化工学报 ›› 2024, Vol. 75 ›› Issue (4): 1284-1301.DOI: 10.11949/0438-1157.20231277
李添翼( ), 武玉泰, 王永胜, 顾佳锐, 宋沂恒, 杨丰铖, 郝广平(
), 武玉泰, 王永胜, 顾佳锐, 宋沂恒, 杨丰铖, 郝广平( )
)
收稿日期:2023-12-04
修回日期:2024-03-20
出版日期:2024-04-25
发布日期:2024-06-06
通讯作者:
郝广平
作者简介:李添翼(1998—),男,博士研究生,tianyili@mail.dlut.edu.cn
基金资助:
Tianyi LI( ), Yutai WU, Yongsheng WANG, Jiarui GU, Yiheng SONG, Fengcheng YANG, Guangping HAO(
), Yutai WU, Yongsheng WANG, Jiarui GU, Yiheng SONG, Fengcheng YANG, Guangping HAO( )
)
Received:2023-12-04
Revised:2024-03-20
Online:2024-04-25
Published:2024-06-06
Contact:
Guangping HAO
摘要:
氢碳氮氧等稳定轻同位素广泛应用于医疗药物、临床诊断、环境地质等领域。如何获得高纯同位素原料并将其标记到目标化合物是同位素产业的核心技术。基于量子效应的同位素吸附分离方法具有选择性高、能耗低等优势,在同位素分离纯化方面展现出应用潜力;而催化同位素交换法和官能团转化法是制备同位素标记化合物的重要手段。基于量子效应的同位素分离与催化标记技术的核心之一是开发高效吸附及催化材料。总结了同位素分离及标记的最新进展,分析了核心材料的发展现状与性能强化方法,并对轻同位素分离纯化及标记方面的挑战和发展趋势进行了展望。
中图分类号:
李添翼, 武玉泰, 王永胜, 顾佳锐, 宋沂恒, 杨丰铖, 郝广平. 轻同位素分离纯化与催化标记研究进展[J]. 化工学报, 2024, 75(4): 1284-1301.
Tianyi LI, Yutai WU, Yongsheng WANG, Jiarui GU, Yiheng SONG, Fengcheng YANG, Guangping HAO. Advances in light isotopes separation and catalytic labeling[J]. CIESC Journal, 2024, 75(4): 1284-1301.
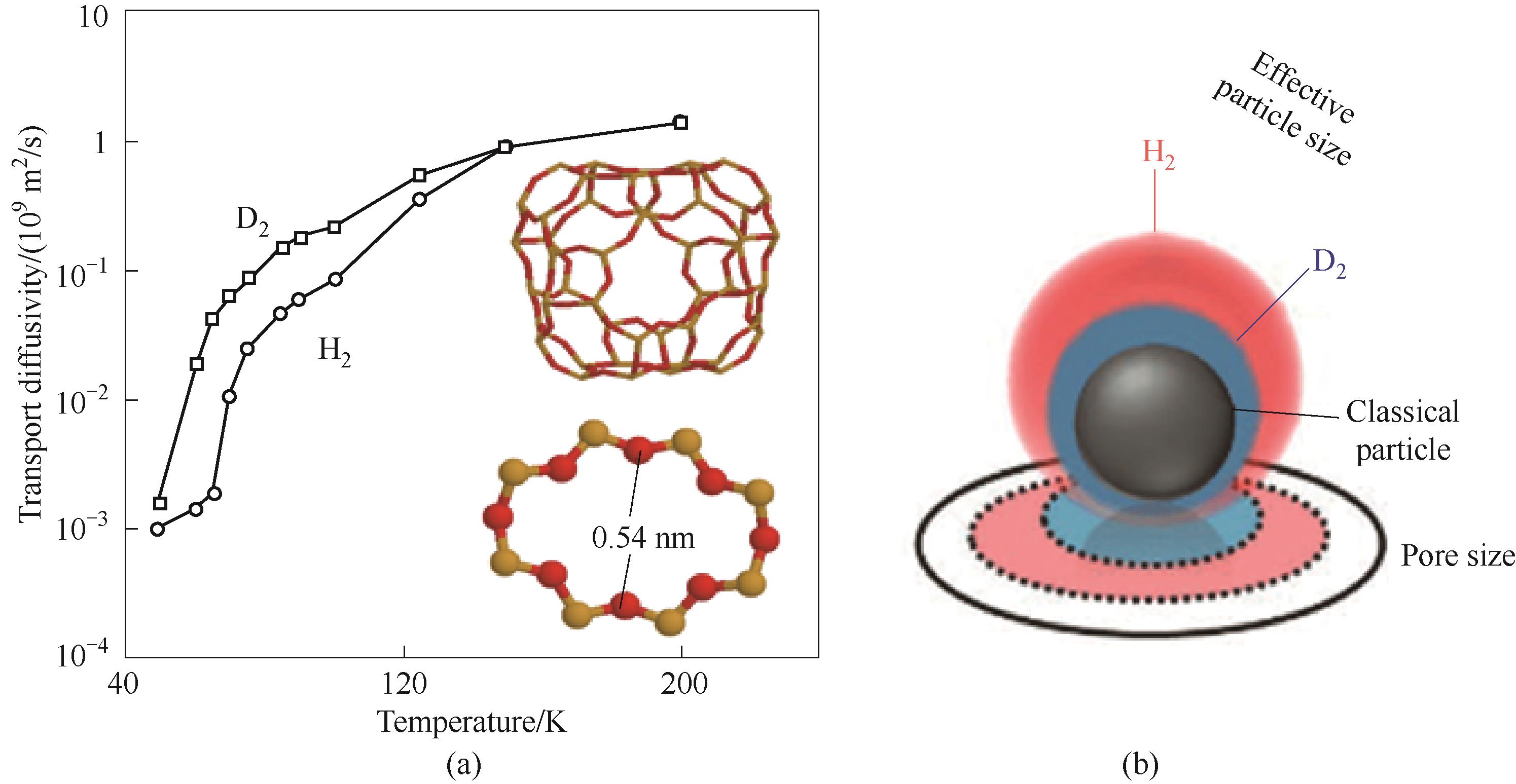
图1 (a)不同温度下H2和D2在RHO分子筛中的扩散速率对比(插图:RHO分子筛的α笼以及连接两个笼之间的8元环孔道)[14];(b)量子效应下H2和D2不同有效直径的示意图[17]
Fig.1 (a) Transport diffusivity of H2 and D2 under different temperature (insets show the one α cage of RHO zeolite and the 8-ring window of RHO zeolites connecting two cages)[14]; (b) Schematic representation for the different effective size of H2 and D2 under quantum effect[17]
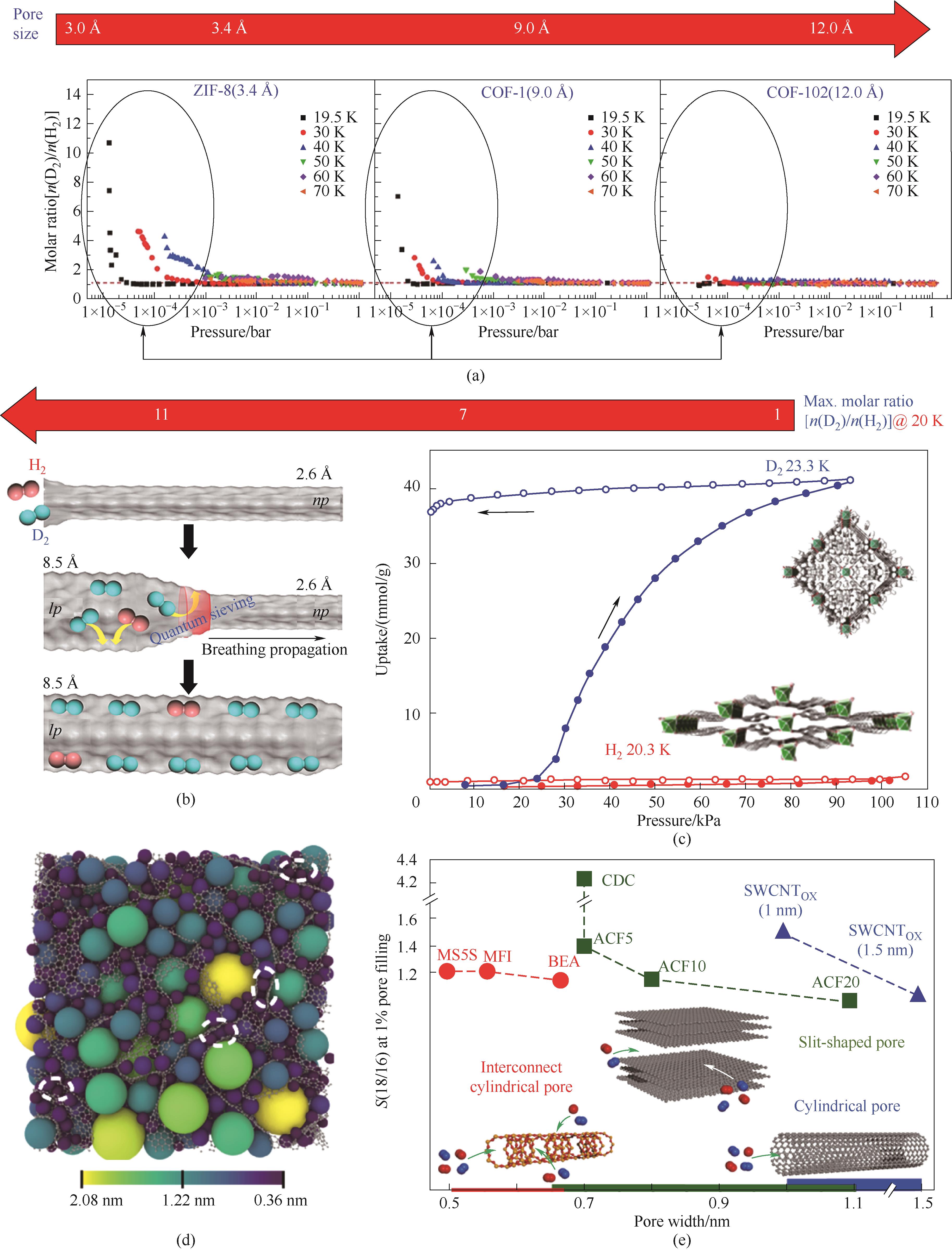
图2 (a)不同孔径的多孔材料的D2/H2分离性能对比[20](1 bar=0.1 MPa);(b)在MIL-53的一维孔道中利用呼吸效应分离D2/H2的示意图[25];(c)DUT-8的H2与D2的吸附等温线[27];(d)碳化物衍生碳的孔道3D示意图(不同颜色的球代表不同尺寸的孔);(e)112 K下不同孔尺寸及构型的18O2/16O2选择性对比[30]
Fig.2 (a) Comparison of the D2/H2 molar ratio as function of the effective pore size of organic frameworks over a temperature range[20]; (b) Schematic view of D2 separation in 1D channel of MIL-53 (Al) during the breathing propagation[25]; (c) H2 and D2 isotherms for DUT-8(Ni) at 20.3 and 23.3 K[27]; (d) 3D rendering of the same slice filled with nonoverlapping spheres (the spheres are colored by diameter, with the values indicated in the color bar); (e) Pore geometry-dependent selectivity of 18O2/16O2 at 112 K[30]
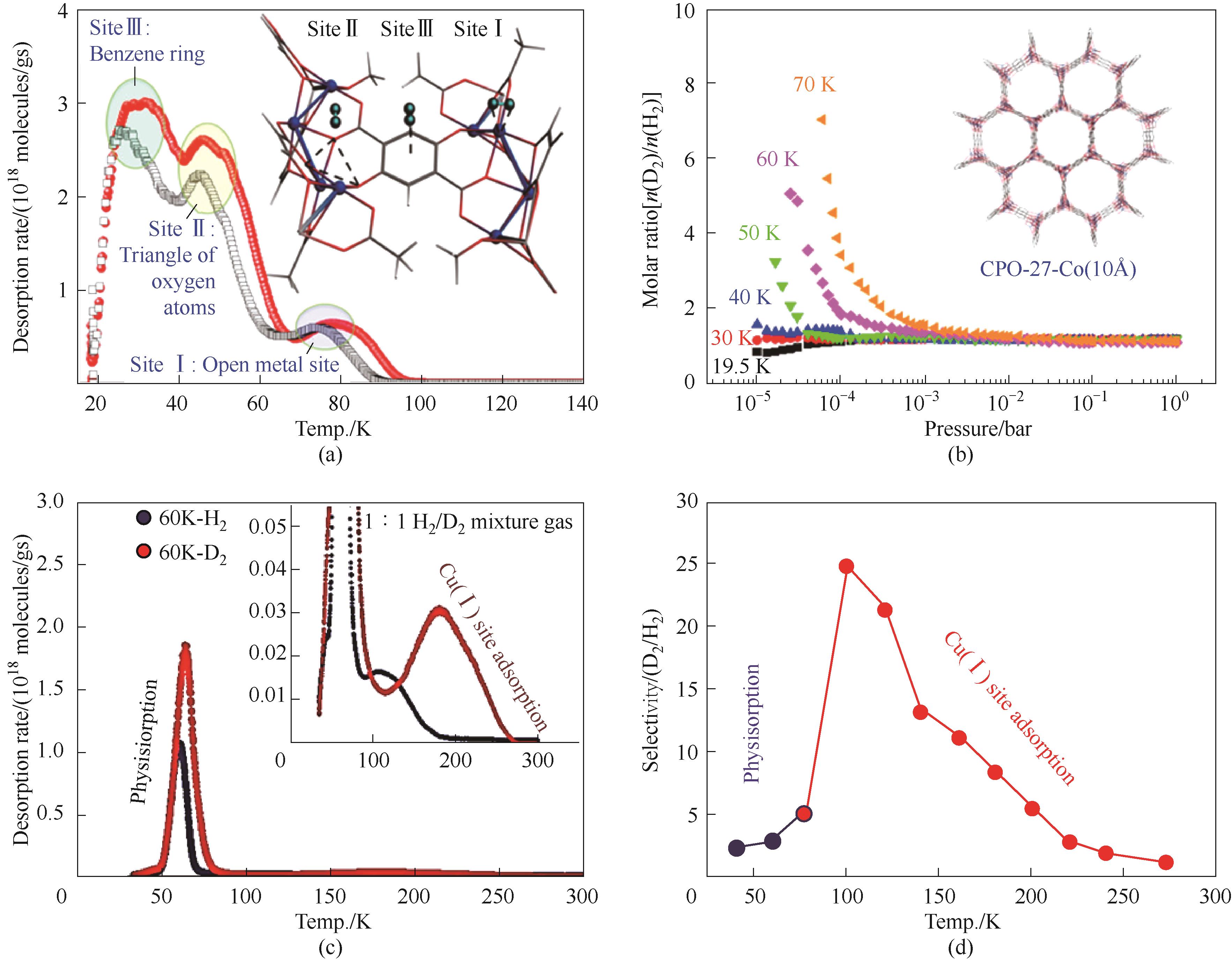
图3 (a)CPO-27-Co对D2和H2的TDS实验图谱(插图表示其对应的吸附位点);(b)CPO-27-Co在不同温度、不同压力下的D2/H2吸附选择性[35];(c)Cu(Ⅰ)-ZSM-5在60 K吸附后的TDS图谱;(d)不同温度下Cu(Ⅰ)-ZSM-5的D2/H2的选择性[37]
Fig.3 (a) TDS spectra of CPO-27-Co for pure gas H2 (open black circle) and D2 (filled red circle); (b) Selectivities of D2/H2 for CPO-27-Co at the temperature range of 19.5—70 K and pressure range of 0—1 bar[35]; (c) TDS spectra of Cu(Ⅰ)-ZSM-5 for pure gas H2 and D2; (d) Selectivities of D2/H2 for Cu(Ⅰ)-ZSM-5 at the temperature range of 23—273 K[37]
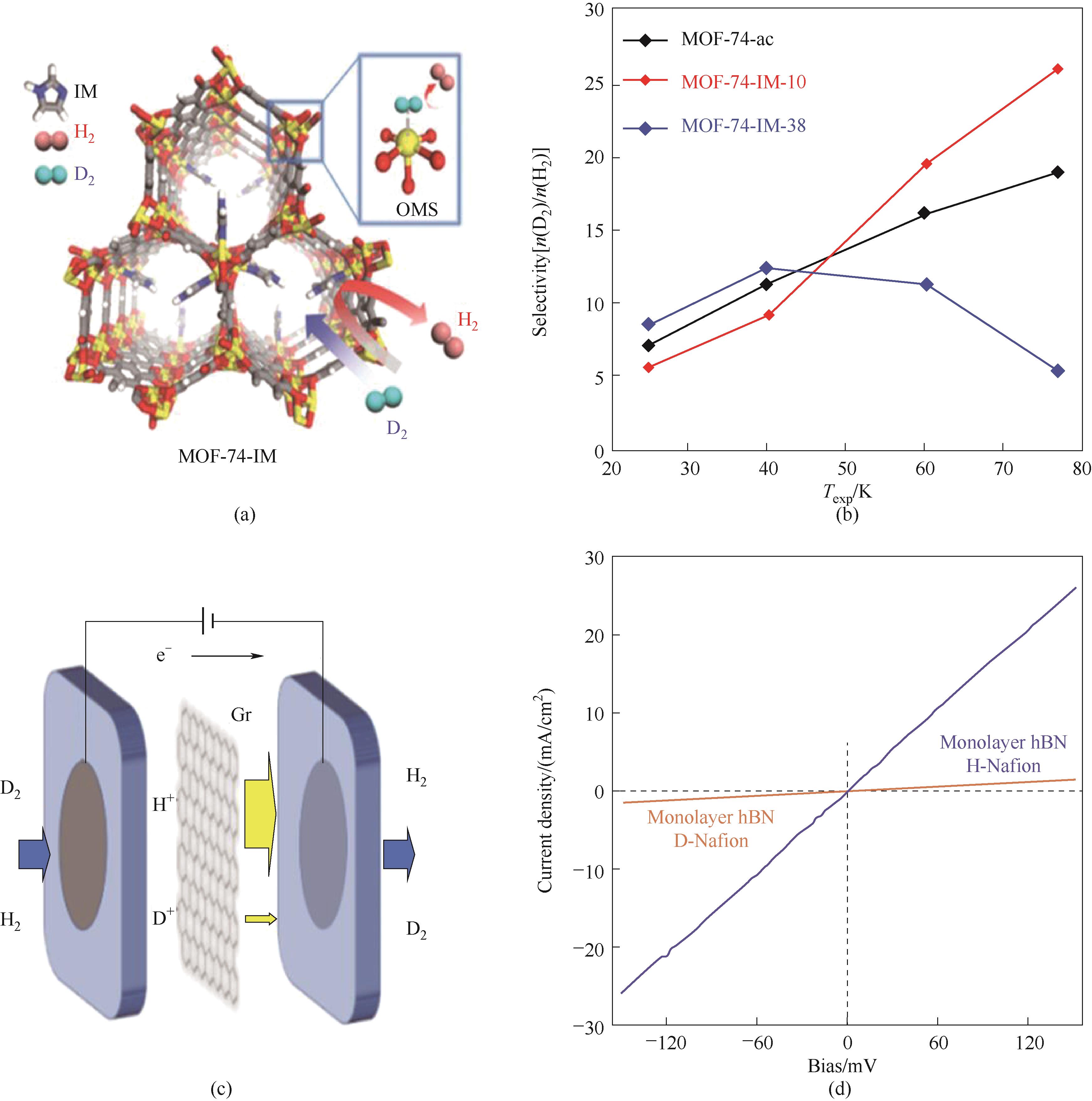
图4 (a)MOF-74-IMs协同效应分离示意图,其中,开放金属位点作为CAQS位点,咪唑(IM)修饰的狭窄孔道作为KQS位点;(b)不同温度下MOF-74-ac和MOF-74-IMs对D2/H2的选择性[39];(c)同位素膜法分离装置示意图[43];(d)H+和D+在透过单层六方氮化硼时的伏安曲线[42]
Fig.4 (a) Illustration of the CAQS and KQS sites in MOF-74-IMs; (b) Selectivity of MOF-74-ac and MOF-74-IMs as a function of exposure temperature[39]; (c) Illustration of selective proton pumping through a graphene sandwich membrane-electrode assembly[43]; (d) Examples of I-V characteristics for H+/D+ transport through monolayers of hBN[42]
