化工学报 ›› 2024, Vol. 75 ›› Issue (4): 1594-1606.DOI: 10.11949/0438-1157.20231388
收稿日期:2023-12-28
修回日期:2024-02-01
出版日期:2024-04-25
发布日期:2024-06-06
通讯作者:
赵振宇,高鑫
作者简介:李昂(1998—),男,硕士研究生,2021207419@tju.edu.cn
基金资助:
Ang LI1( ), Zhenyu ZHAO1(
), Zhenyu ZHAO1( ), Hong LI1, Xin GAO1,2(
), Hong LI1, Xin GAO1,2( )
)
Received:2023-12-28
Revised:2024-02-01
Online:2024-04-25
Published:2024-06-06
Contact:
Zhenyu ZHAO, Xin GAO
摘要:
微波技术作为一种新型过程强化手段,已被广泛应用于材料制备过程。利用微波对吸波型载体的选择性加热,使其产生局部高温诱导催化剂颗粒的沉积,有望构筑高分散钯(Pd)催化剂结构,而这一特殊结构对提高催化剂电催化氧化甲酸活性、提升甲酸燃料电池的性能至关重要。为探究微波诱导高分散钯催化剂便捷制备的可行性,本文首先通过水热法制备了强吸波的空心海胆状磷化铁(FeP)作为催化剂载体,而后分别在常规加热与微波加热条件下通过乙二醇还原法在FeP表面沉积Pd。使用XRD、TEM、SEM技术表征Pd/FeP产品的形貌和微观结构,探究微波加热对催化剂表面金属钯颗粒分散的影响作用。使用循环伏安法和线性伏安法评价所制备催化剂的催化活性,通过探讨催化剂结构与其电催化活性的构效关系,揭示微波合成对Pd/FeP催化剂性能的强化作用机制。研究结果表明,空心海胆状的FeP颗粒具有较强的微波吸收能力,因而在受到微波辐射时,其表面形成的局部过热诱导Pd的原位沉积,使得微波水热法制备的Pd催化剂具有良好的分散特性,然而溶剂主体温度的过高会增加Pd之间的团聚。相对于常规手段合成的催化剂,利用微波水热法在120°C下制备的催化剂电化学活性面积提升了约3.5倍,对甲酸电催化氧化活性提升了约54倍。
中图分类号:
李昂, 赵振宇, 李洪, 高鑫. 微波诱导高分散Pd/FeP催化剂构筑及其电催化性能研究[J]. 化工学报, 2024, 75(4): 1594-1606.
Ang LI, Zhenyu ZHAO, Hong LI, Xin GAO. Microwave induced construction of highly dispersed Pd/FeP catalysts and their electrocatalytic performance[J]. CIESC Journal, 2024, 75(4): 1594-1606.
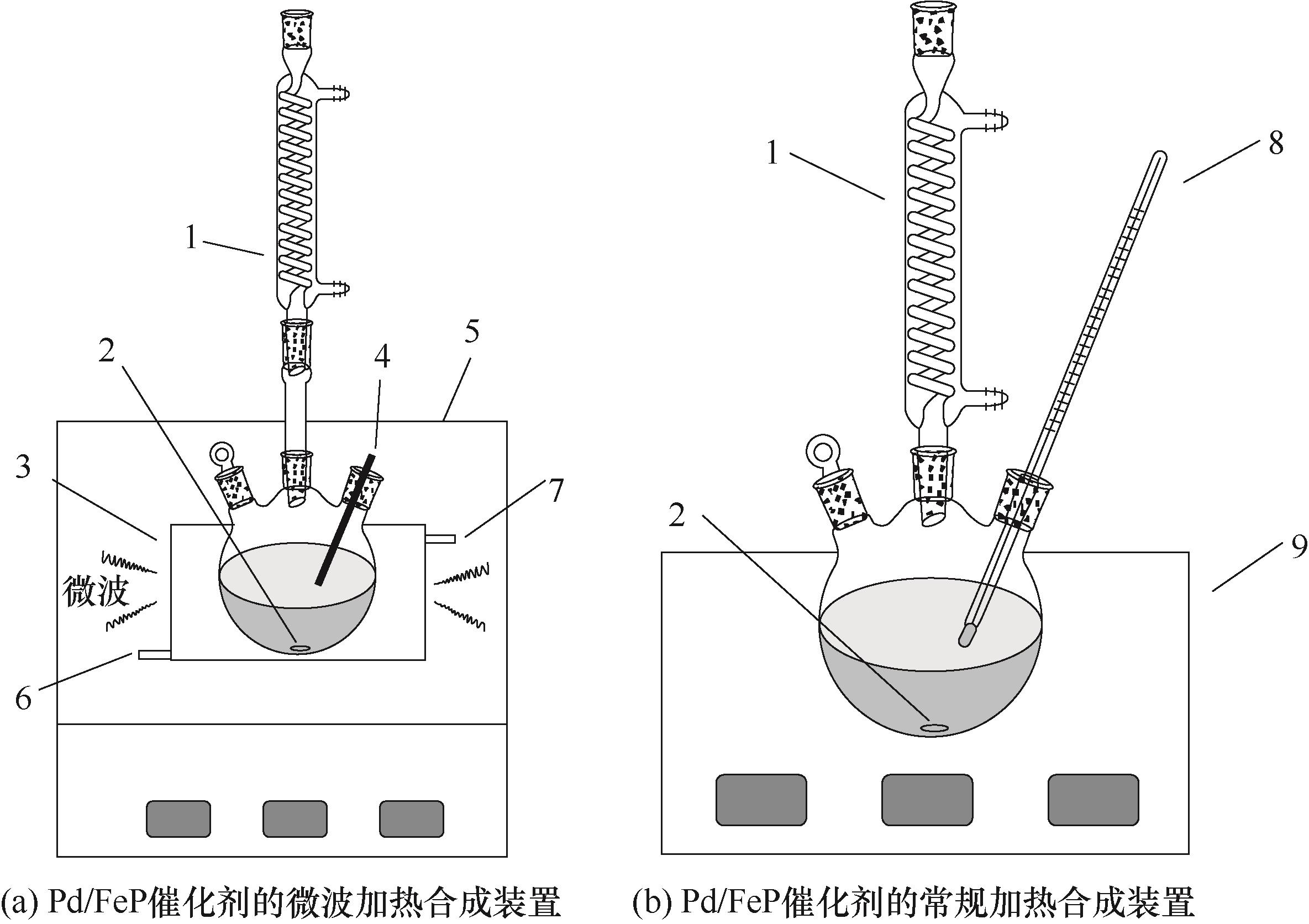
图1 微波和常规加热合成Pd/FeP催化剂装置示意图1—蛇形冷凝管;2—磁子;3—带夹套的微波反应器;4—光纤温度计;5—微波炉;6—硅油入口;7—硅油出口;8—煤油温度计;9—磁力搅拌油浴锅
Fig.1 Schematic diagram of experimental apparatuses for the synthesis of Pd/FeP catalyst under microwave irradiation and conventional heating, respectively
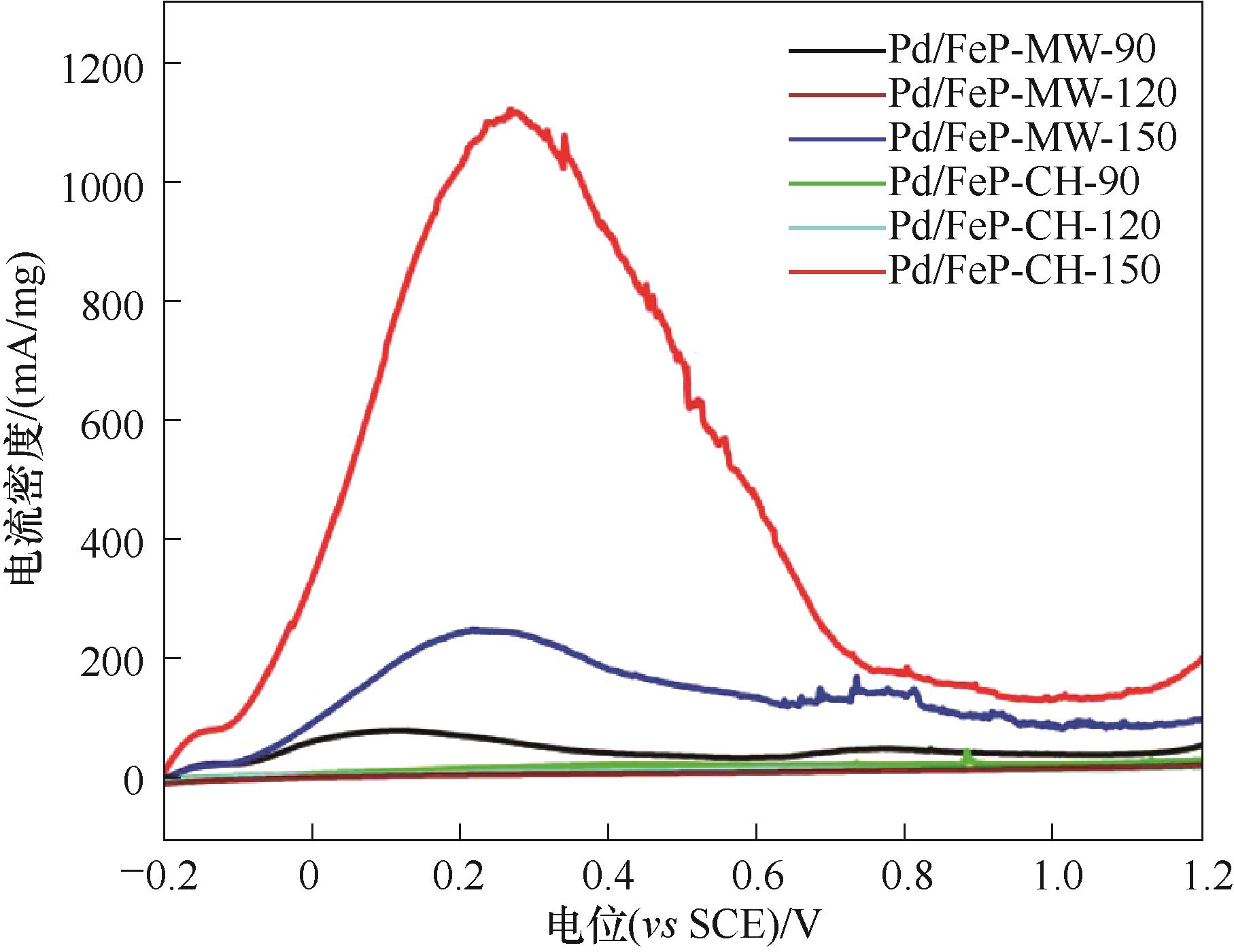
图11 Pd/FeP催化剂在0.5 mol/L H2SO4+0.5 mol/L HCOOH溶液中的线性扫描伏安图
Fig.11 Linear sweep voltammetry of Pd/FeP catalyst in 0.5 mol/L H2SO4+0.5 mol/L HCOOH solution
| 1 | Yang C Z, Wang T Y, Li C C, et al. PdMo bimetallene coupled with MXene nanosheets as efficient bifunctional electrocatalysts for formic acid and methanol oxidation reactions[J]. ACS Applied Materials & Interfaces, 2023, 15(42): 49195-49203. |
| 2 | Hu S Z, Gao Z Q, Gao S J, et al. Atomic ordering effect of intermetallic PdCoNi/rGO catalysts on formic acid electro-oxidation[J]. ACS Applied Energy Materials, 2023, 6(21): 11051-11060. |
