化工学报 ›› 2025, Vol. 76 ›› Issue (7): 3671-3685.DOI: 10.11949/0438-1157.20241430
李同辉( ), 回天力, 郑涛, 张睿, 刘海燕, 刘植昌, 徐春明, 孟祥海(
), 回天力, 郑涛, 张睿, 刘海燕, 刘植昌, 徐春明, 孟祥海( )
)
收稿日期:2024-12-09
修回日期:2025-01-07
出版日期:2025-07-25
发布日期:2025-08-13
通讯作者:
孟祥海
作者简介:李同辉(1995—),男,博士研究生,lthljq@163.com
基金资助:
Tonghui LI( ), Tianli HUI, Tao ZHENG, Rui ZHANG, Haiyan LIU, Zhichang LIU, Chunming XU, Xianghai MENG(
), Tianli HUI, Tao ZHENG, Rui ZHANG, Haiyan LIU, Zhichang LIU, Chunming XU, Xianghai MENG( )
)
Received:2024-12-09
Revised:2025-01-07
Online:2025-07-25
Published:2025-08-13
Contact:
Xianghai MENG
摘要:
开发高效催化剂对于电解水析氢反应(HER)至关重要。在乙酸锰与氯化钠混合溶液刻蚀处理的泡沫镍(NF)上电沉积钯(Pd)纳米颗粒,合成了电解水析氢催化剂(PdMn/NF-45m),Pd含量为0.52%(质量分数)。该催化剂在宽pH范围内表现出优异的HER性能,在1 mol/L KOH、0.5 mol/L H2SO4和1 mol/L磷酸盐缓冲液(PBS)电解液中达到1000 mA/cm²电流密度时所需的过电位仅为302、67和645 mV。溶液刻蚀增加了NF的活性表面积,Mn掺杂降低了Pd的氢吸附自由能。此外,金属氢氧化物促进了水分解为吸附氢,进而与Pd活性位点结合生成氢气,提高了析氢效率。在1000 mA/cm²的电流密度下,该催化剂在1 mol/L和6 mol/L KOH电解液中均可稳定运行240 h;在0.5 mol/L H2SO4溶液中可稳定运行76 h;在1 mol/L PBS溶液中可稳定运行230 h。
中图分类号:
李同辉, 回天力, 郑涛, 张睿, 刘海燕, 刘植昌, 徐春明, 孟祥海. 氢氧化物协同钯双活性位点用于大电流和pH通用析氢反应[J]. 化工学报, 2025, 76(7): 3671-3685.
Tonghui LI, Tianli HUI, Tao ZHENG, Rui ZHANG, Haiyan LIU, Zhichang LIU, Chunming XU, Xianghai MENG. Synergistic palladium double active sites with hydroxide for high current density and pH-universal hydrogen evolution reaction[J]. CIESC Journal, 2025, 76(7): 3671-3685.
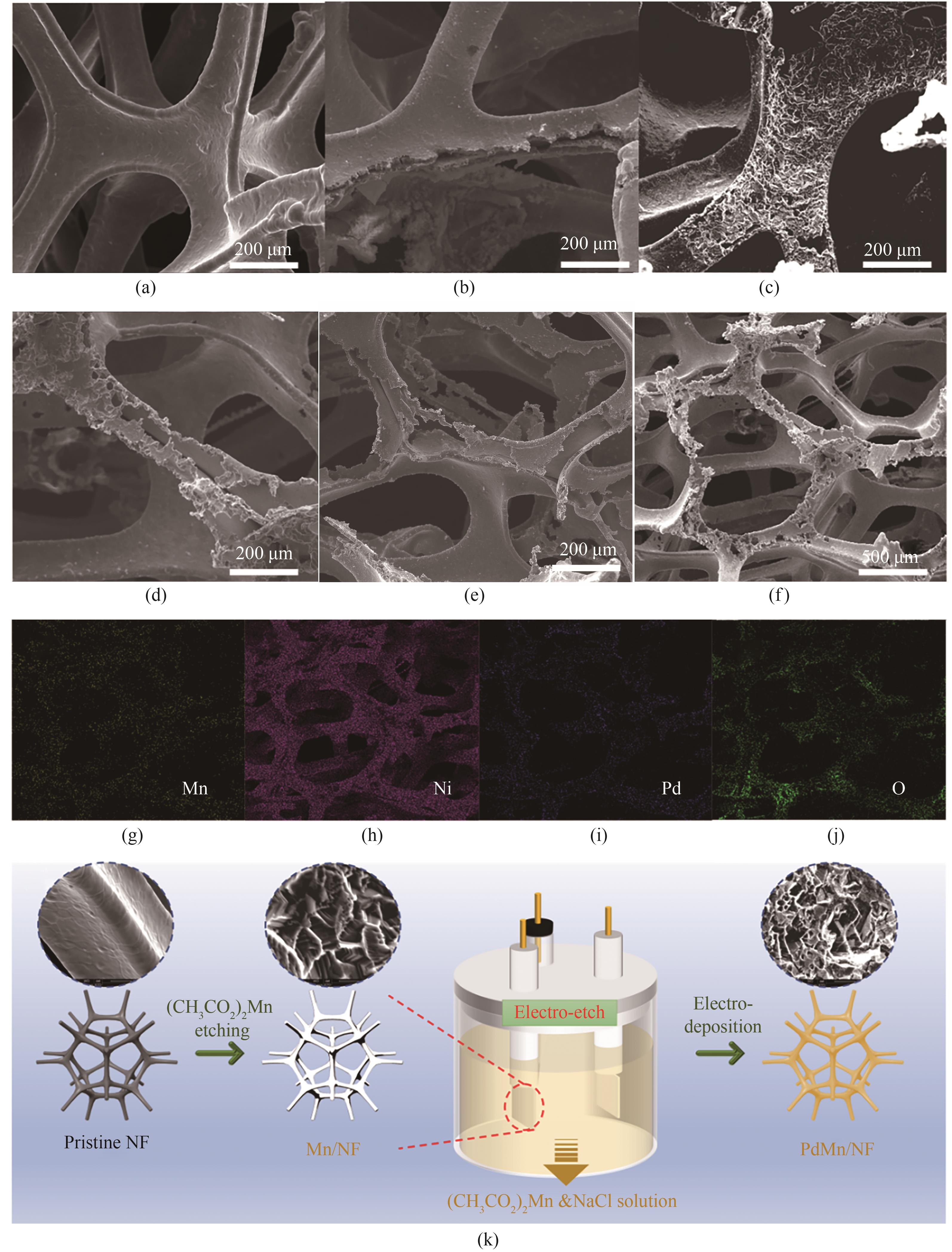
图1 (a) 裸露NF,(b) PdMn/NF-15m,(c) PdMn/NF-30m,(d) PdMn/NF-45m,(e) PdMn/NF-60m的SEM图像;(f) ~ (j) PdMn/NF-45m的SEM图像和EDS元素分布图像;(k) PdMn/NF-45m的制备过程示意图
Fig.1 SEM images of (a) NF, (b) PdMn/NF-15m, (c) PdMn/NF-30m, (d) PdMn/NF-45m and (e) PdMn/NF-60m; (f) — (j) SEM and EDS elemental mapping images of PdMn/NF-45m; (k) Schematic illustration of the fabrication procedure of PdMn/NF-45m