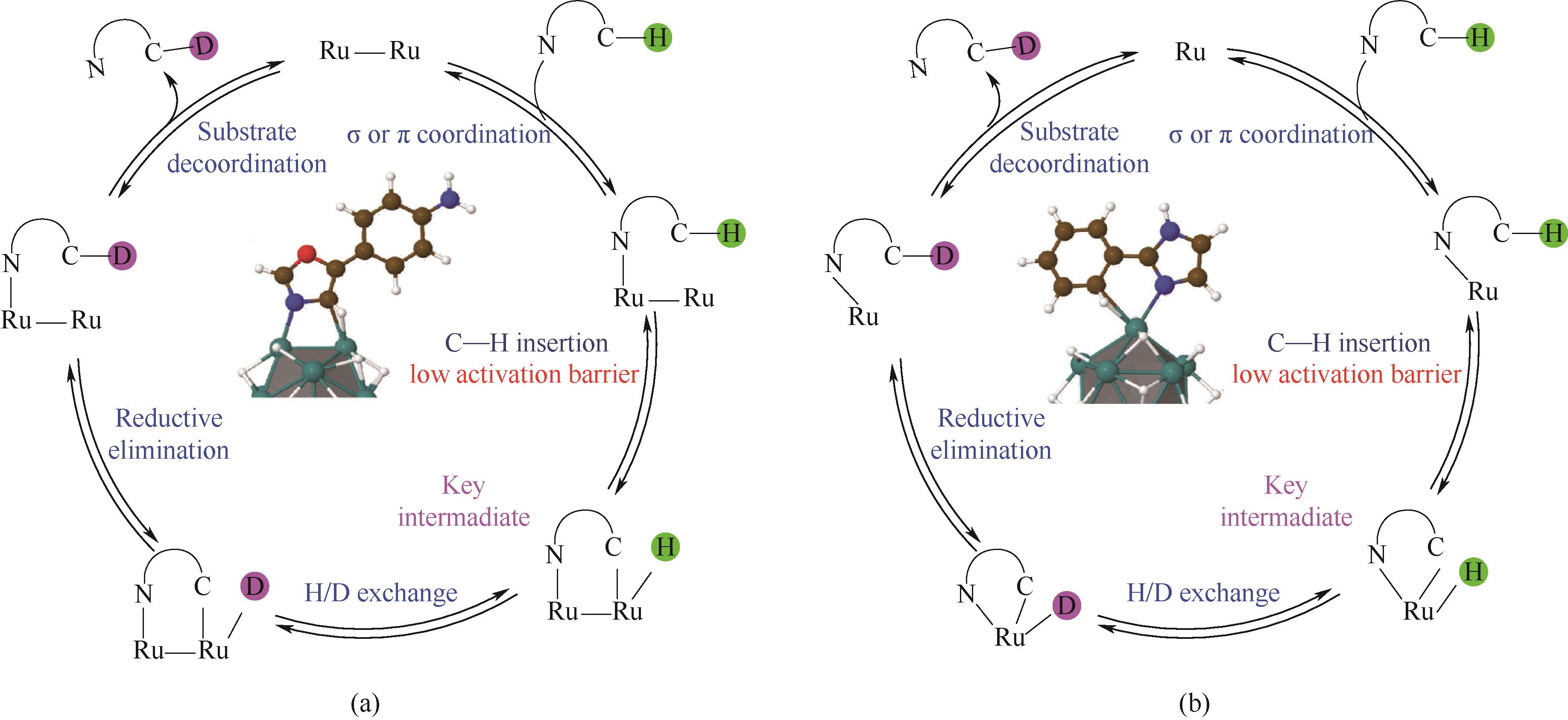
图6 Ru纳米催化剂对N-杂环化合物进行氘标记的一般机制途径:(a)使用双金属环中间体;(b)单金属环中间体[61]
Fig.6 General mechanistic pathway for the deuterium labeling of N-heterocyclic compounds using Ru NPs with dimetallacylic intermediates (a) and with monometallacy intermediates (b)[61]
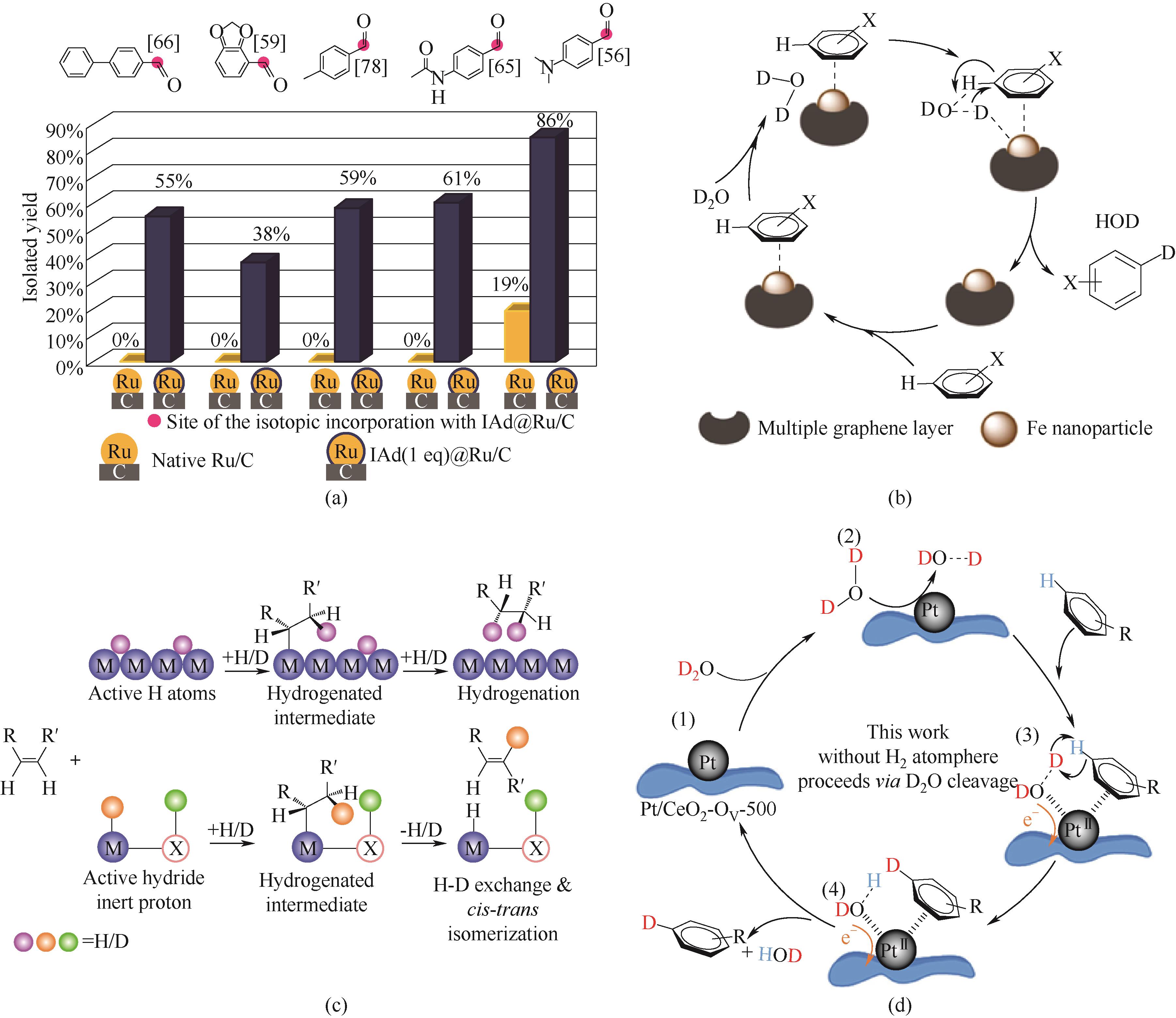
图7 (a)Ru/C和NHC修饰的Ru/C催化剂对不同醛类化合物中甲酰基官能团的氘标记性能对比[63];(b)炭载铁基催化剂催化HIE反应可能的机理途径[64];(c)顺式烯烃与块材金属或单原子分散的金属表面上H2异裂解产生的氢化物和质子进行H/D交换反应示意图[65];(d)Pt/CeO2-D2O无H2体系氘标记苯胺的可能反应路径[69]
Fig.7 (a) Comparison of the deuterium labeling performance for formyl group in various aldehydes using Ru/C and NHC-modified Ru/C catalysts [63]; (b) Possible mechanistic pathways for the catalytic HIE using carbon-supported iron[64]; (c) Schematic illustration of the H/D exchange and reaction of cis-alkene with active H atoms on block metal surface or with hydride and proton derive from the heterolytic dissociation of H2 on atomically dispersed metal catalysts[65]; (d) Possible reaction pathways for the deuteration of aniline in a Pt/CeO2-D2O system without H2[69]

图8 (a)半导体光催化剂上电子和空穴的协同作用实现可控同位素标记的N-甲基化胺的合成[80];(b)多孔CdSe光催化剂催化C—X到C—D转化的自由基途径[83];(c)铜纳米线阵列电催化剂脱卤氘代反应的机制[85];(d)钯膜反应器的氘化反应步骤示意图[93]
Fig.8 (a) Mechanistic proposal of the controllable isotope-labeled N-methylation of amines by the synergistic utilization of electrons and holes on a semiconductor photocatalyst[80]; (b) Porous CdSe photocatalyst catalyzes the radical pathway for C—X to C—D transformation[83]; (c) Mechanism of dehalogenation deuteration reaction of Cu nanowires array electrocatalysts[85]; (d) Schematic diagram of the deuteration reaction steps for the palladium membrane reactor[93]
| 1 | Dayie T K, Olenginski L T, Taiwo K M. Isotope labels combined with solution NMR spectroscopy make visible the invisible conformations of small-to-large RNAs[J]. Chemical Reviews, 2022, 122(10): 9357-9394. |
| 2 | Schellekens R C A, Stellaard F, Woerdenbag H J, et al. Applications of stable isotopes in clinical pharmacology[J]. British Journal of Clinical Pharmacology, 2011, 72(6): 879-897. |
| 3 | Ametamey S M, Honer M, Schubiger P A. Molecular imaging with PET[J]. Chemical Reviews, 2008, 108(5): 1501-1516. |
| 4 | Prokhorov I, Kluge T, Janssen C. Laser absorption spectroscopy of rare and doubly substituted carbon dioxide isotopologues[J]. Analytical Chemistry, 2019, 91(24): 15491-15499. |
| 5 | Douglas P M J, Stolper D A, Eiler J M, et al. Methane clumped isotopes: progress and potential for a new isotopic tracer[J]. Organic Geochemistry, 2017, 113: 262-282. |
| 6 | Stolper D A, Martini A M, Clog M, et al. Distinguishing and understanding thermogenic and biogenic sources of methane using multiply substituted isotopologues[J]. Geochimica et Cosmochimica Acta, 2015, 161: 219-247. |
| 7 | Saud S, Fahad S, Cui G W, et al. Determining nitrogen isotopes discrimination under drought stress on enzymatic activities, nitrogen isotope abundance and water contents of Kentucky bluegrass[J]. Scientific Reports, 2020, 10(1): 6415. |
| 8 | 孙颖, 王和义, 桑革, 等. 反应堆含氚重水提氚关键技术研究进展[J]. 中国工程科学, 2007, 9(5): 1-6. |
