成膜添加剂[J]. 物理化学学报, 2020, 36(11): 142-149. |
| Ran Q, Sun T Y, Han C Y, et al. Natural polyphenol tannic acid as an efficient electrolyte additive for high performance lithium metal anode[J]. Acta Physico-Chimica Sinica, 2020, 36(11): 142-149. |
| [1] | 赵继昊, 唐伟强, 徐小飞, 赵双良, 贺炅皓. 高分子复合材料中键合剂在不同纳米填料表面的吸附能计算[J]. 化工学报, 2022, 73(7): 3174-3181. |
| [2] | 王刚, 车小平, 汪仕勇, 邱介山. 水溶性带电聚合物黏结剂修饰炭电极用于增强电容去离子性能[J]. 化工学报, 2022, 73(4): 1763-1771. |
| [3] | 何鹏鹏, 赵颂, 毛晨岳, 王志, 王纪孝. 耐溶剂复合纳滤膜的研究进展[J]. 化工学报, 2021, 72(2): 727-747. |
| [4] | 王丽明, 杜淼, 单国荣, 卢青, 宋义虎. 低生热橡胶复合体系的研究进展[J]. 化工学报, 2021, 72(2): 863-875. |
| [5] | 彭莉, 吴政奇, 王博轩, 王兴, 顾学红. TMCS修饰MFI分子筛膜的制备及乙醇/水分离稳定性的研究[J]. 化工学报, 2021, 72(1): 569-577. |
| [6] | 原野, 王明, 周云琪, 王志, 王纪孝. 金属有机框架孔径调控进展[J]. 化工学报, 2020, 71(2): 429-450. |
| [7] | 牟帅, 赵长颖, 徐治国. 局部表面改性紫铜方柱阵列池沸腾传热特性和机理[J]. 化工学报, 2019, 70(4): 1291-1301. |
| [8] | 段亚强, 何险峰, 武桐, 张燕萍, 赵志国. 极压石墨烯润滑油添加剂的制备与应用[J]. 化工学报, 2019, 70(1): 360-369. |
| [9] | 周艺璇, 王志, 董晨曦, 王耀, 王纪孝. 双胍基化聚乙烯胺改性制备抗生物污染反渗透膜[J]. 化工学报, 2018, 69(2): 858-865. |
| [10] | 孙雪飞, 高勇强, 赵颂, 张文, 王志, 王晓琳. 胍基聚合物接枝改性制备抗菌抗污染超滤膜[J]. 化工学报, 2018, 69(11): 4869-4878. |
| [11] | 李云, 胡浩威. 润湿性对纳米多孔陶瓷膜输运性能的影响[J]. 化工学报, 2017, 68(9): 3474-3481. |
| [12] | 胡平, 常恬, 陈震宇, 康路, 周宇航, 杨帆, 杨占林, 杜金晶. 纳米Fe3O4磁性颗粒表面改性及其在医学和环保领域的应用[J]. 化工学报, 2017, 68(7): 2641-2652. |
| [13] | 郭凤, 余剑, Tran Tuyet-Suong, 李长明, 许光文. 溶胶-凝胶原位合成钒钨钛催化剂及NH3-SCR性能[J]. 化工学报, 2017, 68(10): 3747-3754. |
| [14] | 郭凤, 余剑, 初茉, 许光文. 溶胶-凝胶原位合成宽活性温度V2O5/TiO2脱硝催化剂[J]. 化工学报, 2014, 65(6): 2098-2105. |
| [15] | 孟胜皓1,闫军1,汪明球1,杜仕国1,王琦1,2. 碳纳米管表面改性及其应用于复合材料的研究现状[J]. 化工进展, 2014, 33(08): 2084-2088. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号