| 3 | Shatla A S, Hassan K M, Abd-El-Latif A A, et al. Poly 1,5-diaminonaphthalene supported Pt, Pd, Pt/Pd and Pd/Pt nanoparticles for direct formic acid oxidation[J]. Journal of Electroanalytical Chemistry, 2019, 833: 231-241. |
| 4 | Zhang L, Wan L, Ma Y R, et al. Crystalline palladium-cobalt alloy nanoassemblies with enhanced activity and stability for the formic acid oxidation reaction[J]. Applied Catalysis B: Environmental, 2013, 138/139: 229-235. |
| 5 | Zhao L, Sui X L, Li J Z, et al. Supramolecular assembly promoted synthesis of three-dimensional nitrogen doped graphene frameworks as efficient electrocatalyst for oxygen reduction reaction and methanol electrooxidation[J]. Applied Catalysis B: Environmental, 2018, 231: 224-233. |
| 6 | Qiu B C, Cai L J, Wang Y, et al. Fabrication of nickel-cobalt bimetal phosphide nanocages for enhanced oxygen evolution catalysis[J]. Advanced Functional Materials, 2018, 28(17): 1706008. |
| 7 | Yang J, Zhang Y, Sun C C, et al. Graphene and cobalt phosphide nanowire composite as an anode material for high performance lithium-ion batteries[J]. Nano Research, 2016, 9(3): 612-621. |
| 8 | Hong W T, Jian C Y, Wang G X, et al. Self-supported nanoporous cobalt phosphosulfate electrodes for efficient hydrogen evolution reaction[J]. Applied Catalysis B: Environmental, 2019, 251: 213-219. |
| 9 | Chang J F, Feng L G, Liu C P, et al. An effective Pd-Ni2P/C anode catalyst for direct formic acid fuel cells[J]. Angewandte Chemie (International Ed. in English), 2014, 53(1): 122-126. |
| 10 | Chang J F, Feng L G, Liu C P, et al. Ni2P enhances the activity and durability of the Pt anode catalyst in direct methanol fuel cells[J]. Energy & Environmental Science, 2014, 7(5): 1628-1632. |
| 11 | Wang F L, Xue H G, Tian Z Q, et al. Fe2P as a novel efficient catalyst promoter in Pd/C system for formic acid electro-oxidation in fuel cells reaction[J]. Journal of Power Sources, 2018, 375: 37-42. |
| 12 | Leng Y, Zhang C J, Liu B, et al. Synergistic activation of palladium nanoparticles by polyoxometalate-attached melem for boosting formic acid dehydrogenation efficiency[J]. ChemSusChem, 2018, 11(19): 3396-3401. |