| Sun Y, Wang H Y, Sang G, et al. Progress in studies on the key technology of tritium extraction from reactor tritiated heavy water[J]. Engineering Science, 2007, 9(5): 1-6. | |
| 9 | 李虎林. 碳、氮、氧稳定同位素生产技术现状及发展趋势[J]. 同位素, 2011, 24(S1): 7-14. |
| Li H L. Production technology status and development trend of stable isotopes C, N and O[J]. Journal of Isotopes, 2011, 24(S1): 7-14. | |
| 10 | Igarashi T, Kambe T, Kihara H. Industrial separation of oxygen isotopes by oxygen distillation[J]. Journal of Labelled Compounds & Radiopharmaceuticals, 2019, 62(12): 865-869. |
| 11 | Oziashvili E D, Egiazarov A S. The separation of stable isotopes of carbon[J]. Russian Chemical Reviews, 1989, 58(4): 325-336. |
| 12 | Malo M, Valle F J, Jiménez F M, et al. Hisotope thermo-diffusion in structural materials[J]. Fusion Engineering and Design, 2017, 124: 924-927. |
| 13 | Park J Y, Adhikary A, Moon H R. Progress in the development of flexible metal-organic frameworks for hydrogen storage and selective separation of its isotopes[J]. Coordination Chemistry Reviews, 2023, 497(15): 215402. |
| 14 | Anil Kumar A V, Bhatia S K. Quantum effect induced reverse kinetic molecular sieving in microporous materials[J]. Physical Review Letters, 2005, 95(24): 245901. |
| 15 | Hathorn B, Sumpter B, Noid D. Contribution of restricted rotors to quantum sieving of hydrogen isotopes[J]. Physical Review A, 2001, 64(2): 022903. |
| 16 | Kidambi P R, Chaturvedi P, Moehring N K. Subatomic species transport through atomically thin membranes: present and future applications[J]. Science, 2021, 374(6568): eabd7687. |
| 17 | Kim J Y, Oh H, Moon H R. Hydrogen isotope separation in confined nanospaces: carbons, zeolites, metal-organic frameworks, and covalent organic frameworks[J]. Advanced Materials, 2019, 31(20): e1805293. |
| 18 | Kopf S, Bourriquen F, Li W, et al. Recent developments for the deuterium and tritium labeling of organic molecules[J]. Chemical Reviews, 2022, 122(6): 6634-6718. |
| 19 | Beenakker J J M, Borman V D, Krylov S Y. Molecular transport in subnanometer pores: zero-point energy, reduced dimensionality and quantum sieving[J]. Chemical Physics Letters, 1995, 232(4): 379-382. |
| 20 | Oh H, Park K S, Kalidindi S B, et al. Quantum cryo-sieving for hydrogen isotope separation in microporous frameworks: an experimental study on the correlation between effective quantum sieving and pore size[J]. Journal of Materials Chemistry A, 2013, 1(10): 3244-3248. |
| 21 | Xing Y L, Cai J J, Li L J, et al. An exceptional kinetic quantum sieving separation effect of hydrogen isotopes on commercially available carbon molecular sieves[J]. Physical Chemistry Chemical Physics, 2014, 16(30): 15800-15805. |
| 22 | Liu M, Zhang L D, Little M A, et al. Barely porous organic cages for hydrogen isotope separation[J]. Science, 2019, 366(6465): 613-620. |
| 23 | Si Y N, He X, Jiang J, et al. Highly effective H2/D2 separation in a stable Cu-based metal-organic framework[J]. Nano Research, 2021, 14(2): 518-525. |
| 24 | Yan Q Q, Wang J, Zhang L D, et al. A squarate-pillared titanium oxide quantum sieve towards practical hydrogen isotope separation[J]. Nature Communications, 2023, 14(1): 4189. |