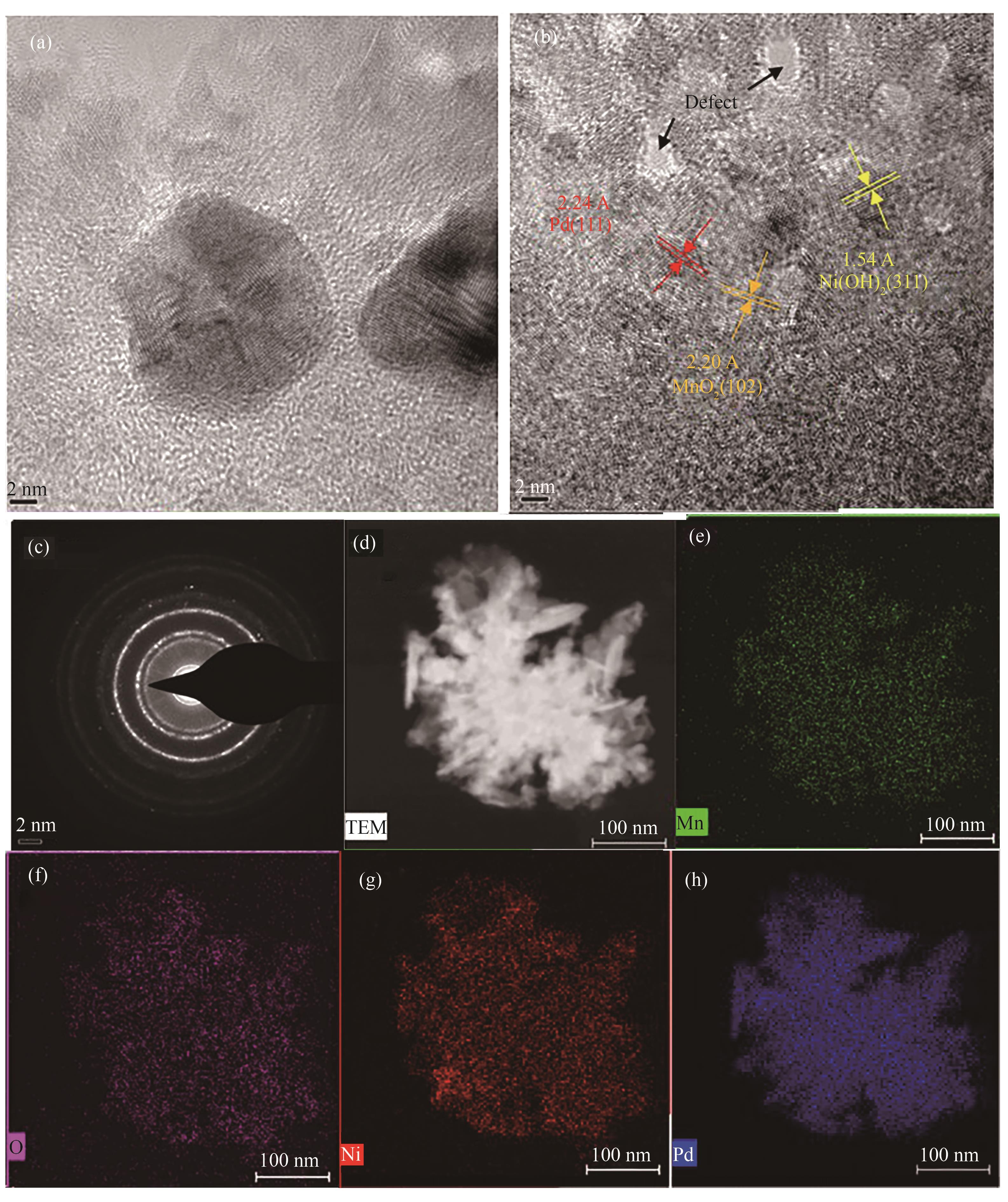
图2 (a),(b) PdMn/NF-45m的TEM图像;(c) PdMn/NF-45m的SAED图像;(d) ~ (h)PdMn/NF-45m的TEM-EDS图
Fig.2 (a), (b) TEM images of PdMn/NF-45m; (c) SAED image of PdMn/NF-45m; (d) — (h) TEM-EDS images of PdMn/NF-45m
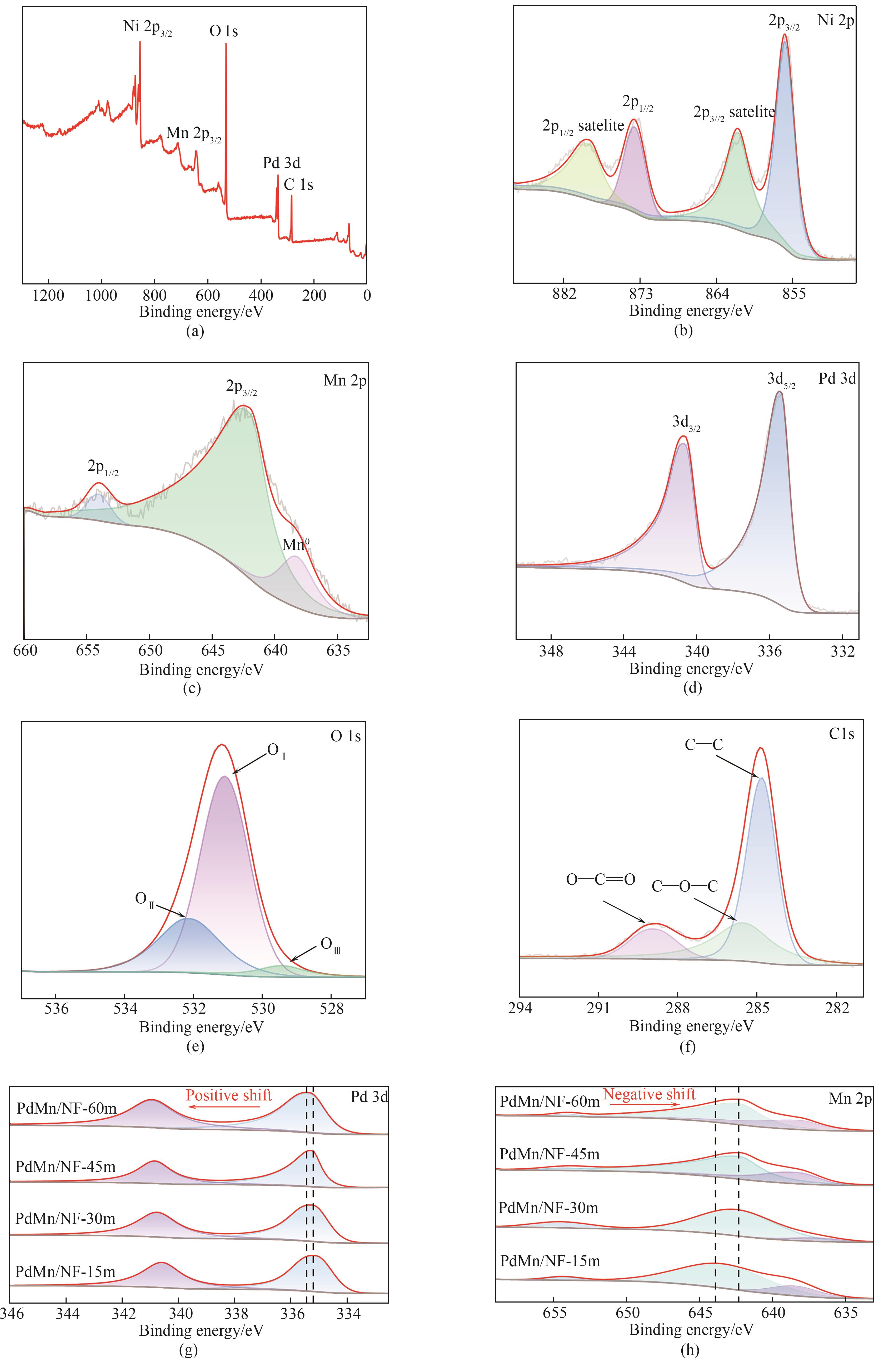
图4 PdMn/NF-45m XPS光谱的(a) 全谱,(b) Ni 2p,(c) Mn 2p,(d) Pd 3d,(e)O 1s,(f) C 1s;PdMn/NF-15m、PdMn/NF-30m、PdMn/NF-45m和PdMn/NF-60m的(g)Pd 3d和(h)Mn 2p XPS光谱
Fig.4 (a) Full survey spectrum, (b) Ni 2p, (c) Mn 2p, (d) Pd 3d, (e) O 1s, (f) C 1s XPS spectra of PdMn/NF-45m; (g) Pd 3d and (h) Mn 2p XPS spectra of PdMn/NF-15m, PdMn/NF-30m, PdMn/NF-45m and PdMn/NF-60m
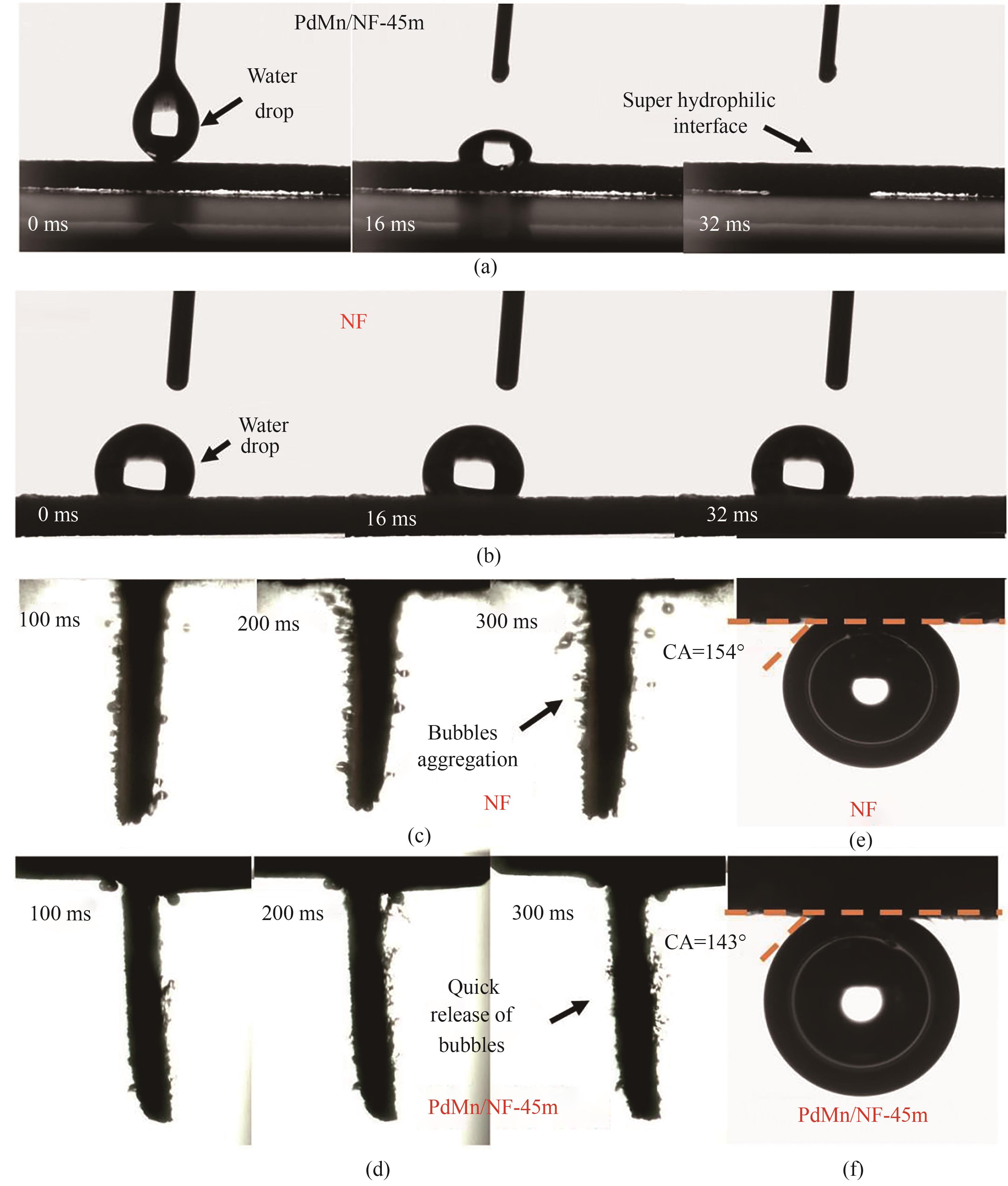
图5 (a) PdMn/NF-45m和(b) NF的固液接触角;在1 mol/L KOH溶液中,以500 mA/cm²的电流密度对(e)裸露NF和(d)PdMn/NF-45m电极表面氢气释放行为的原位观察;(e) NF和(f) PdMn/NF-45m的固气接触角
Fig.5 Solid-liquid contact angle of (a) PdMn/NF-45m and (b) pristine NF; Insitu observation of hydrogen evolution behavior on the surface of (c)pristine NF and (d) PdMn/NF-45m electrodes under a current density of 500 mA/cm² in 1 mol/L KOH solution; Solid-gas contact angle of (e) pristine NF and (f) PdMn/NF-45m
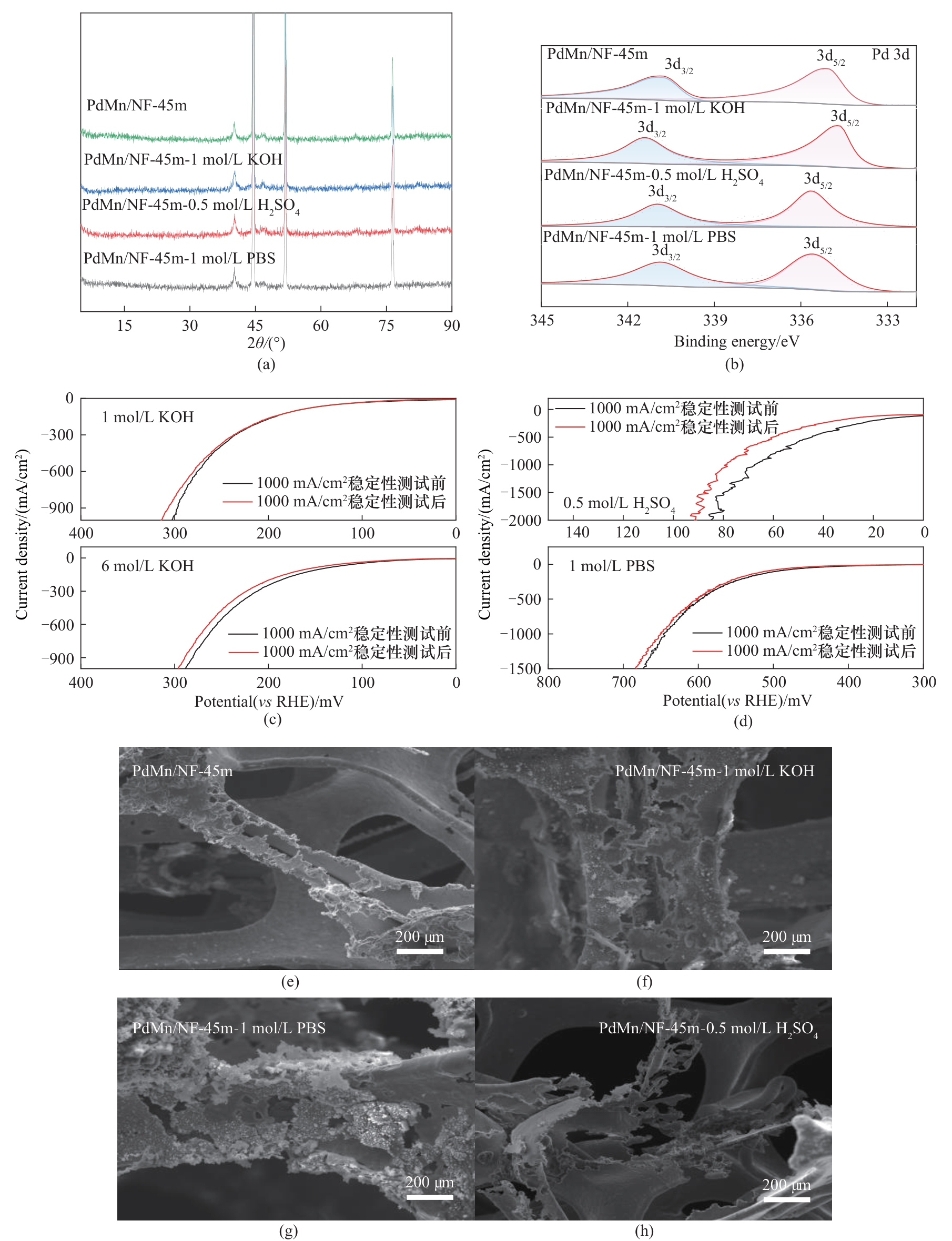
图9 催化剂在碱性、酸性和中性稳定性测试后XRD、XPS和SEM对比图
Fig.9 Comparison of XRD, XPS and SEM after stability tests of the catalysts under alkaline, acidic and neutral conditions
| [1] | Liu S J, Wei Y, Wang M K, et al. The future of alkaline water splitting from the perspective of electrocatalysts-seizing today's opportunities[J]. Coordination Chemistry Reviews, 2025, 522: 216190. |
| [2] | Zhang W Z, Liu M H, Gu X, et al. Water electrolysis toward elevated temperature: advances, challenges and frontiers[J]. Chemical Reviews, 2023, 123(11): 7119-7192. |
