化工学报 ›› 2025, Vol. 76 ›› Issue (2): 519-531.DOI: 10.11949/0438-1157.20240806
收稿日期:2024-07-17
修回日期:2024-09-09
出版日期:2025-02-25
发布日期:2025-03-10
通讯作者:
王宇新
作者简介:钟晓航(1999—),女,硕士研究生,zhongxiaohang@tju.edu.cn
Xiaohang ZHONG1( ), Wei XU2, Wen ZHANG1, Li XU1, Yuxin WANG1(
), Wei XU2, Wen ZHANG1, Li XU1, Yuxin WANG1( )
)
Received:2024-07-17
Revised:2024-09-09
Online:2025-02-25
Published:2025-03-10
Contact:
Yuxin WANG
摘要:
碱性水电解是利用可再生能源制氢的重要技术。由于碱性水电解槽能够使用相对廉价的结构材料和非贵金属电极催化剂,因此在制氢成本方面具有竞争优势。早前就有研究发现,工业碱性水电解槽的结构件或管路中的铁会部分溶解至电解液中,并沉积于电极和隔膜上,但铁杂质对水电解性能的具体影响却研究较少。近年来,随着氢能受到越来越多的关注,人们对碱性水电解制氢中铁杂质影响的研究也随之加强,并取得了较多的成果。本文旨在综述与此相关的研究,特别是铁杂质对碱性水电解过程中镍基析氧阳极和镍基析氢阴极的影响。
中图分类号:
钟晓航, 许卫, 张文, 许莉, 王宇新. 碱性水电解制氢中铁杂质的影响研究进展[J]. 化工学报, 2025, 76(2): 519-531.
Xiaohang ZHONG, Wei XU, Wen ZHANG, Li XU, Yuxin WANG. A critical review on the effects of Fe impurity on H2 production via alkaline water electrolysis[J]. CIESC Journal, 2025, 76(2): 519-531.
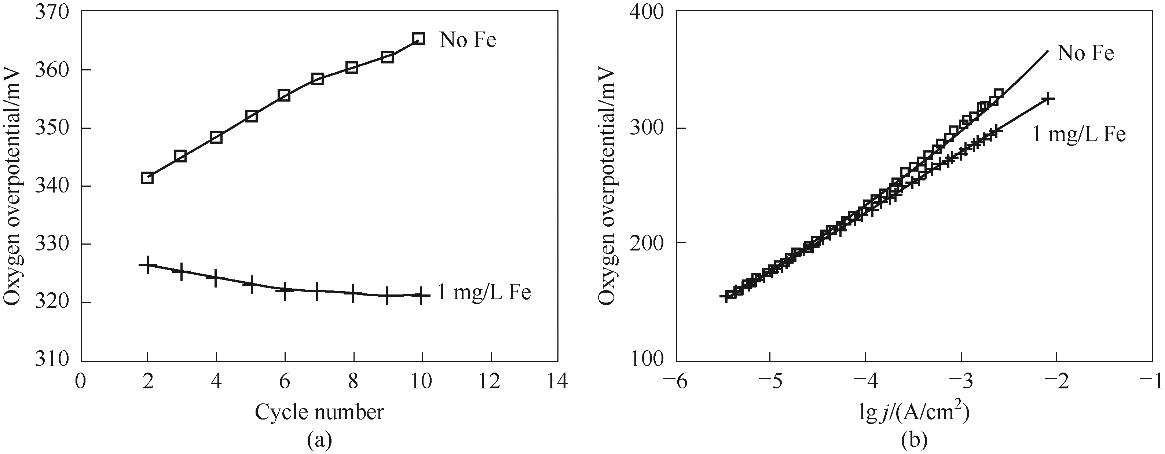
图1 电解液中Fe对水合氧化镍电极OER过电位(a)和Tafel斜率(b)的影响[34]
Fig.1 Effect of Fe in electrolyte on OER overpotential (a) and Tafel slope (b) of hydrous nickel oxide electrode[34]
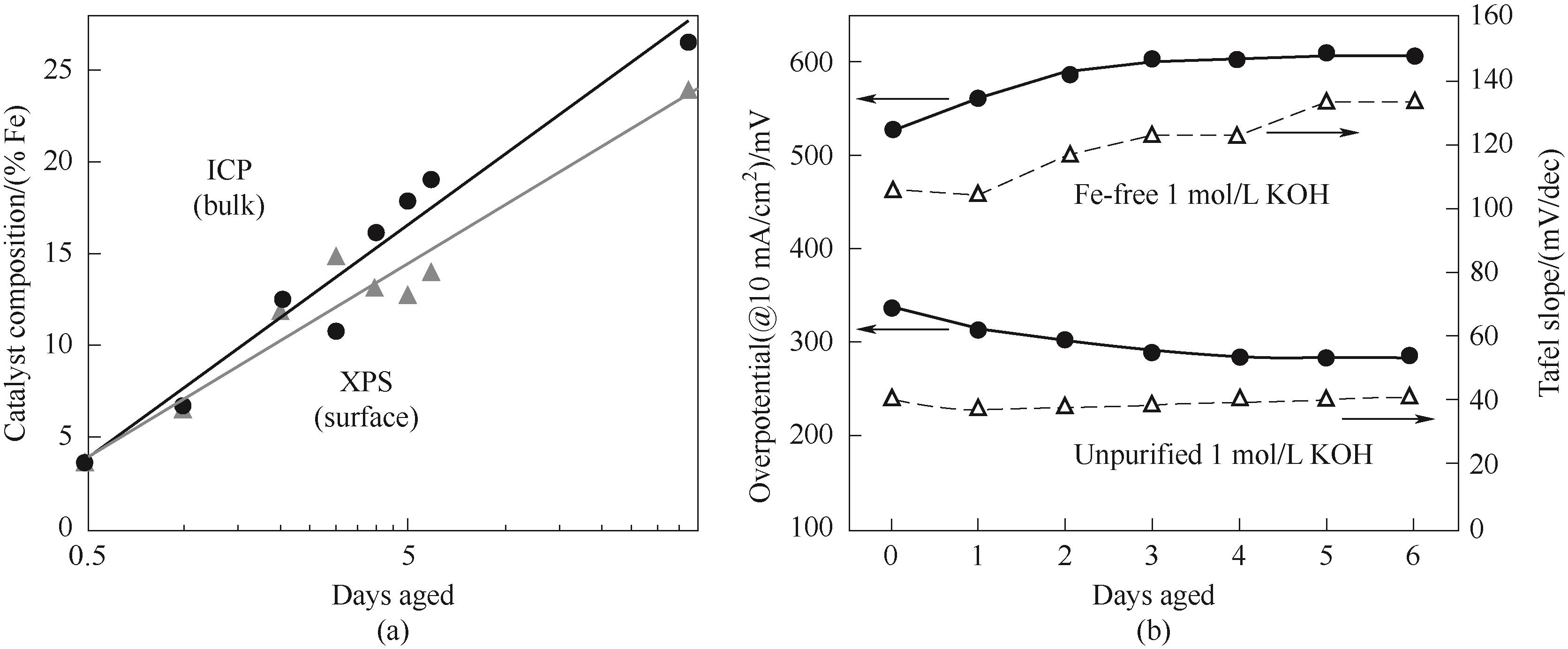
图2 (a) Ni(OH)2薄膜的Fe含量与在未纯化的1 mol/L KOH中老化天数的关系;(b) 在无Fe和未纯化的1 mol/L KOH中,Ni(OH)2薄膜在10 mA/cm2下的老化过电位和 Tafel 斜率[46]
Fig.2 (a) Fe content of Ni(OH)2 films versus days of aging in unpurified 1 mol/L KOH;(b) Overpotentials and Tafel slopes of Ni(OH)2 films in Fe-free and unpurified 1 mol/L KOH at 10 mA/cm2[46]
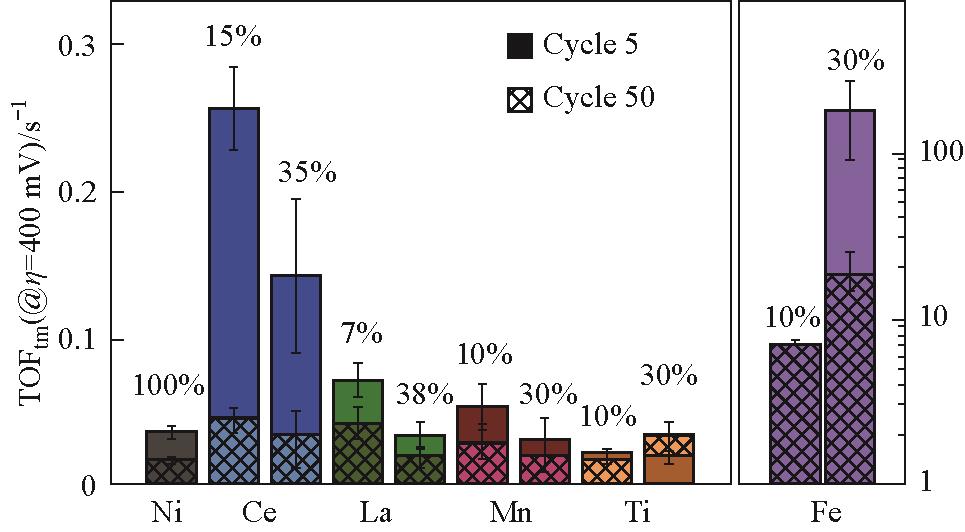
图3 在无Fe的1 mol/L KOH溶液中,Ni1-z M z O x H y 薄膜在400 mV过电位下,第5个循环和第50个循环的OER转化频率;报告值为三个样品的平均值,误差条为标准偏差;Ni0.9Fe0.1O x H y 的误差条较小,在此刻度上不明显;TOFtm的计算假设所有金属阳离子都是活性的(因此是下限)[53]
Fig.3 OER turnover frequency of Ni1-z M z O x H y films at 400 mV overpotential at cycle 5 and cycle 50 in Fe-free 1 mol/L KOH; Values reported are the average, and error bars are the standard deviation of three samples; The error bars of Ni0.9Fe0.1O x H y are small and not visible on this scale; TOFtm are calculated assuming all metal cations are active (and thus are lower limits)[53]
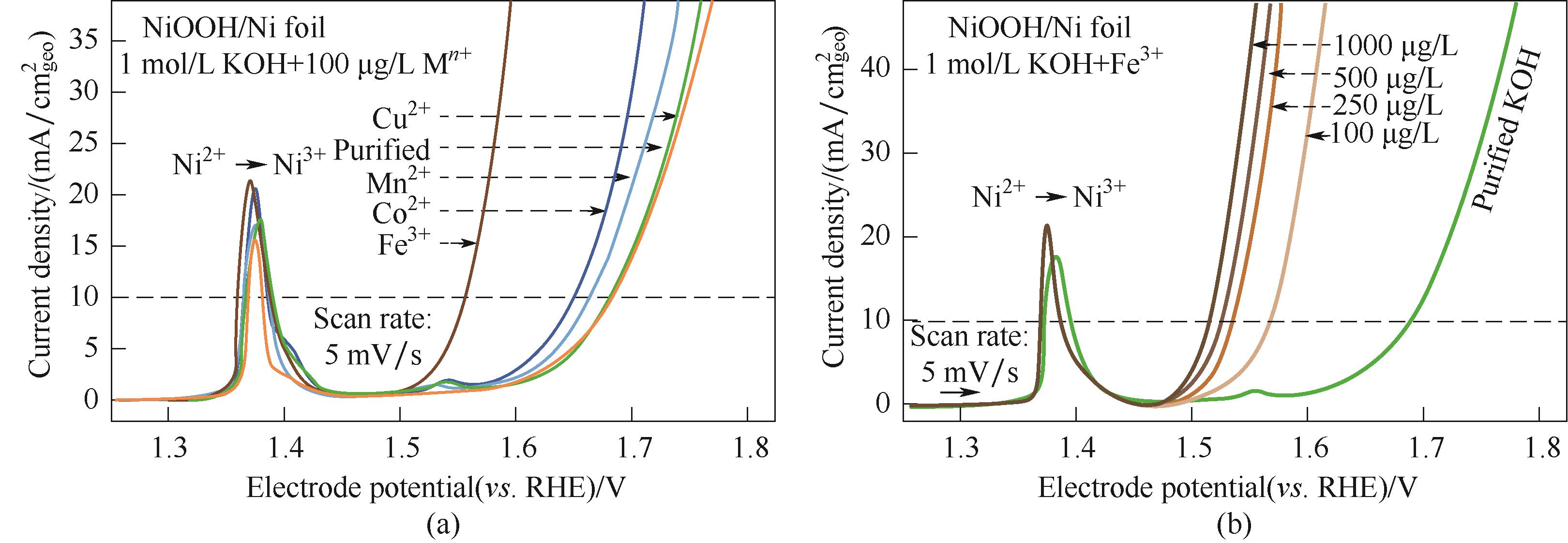
图4 线性扫描伏安图:(a) NF-NiOOH电极在添加过渡金属阳离子(100 μg/L M n+)的纯化1 mol/L KOH电解液中进行CP调节后的OER性能变化;(b) Fe3+浓度对NF-NiOOH电极电化学性能的影响[55]
Fig.4 Linear sweep voltammograms: (a) variations of OER performance of NF-NiOOH electrodes after CP conditioning in purified 1 mol/L KOH electrolyte spiked with transition metal cations (100 μg/L M n+); (b) Influence of the Fe3+ concentration on the electrochemical performance of NF-NiOOH electrodes[55]
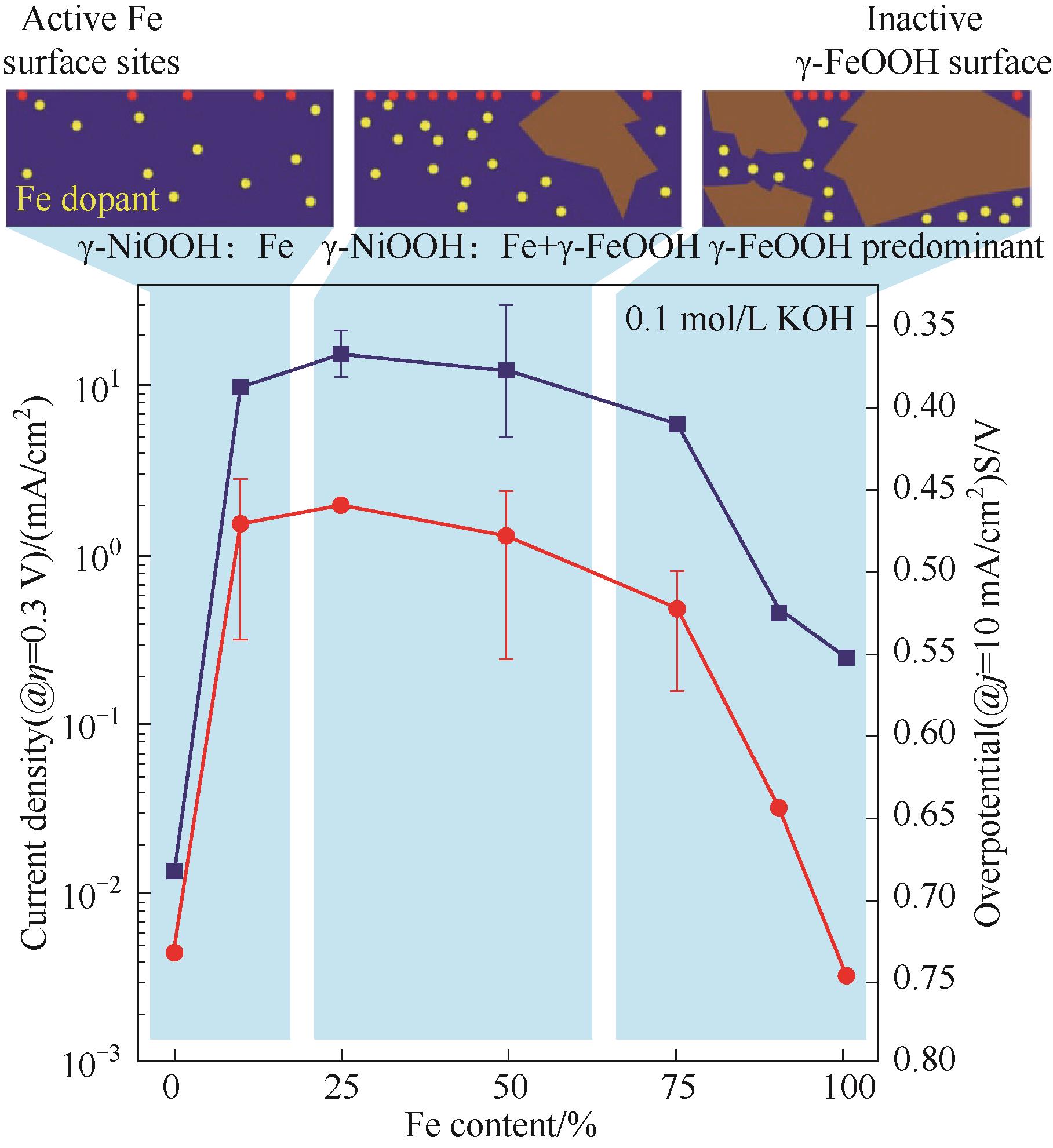
图5 在0.1 mol/L KOH中,混合Ni-Fe催化剂的OER活性随Fe含量变化的测量结果;对于0%Fe催化剂,测试在除Fe后的电解液中进行;上图:Fe含量对在γ-NiOOH中竞争形成高活性Fe位点和相分离的低活性γ-FeOOH 的影响[56]