| 25 | Kim J Y, Zhang L D, Balderas-Xicohténcatl R, et al. Selective hydrogen isotope separation via breathing transition in MIL-53(Al)[J]. Journal of the American Chemical Society, 2017, 139(49): 17743-17746. |
| 26 | Jung M, Park J, Muhammad R, et al. Elucidation of diffusivity of hydrogen isotopes in flexible MOFs by quasi-elastic neutron scattering[J]. Advanced Materials, 2021, 33(20): e2007412. |
| 27 | Bondorf L, Fiorio J L, Bon V, et al. Isotope-selective pore opening in a flexible metal-organic framework[J]. Science Advances, 2022, 8(15): eabn7035. |
| 28 | Zhang L D, Jee S, Park J, et al. Exploiting dynamic opening of apertures in a partially fluorinated MOF for enhancing H2 desorption temperature and isotope separation[J]. Journal of the American Chemical Society, 2019, 141(50): 19850-19858. |
| 29 | Garberoglio G, Johnson J K. Hydrogen isotope separation in carbon nanotubes: calculation of coupled rotational and translational states at high densities[J]. ACS Nano, 2010, 4(3): 1703-1715. |
| 30 | Ujjain S K, Bagusetty A, Matsuda Y, et al. Adsorption separation of heavier isotope gases in subnanometer carbon pores[J]. Nature Communications, 2021, 12(1): 546. |
| 31 | Wulf T, Heine T. Toward separation of hydrogen isotopologues by exploiting zero-point energy difference at strongly attractive adsorption site models[J]. International Journal of Quantum Chemistry, 2018, 118(9): e25545. |
| 32 | So S H, Oh H. A mini-review of the current progress and future challenges of zeolites for hydrogen isotopes separation through a quantum effect[J]. International Journal of Hydrogen Energy, 2024, 50: 539-560. |
| 33 | 李沐紫, 贾国伟, 赵砚珑, 等. 金属有机框架材料对非二氧化碳温室气体捕捉研究进展[J]. 化工学报, 2023, 74(1): 365-379. |
| Li M Z, Jia G W, Zhao Y L, et al. The progress of metal-organic frameworks for non-CO2 greenhouse gases capture[J]. CIESC Journal, 2023, 74(1): 365-379. | |
| 34 | FitzGerald S A, Pierce C J, Rowsell J L C, et al. Highly selective quantum sieving of D2 from H2 by a metal-organic framework as determined by gas manometry and infrared spectroscopy[J]. Journal of the American Chemical Society, 2013, 135(25): 9458-9464. |
| 35 | Oh H, Savchenko I, Mavrandonakis A, et al. Highly effective hydrogen isotope separation in nanoporous metal-organic frameworks with open metal sites: direct measurement and theoretical analysis[J]. ACS Nano, 2014, 8(1): 761-770. |
| 36 | Weinrauch I, Savchenko I, Denysenko D, et al. Capture of heavy hydrogen isotopes in a metal-organic framework with active Cu(Ⅰ) sites[J]. Nature Communications, 2017, 8: 14496. |
| 37 | Xiong R, Zhang L, Li P, et al. Highly effective hydrogen isotope separation through dihydrogen bond on Cu(Ⅰ)-exchanged zeolites well above liquid nitrogen temperature[J]. Chemical Engineering Journal, 2020, 391: 123485. |
| 38 | Giraudet M, Bezverkhyy I, Weber G, et al. D2/H2 adsorption selectivity on FAU zeolites at 77.4 K: influence of Si/Al ratio and cationic composition[J]. Microporous and Mesoporous Materials, 2018, 270: 211-219. |
| 39 | Kim J Y, Balderas-Xicohténcatl R, Zhang L D, et al. Exploiting diffusion barrier and chemical affinity of metal-organic frameworks for efficient hydrogen isotope separation[J]. Journal of the American Chemical Society, 2017, 139(42): 15135-15141. |