| [3] | Karuppasamy L, Gurusamy L, Anandan S, et al. Graphene nanosheets supported high-defective Pd nanocrystals as an efficient electrocatalyst for hydrogen evolution reaction[J]. Chemical Engineering Journal, 2021, 425: 131526. |
| [4] | Kou Z H, Liu Y N, Cui W J, et al. Electronic structure optimization of metal-phthalocyanine via confining atomic Ru for all-pH hydrogen evolution[J]. Energy & Environmental Science, 2024, 17(4): 1540-1548. |
| [5] | 陈佳丽, 陈丽娟, 郭晓慧, 等. 钌基析氢电催化剂的研究进展[J]. 材料研究与应用, 2024, 18(1): 51-61. |
| Chen J L, Chen L J, Guo X H, et al. Recent progress on Ru-based electrocatalysts for hydrogen evolution[J]. Materials Research and Application, 2024, 18(1): 51-61. | |
| [6] | Shi G Y, Tano T, Tryk D A, et al. Nanostructured Pt-NiFe oxide catalyst for hydrogen evolution reaction in alkaline electrolyte membrane water electrolyzers[J]. ACS Catalysis, 2024, 14(12): 9460-9468. |
| [7] | 陈凯, 吴锋顺, 肖顺, 等. PtRu/N掺杂碳电催化甲醇氧化及电解水析氢性能[J]. 无机化学学报, 2024, 40(7): 1357-1367. |
| Chen K, Wu F S, Xiao S, et al. PtRu/nitrogen-doped carbon for electrocatalytic methanol oxidation and hydrogen evolution by water electrolysis[J]. Chinese Journal of Inorganic Chemistry, 2024, 40(7): 1357-1367. | |
| [8] | Li Z Y, Wang C Y, Liang Y Q, et al. Boosting hydrogen evolution performance of nanoporous Fe-Pd alloy electrocatalyst by metastable phase engineering[J]. Applied Catalysis B: Environment and Energy, 2024, 345: 123677. |
| [9] | Bawab B, Thalluri S M, Kolíbalová E, et al. Synergistic effect of Pd single atoms and nanoparticles deposited on carbon supports by ALD boosts alkaline hydrogen evolution reaction[J]. Chemical Engineering Journal, 2024, 482: 148959. |
| [10] | Xia B Y, Wu H B, Yan Y, et al. Ultrathin and ultralong single-crystal platinum nanowire assemblies with highly stable electrocatalytic activity[J]. Journal of the American Chemical Society, 2013, 135(25): 9480-9485. |