Fig.5 Measured OER activity of mixed Ni-Fe catalysts as a function of Fe content in 0.1 mol/L KOH; For 0% Fe, measurements were performed in electrolyte which was carefully purified to remove any Fe contamination; Top: a schematic illustrating the influence of Fe content on the competing formation of highly active Fe sites in γ-NiOOH and of phase-separated low-activity γ-FeOOH[56]
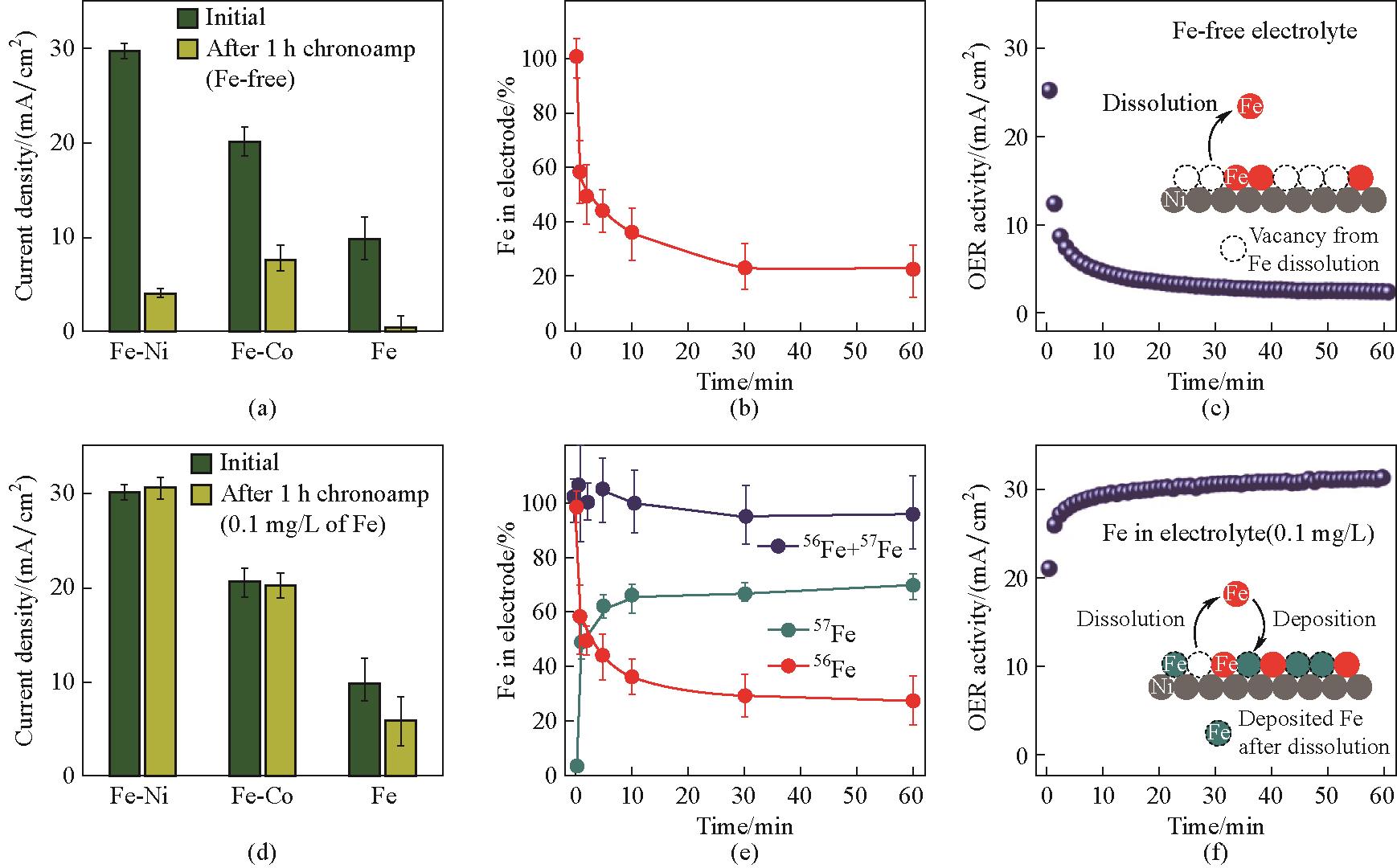
图6 Fe-MO x H y 的活性-稳定性趋势以及同位素标记实验对动态Fe交换的观察:在1.7 V的电压下,Fe-MO x H y 在‘无Fe’KOH (a) 与含0.1 mg/L Fe的KOH溶液 (d) 中进行1 h计时电流实验的活动性-稳定性结果;Fe-NiO x H y 电极在1.7 V下的总Fe含量 [(b),(e)] 及OER活性 [(c),(f)] 随时间变化;(c)中的示意图描述了溶解过程;在含0.1 mg/L 57Fe电解质中进行的类似恒电位测试 [(e),(f)] 显示了OER催化过程中的Fe动态交换(溶解与再沉积);(f)中的示意图描述了Fe溶解和再沉积过程[26]
Fig.6 Activity-stability trend of Fe-M hydr(oxy)oxides and observation of dynamic Fe exchange by isotopic labelling experiments: summary of the results of the activity-stability study of Fe-M hydr(oxy)oxides during chronoamperometry experiments at 1.7 V for 1 h in ‘Fe-free’ purified KOH (a) and in a KOH solution containing 0.1 mg/L Fe (d); the total amount of Fe in the Fe-NiO x H y electrode [(b),(e)] and OER activity [(c),(f)] during chronoamperometry measurements at 1.7 V; the schematic diagram in (c) depicts the dissolution process; similar chronoamperometry experiments performed in electrolyte containing 0.1 mg/L 57Fe[(e),(f)] reveal Fe dynamic exchange (dissolution and redeposition) at the interface during OER catalysis; the schematic diagram in (f) depicts both the Fe dissolution and redeposition processes[26]
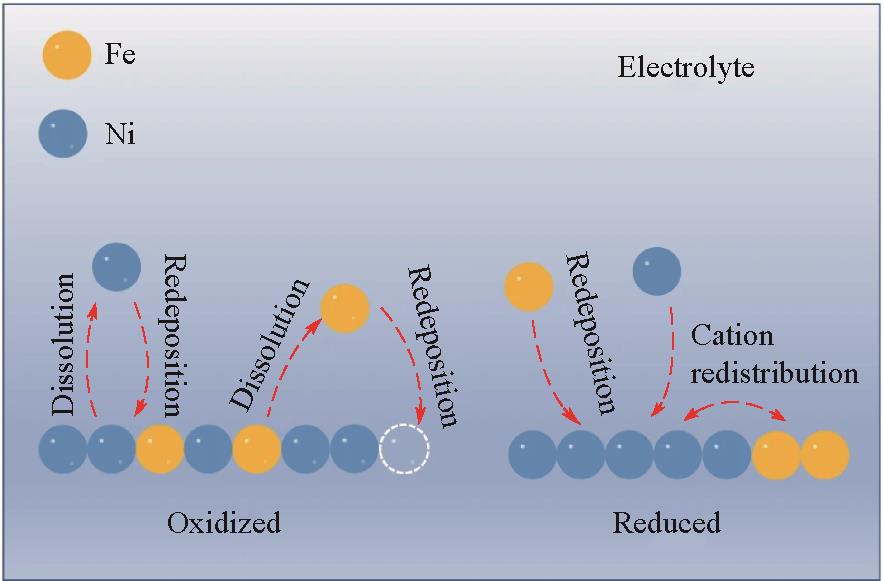
图7 可逆相偏析机制:在氧化还原电位下,Fe的溶解和Fe位点选择性再沉积导致了相偏析,而在还原态下,金属阳离子的再分布和均匀的Fe再沉积缓解了相偏析[59]
Fig.7 Mechanism for reversible phase segregation: at the OER potential, the dissolution of Fe and site-selective redeposition of Fe lead to phase segregation, whereas at the reduction state, the redistribution of metal cations and homogeneous Fe redeposition alleviate the phase segregation[59]
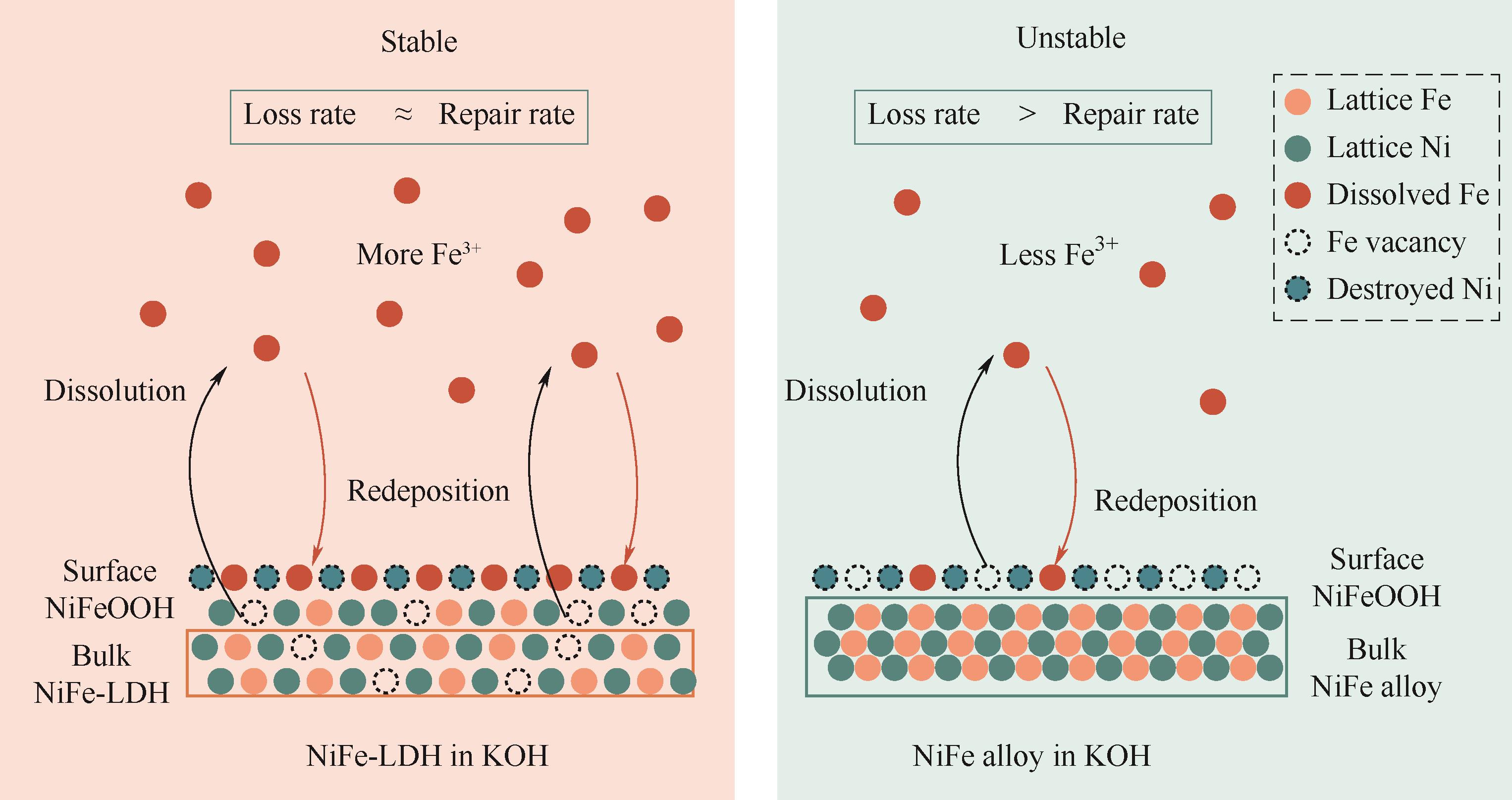
图8 OER过程中NiFe-LDH和NiFe合金在KOH中的Fe溶解/再沉积示意图[61]
Fig.8 Schematic diagram of the Fe dissolution/redeposition for NiFe-LDH and NiFe alloys in KOH during the OER process[61]
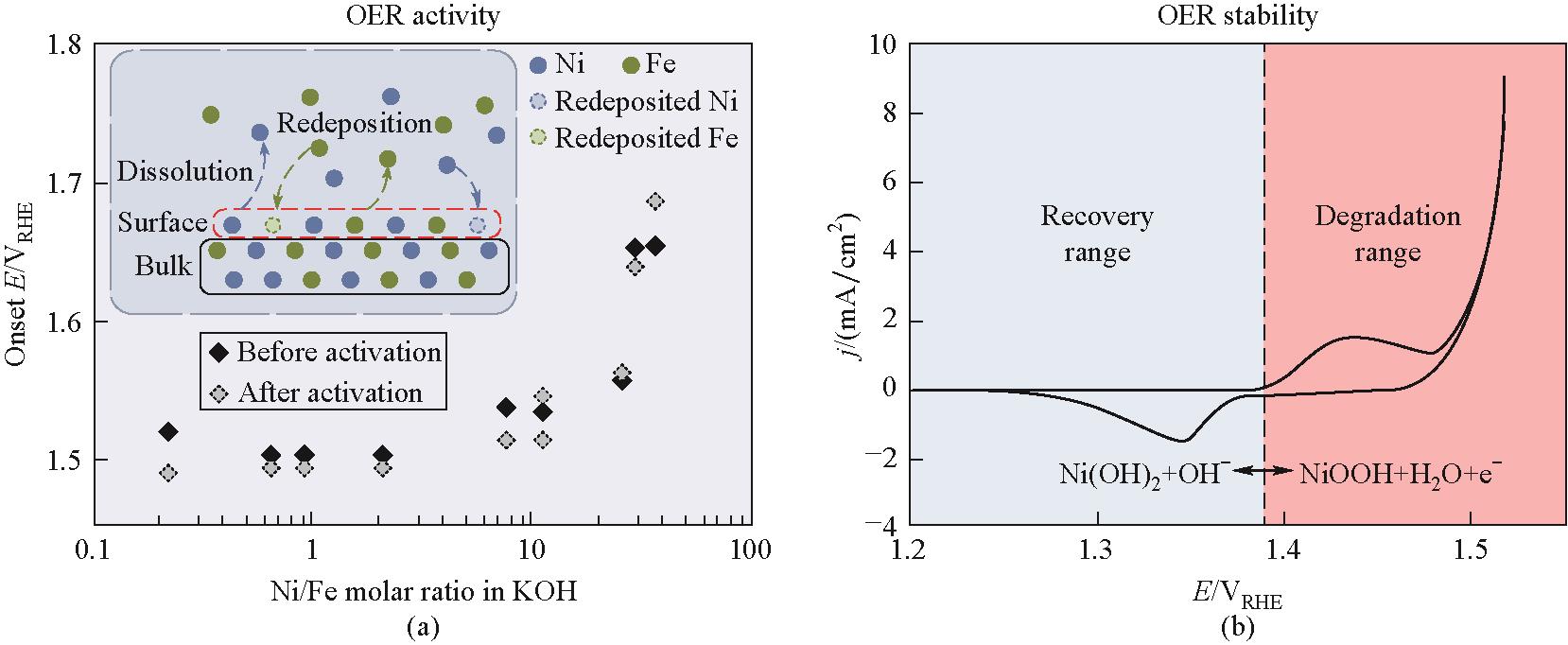
图9 (a)KOH溶液中Ni/Fe摩尔比与Ni-Fe合金电极OER起始电位关系;(b)电极衰减范围和恢复范围示意[62]
Fig.9 (a)Change of OER onset potential with Ni/Fe molar ratio in KOH solution; (b)Schematic of electrode degradation and recovery potential ranges[62]
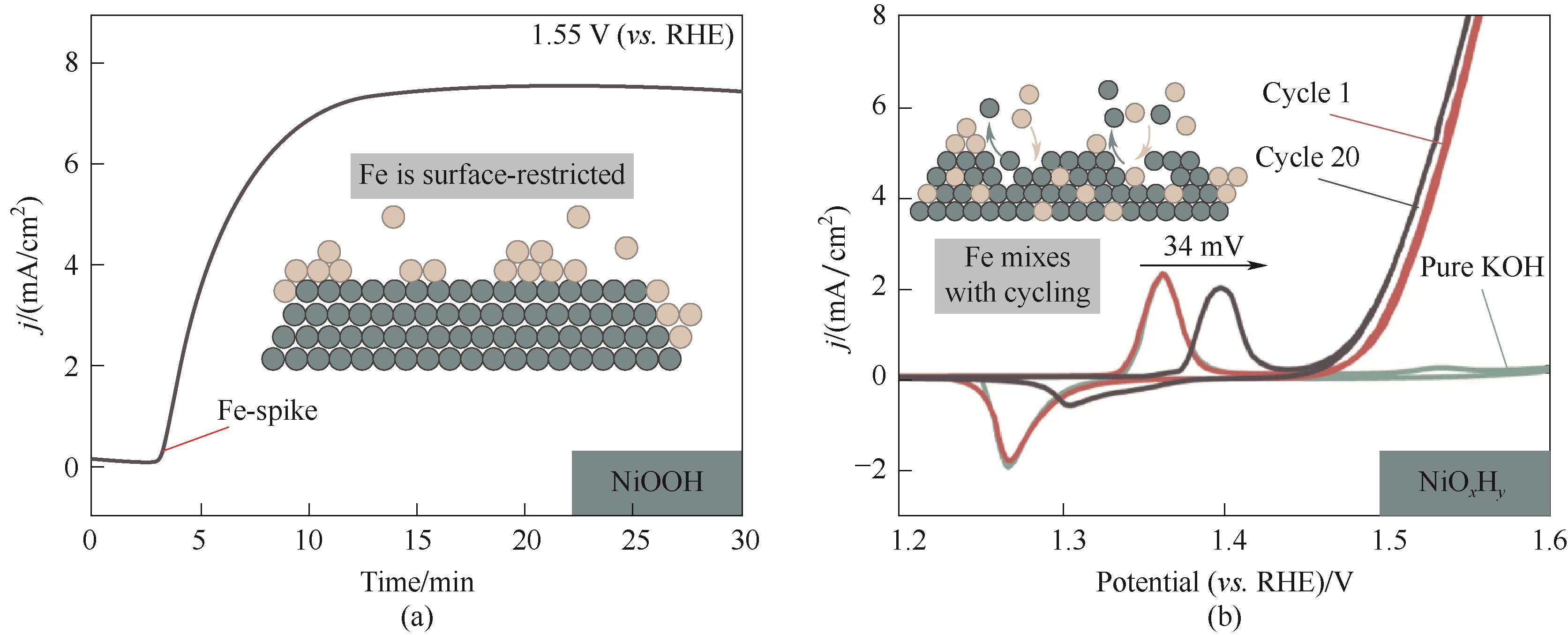
图10 (a) NiOOH在1.55 V (vs. RHE)的CA测试,在除Fe后的1 mol/L KOH中开始测量,后加Fe(NO3)3到0.1 mg/L;(b) 在 (a) 中Fe掺杂达到峰值后,进行CV测试[58]
Fig.10 (a) CA measurements of NiOOH at 1.55 V (vs. RHE), after starting the measurement in purified Fe-free 1 mol/L KOH electrolyte, aqueous Fe(NO3)3 was added to a concentration of 0.1 mg/L; (b) CV test after peak Fe doping in (a)[58]
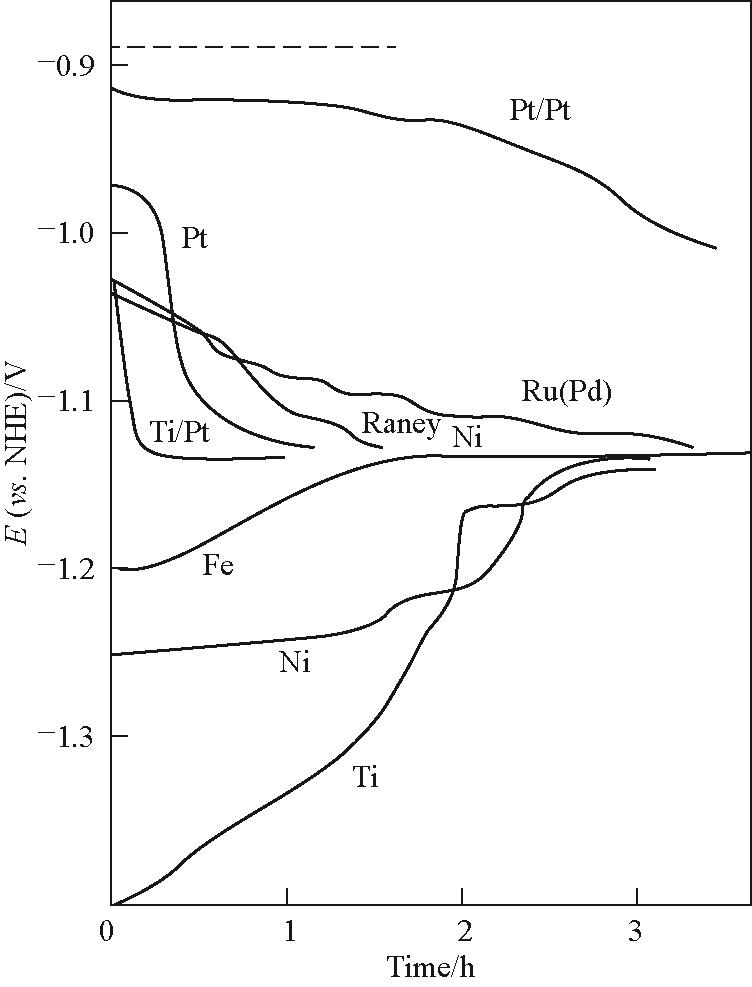
图11 在90℃、3 kA/m²、含10 mg/L Fe的33%(质量分数) NaOH条件下,阴极HER电位随时间变化;虚线表示可逆电位[63]
Fig.11 Variation of the cathode potential with time during the course of the HER from 33%(mass) NaOH at 90℃ and 3 kA/m2 in the presence of 10 mg/L Fe; The dashed line represents the reversible potential[63]