| 40 | Hu X Y, Ding F Y, Xiong R J, et al. Highly effective H2/D2 separation within the stable Cu(Ⅰ)Cu(Ⅱ)-BTC: the effect of Cu(Ⅰ) structure on quantum sieving[J]. ACS Applied Materials & Interfaces, 2023, 15(3): 3941-3952. |
| 41 | Hu S, Lozada-Hidalgo M, Wang F C, et al. Proton transport through one-atom-thick crystals[J]. Nature, 2014, 516(7530): 227-230. |
| 42 | Lozada-Hidalgo M, Hu S, Marshall O, et al. Sieving hydrogen isotopes through two-dimensional crystals[J]. Science, 2016, 351(6268): 68-70. |
| 43 | Bukola S, Liang Y, Korzeniewski C, et al. Selective proton/deuteron transport through Nafion|graphene|Nafion sandwich structures at high current density[J]. Journal of the American Chemical Society, 2018, 140(5): 1743-1752. |
| 44 | Yasuda S, Matsushima H, Harada K, et al. Efficient hydrogen isotope separation by tunneling effect using graphene-based heterogeneous electrocatalysts in electrochemical hydrogen isotope pumping[J]. ACS Nano, 2022, 16(9): 14362-14369. |
| 45 | Labiche A, Malandain A, Molins M, et al. Modern strategies for carbon isotope exchange[J]. Angewandte Chemie International Edition, 2023, 62(36): e202303535. |
| 46 | Zhou L, Bian X Y, Yang S Z, et al. A two-step synthesis of deuterium labeled 8,8,9,9-d4-hexadecane from nonanoic acid[J]. Journal of Labelled Compounds and Radiopharmaceuticals, 2012, 55(4): 158-160. |
| 47 | Müller K, Seubert A. Synthesis of deuterium-labelled fluorobenzoic acids to be used as internal standards in isotope dilution mass spectrometry[J]. Isotopes in Environmental and Health Studies, 2014, 50(1): 88-93. |
| 48 | Mohrig J R, Reiter N J, Kirk R, et al. Effect of buffer general acid-base catalysis on the stereoselectivity of ester and thioester H/D exchange in D2O[J]. Journal of the American Chemical Society, 2011, 133(13): 5124-5128. |
| 49 | Li F, Chen Q, Liu C C, et al. Hydrogen-deuterium exchange reaction of 2-benzylthio-5-methyl-1,2,4-triazolo[1,5-a]pyrimidine under basic conditions[J]. Applied Magnetic Resonance, 2012, 42(2): 169-177. |
| 50 | Wang L, Murai Y, Yoshida T, et al. Hydrogen/deuterium exchange of cross-linkable α-amino acid derivatives in deuterated triflic acid[J]. Bioscience, Biotechnology, and Biochemistry, 2014, 78(7): 1129-1134. |
| 51 | Duttwyler S, Butterfield A M, Siegel J S. Arenium acid catalyzed deuteration of aromatic hydrocarbons[J]. The Journal of Organic Chemistry, 2013, 78(5): 2134-2138. |
| 52 | Sakamoto T, Mori K, Akiyama T. Chiral phosphoric acid catalyzed enantioselective transfer deuteration of ketimines by use of benzothiazoline as a deuterium donor: synthesis of optically active deuterated amines[J]. Organic Letters, 2012, 14(13): 3312-3315. |
| 53 | Hu Y, Liang L, Wei W T, et al. A convenient synthesis of deuterium labeled amines and nitrogen heterocycles with KOt-Bu/DMSO-d6 [J]. Tetrahedron, 2015, 71(9): 1425-1430. |
| 54 | Zhan M, Xu R X, Tian Y, et al. A simple and cost-effective method for the regioselective deuteration of phenols[J]. European Journal of Organic Chemistry, 2015, 2015(15): 3370-3373. |
| 55 | Sajiki H, Sawama Y, Monguchi Y. Efficient H-D exchange reactions using heterogeneous platinum-group metal on carbon-H2-D2O system[J]. Synlett, 2012, 23(7): 959-972. |