| [11] | Zhang H, Jin M S, Liu H Y, et al. Facile synthesis of Pd-Pt alloy nanocages and their enhanced performance for preferential oxidation of CO in excess hydrogen[J]. ACS Nano, 2011, 5(10): 8212-8222. |
| [12] | Guo S J, Dong S J, Wang E K. Three-dimensional Pt-on-Pd bimetallic nanodendrites supported on graphene nanosheet: facile synthesis and used as an advanced nanoelectrocatalyst for methanol oxidation[J]. ACS Nano, 2010, 4(1): 547-555. |
| [13] | Yin A X, Min X Q, Zhang Y W, et al. Shape-selective synthesis and facet-dependent enhanced electrocatalytic activity and durability of monodisperse sub-10 nm Pt-Pd tetrahedrons and cubes[J]. Journal of the American Chemical Society, 2011, 133(11): 3816-3819. |
| [14] | Li Q, Chen C, Luo W S, et al. In situ active site refreshing of electro-catalytic materials for ultra-durable hydrogen evolution at elevated current density[J]. Advanced Energy Materials, 2024, 14(17): 2304099. |
| [15] | Zhang Q, Jiao S H, Wang B R, et al. Accelerate the alkaline hydrogen evolution reaction of the heterostructural Ni2P@Ni(OH)2/NF by dispersing a trifle of Ru on the surface[J]. International Journal of Hydrogen Energy, 2021, 46(52): 26329-26339. |
| [16] | 柴亚婷, 路家伟, 王蕊欣, 等. 碳纸自支撑N掺杂碳纳米管复合MoC/NiCo异质结构的电解水析氧性能[J]. 化工学报, 2023, 74(12): 4904-4913. |
| Chai Y T, Lu J W, Wang R X, et al. Carbon paper self-supported N-doped carbon nanotubes with MoC/NiCo heterostructures for electrolytic water oxygen evolution reaction[J]. CIESC Journal, 2023, 74(12): 4904-4913. | |
| [17] | Meng L X, Shang S Y, Liu S Y, et al. Cooperative effects between NiMo alloy enable highly efficient for all-pH-value and alkaline seawater hydrogen evolution[J]. Applied Catalysis B: Environment and Energy, 2024, 358: 124388. |
