化工学报 ›› 2024, Vol. 75 ›› Issue (4): 1256-1269.DOI: 10.11949/0438-1157.20231215
收稿日期:2023-11-22
修回日期:2024-03-11
出版日期:2024-04-25
发布日期:2024-06-06
通讯作者:
牛志强
作者简介:孙铭泽(1998—),男,博士研究生,smz20@mails.tsinghua.edu.cn基金资助:
Mingze SUN( ), Helai HUANG(
), Helai HUANG( ), Zhiqiang NIU(
), Zhiqiang NIU( )
)
Received:2023-11-22
Revised:2024-03-11
Online:2024-04-25
Published:2024-06-06
Contact:
Zhiqiang NIU
摘要:
开发低成本、高性能的氧还原铂基催化剂仍然是目前推动质子交换膜燃料电池(PEMFC)商业化进程的重要方向。在拓展单晶电极表面的相关研究中,活性金属铂的原子排布、应力应变、周边配位环境等因素都被认为对氧还原的性能具有重要影响。然而,在规整表面的单晶电极上得到的经验并不能完全指导纳米催化剂的设计,这是因为纳米颗粒存在着尺寸效应带来的活性-利用率的矛盾关系。通过在纳米尺度上模拟单晶电极的性质,构造纳米薄膜材料及二维晶面可控的纳米材料,可以一定程度上实现拓展表面性质。结合本课题组的研究工作,本文总结了拓展表面催化剂用于氧还原反应的理论和实验结果,探讨了纳米催化剂的发展和目前存在的问题,并对今后的研究方向进行了展望。
中图分类号:
孙铭泽, 黄鹤来, 牛志强. 铂基氧还原催化剂:从单晶电极到拓展表面纳米材料[J]. 化工学报, 2024, 75(4): 1256-1269.
Mingze SUN, Helai HUANG, Zhiqiang NIU. Pt-based oxygen reduction reaction catalysts: from single crystal electrode to nanostructured extended surface[J]. CIESC Journal, 2024, 75(4): 1256-1269.
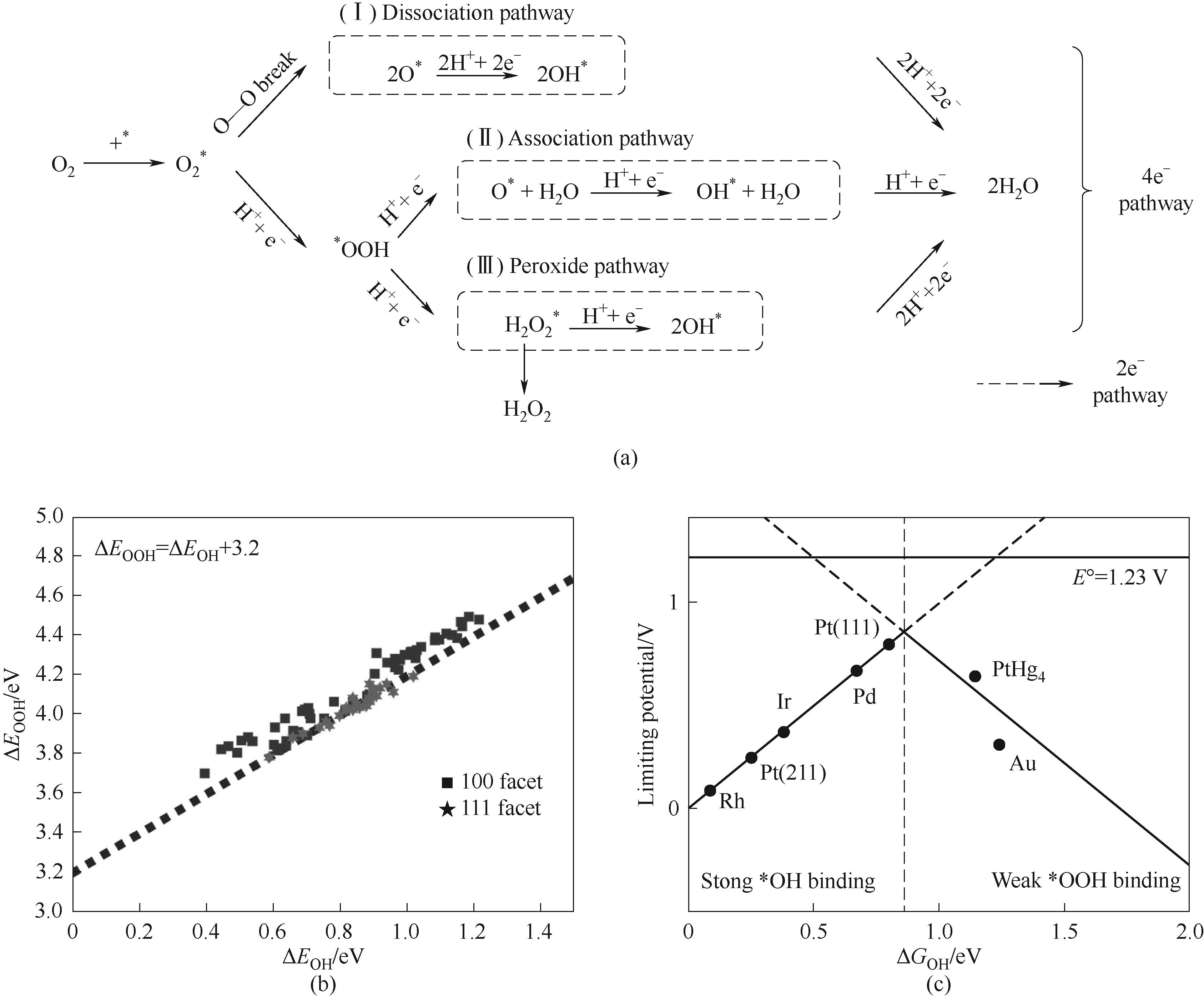
图1 酸性条件下二电子和四电子ORR基本步骤(a);*OOH和*OH中间体的线性相关关系(b)[27];ORR过程的“火山型曲线”关系(c)[28]
Fig.1 2e- and 4e- mechanisms of ORR (a); Scaling relationship of *OOH and *OH intermediates (b)[27]; Volcano plot of ORR (c)[28]
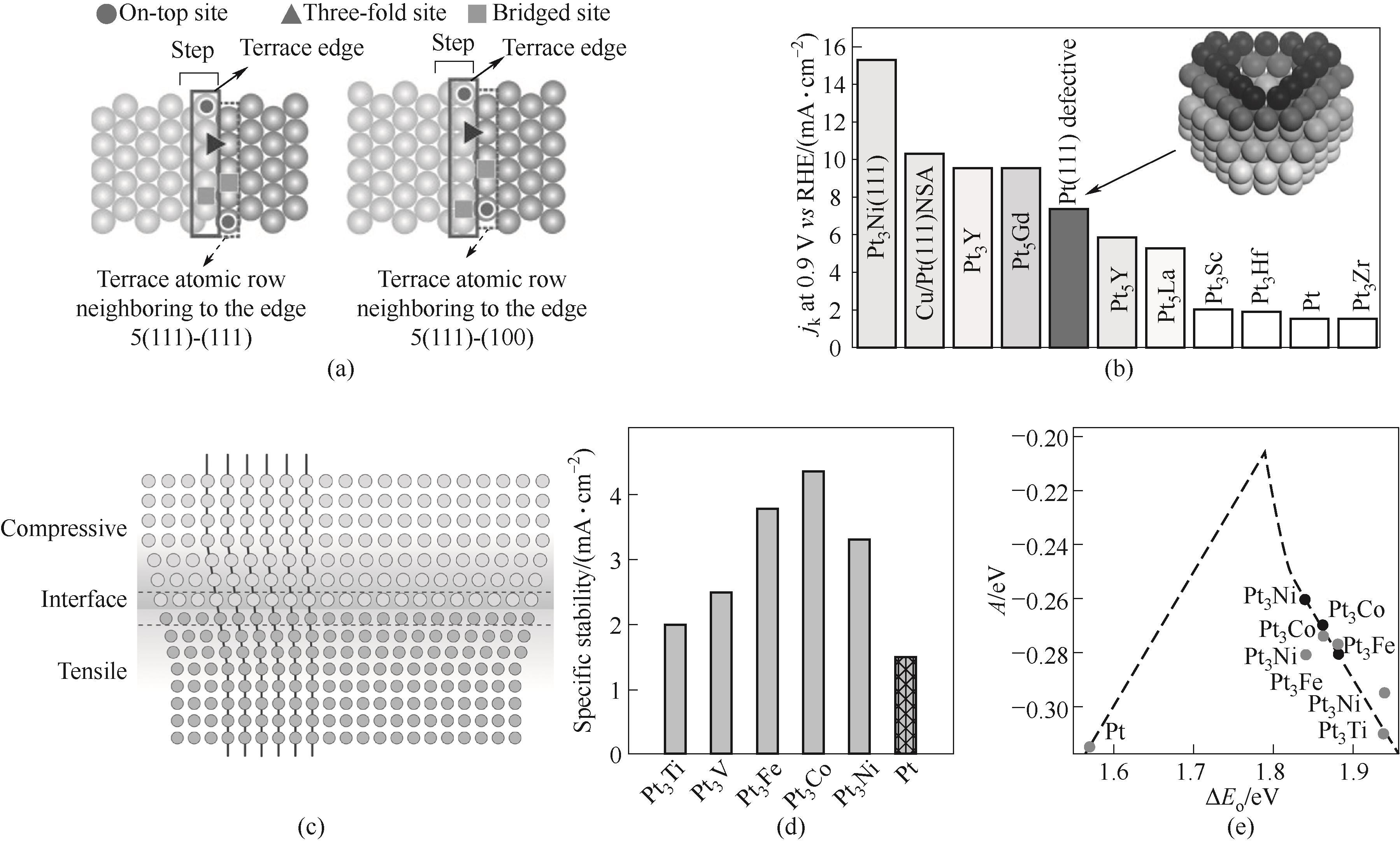
图2 台阶位点ORR提升的来源(a)[39];Pt(111)腔体凹面结构会提升ORR活性(b)[40];应变效应示意图(c)[44];Pt与3d-过渡金属形成的合金薄膜材料ORR活性的实验(d)以及理论计算结果(e)[45]
Fig.2 Origin of the increase of ORR activity by step sites (a)[39]; Increase of ORR activities for defective Pt(111) cavity (b)[40]; Schematic illustration of strain effect (c)[44]; Specific activity (d) as well as theory calculation (e) of Pt and Pt3M electrodes[45]
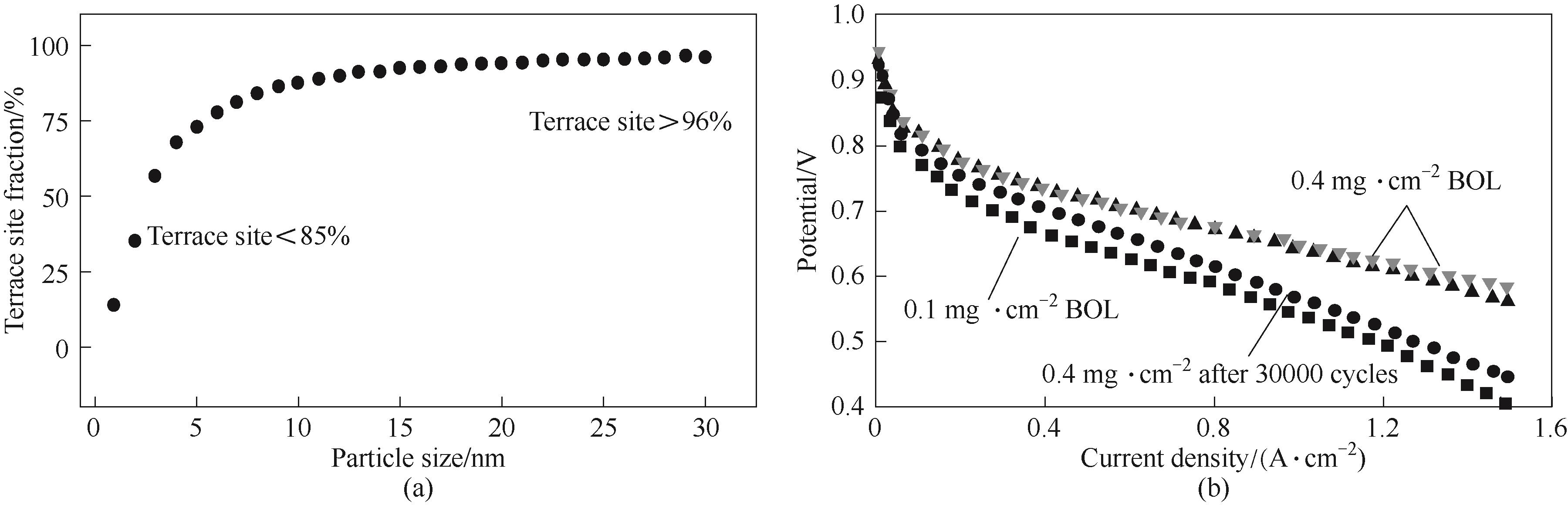
图3 八面体颗粒的尺寸与平台位点比例关系(a)[71];在高电流密度下降低载量对催化剂性能的影响(b)[72]
Fig.3 Relationship between particle size and terrace site fraction (a)[71]; Influence of low Pt loading for ORR activity especially at high current density (b)[72]
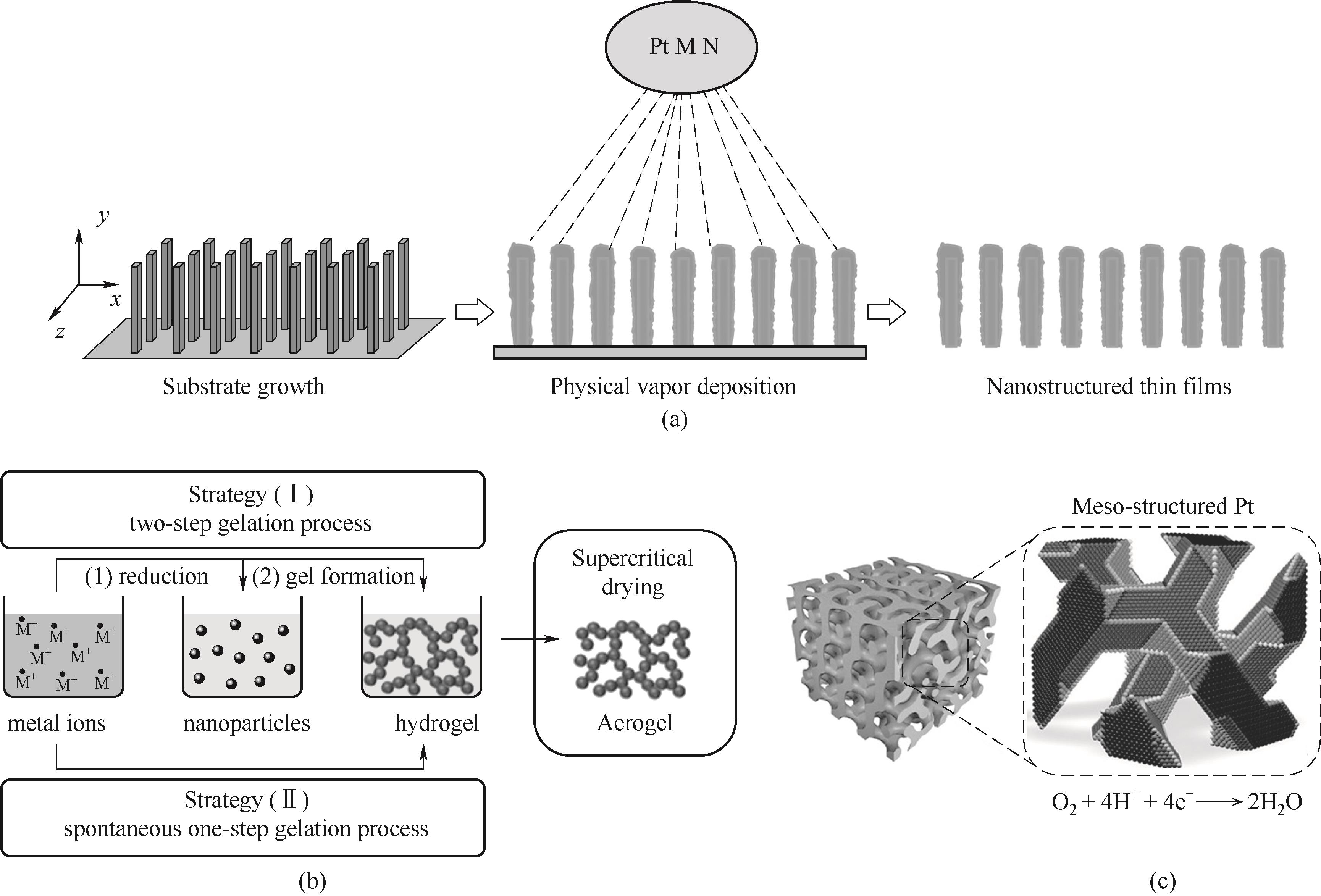
图4 NSTF催化剂的合成方法示意图(a)[84];纳米气凝胶催化剂的合成示意图(b)[85];螺旋式介孔网络Pt薄膜的合成示意图(c)[89]
Fig.2 Schematic illustration of the preperation and structure of NSTF catalyst (a)[84]; Schematic illustration of the synthesis of aerogel (b)[85]; Schematic illustration of the synthesis of meso-structured Pt (c)[89]
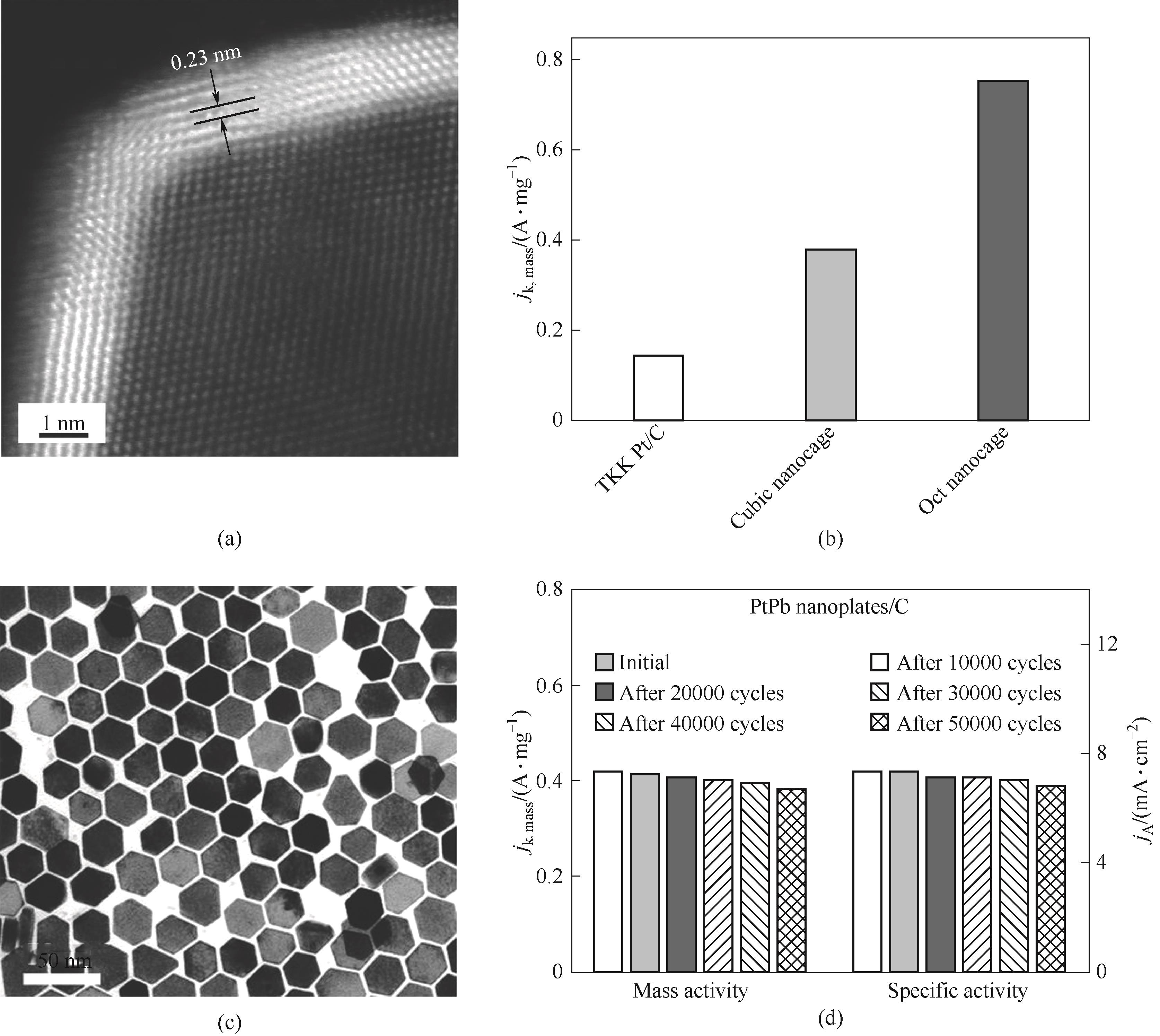
图5 单个八面体纳米笼的HAADF-STEM图像(a);八面体纳米笼、立方体纳米笼和TKK Pt/C在0.9 V时的SA和MA(b)[96];PtPb/Pt六方纳米板的TEM图像(c);稳定性测试后PtPb/Pt六方纳米板催化剂的SA和MA的变化(d)[97]
Fig.5 HAADF-STEM image of an individual octahedral nanocage (a); Specific activities and mass activities at 0.9 V (RHE) of octahedral nanocages, cubic nanocages, and TKK Pt/C (b)[96]; TEM image of PtPb/Pt hexagonal nanoplates (c); Specific activities and mass activities of hexagonal nanoplates after stability test (d)[97]

图6 PtCuNi 纳米管的合成示意图(a);拓展面催化剂透射电镜表征 (b);EDS mapping 呈现出Cu@PtNi的核壳结构 (c);PtCuNiAu纳米管、PtCuNi 纳米管、PtCuNi NPs 和商用Pt/C在0.9 V时的SA和MA(d);PtCuNi纳米管和商用Pt/C的H2-空气燃料电池极化曲线和功率密度(e)[102]