| 56 | Sawama Y, Nakano A, Matsuda T, et al. H-D exchange deuteration of arenes at room temperature[J]. Organic Process Research & Development, 2019, 23(4): 648-653. |
| 57 | Yamada T, Sawama Y, Shibata K, et al. Multiple deuteration of alkanes synergistically-catalyzed by platinum and rhodium on carbon as a mixed catalytic system[J]. RSC Advances, 2015, 5(18): 13727-13732. |
| 58 | Pery T, Pelzer K, Buntkowsky G, et al. Direct NMR evidence for the presence of mobile surface hydrides on ruthenium nanoparticles[J]. ChemPhysChem, 2005, 6(4): 605-607. |
| 59 | Pieters G, Taglang C, Bonnefille E, et al. Regioselective and stereospecific deuteration of bioactive aza compounds by the use of ruthenium nanoparticles[J]. Angewandte Chemie International Edition, 2014, 53(1): 230-234. |
| 60 | Palazzolo A, Feuillastre S, Pfeifer V, et al. Efficient access to deuterated and tritiated nucleobase pharmaceuticals and oligonucleotides using hydrogen-isotope exchange[J]. Angewandte Chemie International Edition, 2019, 58(15): 4891-4895. |
| 61 | Pfeifer V, Certiat M, Bouzouita D, et al. Hydrogen isotope exchange catalyzed by Ru nanocatalysts: labelling of complex molecules containing n-heterocycles and reaction mechanism insights[J]. Chemistry, 2020, 26(22): 4988-4996. |
| 62 | Valero M, Bouzouita D, Palazzolo A, et al. NHC-stabilized iridium nanoparticles as catalysts in hydrogen isotope exchange reactions of anilines[J]. Angewandte Chemie International Edition, 2020, 59(9): 3517-3522. |
| 63 | Palazzolo A, Naret T, Daniel-Bertrand M, et al. Tuning the reactivity of a heterogeneous catalyst using n-heterocyclic carbene ligands for C—H activation reactions[J]. Angewandte Chemie International Edition, 2020, 59(47): 20879-20884. |
| 64 | Li W, Rabeah J, Bourriquen F, et al. Scalable and selective deuteration of (hetero)arenes[J]. Nature Chemistry, 2022, 14(3): 334-341. |
| 65 | Liu K L, Qin R X, Li K J, et al. Atomically dispersed palladium catalyzes H/D exchange and isomerization of alkenes via reversible insertion and elimination[J]. Chem Catalysis, 2021, 1(7): 1480-1492. |
| 66 | Ren Y Q, Chang R P, Hu X, et al. Layer-by-layer enriching active Ni-N3C sites in nickel-nitrogen-carbon electrocatalysts for enhanced CO2-to-CO reduction[J]. Chinese Chemical Letters, 2023, 34(12): 108634. |
| 67 | Tang S Y, Wang Y S, Yuan Y F, et al. Hydrophilic carbon monoliths derived from metal-organic frameworks@resorcinol-formaldehyde resin for atmospheric water harvesting[J]. New Carbon Materials, 2022, 37(1): 237-244. |
| 68 | Jiang W J, Shao F J, Cheng J X, et al. Calcium aluminate induced Pt0-Pt δ + coupling boost catalyzed H-D exchange reaction of arenes with deuterium oxide[J]. Asian Journal of Organic Chemistry, 2023, 12(4): e202200662. |
| 69 | Jiang W J, Shao F J, Xu Z P, et al. Oxygen vacancy of CeO2 modulated Pt catalyst facilitates H-D exchange of aniline aromatics by cleaving D2O[J]. AIChE Journal, 2024, 70(4): e18331. |
| 70 | Maegawa T, Fujiwara Y, Inagaki Y, et al. Mild and efficient H/D exchange of alkanes based on C—H activation catalyzed by rhodium on charcoal[J]. Angewandte Chemie International Edition, 2008, 47(29): 5394-5397. |