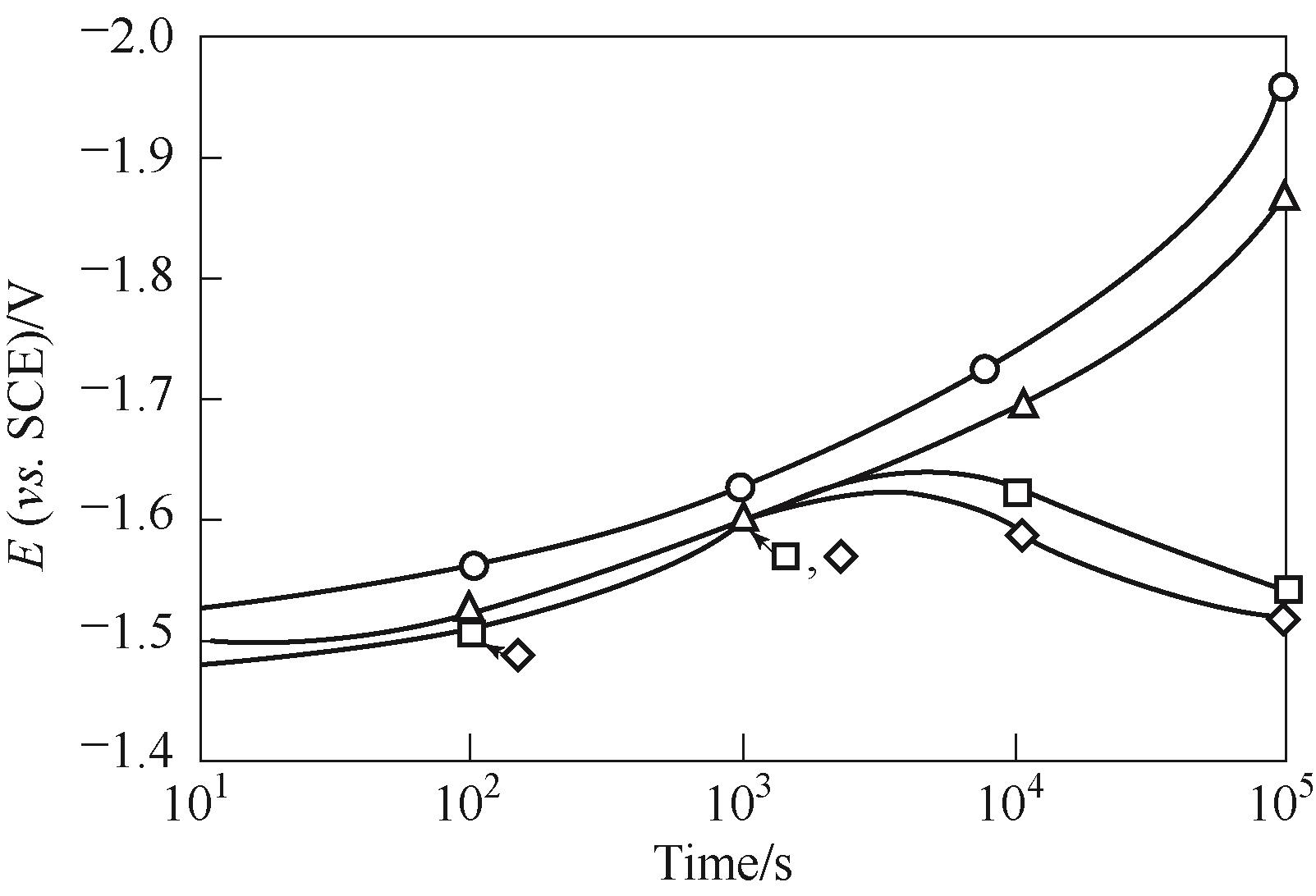
图12 在 37℃、30%(质量分数) KOH、250 mA/cm2条件下,Ni阴极电位随时间变化的情况(: 0.03 mg/L Fe;:3.0 mg/L Fe)[64]
Fig.12 Nickel cathode potential behavior with time under galvanostatic control of 250 mA/cm2 in 30%(mass) KOH at 37℃ ( for 0.03 mg/L dissolved iron; for 3.0 mg/L dissolved iron)[64]
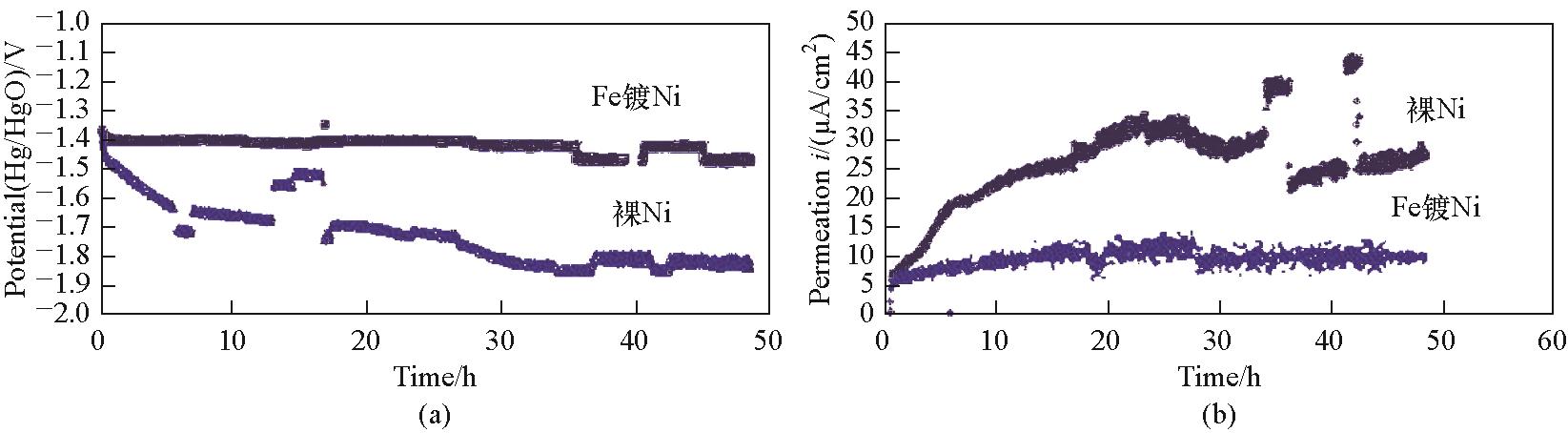
图13 (a) 长期实验中的阴极极化电位;(b) 长期实验中的氢渗透电流;温度:70℃,充电电流:100 mA/cm²,裸Ni和Fe镀Ni的三个重复实验的100 s平均电流[67]
Fig.13 (a) Cathode polarization potentials during longer term experiments; (b) Hydrogen permeation currents during longer term experiments; T = 70℃, charging current = 100 mA/cm2, 100 s averaged currents from three replicate experiments for bare Ni and Ni with Fe coating[67]
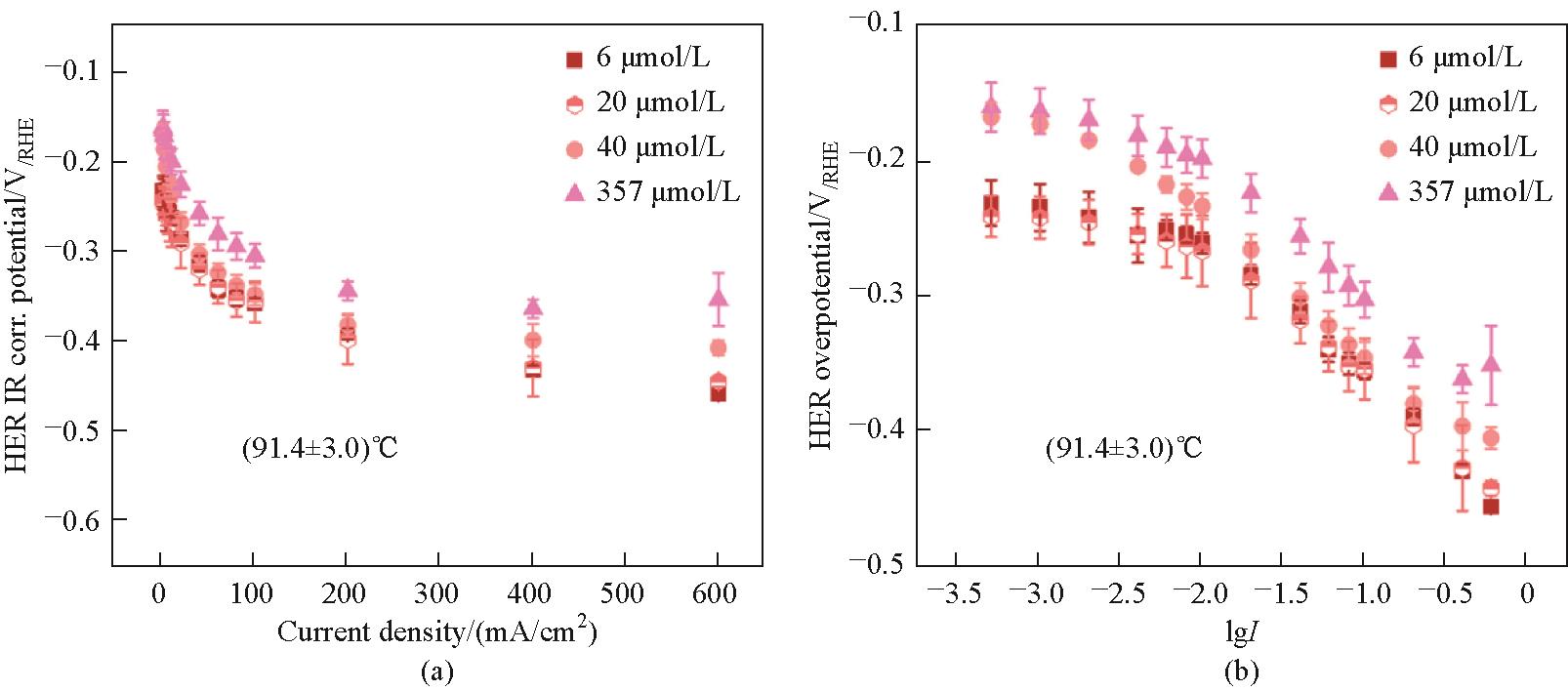
图14 91℃下,电解液中Fe浓度分别为6、20、40和357 μmol/L时IR校正后的HER电位I-V曲线(a)和HER Tafel图(b);Tafel图中采用的是电流密度(单位是A/cm²)的对数值,数据以平均值±标准差的形式呈现[68]
Fig.14 I-V curves for internal resistance corrected HER potential (a) and Tafel plots (b) for HER at 91℃ for Fe electrolyte concentrations of 6, 20, 40, and 357 μmol/L; Note that for the Tafel plots the logarithmic value of the current density was used in A/cm2; Data are represented as mean ± standard deviations[68]
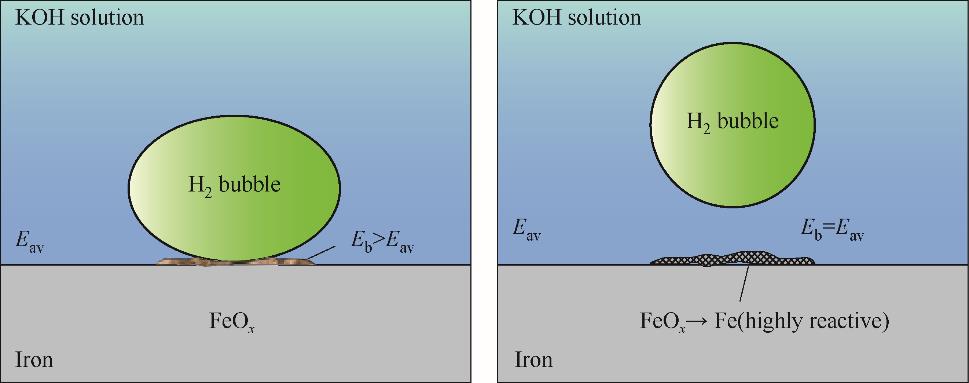
图15 氢气泡内/下腐蚀产物的形成及其在脱离氢气泡后还原成金属的示意图[69]
Fig.15 Schematic diagram of the formation of corrosion products at/under hydrogen bubble and their reduction to metal upon detachment of the bubble[69]
| 1 | Johnston B, Mayo M C, Khare A. Hydrogen: the energy source for the 21st century[J]. Technovation, 2005, 25(6): 569-585. |
| 2 | Hassan Q, Sameen A Z, Salman H M, et al. Hydrogen energy future: advancements in storage technologies and implications for sustainability[J]. Journal of Energy Storage, 2023, 72: 108404. |
| 3 | Thomas J M, Edwards P P, Dobson P J, et al. Decarbonising energy: the developing international activity in hydrogen technologies and fuel cells[J]. Journal of Energy Chemistry, 2020, 51: 405-415. |
| 4 | Rissman J, Bataille C, Masanet E, et al. Technologies and policies to decarbonize global industry: review and assessment of mitigation drivers through 2070[J]. Applied Energy, 2020, 266: 114848. |
| 5 | Sikiru S, Oladosu T L, Amosa T I, et al. Hydrogen-powered horizons: transformative technologies in clean energy generation, distribution, and storage for sustainable innovation[J]. International Journal of Hydrogen Energy, 2024, 56: 1152-1182. |
| 6 | Zhou Q, Liao L, Zhou H, et al. Innovative strategies in design of transition metal-based catalysts for large-current-density alkaline water/seawater electrolysis[J]. Materials Today Physics, 2022, 26: 100727. |
| 7 | Zeng K, Zhang D K. Recent progress in alkaline water electrolysis for hydrogen production and applications[J]. Progress in Energy and Combustion Science, 2010, 36(3): 307-326. |