Fig.6 Schematic illustration of the synthesis of extended PtCuNi catalyst (a); Representative HAADF-STEM image of Cu@PtNi core-shell nanowire (b); EDS elemental mapping of Cu@PtNi core-shell nanowire (c); Specific activities and mass activities at 0.9 V (RHE) of extended PtCuNiAu, extended PtCuNi, PtCuNi NPs and commercial Pt/C (d); H2-air fuel cell polarization and power density plots of commercial Pt/C and extended PtCuNi (e)[102]
| 1 | Hassan Q, Sameen A Z, Salman H M, et al. Hydrogen energy future: advancements in storage technologies and implications for sustainability[J]. Journal of Energy Storage, 2023, 72: 108404. |
| 2 | Pathak P K, Yadav A K, Padmanaban S. Transition toward emission-free energy systems by 2050: potential role of hydrogen[J]. International Journal of Hydrogen Energy, 2023, 48(26): 9921-9927. |
| 3 | Abe J O, Popoola A P I, Ajenifuja E, et al. Hydrogen energy, economy and storage: review and recommendation[J]. International Journal of Hydrogen Energy, 2019, 44(29): 15072-15086. |
| 4 | Yusaf T, Faisal Mahamude A S, Kadirgama K, et al. Sustainable hydrogen energy in aviation—a narrative review[J]. International Journal of Hydrogen Energy, 2024, 52: 1026-1045. |
| 5 | Dyantyi N, Parsons A, Bujlo P, et al. Behavioural study of PEMFC during start-up/shutdown cycling for aeronautic applications[J]. Materials for Renewable and Sustainable Energy, 2019, 8(1): 4. |
| 6 | Wang X Y, Zhu J Z, Han M F. Industrial development status and prospects of the marine fuel cell: a review[J]. Journal of Marine Science and Engineering, 2023, 11(2): 238. |
| 7 | Zhan Z P. Current status and future development of fuel cell ships in China[J]. Journal of Physics: Conference Series, 2022, 2160(1): 012061. |
| 8 | Debe M K. Electrocatalyst approaches and challenges for automotive fuel cells[J]. Nature, 2012, 486: 43-51. |
| 9 | Zhu X Y, Gui P P, Zhang X X, et al. Multi-objective optimization of a hybrid energy system integrated with solar-wind-PEMFC and energy storage[J]. Journal of Energy Storage, 2023, 72: 108562. |
| 10 | Shao M H, Chang Q W, Dodelet J P, et al. Recent advances in electrocatalysts for oxygen reduction reaction[J]. Chemical Reviews, 2016, 116(6): 3594-3657. |
| 11 | Hu X Y, Yang B Z, Ke S R, et al. Review and perspectives of carbon-supported platinum-based catalysts for proton exchange membrane fuel cells[J]. Energy & Fuels, 2023, 37(16): 11532-11566. |
| 12 | Zhang X, Xie Y, Wang L. Progress and prospect of Pt-based catalysts for electrocatalytic hydrogen oxidation reactions[J]. Nano Research, 2024, 17(3): 960-981. |
| 13 | James B, Huya-Kouadio J, Houchins C, et al. DOE Hydrogen and Fuel Cells Program. Heavy-Duty Fuel Cell System Cost - 2022[EB/OL]. Dimitrios Papageorgopoulos and Sunita Satyapal (DOE), 2023[2024-04-10]. |
| 14 | Duan Z Y, Wang G F. Comparison of reaction energetics for oxygen reduction reactions on Pt(100), Pt(111), Pt/Ni(100), and Pt/Ni(111) surfaces: a first-principles study[J]. The Journal of Physical Chemistry C, 2013, 117(12): 6284-6292. |
| 15 | Dong J C, Zhang X G, Briega-Martos V, et al. In situ Raman spectroscopic evidence for oxygen reduction reaction intermediates at platinum single-crystal surfaces[J]. Nature Energy, 2019, 4: 60-67. |
