化工学报 ›› 2025, Vol. 76 ›› Issue (8): 3753-3771.DOI: 10.11949/0438-1157.20250058
• 综述与专论 • 下一篇
王御风1( ), 罗小雪1(
), 罗小雪1( ), 范鸿亮1, 吴白婧1, 李存璞1,2(
), 范鸿亮1, 吴白婧1, 李存璞1,2( ), 魏子栋1,2(
), 魏子栋1,2( )
)
收稿日期:2025-01-14
修回日期:2025-03-11
出版日期:2025-08-25
发布日期:2025-09-17
通讯作者:
李存璞,魏子栋
作者简介:王御风(2000—),男,硕士研究生,30406870@qq.com基金资助:
Yufeng WANG1( ), Xiaoxue LUO1(
), Xiaoxue LUO1( ), Hongliang FAN1, Baijing WU1, Cunpu LI1,2(
), Hongliang FAN1, Baijing WU1, Cunpu LI1,2( ), Zidong WEI1,2(
), Zidong WEI1,2( )
)
Received:2025-01-14
Revised:2025-03-11
Online:2025-08-25
Published:2025-09-17
Contact:
Cunpu LI, Zidong WEI
摘要:
随着全球能源危机和环境污染问题日益加剧,电解水制氢作为一种清洁、高效的制氢技术备受关注。然而,传统电解水阳极析氧反应存在动力学迟缓、过电位高以及氧气副产物等问题,限制了技术的进一步发展。近年来,研究人员发展了利用电解水阳极生成的活性氧物种(ROS)(*OOH、*OH和*O等)实现有机物选择性氧化的系列策略,可以有效提升电解水的能量、原子利用率,减少能耗,提升生产附加值。电极界面作为电化学反应的场所,电极材料的多尺度结构、反应底物的吸附行为等,都影响着耦合电解水制氢的绿色有机氧化效率和选择性。因此,本文综述了利用活性氧物种氧化有机物耦合电解水制氢的电极表面调控策略,重点讨论了电极强化“界面-底物”过程的作用、电极控制荷质传递过程以及电极调控反应作用机理等三个方面。通过掺杂、构筑应变、引入空位和异质结等手段调控电极电子结构,能够促进ROS生成和反应底物吸附,提升反应选择性与效率;通过采用恒电位和脉冲电解等多种电化学方法,可以调控电极材料的金属价态和电子传递速率,优化反应动力学;通过引入介体或添加剂、开发双效催化剂以及表面插层等手段,可以有效调控电极表面对ROS的吸附与利用,同时调节电极对反应底物的吸附特性。未来研究将集中于电极表面催化形态精准调控、催化剂活性位点优化以及机理研究,推动电解水制氢与绿色有机电化学合成的协同发展,为新能源和绿色化学的应用提供新思路。
中图分类号:
王御风, 罗小雪, 范鸿亮, 吴白婧, 李存璞, 魏子栋. 耦合电解水制氢的绿色有机电合成——电极界面调控策略综述[J]. 化工学报, 2025, 76(8): 3753-3771.
Yufeng WANG, Xiaoxue LUO, Hongliang FAN, Baijing WU, Cunpu LI, Zidong WEI. Green organic electrosynthesis coupled with water electrolysis to produce hydrogen—overview of electrode interface regulation strategies[J]. CIESC Journal, 2025, 76(8): 3753-3771.