| 8 | Smolinka T, Bergmann H, Garche J, et al. The history of water electrolysis from its beginnings to the present[M]//Electrochemical Power Sources: Fundamentals, Systems, and Applications. Amsterdam: Elsevier, 2022: 83-164. |
| 9 | Santos D M F, Sequeira C A C, Figueiredo J L. Hydrogen production by alkaline water electrolysis[J]. Química Nova, 2013, 36(8): 1176-1193. |
| 10 | Kreuter W, Hofmann H. Electrolysis: the important energy transformer in a world of sustainable energy[J]. International Journal of Hydrogen Energy, 1998, 23(8): 661-666. |
| 11 | Wan L, Xu Z, Xu Q, et al. Key components and design strategy of the membrane electrode assembly for alkaline water electrolysis[J]. Energy & Environmental Science, 2023, 16(4): 1384-1430. |
| 12 | Chen Y, Min L, Zhang W, et al. Crown ether as a bifunctional booster in electrochemical water splitting[J]. International Journal of Hydrogen Energy, 2024, 51: 1534-1543. |
| 13 | Song C, Min L, Zhang W, et al. A benzimidazole-linked polymer membrane in alkaline water electrolysis[J]. Journal of Membrane Science, 2023, 683: 121883. |
| 14 | Abbasi R, Setzler B P, Lin S S, et al. A roadmap to low-cost hydrogen with hydroxide exchange membrane electrolyzers[J]. Advanced Materials, 2019, 31(31): 1805876. |
| 15 | Ursúa A, Gandía L M, Sanchis P. Hydrogen production from water electrolysis: current status and future trends[C]//Proceedings of the IEEE. IEEE, 2012: 410-426. |
| 16 | El-Shafie M. Hydrogen production by water electrolysis technologies: a review[J]. Results in Engineering, 2023, 20: 101426. |
| 17 | Liu R T, Xu Z L, Li F M, et al. Recent advances in proton exchange membrane water electrolysis[J]. Chemical Society Reviews, 2023, 52(16): 5652-5683. |
| 18 | Zhang W, Liu M, Gu X, et al. Water electrolysis toward elevated temperature: advances, challenges and frontiers[J]. Chemical Reviews, 2023, 123(11): 7119-7192. |
| 19 | Baratov S, Filonova E, Ivanova A, et al. Current and further trajectories in designing functional materials for solid oxide electrochemical cells: a review of other reviews[J]. Journal of Energy Chemistry, 2024, 94: 302-331. |
| 20 | Santoro C, Lavacchi A, Mustarelli P, et al. What is next in anion-exchange membrane water electrolyzers? Bottlenecks, benefits, and future[J]. ChemSusChem, 2022, 15(8): e202200027. |
| 21 | Du N Y, Roy C, Peach R, et al. Anion-exchange membrane water electrolyzers[J]. Chemical Reviews, 2022, 122(13): 11830-11895. |
| 22 | de Groot M T. Alkaline water electrolysis: with or without iron in the electrolyte?[J]. Current Opinion in Chemical Engineering, 2023, 42: 100981. |
| 23 | Bailleux C, Damien A, Montet A. Alkaline electrolysis of water-EGF activity in electrochemical engineering from 1975 to 1982[J]. International Journal of Hydrogen Energy, 1983, 8(7): 529-538. |