| 16 | Wang T, Zhang Y R, Huang B T, et al. Enhancing oxygen reduction electrocatalysis by tuning interfacial hydrogen bonds[J]. Nature Catalysis, 2021, 4: 753-762. |
| 17 | Chen C H, Meadows K E, Cuharuc A, et al. High resolution mapping of oxygen reduction reaction kinetics at polycrystalline platinum electrodes[J]. Physical Chemistry Chemical Physics, 2014, 16(34): 18545-18552. |
| 18 | Temmel S E, Fabbri E, Pergolesi D, et al. Investigating the role of strain toward the oxygen reduction activity on model thin film Pt catalysts[J]. ACS Catalysis, 2016, 6(11): 7566-7576. |
| 19 | Ahluwalia R K, Wang X, Lajunen A, et al. Kinetics of oxygen reduction reaction on nanostructured thin-film platinum alloy catalyst[J]. Journal of Power Sources, 2012, 215: 77-88. |
| 20 | Zhang J M, Yang H B, Zhou D J, et al. Adsorption energy in oxygen electrocatalysis[J]. Chemical Reviews, 2022, 122(23): 17028-17072. |
| 21 | Zhao J Y, Lian J X, Zhao Z, et al. A review of In-situ techniques for probing active sites and mechanisms of electrocatalytic oxygen reduction reactions[J]. Nano-Micro Letters, 2022, 15(1): 19. |
| 22 | Tang C, Chen L, Li H J, et al. Tailoring acidic oxygen reduction selectivity on single-atom catalysts via modification of first and second coordination spheres[J]. Journal of the American Chemical Society, 2021, 143(20): 7819-7827. |
| 23 | Huang H L, Sun M Z, Li M, et al. Recent advances in single-atom catalysts for electrocatalytic synthesis of hydrogen peroxide[J]. Green Energy and Resources, 2023, 1(3): 100031. |
| 24 | Zhang Y X, Zhang S B, Huang H L, et al. General synthesis of a diatomic catalyst library via a macrocyclic precursor-mediated approach[J]. Journal of the American Chemical Society, 2023, 145(8): 4819-4827. |
| 25 | Sun M Z, Gong S Y, Zhang Y-X, et al. A perspective on the PGM-free metal-nitrogen-carbon catalysts for PEMFC[J]. Journal of Energy Chemistry, 2022, 67: 250-254. |
| 26 | Zhang S B, Wu Y F, Zhang Y X, et al. Dual-atom catalysts: controllable synthesis and electrocatalytic applications[J]. Science China Chemistry, 2021, 64(11): 1908-1922. |
| 27 | Viswanathan V, Hansen H A, Rossmeisl J, et al. Universality in oxygen reduction electrocatalysis on metal surfaces[J]. ACS Catalysis, 2012, 2(8): 1654-1660. |
| 28 | Xie C L, Niu Z Q, Kim D, et al. Surface and interface control in nanoparticle catalysis[J]. Chemical Reviews, 2020, 120(2): 1184-1249. |
| 29 | Gao G P, Waclawik E R, Du A J. Computational screening of two-dimensional coordination polymers as efficient catalysts for oxygen evolution and reduction reaction[J]. Journal of Catalysis, 2017, 352: 579-585. |
| 30 | Calle-Vallejo F, Krabbe A, García-Lastra J M. How covalence breaks adsorption-energy scaling relations and solvation restores them[J]. Chemical Science, 2017, 8(1): 124-130. |
| 31 | Tritsaris G A, Greeley J, Rossmeisl J, et al. Atomic-scale modeling of particle size effects for the oxygen reduction reaction on Pt[J]. Catalysis Letters, 2011, 141(7): 909-913. |
| 32 | Hammer B, Nørskov J K. Why gold is the noblest of all the metals[J]. Nature, 1995, 376: 238-240. |