| [18] | Li S L, Zhou Z X, Liu G Q, et al. Confining ruthenium nanoparticles in MOF pores for high-performance hydrogen evolution reaction[J]. Chemical Engineering Journal, 2024, 493: 152820. |
| [19] | Hou S, Xu Y F, Chen Z G, et al. Ru single atoms tailoring the acidity of metallic tungsten dioxide for a boosted alkaline hydrogen evolution reaction[J]. ACS Catalysis, 2024, 14(11): 8238-8251. |
| [20] | Fan K C, Zong L B, Liu J X, et al. In situ reconstruction to surface sulfide adsorbed metal scaffold for enhanced electrocatalytic hydrogen evolution activity[J]. Advanced Energy Materials, 2024, 14(23): 2400052. |
| [21] | Huang T X, Cong X, Wu S S, et al. Visualizing the structural evolution of individual active sites in MoS2 during electrocatalytic hydrogen evolution reaction[J]. Nature Catalysis, 2024, 7: 646-654. |
| [22] | Liao Q D, You T, Liu X S, et al. Coupled plasma etching and electrodeposition of CoP/NiO nanosheets with surface reconstruction for water-splitting[J]. Journal of Materials Chemistry A, 2024, 12(16): 9830-9840. |
| [23] | Al-Salihy A, Liang C, Salah A, et al. Ultralow Ru-doped NiMoO4@Ni3(PO4)2 core-shell nanostructures for improved overall water splitting[J]. Chinese Journal of Catalysis, 2024, 60: 360-375. |
| [24] | Jiang H, Sun M Z, Wu S L, et al. Oxygen-incorporated NiMoP nanotube arrays as efficient bifunctional electrocatalysts for urea-assisted energy-saving hydrogen production in alkaline electrolyte[J]. Advanced Functional Materials, 2021, 31(43): 2104951. |
| [25] | Zhang X, Luo Z M, Yu P, et al. Lithiation-induced amorphization of Pd3P2S8 for highly efficient hydrogen evolution[J]. Nature Catalysis, 2018, 1: 460-468. |