| 24 | Davalos Monteiro R, van de Wetering J, Krawczyk B, et al. Corrosion behaviour of type 316L stainless steel in hot caustic aqueous environments[J]. Metals and Materials International, 2020, 26(5): 630-640. |
| 25 | Todoroki N, Wadayama T. Dissolution of constituent elements from various austenitic stainless steel oxygen evolution electrodes under potential cycle loadings[J]. International Journal of Hydrogen Energy, 2022, 47(77): 32753-32762. |
| 26 | Chung D Y, Lopes P P, Farinazzo Bergamo Dias Martins P, et al. Dynamic stability of active sites in hydr(oxy)oxides for the oxygen evolution reaction[J]. Nature Energy, 2020, 5: 222-230. |
| 27 | Tyndall D, Craig M J, Gannon L, et al. Demonstrating the source of inherent instability in NiFe LDH-based OER electrocatalysts[J]. Journal of Materials Chemistry A, 2023, 11(8): 4067-4077. |
| 28 | Liu W, Ding X Q, Cheng J J, et al. Inhibiting dissolution of active sites in 80℃ alkaline water electrolysis by oxyanion engineering[J]. Angewandte Chemie International Edition, 2024, 63(32): e202406082. |
| 29 | Trotochaud L, Young S L, Ranney J K, et al. Nickel-iron oxyhydroxide oxygen-evolution electrocatalysts: the role of intentional and incidental iron incorporation[J]. Journal of the American Chemical Society, 2014, 136(18): 6744-6753. |
| 30 | Cameron D S, Phillips R L, Willis P M. Poison tolerant platinum catalysed cathodes for membrane cells[M]//Modern Chlor-Alkali Technology. Dordrecht: Springer Netherlands, 1990: 95-107. |
| 31 | Rodríguez J, Palmas S, Sánchez-Molina M, et al. Simple and precise approach for determination of ohmic contribution of diaphragms in alkaline water electrolysis[J]. Membranes, 2019, 9(10): 129. |
| 32 | O'Brien T F, Bommaraju T V, Hine F. Handbook of Chlor-Alkali Technology[M]. Boston, MA: Springer US, 2005. |
| 33 | Spanos I, Masa J, Zeradjanin A, et al. The effect of iron impurities on transition metal catalysts for the oxygen evolution reaction in alkaline environment: activity mediators or active sites?[J]. Catalysis Letters, 2021, 151(7): 1843-1856. |
| 34 | Corrigan D A. The catalysis of the oxygen evolution reaction by iron impurities in thin film nickel oxide electrodes[J]. Journal of the Electrochemical Society, 1987, 134(2): 377. |
| 35 | Lyons M E G, Brandon M P. The oxygen evolution reaction on passive oxide covered transition metal electrodes in aqueous alkaline solution(Part 1): Nickel[J]. International Journal of Electrochemical Science, 2008, 3(12): 1386-1424. |
| 36 | Kostecki R, McLarnon F. Electrochemical and in situ Raman spectroscopic characterization of nickel hydroxide electrodes(Ⅰ): Pure nickel hydroxide[J]. Journal of the Electrochemical Society, 1997, 144(2): 485-493. |
| 37 | Yeo B S, Bell A T. In situ Raman study of nickel oxide and gold-supported nickel oxide catalysts for the electrochemical evolution of oxygen[J]. The Journal of Physical Chemistry C, 2012, 116(15): 8394-8400. |