| 33 | Hammer B, Nørskov J K. Theoretical Surface Science and Catalysis—Calculations and Concepts[M]//Advances in Catalysis. Amsterdam: Elsevier, 2000: 71-129. |
| 34 | Nilsson A, Pettersson L G M, Hammer B, et al. The electronic structure effect in heterogeneous catalysis[J]. Catalysis Letters, 2005, 100(3): 111-114. |
| 35 | Zhu X J, Guo Q S, Sun Y F, et al. Optimising surface d charge of AuPd nanoalloy catalysts for enhanced catalytic activity[J]. Nature Communications, 2019, 10: 1428. |
| 36 | Kitchin J R, Nørskov J K, Barteau M A, et al. Modification of the surface electronic and chemical properties of Pt(111) by subsurface 3d transition metals[J]. The Journal of Chemical Physics, 2004, 120(21): 10240-10246. |
| 37 | Marković N M, Adžić R R, Cahan B D, et al. Structural effects in electrocatalysis: oxygen reduction on platinum low index single-crystal surfaces in perchloric acid solutions[J]. Journal of Electroanalytical Chemistry, 1994, 377(1/2): 249-259. |
| 38 | Marković N M, Gasteiger H A, Ross P N. Oxygen reduction on platinum low-index single-crystal surfaces in sulfuric acid solution: rotating ring-Pt(hkl) disk studies[J]. The Journal of Physical Chemistry, 1995, 99(11): 3411-3415. |
| 39 | Hoshi N, Nakamura M, Hitotsuyanagi A. Active sites for the oxygen reduction reaction on the high index planes of Pt[J]. Electrochimica Acta, 2013, 112: 899-904. |
| 40 | Calle-Vallejo F, Tymoczko J, Colic V, et al. Finding optimal surface sites on heterogeneous catalysts by counting nearest neighbors[J]. Science, 2015, 350(6257): 185-189. |
| 41 | Calle-Vallejo F, Martínez J I, García-Lastra J M, et al. Fast prediction of adsorption properties for platinum nanocatalysts with generalized coordination numbers[J]. Angewandte Chemie International Edition, 2014, 53(32): 8316-8319. |
| 42 | Calle-Vallejo F, Pohl M D, Reinisch D, et al. Why conclusions from platinum model surfaces do not necessarily lead to enhanced nanoparticle catalysts for the oxygen reduction reaction[J]. Chemical Science, 2017, 8(3): 2283-2289. |
| 43 | Calle-Vallejo F, Bandarenka A S. Enabling generalized coordination numbers to describe strain effects[J]. ChemSusChem, 2018, 11(11): 1824-1828. |
| 44 | Luo M C, Guo S J. Strain-controlled electrocatalysis on multimetallic nanomaterials[J]. Nature Reviews Materials, 2017, 2(11): 17059. |
| 45 | Stamenkovic V, Mun B S, Mayrhofer K J J, et al. Changing the activity of electrocatalysts for oxygen reduction by tuning the surface electronic structure[J]. Angewandte Chemie International Edition, 2006, 118(18): 2963-2967. |
| 46 | Strasser P, Koh S, Anniyev T, et al. Lattice-strain control of the activity in dealloyed core-shell fuel cell catalysts[J]. Nature Chemistry, 2010, 2: 454-460. |
| 47 | Wang Z C, Chen S H, Wu W, et al. Tailored lattice compressive strain of Pt-skins by the L12-Pt3M intermetallic core for highly efficient oxygen reduction[J]. Advanced Materials, 2023, 35(36): e2301310. |