| 13 | Bao Y F, Wang F L, Gu X C, et al. Core-shell structured PtRu nanoparticles@FeP promoter with an efficient nanointerface for alcohol fuel electrooxidation[J]. Nanoscale, 2019, 11(40): 18866-18873. |
| 14 | Zhang L L, Ding L X, Luo Y R, et al. PdO/Pd-CeO2 hollow spheres with fresh Pd surface for enhancing formic acid oxidation[J]. Chemical Engineering Journal, 2018, 347: 193-201. |
| 15 | Wang H W, Gu X K, Zheng X S, et al. Disentangling the size-dependent geometric and electronic effects of palladium nanocatalysts beyond selectivity[J]. Science Advances, 2019, 5(1): eaat6413. |
| 16 | Zhou W J, Li M, Ding O L, et al. Pd particle size effects on oxygen electrochemical reduction[J]. International Journal of Hydrogen Energy, 2014, 39(12): 6433-6442. |
| 17 | Wei M, Zhao L, Jiang L W, et al. Development of high-throughput rapid heat-treatment and characterization process[J]. Acta Materialia, 2023, 261: 119365. |
| 18 | Kappe C O. Controlled microwave heating in modern organic synthesis[J]. Angewandte Chemie (International Ed. in English), 2004, 43(46): 6250-6284. |
| 19 | Varma R S. Journey on greener pathways: from the use of alternate energy inputs and benign reaction media to sustainable applications of nano-catalysts in synthesis and environmental remediation[J]. Green Chemistry, 2014, 16(4): 2027-2041. |
| 20 | Breeden S W, Clark J H, Farmer T J, et al. Microwave heating for rapid conversion of sugars and polysaccharides to 5-chloromethyl furfural[J]. Green Chemistry, 2013, 15(1): 72-75. |
| 21 | Demirskyi D, Agrawal D, Ragulya A. Neck growth kinetics during microwave sintering of copper[J]. Scripta Materialia, 2010, 62(8): 552-555. |
| 22 | Upadhyaya A, Tiwari S K, Mishra P. Microwave sintering of W-Ni-Fe alloy[J]. Scripta Materialia, 2007, 56(1): 5-8. |
| 23 | Ebadzadeh T, Sarrafi M H, Salahi E. Microwave-assisted synthesis and sintering of mullite[J]. Ceramics International, 2009, 35(8): 3175-3179. |
| 24 | Fidalgo B, Fernández Y, Zubizarreta L, et al. Growth of nanofilaments on carbon-based materials from microwave-assisted decomposition of CH4 [J]. Applied Surface Science, 2008, 254(11): 3553-3557. |