| 38 | Doyle R L, Lyons M E G. Kinetics and mechanistic aspects of the oxygen evolution reaction at hydrous iron oxide films in base[J]. Journal of the Electrochemical Society, 2013, 160(2): H142-H154. |
| 39 | Oliva P, Leonardi J, Laurent J F, et al. Review of the structure and the electrochemistry of nickel hydroxides and oxy-hydroxides[J]. Journal of Power Sources, 1982, 8(2): 229-255. |
| 40 | Wehrens-Dijksma M, Notten P H L. Electrochemical quartz microbalance characterization of Ni(OH)2-based thin film electrodes[J]. Electrochimica Acta, 2006, 51(18): 3609-3621. |
| 41 | Godwin I J, Lyons M E G. Enhanced oxygen evolution at hydrous nickel oxide electrodes via electrochemical ageing in alkaline solution[J]. Electrochemistry Communications, 2013, 32: 39-42. |
| 42 | Lu P W T, Srinivasan S. Electrochemical-ellipsometric studies of oxide film formed on nickel during oxygen evolution[J]. Journal of the Electrochemical Society, 1978, 125(9): 1416. |
| 43 | Bernard M C, Bernard P, Keddam M, et al. Characterisation of new nickel hydroxides during the transformation of α-Ni(OH)2 to β-Ni(OH)2 by ageing[J]. Electrochimica Acta, 1996, 41(1): 91-93. |
| 44 | Kim M S, Kim K B. A study on the phase transformation of electrochemically precipitated nickel hydroxides using an electrochemical quartz crystal microbalance[J]. Journal of the Electrochemical Society, 1998, 145(2): 507-511. |
| 45 | Louie M W, Bell A T. An investigation of thin-film Ni-Fe oxide catalysts for the electrochemical evolution of oxygen[J]. Journal of the American Chemical Society, 2013, 135(33): 12329-12337. |
| 46 | Klaus S, Cai Y, Louie M W, et al. Effects of Fe electrolyte impurities on Ni(OH)2/NiOOH structure and oxygen evolution activity[J]. The Journal of Physical Chemistry C, 2015, 119(13): 7243-7254. |
| 47 | Deng J, Nellist M R, Stevens M B, et al. Morphology dynamics of single-layered Ni(OH)2/NiOOH nanosheets and subsequent Fe incorporation studied by in situ electrochemical atomic force microscopy[J]. Nano Letters, 2017, 17(11): 6922-6926. |
| 48 | Burke M S, Kast M G, Trotochaud L, et al. Cobalt-iron (oxy)hydroxide oxygen evolution electrocatalysts: the role of structure and composition on activity, stability, and mechanism[J]. Journal of the American Chemical Society, 2015, 137(10): 3638-3648. |
| 49 | Zhang T, Nellist M R, Enman L J, et al. Modes of Fe incorporation in Co-Fe (oxy)hydroxide oxygen evolution electrocatalysts[J]. ChemSusChem, 2019, 12(9): 2015-2021. |
| 50 | Stevens M B, Enman L J, Korkus E H, et al. Ternary Ni-Co-Fe oxyhydroxide oxygen evolution catalysts: intrinsic activity trends, electrical conductivity, and electronic band structure[J]. Nano Research, 2019, 12(9): 2288-2295. |