| 71 | Gao J, Ma R, Feng L, et al. Ambient hydrogenation and deuteration of alkenes using a nanostructured Ni-core-shell catalyst[J]. Angewandte Chemie International Edition, 2021, 60(34): 18591-18598. |
| 72 | Tuokko S, Pihko P M. Palladium on charcoal as a catalyst for stoichiometric chemo- and stereoselective hydrosilylations and hydrogenations with triethylsilane[J]. Organic Process Research & Development, 2014, 18(12): 1740-1751. |
| 73 | Sawama Y, Park K, Yamada T, et al. New gateways to the platinum group metal-catalyzed direct deuterium-labeling method utilizing hydrogen as a catalyst activator[J]. Chemical & Pharmaceutical Bulletin, 2018, 66(1): 21-28. |
| 74 | Zhou B, Chandrashekhar V G, Ma Z, et al. Development of a general and selective nanostructured cobalt catalyst for the hydrogenation of benzofurans, indoles and benzothiophenes[J]. Angewandte Chemie International Edition, 2023, 62(10): e202215699. |
| 75 | Li W, Qu R Y, Liu W P, et al. Copper-catalysed low-temperature water-gas shift reaction for selective deuteration of aryl halides[J]. Chemical Science, 2021, 12(42): 14033-14038. |
| 76 | 董灵玉, 葛睿, 原亚飞, 等. 多孔炭基二氧化碳电催化材料研究进展[J]. 化工学报, 2020, 71(6): 2492-2509. |
| Dong L Y, Ge R, Yuan Y F, et al. Recent advances in porous carbon-based carbon dioxide electrocatalytic materials[J]. CIESC Journal, 2020, 71(6): 2492-2509. | |
| 77 | Dong L Y, Guan M H, Ren Y Q, et al. Mesoporous Fe-doped carbon electrocatalysts with highly exposed active sites for efficient synthesis of hydrogen peroxide and tandem epoxidation of propene[J]. Renewables, 2023, 1(5): 562-571. |
| 78 | Ou W, Qiu C T, Su C L. Photo- and electro-catalytic deuteration of feedstock chemicals and pharmaceuticals: a review[J]. Chinese Journal of Catalysis, 2022, 43(4): 956-970. |
| 79 | Qiu C T, Xu Y S, Fan X, et al. Highly crystalline K-intercalated polymeric carbon nitride for visible-light photocatalytic alkenes and alkynes deuterations[J]. Advanced Science, 2018, 6(1): 1801403. |
| 80 | Zhang Z F, Qiu C T, Xu Y S, et al. Semiconductor photocatalysis to engineering deuterated n-alkyl pharmaceuticals enabled by synergistic activation of water and alkanols[J]. Nature Communications, 2020, 11(1): 4722. |
| 81 | Nan X L, Wang Y, Li X B, et al. Site-selective D2O-mediated deuteration of diaryl alcohols via quantum dots photocatalysis[J]. Chemical Communications, 2021, 57(55): 6768-6771. |
| 82 | Han C, Han G Q, Yao S K, et al. Defective ultrathin ZnIn2S4 for photoreductive deuteration of carbonyls using D2O as the deuterium source[J]. Advanced Science, 2022, 9(3): e2103408. |
| 83 | Liu C B, Chen Z X, Su C L, et al. Controllable deuteration of halogenated compounds by photocatalytic D2O splitting[J]. Nature Communications, 2018, 9(1): 80. |
| 84 | Luo T, Wang Z, Chen Y L, et al. Photocatalytic dehalogenative deuteration of halides over a robust metal-organic framework[J]. Angewandte Chemie International Edition, 2023, 62(48): e202306267. |
| 85 | Liu C B, Han S Y, Li M Y, et al. Electrocatalytic deuteration of halides with D2O as the deuterium source over a copper nanowire arrays cathode[J]. Angewandte Chemie International Edition, 2020, 59(42): 18527-18531. |