| [26] | Ding J Q, Jiang X, Wang C K, et al. Epitaxial growth triggered core-shell Pd@RuP nanorods for high-efficiency electrocatalytic hydrogen evolution[J]. Journal of Energy Chemistry, 2023, 86: 510-517. |
| [27] | Jiang T, Yu L Y, Zhao Z J, et al. Regulating the intermediate affinity on Pd nanoparticles through the control of inserted-B atoms for alkaline hydrogen evolution[J]. Chemical Engineering Journal, 2022, 433: 133525. |
| [28] | Xu Y, Liu M Y, Ren T L, et al. Regulation of the surface micro-structure and crystal phase of Pd2B mesoporous nanoparticles for enhanced hydrogen evolution electrocatalysis[J]. Journal of Materials Chemistry A, 2021, 9(37): 21123-21131. |
| [29] | Zhao Z F, Yao X Y, Yu R P, et al. Enhanced electrocatalytic hydrodechlorination by modulating metal-support interaction and H generation of single-Pd-atom anchored NiFeP electrode[J]. Chemical Engineering Journal, 2024, 492: 152340. |
| [30] | Xiao M Y, Jiao L W, Xiang D Y, et al. Synergistic electronic structure tuning of self-assembled hollow hierarchical Ni-Fe-Co-P nanowire arrays on carbon cloth for enhanced water splitting[J]. Journal of Alloys and Compounds, 2023, 968: 171883. |
| [31] | Anantharaj S, Noda S, Jothi V R, et al. Strategies and perspectives to catch the missing pieces in energy-efficient hydrogen evolution reaction in alkaline media[J]. Angewandte Chemie International Edition, 2021, 60(35): 18981-19006. |
| [32] | Zhu Z J, Yin H J, He C T, et al. Ultrathin transition metal dichalcogenide/3D metal hydroxide hybridized nanosheets to enhance hydrogen evolution activity[J]. Advanced Materials, 2018, 30(28): 1801171. |
| [33] | Wang C D, Humayun M, Debecker D P, et al. Electrocatalytic water oxidation with layered double hydroxides confining single atoms[J]. Coordination Chemistry Reviews, 2023, 478: 214973. |