| 51 | Spanos I, Tesch M F, Yu M Q, et al. Facile protocol for alkaline electrolyte purification and its influence on a Ni-Co oxide catalyst for the oxygen evolution reaction[J]. ACS Catalysis, 2019, 9(9): 8165-8170. |
| 52 | Zhou Y C, López N. The role of Fe species on NiOOH in oxygen evolution reactions[J]. ACS Catalysis, 2020, 10(11): 6254-6261. |
| 53 | Enman L J, Burke M S, Batchellor A S, et al. Effects of intentionally incorporated metal cations on the oxygen evolution electrocatalytic activity of nickel (oxy)hydroxide in alkaline media[J]. ACS Catalysis, 2016, 6(4): 2416-2423. |
| 54 | Stevens M B, Trang C D M, Enman L J, et al. Reactive Fe-sites in Ni/Fe (oxy)hydroxide are responsible for exceptional oxygen electrocatalysis activity[J]. Journal of the American Chemical Society, 2017, 139(33): 11361-11364. |
| 55 | Marquez R A, Kalokowski E, Espinosa M, et al. Transition metal incorporation: electrochemical, structure, and chemical composition effects on nickel oxyhydroxide oxygen-evolution electrocatalysts[J]. Energy & Environmental Science, 2024, 17(5): 2028-2045. |
| 56 | Friebel D, Louie M W, Bajdich M, et al. Identification of highly active Fe sites in (Ni, Fe)OOH for electrocatalytic water splitting[J]. Journal of the American Chemical Society, 2015, 137(3): 1305-1313. |
| 57 | Farhat R, Dhainy J, Halaoui L I. OER catalysis at activated and codeposited NiFe-oxo/hydroxide thin films is due to postdeposition surface-Fe and is not sustainable without Fe in solution[J]. ACS Catalysis, 2020, 10(1): 20-35. |
| 58 | Ou Y Q, Twight L P, Samanta B, et al. Cooperative Fe sites on transition metal (oxy)hydroxides drive high oxygen evolution activity in base[J]. Nature Communications, 2023, 14(1): 7688. |
| 59 | Kuai C G, Xu Z R, Xi C, et al. Phase segregation reversibility in mixed-metal hydroxide water oxidation catalysts[J]. Nature Catalysis, 2020, 3: 743-753. |
| 60 | Zou S H, Burke M S, Kast M G, et al. Fe (oxy)hydroxide oxygen evolution reaction electrocatalysis: intrinsic activity and the roles of electrical conductivity, substrate, and dissolution[J]. Chemistry of Materials, 2015, 27(23): 8011-8020. |
| 61 | Zhang Q, Xiao W, Fu H C, et al. Unraveling the mechanism of self-repair of NiFe-based electrocatalysts by dynamic exchange of iron during the oxygen evolution reaction[J]. ACS Catalysis, 2023, 13(22): 14975-14986. |
| 62 | Bao F X, Kemppainen E, Dorbandt I, et al. Host, suppressor, and promoter-the roles of Ni and Fe on oxygen evolution reaction activity and stability of NiFe alloy thin films in alkaline media[J]. ACS Catalysis, 2021, 11(16): 10537-10552. |
| 63 | Nidola A, Schira R. Deactivation of low hydrogen overvoltage cathodes in chlor-alkali membrane cell technology by metallic impurities[C]//Proceedings of the Symposium on Advances in the Chlor-Alkali and Chlorate Industry. Electrochemical Society, Industrial Electrolytic Division, 1984: F: 206-221. |