| 48 | Yang W H, Zou L L, Huang Q H, et al. Lattice contracted ordered intermetallic core-shell PtCo@Pt nanoparticles: synthesis, structure and origin for enhanced oxygen reduction reaction[J]. Journal of the Electrochemical Society, 2017, 164(6): H331-H337. |
| 49 | Stephens I E L, Bondarenko A S, Perez-Alonso F J, et al. Tuning the activity of Pt(111) for oxygen electroreduction by subsurface alloying[J]. Journal of the American Chemical Society, 2011, 133(14): 5485-5491. |
| 50 | Hernandez-Fernandez P, Masini F, McCarthy D N, et al. Mass-selected nanoparticles of Pt x Y as model catalysts for oxygen electroreduction[J]. Nature Chemistry, 2014, 6: 732-738. |
| 51 | Escudero-Escribano M, Malacrida P, Hansen M H, et al. Tuning the activity of Pt alloy electrocatalysts by means of the lanthanide contraction[J]. Science, 2016, 352(6281): 73-76. |
| 52 | Li J, Li L, Wang M J, et al. Alloys with Pt-skin or Pt-rich surface for electrocatalysis[J]. Current Opinion in Chemical Engineering, 2018, 20: 60-67. |
| 53 | van der Vliet D F, Wang C, Li D G, et al. Unique electrochemical adsorption properties of Pt-skin surfaces[J]. Angewandte Chemie International Edition, 2012, 51(13): 3139-3142. |
| 54 | Wang C, Chi M F, Li D G, et al. Design and synthesis of bimetallic electrocatalyst with multilayered Pt-skin surfaces[J]. Journal of the American Chemical Society, 2011, 133(36): 14396-14403. |
| 55 | Stamenkovic V R, Mun B S, Mayrhofer K J J, et al. Effect of surface composition on electronic structure, stability, and electrocatalytic properties of Pt-transition metal alloys: Pt-skin versus Pt-skeleton surfaces[J]. Journal of the American Chemical Society, 2006, 128(27): 8813-8819. |
| 56 | Wang G W, Huang B, Xiao L, et al. Pt skin on AuCu intermetallic substrate: a strategy to maximize Pt utilization for fuel cells[J]. Journal of the American Chemical Society, 2014, 136(27): 9643-9649. |
| 57 | Stamenkovic V R, Fowler B, Mun B S, et al. Improved oxygen reduction activity on Pt3Ni(111) via increased surface site availability[J]. Science, 2007, 315(5811): 493-497. |
| 58 | Huang X Q, Zhao Z P, Cao L, et al. High-performance transition metal-doped Pt3Ni octahedra for oxygen reduction reaction[J]. Science, 2015, 348(6240): 1230-1234. |
| 59 | Wang S J, Sheng T, Yuan Q. Low-Pt octahedral PtCuCo nanoalloys: “one stone, four birds” for oxygen reduction and methanol oxidation reactions[J]. Inorganic Chemistry, 2023, 62(29): 11581-11588. |
| 60 | Kong F P, Ren Z H, Norouzi Banis M, et al. Active and stable Pt-Ni alloy octahedra catalyst for oxygen reduction via near-surface atomical engineering[J]. ACS Catalysis, 2020, 10(7): 4205-4214. |
| 61 | Luo Y Y, Lou W H, Feng H Y, et al. Ultra-small nanoparticles of Pd-Pt-Ni alloy octahedra with high lattice strain for efficient oxygen reduction reaction[J]. Catalysts, 2023, 13(1): 97. |
| 62 | 常丰瑞, 黄俭标, 马建新, 等. PEMFC用Pt纳米线阴极催化剂的制备及在电堆中的应用[J]. 化工学报, 2014, 65(10): 3891-3898. |
| Chang F R, Huang J B, Ma J X, et al. Preparation of Pt nanowires as cathode catalyst for PEMFC and its application in stack[J]. CIESC Journal, 2014, 65(10): 3891-3898. | |