| 25 | Schwenke A M, Hoeppener S, Schubert U S. Synthesis and modification of carbon nanomaterials utilizing microwave heating[J]. Advanced Materials, 2015, 27(28): 4113-4141. |
| 26 | 陈婷, 胡泽浩, 秦喆, 等. 有机相微波合成AgInS2量子点及其白光发光二极管应用研究[J]. 化工学报, 2022,73(11): 5167-5176. |
| Chen T, Hu Z H, QIN Z, et al. Microwave synthesis of AgInS2 quantum dots in organic solvent and application for white light-emitting diodes[J]. CIESC Journal, 2022, 73(11): 5167-5176. | |
| 27 | Xu W T, Zhou J C, Su Z M, et al. Microwave catalytic effect: a new exact reason for microwave-driven heterogeneous gas-phase catalytic reactions[J]. Catalysis Science & Technology, 2016, 6(3): 698-702. |
| 28 | Luo M D, Zhou J C, Xu W T, et al. Development of composite microwave catalysts (ABS x /CNTs, A = Co, B = Ni, Mo) for the highly effective direct decomposition of H2S into H2 and S[J]. Fuel, 2020, 281: 118729. |
| 29 | Rodríguez A M, Prieto P, de la Hoz A, et al. Influence of polarity and activation energy in microwave-assisted organic synthesis (MAOS)[J]. ChemistryOpen, 2015, 4(3): 308-317. |
| 30 | Dudley G B, Richert R, Stiegman A E. On the existence of and mechanism for microwave-specific reaction rate enhancement[J]. Chemical Science, 2015, 6(4): 2144-2152. |
| 31 | Horikoshi S, Serpone N. Role of microwaves in heterogeneous catalytic systems[J]. Catalysis Science & Technology, 2014, 4(5): 1197-1210. |
| 32 | Horikoshi S, Osawa A, Abe M, et al. On the generation of hot-spots by microwave electric and magnetic fields and their impact on a microwave-assisted heterogeneous reaction in the presence of metallic Pd nanoparticles on an activated carbon support[J]. The Journal of Physical Chemistry C, 2011, 115(46): 23030-23035. |
| 33 | Zhao Z Y, Shen X, Li H, et al. Watching microwave-induced microscopic hot spots via the thermosensitive fluorescence of europium/terbium mixed-metal organic complexes[J]. Angewandte Chemie (International Ed. in English), 2022, 61(6): e202114340. |
| 34 | Mu S Y, Liu K, Li H, et al. Microwave-assisted synthesis of highly dispersed ZrO2 on CNTs as an efficient catalyst for producing 5-hydroxymethylfurfural (5-HMF)[J]. Fuel Processing Technology, 2022, 233: 107292. |