| 86 | Wu Y M, Liu C B, Wang C H, et al. Selective transfer semihydrogenation of alkynes with H2O (D2O) as the H (D) source over a Pd-P cathode[J]. Angewandte Chemie International Edition, 2020, 59(47): 21170-21175. |
| 87 | Wu Y M, Liu C B, Wang C H, et al. Converting copper sulfide to copper with surface sulfur for electrocatalytic alkyne semi-hydrogenation with water[J]. Nature Communications, 2021, 12(1): 3881. |
| 88 | Guo S S, Wu Y M, Wang C H, et al. Electrocatalytic hydrogenation of quinolines with water over a fluorine-modified cobalt catalyst[J]. Nature Communications, 2022, 13(1): 5297. |
| 89 | Li H Z, Gao Y, Wu Y M, et al. σ-Alkynyl adsorption enables electrocatalytic semihydrogenation of terminal alkynes with easy-reducible/passivated groups over amorphous PdS x nanocapsules[J]. Journal of the American Chemical Society, 2022, 144(42): 19456-19465. |
| 90 | Song Z Y, Cheng C Q, Wang C H, et al. Interstitial modification of palladium nanocubes with nitrogen atoms promotes aqueous electrocatalytic alkyne semihydrogenation[J]. ACS Materials Letters, 2023, 5(11): 3068-3073. |
| 91 | Li R, Wu Y M, Wang C H, et al. One-pot H/D exchange and low-coordinated iron electrocatalyzed deuteration of nitriles in D2O to α, β-deuterio aryl ethylamines[J]. Nature Communications, 2022, 13(1): 5951. |
| 92 | Wu Y M, Li M Y, Li T L, et al. Electrosynthesis of 15N-labeled amino acids from 15N-nitrite and ketonic acids[J]. Science China Chemistry, 2023, 66(6): 1854-1859. |
| 93 | Kurimoto A, Sherbo R S, Cao Y, et al. Electrolytic deuteration of unsaturated bonds without using D2 [J]. Nature Catalysis, 2020, 3: 719-726. |
| 94 | Ou W, Xiang X D, Zou R, et al. Room-temperature palladium-catalyzed deuterogenolysis of carbon oxygen bonds towards deuterated pharmaceuticals[J]. Angewandte Chemie International Edition, 2021, 60(12): 6357-6361. |
| [1] | 范以薇, 刘威, 李盈盈, 王培霞, 张吉松. 有机液体储氢中全氢化乙基咔唑催化脱氢研究进展[J]. 化工学报, 2024, 75(4): 1198-1208. |
| [2] | 贾旭东, 杨博龙, 程前, 李雪丽, 向中华. 分步负载金属法制备铁钴双金属位点高效氧还原电催化剂[J]. 化工学报, 2024, 75(4): 1578-1593. |
| [3] | 严孝清, 赵瑛, 张宇哲, 欧鸿辉, 黄起中, 胡华贵, 杨贵东. 五重孪晶铜纳米线@聚吡咯制备及其电催化硝酸盐还原制氨[J]. 化工学报, 2024, 75(4): 1519-1532. |
| [4] | 韩宇, 周乐, 张鑫, 罗勇, 孙宝昌, 邹海魁, 陈建峰. 高黏附性Pd/SiO2/NF整体式催化剂的制备及加氢性能研究[J]. 化工学报, 2024, 75(4): 1533-1542. |
| [5] | 李昂, 赵振宇, 李洪, 高鑫. 微波诱导高分散Pd/FeP催化剂构筑及其电催化性能研究[J]. 化工学报, 2024, 75(4): 1594-1606. |
| [6] | 刘莹, 郑芳, 杨启炜, 张治国, 任其龙, 鲍宗必. 二甲苯异构体吸附分离研究进展[J]. 化工学报, 2024, 75(4): 1081-1095. |
| [7] | 孙铭泽, 黄鹤来, 牛志强. 铂基氧还原催化剂:从单晶电极到拓展表面纳米材料[J]. 化工学报, 2024, 75(4): 1256-1269. |
| [8] | 程骁恺, 历伟, 王靖岱, 阳永荣. 镍催化可控/活性自由基聚合反应研究进展[J]. 化工学报, 2024, 75(4): 1105-1117. |
| [9] | 陈志明, 王泽凤, 马高琪, 王良波, 余承涛, 潘鹏举. 基于亚锡灭活及链端改性提高聚乳酸热稳定性的研究进展[J]. 化工学报, 2024, 75(3): 760-767. |
| [10] | 张天永, 张晶怡, 姜爽, 李彬, 吕东军, 陈都民, 陈雪. 弱酸性蓝AS染料排放的废盐制碳基吸附剂及利用[J]. 化工学报, 2024, 75(3): 890-899. |
| [11] | 卫月星, 贺子岳, 燕可洲, 李林玉, 秦育红, 贺冲, 焦路畅. 改性煤气化渣催化降解双酚A的性能研究[J]. 化工学报, 2024, 75(3): 877-889. |
| [12] | 王宝凤, 王术高, 程芳琴. 固废基硫掺杂多孔炭材料制备及其对CO2吸附性能研究进展[J]. 化工学报, 2024, 75(2): 395-411. |
| [13] | 盖星宇, 岳玉学, 杨春华, 张子龙, 蔡天姿, 张海丰, 王柏林, 李小年. 碳负载Cs和Cu基催化剂用于1,1,2-三氯乙烷的气相脱氯化氢[J]. 化工学报, 2024, 75(2): 575-583. |
| [14] | 张强, 王宪飞, 王凯, 骆广生, 路忠凯. 非金属催化剂在环氧化物和环状酸酐共聚中的研究进展[J]. 化工学报, 2024, 75(1): 60-73. |
| [15] | 王欣雨, 王永涛, 姚加, 李浩然. 电子顺磁共振技术在化工基础研究中的应用进展[J]. 化工学报, 2024, 75(1): 74-82. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号