| 63 | Inaba M, Zana A, Quinson J, et al. The oxygen reduction reaction on Pt: why particle size and interparticle distance matter[J]. ACS Catalysis, 2021, 11(12): 7144-7153. |
| 64 | Gan J, Luo W, Chen W Y, et al. Mechanistic understanding of size-dependent oxygen reduction activity and selectivity over Pt/CNT nanocatalysts[J]. European Journal of Inorganic Chemistry, 2019, 2019(27): 3210-3217. |
| 65 | van Helden P, Ciobîca I M, Coetzer R L J. The size-dependent site composition of FCC cobalt nanocrystals[J]. Catalysis Today, 2016, 261: 48-59. |
| 66 | Kodama K, Nagai T, Kuwaki A, et al. Challenges in applying highly active Pt-based nanostructured catalysts for oxygen reduction reactions to fuel cell vehicles[J]. Nature Nanotechnology, 2021, 16: 140-147. |
| 67 | Yuan Y, Yan N, Dyson P J. Advances in the rational design of rhodium nanoparticle catalysts: control via manipulation of the nanoparticle core and stabilizer[J]. ACS Catalysis, 2012, 2(6): 1057-1069. |
| 68 | Fan L H, Deng H, Zhang Y G, et al. Towards ultralow platinum loading proton exchange membrane fuel cells[J]. Energy & Environmental Science, 2023, 16(4): 1466-1479. |
| 69 | Patil V, Reshmi P V, Prajna S, et al. Degradation mechanisms in PEM fuel cells: a brief review[J]. Materials Today: Proceedings, DOI:10.1016/j.matpr.2023.03.603 . |
| 70 | Miao Z P, Li S Z, Priest C, et al. Effective approaches for designing stable M-N x /C oxygen-reduction catalysts for proton-exchange-membrane fuel cells[J]. Advanced Materials, 2022, 34(52): e2200595. |
| 71 | Sun M Z, Gong S Y, Li Z W, et al. Terrace-rich ultrathin PtCu surface on earth-abundant metal for oxygen reduction reaction[J]. ACS Nano, 2023, 17(19): 19421-19430. |
| 72 | Greszler T A, Caulk D, Sinha P. The impact of platinum loading on oxygen transport resistance[J]. Journal of the Electrochemical Society, 2012, 159(12): F831-F840. |
| 73 | Zhao Z P, Chen C L, Liu Z Y, et al. Pt-based nanocrystal for electrocatalytic oxygen reduction[J]. Advanced Materials, 2019, 31(31): e1808115. |
| 74 | Kongkanand A, Mathias M F. The priority and challenge of high-power performance of low-platinum proton-exchange membrane fuel cells[J]. The Journal of Physical Chemistry Letters, 2016, 7(7): 1127-1137. |
| 75 | Pan L J, Ott S, Dionigi F, et al. Current challenges related to the deployment of shape-controlled Pt alloy oxygen reduction reaction nanocatalysts into low Pt-loaded cathode layers of proton exchange membrane fuel cells[J]. Current Opinion in Electrochemistry, 2019, 18: 61-71. |
| 76 | Cao F, Ding R, Rui Z Y, et al. Advances in low Pt loading membrane electrode assembly for proton exchange membrane fuel cells[J]. Molecules, 2023, 28(2): 773. |
| 77 | Zaman S, Douka A I, Noureen L, et al. Oxygen reduction performance measurements: discrepancies against benchmarks[J]. Battery Energy, 2023, 2(3): 20220060. |
| 78 | Zaman S, Tian X L, Xia B Y. Bridging oxygen reduction performance gaps in half and full cells: challenges and perspectives[J]. Materials Chemistry Frontiers, 2023, 7(20): 4605-4612. |
| 79 | Li J R, Liu M X, Liu X, et al. The recent progress of oxygen reduction electrocatalysts used at fuel cell level[J]. Small Methods, 2024, 8(3): e2301249. |