| 35 | Buinachev S, Mashkovtsev M A, Zhirenkina N, et al. A new approach for the synthesis of monodisperse zirconia powders with controlled particle size[J]. International Journal of Hydrogen Energy, 2021, 46(32): 16878-16887. |
| 36 | Xiao S N, Zhou C, Ye X Y, et al. Solid-phase microwave reduction of WO3 by GO for enhanced synergistic photo-Fenton catalytic degradation of bisphenol A[J]. ACS Applied Materials & Interfaces, 2020, 12(29): 32604-32614. |
| 37 | Huang H W, Zhou S, Yu C, et al. Rapid and energy-efficient microwave pyrolysis for high-yield production of highly-active bifunctional electrocatalysts for water splitting[J]. Energy & Environmental Science, 2020, 13(2): 545-553. |
| 38 | Zhao B, Shao G, Fan B B, et al. Synthesis of flower-like CuS hollow microspheres based on nanoflakes self-assembly and their microwave absorption properties[J]. Journal of Materials Chemistry A, 2015, 3(19): 10345-10352. |
| 39 | Wen C Y, Li X, Zhang R X, et al. High-density anisotropy magnetism enhanced microwave absorption performance in Ti3C2T x MXene@Ni microspheres[J]. ACS Nano, 2022, 16(1): 1150-1159. |
| 40 | Li B D, Zeng Z H, Qiao J, et al. Hollow ZnO/Fe3O4@C nanofibers for efficient electromagnetic wave absorption[J]. ACS Applied Nano Materials, 2022, 5(8): 11617-11626. |
| 41 | Elhassan A, Abdalla I, Yu J Y, et al. Microwave-assisted fabrication of sea cucumber-like hollow structured composite for high-performance electromagnetic wave absorption[J]. Chemical Engineering Journal, 2020, 392: 123646. |
| 42 | Zheng Q, Cao W Q, Zhai H Z, et al. Tailoring carbon-based nanofiber microstructures for electromagnetic absorption, shielding, and devices[J]. Materials Chemistry Frontiers, 2023, 7(9): 1737-1759. |
| 43 | Ji T, Tu R, Mu L W, et al. Structurally tuning microwave absorption of core/shell structured CNT/polyaniline catalysts for energy efficient saccharide-HMF conversion[J]. Applied Catalysis B: Environmental, 2018, 220: 581-588. |
| 44 | Wang F L, Yang X D, Dong B X, et al. A FeP powder electrocatalyst for the hydrogen evolution reaction[J]. Electrochemistry Communications, 2018, 92: 33-38 |