| [34] | Shibli S M A, Sha M A. Development and characterization of electro active CeO2-RuO2 mixed oxide and its role in alkaline hydrogen evolution reaction[J]. Journal of Alloys and Compounds, 2018, 749: 250-261. |
| [35] | Chen X F, Li D, Wen Y, et al. Favorable surface etching of NiRuFe(OH) x in neutral hydrogen evolution reaction[J]. Catalysis Today, 2022, 400: 1-5. |
| [36] | Guo W, Kim J, Kim H, et al. Boosting hydrogen evolution on NiFeZn electrocatalyst by defect surface modulation using alkali etching[J]. International Journal of Hydrogen Energy, 2023, 48(6): 2090-2100. |
| [37] | Jiang J H, Dou H J, Cao M R, et al. Amorphous nickel oxide electrodes with high-current-density electrocatalytic performance for hydrogen evolution[J]. International Journal of Hydrogen Energy, 2024, 51: 887-894. |
| [38] | Xue M Y, Bao Y K, Xu X, et al. Eco-friendly and large-scale fabrication of NiMoN/Ni(OH)2/NF as highly efficient and stable alkaline hydrogen evolution reaction electrode[J]. International Journal of Hydrogen Energy, 2024, 82: 655-661. |
| [39] | Wang H J, Chen H Y, Mao Q Q, et al. A PdMn bimetallene for low-energy electrocatalytic hydrogen generation coupled with formate oxidation[J]. Chemical Communications, 2022, 58(94): 13115-13118. |
| [40] | 原荷峰, 马自在, 王淑敏, 等. 富氧空位Co3O4纳米线的制备及其电解水性能研究[J]. 化工学报, 2020, 71(12): 5831-5841. |
| Yuan H F, Ma Z Z, Wang S M, et al. Engineering oxygen vacancy-rich Co3O4 nanowire as high-efficiency and durable bifunctional electrocatalyst for overall alkaline water splitting[J]. CIESC Journal, 2020, 71(12): 5831-5841. | |
| [41] | 赵娟, 吴梦成, 雷惊雷, 等. 一步水热法制备电解水析氧反应Ni3S2@Mo2S3高效催化剂[J]. 化工学报, 2022, 73(4): 1575-1584. |