| 80 | Luo M C, Guo S J. Multimetallic electrocatalyst stabilized by atomic ordering[J]. Joule, 2019, 3(1): 9-10. |
| 81 | Liu J Y, Liu S Y, Yan F Z, et al. Ultrathin nanotube structure for mass-efficient and durable oxygen reduction reaction catalysts in PEM fuel cells[J]. Journal of the American Chemical Society, 2022, 144(41): 19106-19114. |
| 82 | Li J L, Liu H Y, Zhang W Q, et al. In-situ preparation of low Pt loading multi rhombic-pyramidal Pt-Pd catalyst layer for high-performance proton exchange membrane fuel cells[J]. Journal of Power Sources, 2023, 556: 232445. |
| 83 | van der Vliet D, Wang C, Debe M, et al. Platinum-alloy nanostructured thin film catalysts for the oxygen reduction reaction[J]. Electrochimica Acta, 2011, 56(24): 8695-8699. |
| 84 | van der Vliet D F, Wang C, Tripkovic D, et al. Mesostructured thin films as electrocatalysts with tunable composition and surface morphology[J]. Nature Materials, 2012, 11: 1051-1058. |
| 85 | Liu W, Herrmann A K, Bigall N C, et al. Noble metal aerogels-synthesis, characterization, and application as electrocatalysts[J]. Accounts of Chemical Research, 2015, 48(2): 154-162. |
| 86 | Liu W, Rodriguez P, Borchardt L, et al. Bimetallic aerogels: high-performance electrocatalysts for the oxygen reduction reaction[J]. Angewandte Chemie International Edition, 2013, 52(37): 9849-9852. |
| 87 | Liu W, Herrmann A K, Geiger D, et al. High-performance electrocatalysis on palladium aerogels[J]. Angewandte Chemie International Edition, 2012, 51(23): 5743-5747. |
| 88 | Hwang C K, Kim J M, Hwang S, et al. Porous strained Pt nanostructured thin-film electrocatalysts via dealloying for PEM fuel cells[J]. Advanced Materials Interfaces, 2020, 7(2): 1901326. |
| 89 | Kibsgaard J, Gorlin Y, Chen Z B, et al. Meso-structured platinum thin films: active and stable electrocatalysts for the oxygen reduction reaction[J]. Journal of the American Chemical Society, 2012, 134(18): 7758-7765. |
| 90 | Beermann V, Gocyla M, Willinger E, et al. Rh-doped Pt-Ni octahedral nanoparticles: understanding the correlation between elemental distribution, oxygen reduction reaction, and shape stability[J]. Nano Letters, 2016, 16(3): 1719-1725. |
| 91 | Meier J C, Galeano C, Katsounaros I, et al. Design criteria for stable Pt/C fuel cell catalysts[J]. Beilstein Journal of Nanotechnology, 2014, 5: 44-67. |
| 92 | Mayrhofer K J J, Blizanac B B, Arenz M, et al. The impact of geometric and surface electronic properties of Pt-catalysts on the particle size effect in electrocatalysis[J]. The Journal of Physical Chemistry B, 2005, 109(30): 14433-14440. |
| 93 | Han B H, Carlton C E, Kongkanand A, et al. Record activity and stability of dealloyed bimetallic catalysts for proton exchange membrane fuel cells[J]. Energy & Environmental Science, 2015, 8(1): 258-266. |
| 94 | Marković N M, Ross P N. Surface science studies of model fuel cell electrocatalysts[J]. Surface Science Reports, 2002, 45(4): 117-229. |