| 45 | Tian L H, Yan X D, Chen X B. Electrochemical activity of iron phosphide nanoparticles in hydrogen evolution reaction[J]. ACS Catalysis, 2016, 6(8): 5441-5448. |
| 46 | Wang B, Wu H B, Yu L, et al. Template-free formation of uniform urchin-like α-FeOOH hollow spheres with superior capability for water treatment[J]. Advanced Materials, 2012, 24(8): 1111-1116. |
| 47 | 王亭杰, 金涌, 汪展文, 等. 针状羟基氧化铁的成核与生长过程[J]. 化工学报, 1998, 49(2): 201-207. |
| Wang T J, Jin Y, Wang Z W, et al. Nucleation and growth process of the needle-like goethite crystals[J]. Journal of Chemical Industry and Engineering (China), 1998, 49(2): 201-207. |
| [1] | 韩宇, 周乐, 张鑫, 罗勇, 孙宝昌, 邹海魁, 陈建峰. 高黏附性Pd/SiO2/NF整体式催化剂的制备及加氢性能研究[J]. 化工学报, 2024, 75(4): 1533-1542. |
| [2] | 李云璇, 刘新悦, 陈熙, 刘文, 周明月, 蓝兴英. 基于固液氧化还原靶向反应的能量存储技术:材料、器件及动力学[J]. 化工学报, 2024, 75(4): 1222-1240. |
| [3] | 范以薇, 刘威, 李盈盈, 王培霞, 张吉松. 有机液体储氢中全氢化乙基咔唑催化脱氢研究进展[J]. 化工学报, 2024, 75(4): 1198-1208. |
| [4] | 贾旭东, 杨博龙, 程前, 李雪丽, 向中华. 分步负载金属法制备铁钴双金属位点高效氧还原电催化剂[J]. 化工学报, 2024, 75(4): 1578-1593. |
| [5] | 严孝清, 赵瑛, 张宇哲, 欧鸿辉, 黄起中, 胡华贵, 杨贵东. 五重孪晶铜纳米线@聚吡咯制备及其电催化硝酸盐还原制氨[J]. 化工学报, 2024, 75(4): 1519-1532. |
| [6] | 程骁恺, 历伟, 王靖岱, 阳永荣. 镍催化可控/活性自由基聚合反应研究进展[J]. 化工学报, 2024, 75(4): 1105-1117. |
| [7] | 吴希, 孙博, 刘银东, 齐传磊, 陈凯毅, 王路海, 许崇, 李永峰. 钠离子电池沥青基碳负极材料制备技术研究进展[J]. 化工学报, 2024, 75(4): 1270-1283. |
| [8] | 李添翼, 武玉泰, 王永胜, 顾佳锐, 宋沂恒, 杨丰铖, 郝广平. 轻同位素分离纯化与催化标记研究进展[J]. 化工学报, 2024, 75(4): 1284-1301. |
| [9] | 孙铭泽, 黄鹤来, 牛志强. 铂基氧还原催化剂:从单晶电极到拓展表面纳米材料[J]. 化工学报, 2024, 75(4): 1256-1269. |
| [10] | 吴吉昊, 陈涛, 刘思宇, 刘梦柯, 杨卷. 双功能活化制备沥青基硬炭用于钠离子电池负极[J]. 化工学报, 2024, 75(3): 1019-1027. |
| [11] | 陈志明, 王泽凤, 马高琪, 王良波, 余承涛, 潘鹏举. 基于亚锡灭活及链端改性提高聚乳酸热稳定性的研究进展[J]. 化工学报, 2024, 75(3): 760-767. |
| [12] | 潘娜, 田昌, 怀兰坤, 刘玉玉, 张芬芬, 高晓梅, 刘伟, 闫良国, 赵艳侠. 聚合铝钛基絮凝剂的合成与应用[J]. 化工学报, 2024, 75(3): 1009-1018. |
| [13] | 卫月星, 贺子岳, 燕可洲, 李林玉, 秦育红, 贺冲, 焦路畅. 改性煤气化渣催化降解双酚A的性能研究[J]. 化工学报, 2024, 75(3): 877-889. |
| [14] | 盖星宇, 岳玉学, 杨春华, 张子龙, 蔡天姿, 张海丰, 王柏林, 李小年. 碳负载Cs和Cu基催化剂用于1,1,2-三氯乙烷的气相脱氯化氢[J]. 化工学报, 2024, 75(2): 575-583. |
| [15] | 尹玉华, 方灿, 易清风, 李广. 不同碳导电剂对铁-空气电池性能的影响[J]. 化工学报, 2024, 75(2): 685-694. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号