| 64 | Riley M A, Moran P J. The influence of iron deposition on the voltage-time behavior of nickel cathodes in alkaline water electrolysis[J]. Journal of the Electrochemical Society, 1986, 133(4): 760-761. |
| 65 | Rommal H E G, Moran P J. Time-dependent energy efficiency losses at nickel cathodes in alkaline water electrolysis systems[J]. Journal of the Electrochemical Society, 1985, 132(2): 325-329. |
| 66 | Brossard L. Electrocatalytic performance for alkaline water electrolysis of Ni electrodes electrocoated with Fe or Fe/Mo[J]. International Journal of Hydrogen Energy, 1991, 16(1): 13-21. |
| 67 | Mauer A E, Kirk D W, Thorpe S J. The role of iron in the prevention of nickel electrode deactivation in alkaline electrolysis[J]. Electrochimica Acta, 2007, 52(11): 3505-3509. |
| 68 | Demnitz M, Lamas Y M, Garcia Barros R L, et al. Effect of iron addition to the electrolyte on alkaline water electrolysis performance[J]. iScience, 2024, 27(1): 108695. |
| 69 | Flis-Kabulska I, Flis J, Sun Y, et al. Hydrogen evolution on plasma carburised nickel and effect of iron deposition from the electrolyte in alkaline water electrolysis[J]. Electrochimica Acta, 2015, 167: 61-68. |
| 70 | Flis-Kabulska I, Flis J. Hydrogen evolution and corrosion products on iron cathodes in hot alkaline solution[J]. International Journal of Hydrogen Energy, 2014, 39(8): 3597-3605. |
| [1] | 殷梦凡, 王倩, 郑涛, 姬奎, 王绍贵, 郭辉, 林志强, 张睿, 孙晖, 刘海燕, 刘植昌, 徐春明, 孟祥海, 王月平. 可再生能源电解水制氢-低温低压合成氨万吨级工业示范流程设计[J]. 化工学报, 2025, 76(2): 825-834. |
| [2] | 白谨豪, 管小平, 杨宁. 压滤式水电解槽乳突板内的流动特性分析与优化[J]. 化工学报, 2025, 76(2): 584-595. |
| [3] | 张珂, 任维杰, 王梦娜, 范凯锋, 常丽萍, 李佳斌, 马涛, 田晋平. Bunsen反应产物在微通道中的液-液两相混合特性[J]. 化工学报, 2025, 76(2): 623-636. |
| [4] | 何传超, 周静红, 曹约强, 施尧, 周兴贵. Ag/SiO2催化草酸酯加氢制乙醇酸甲酯的床层-颗粒双尺度耦合模拟研究[J]. 化工学报, 2025, 76(2): 654-666. |
| [5] | 邹吉军, 刘宝宏, 史成香, 潘伦, 张香文. 综纤维素衍生物转化合成生物航空燃料的非均相催化剂研究进展[J]. 化工学报, 2025, 76(1): 1-17. |
| [6] | 杨晨, 毛伟, 董兴宗, 田松, 赵锋伟, 吕剑. 选择性加氢脱氯合成烯烃研究进展[J]. 化工学报, 2025, 76(1): 53-70. |
| [7] | 纪之骄, 张晓方, 甘汶, 薛云鹏. 载体对单原子电催化剂合成氨性能的影响与调控策略[J]. 化工学报, 2025, 76(1): 18-39. |
| [8] | 郭珊, 田雨, 徐永滨, 王朋, 刘治明. 废旧电池再资源化制备高性能中熵合金催化剂及其性能研究[J]. 化工学报, 2025, 76(1): 231-240. |
| [9] | 石美琳, 赵连达, 邓行健, 王静松, 左海滨, 薛庆国. 催化甲烷重整工艺的研究进展[J]. 化工学报, 2024, 75(S1): 25-39. |
| [10] | 王树振, 王玉婷, 马梦茜, 张巍, 向江南, 鲁海莹, 王琰, 范彬彬, 郑家军, 代卫炯, 李瑞丰. 两步晶化合成ZSM-22分子筛及其临氢异构反应性能[J]. 化工学报, 2024, 75(9): 3176-3187. |
| [11] | 王军锋, 张俊杰, 张伟, 王家乐, 双舒炎, 张亚栋. 液相放电等离子体分解甲醇制氢:电极配置的优化[J]. 化工学报, 2024, 75(9): 3277-3286. |
| [12] | 王冉, 王焕, 熊晓云, 关慧敏, 郑云锋, 陈彩琳, 秦玉才, 宋丽娟. FCC催化剂传质强化活性位利用效率的可视化分析[J]. 化工学报, 2024, 75(9): 3198-3209. |
| [13] | 刘亚超, 谭晓杰, 李旭东, 王瑞, 王慧, 韩璇, 赵青山. DES合成高活性CoCO3纳米片及析氧反应性能研究[J]. 化工学报, 2024, 75(9): 3320-3328. |
| [14] | 张梦婷, 王书林, 桑熙, 元兴昊, 徐刚. 人工Cu-TM1459金属酶催化不对称迈克尔加成反应[J]. 化工学报, 2024, 75(9): 3255-3265. |
| [15] | 罗欣怡, 徐强, 佘永璐, 聂腾飞, 郭烈锦. 光电分解水制氢气泡动力学特性及其传质机理研究[J]. 化工学报, 2024, 75(9): 3083-3093. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号