| 95 | Todoroki N, Kato T, Hayashi T, et al. Pt-Ni nanoparticle-stacking thin film: highly active electrocatalysts for oxygen reduction reaction[J]. ACS Catalysis, 2015, 5(4): 2209-2212. |
| 96 | Zhang L, Roling L T, Wang X, et al. Platinum-based nanocages with subnanometer-thick walls and well-defined, controllable facets[J]. Science, 2015, 349(6246): 412-416. |
| 97 | Bu L Z, Zhang N, Guo S J, et al. Biaxially strained PtPb/Pt core/shell nanoplate boosts oxygen reduction catalysis[J]. Science, 2016, 354(6318): 1410-1414. |
| 98 | Xie M H, Lyu Z H, Chen R H, et al. Pt-Co@Pt octahedral nanocrystals: enhancing their activity and durability toward oxygen reduction with an intermetallic core and an ultrathin shell[J]. Journal of the American Chemical Society, 2021, 143(22): 8509-8518. |
| 99 | Li Z, Ji S F, Liu Y W, et al. Well-defined materials for heterogeneous catalysis: from nanoparticles to isolated single-atom sites[J]. Chemical Reviews, 2020, 120(2): 623-682. |
| 100 | Gong S Y, Zhang Y X, Niu Z Q. Recent advances in earth-abundant core/noble-metal shell nanoparticles for electrocatalysis[J]. ACS Catalysis, 2020, 10(19): 10886-10904. |
| 101 | Ding H, Wang P, Su C J, et al. Epitaxial growth of ultrathin highly crystalline Pt-Ni nanostructure on a metal carbide template for efficient oxygen reduction reaction[J]. Advanced Materials, 2022, 34(12): e2109188. |
| 102 | Gong S Y, Sun M Z, Lee Y Y, et al. Bulk-like Pt(100)-oriented ultrathin surface: combining the merits of single crystals and nanoparticles to boost oxygen reduction reaction[J]. Angewandte Chemie International Edition, 2023, 62(4): 2214516. |
| [1] | 李云璇, 刘新悦, 陈熙, 刘文, 周明月, 蓝兴英. 基于固液氧化还原靶向反应的能量存储技术:材料、器件及动力学[J]. 化工学报, 2024, 75(4): 1222-1240. |
| [2] | 范以薇, 刘威, 李盈盈, 王培霞, 张吉松. 有机液体储氢中全氢化乙基咔唑催化脱氢研究进展[J]. 化工学报, 2024, 75(4): 1198-1208. |
| [3] | 冯彬彬, 卢明佳, 黄志宏, 常译文, 崔志明. 碳载体在质子交换膜燃料电池中的应用及优化[J]. 化工学报, 2024, 75(4): 1469-1484. |
| [4] | 贾旭东, 杨博龙, 程前, 李雪丽, 向中华. 分步负载金属法制备铁钴双金属位点高效氧还原电催化剂[J]. 化工学报, 2024, 75(4): 1578-1593. |
| [5] | 严孝清, 赵瑛, 张宇哲, 欧鸿辉, 黄起中, 胡华贵, 杨贵东. 五重孪晶铜纳米线@聚吡咯制备及其电催化硝酸盐还原制氨[J]. 化工学报, 2024, 75(4): 1519-1532. |
| [6] | 韩宇, 周乐, 张鑫, 罗勇, 孙宝昌, 邹海魁, 陈建峰. 高黏附性Pd/SiO2/NF整体式催化剂的制备及加氢性能研究[J]. 化工学报, 2024, 75(4): 1533-1542. |
| [7] | 吴希, 孙博, 刘银东, 齐传磊, 陈凯毅, 王路海, 许崇, 李永峰. 钠离子电池沥青基碳负极材料制备技术研究进展[J]. 化工学报, 2024, 75(4): 1270-1283. |
| [8] | 张劲, 郭志斌, 罗来明, 卢善富, 相艳. 5 kW重整甲醇高温质子交换膜燃料电池系统设计与性能[J]. 化工学报, 2024, 75(4): 1697-1704. |
| [9] | 董霄, 白志山, 杨晓勇, 殷伟, 刘宁普, 于启凡. CHPPO工艺氧化液耦合除杂技术的研究与工业应用[J]. 化工学报, 2024, 75(4): 1630-1641. |
| [10] | 李昂, 赵振宇, 李洪, 高鑫. 微波诱导高分散Pd/FeP催化剂构筑及其电催化性能研究[J]. 化工学报, 2024, 75(4): 1594-1606. |
| [11] | 潘娜, 田昌, 怀兰坤, 刘玉玉, 张芬芬, 高晓梅, 刘伟, 闫良国, 赵艳侠. 聚合铝钛基絮凝剂的合成与应用[J]. 化工学报, 2024, 75(3): 1009-1018. |
| [12] | 张领先, 刘斌, 邓琳, 任宇航. 基于改进TSO优化Xception的PEMFC故障诊断[J]. 化工学报, 2024, 75(3): 945-955. |
| [13] | 谭耀文, 姜攀星, 杜青, 余婉秋, 温小飞, 詹志刚. 工作电压对PEMFC膜电极衰退影响模拟研究[J]. 化工学报, 2024, 75(3): 974-986. |
| [14] | 吴吉昊, 陈涛, 刘思宇, 刘梦柯, 杨卷. 双功能活化制备沥青基硬炭用于钠离子电池负极[J]. 化工学报, 2024, 75(3): 1019-1027. |
| [15] | 王沛, 段睿明, 张广儒, 金万勤. 光热驱动的膜分离生物甲烷制氢过程建模与仿真分析[J]. 化工学报, 2024, 75(3): 967-973. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号