| Zhao J, Wu M C, Lei J L, et al. One-step hydrothermal method toward preparation of Ni3S2@Mo2S3 high-efficient catalyst for oxygen evolution reaction in water electrolysis[J]. CIESC Journal, 2022, 73(4): 1575-1584. | |
| [42] | Lv H, Xu D D, Sun L Z, et al. Ternary palladium-boron-phosphorus alloy mesoporous nanospheres for highly efficient electrocatalysis[J]. ACS Nano, 2019, 13(10): 12052-12061. |
| [1] | 钟晓航, 许卫, 张文, 许莉, 王宇新. 碱性水电解制氢中铁杂质的影响研究进展[J]. 化工学报, 2025, 76(2): 519-531. |
| [2] | 王金山, 王世学, 朱禹. 冷却表面温差对高温质子交换膜燃料电池性能的影响[J]. 化工学报, 2024, 75(5): 2026-2035. |
| [3] | 向千禧, 杨小康, 孙嘉琦, 谢峰, 邵志刚. 质子交换膜水电解池分布特性研究[J]. 化工学报, 2024, 75(11): 4359-4368. |
| [4] | 马佳欢, 杨微微, 白羽, 孙克宁. 二维金属有机框架及其衍生物用于电催化分解水的研究进展[J]. 化工学报, 2020, 71(9): 4006-4030. |
| [5] | 李国荣, 邹翔达, 王启凡, 王欣, 唐中帜, 周扬杰, 唐电. 高Si掺杂RuO2材料的晶体结构、电子结构和导电性[J]. 化工学报, 2018, 69(8): 3717-3723. |
| [6] | 王学科, 王树博, 潘元, 谢晓峰, 黄海燕, 朱彤. 阳极进气湿度对质子交换膜水含量及电流密度分布影响[J]. 化工学报, 2015, 66(S2): 342-348. |
| [7] | 岳雯婷, 张丽, 刘秀明, 刘国桢, 刘云义. 电流密度对氯碱工业离子膜电解槽传递特性影响[J]. 化工学报, 2015, 66(3): 915-923. |
| [8] | 马洪运, 范永生, 王保国. 锌-空气电池电解液Zn2+浓度对析氢过程的影响[J]. 化工学报, 2014, 65(7): 2843-2848. |
| [9] | 王宏智, 黄波, 张卫国, 刘祥亭, 姚素薇. 深共融溶剂中电沉积Ni-Mo合金及其催化析氢性能[J]. 化工学报, 2014, 65(11): 4524-4529. |
| [10] | 曹梅娟, 于艳敏, 付海燕, 佘远斌. 取代基及中心金属离子对卟啉电子结构及催化活性的影响[J]. 化工学报, 2013, 64(S1): 88-97. |
| [11] | 李雪, 谢晓峰. Li2MnO3·LiMO2(M=Co,Ni)的电子结构与性能[J]. 化工学报, 2013, 64(S1): 188-193. |
| [12] | 李英, 周勤文, 周晓慧. PEMFC阴极扩散层结构特性对水淹影响的数值分析[J]. 化工学报, 2013, 64(4): 1424-1430. |
| [13] | 唐文超1,2,林 瑞1,2,黄 真1,2,曹春晖1,2,马建新1,2. 在线分区测试燃料电池内部电流密度分布研究进展[J]. 化工进展, 2013, 32(10): 2324-2335. |
| [14] | 曹涛锋, 林鸿, 陶文铨. 质子交换膜燃料电池温度和电流分布同步测定 [J]. 化工学报, 2011, 62(S1): 174-178. |
| [15] | 杨卫身;毕会锋;王斌;杨凤林 . 蒽醌染料活性艳蓝KN-R的ACF电极成对电解脱色 [J]. CIESC Journal, 2006, 57(11): 2714-2719. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号