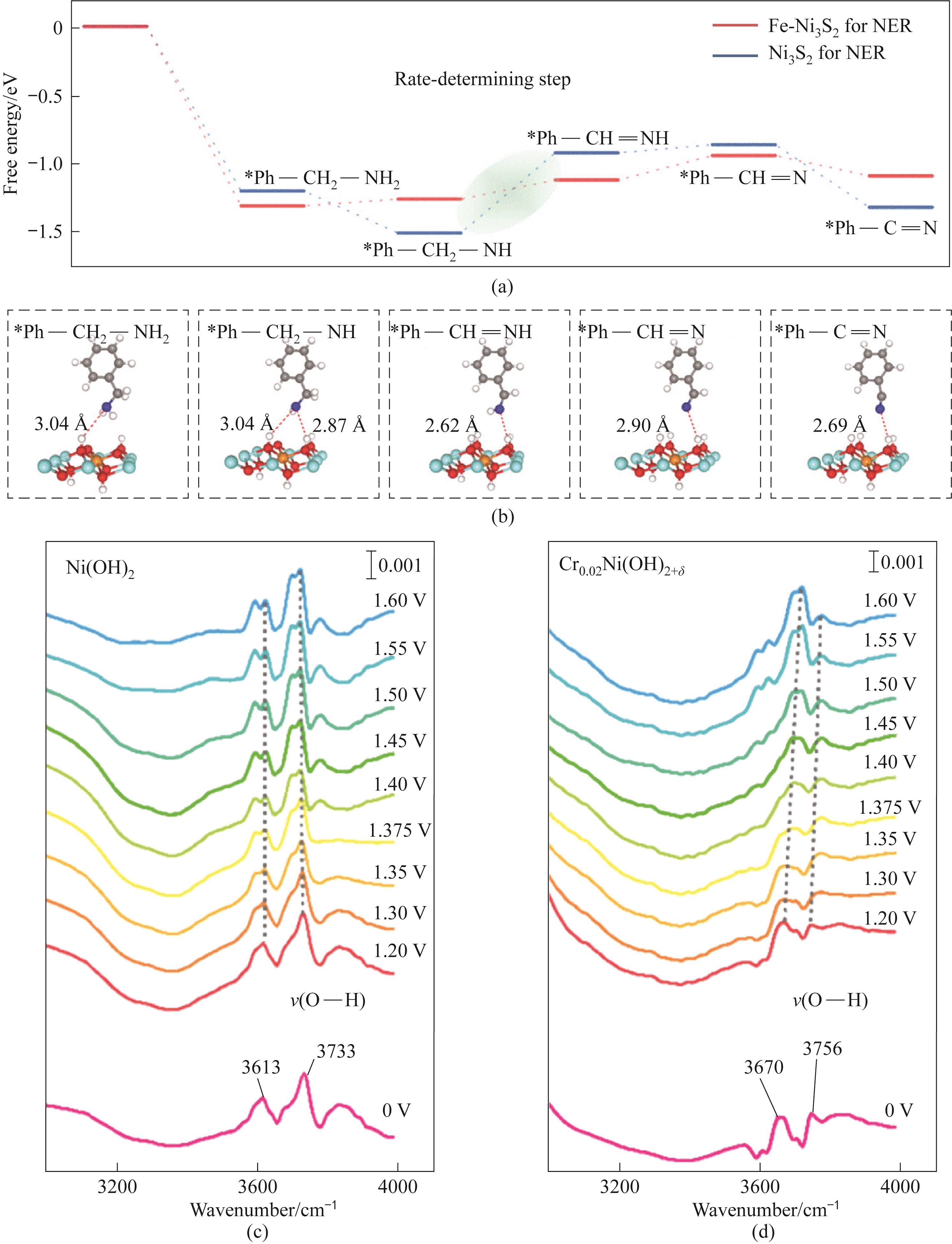
图2 (a) Fe-Ni3S2和Ni3S2自重构后BA电氧化的反应路径[通过Fe掺杂,决速步骤的ΔG(*Ph—CH2—NH到*Ph—CH2NH)从0.59 eV降低到0.14 eV];(b) BA电氧化吸附中间体结构(1 Å=0.1 nm);(c) Ni(OH)2和(d) Cr0.02Ni(OH)2+δ 在不同电位下的原位ATR-FTIR光谱[51-52]
Fig.2 (a) Reaction pathways of BA electro oxidation after Fe-Ni3S2 and Ni3S2 self reconstruction; (b) BA electro oxidation adsorption intermediate structure; In situ ATR-FTIR spectra of (c) Ni (OH)2 and (d) Cr0.02Ni (OH)2+δ at different potentials[51-52]

图3 (a) S-NiCo-LDH LSV曲线;Mn-Co-S/NF和Mn-Co/NF的(b) Co 2p和(c) Mn 2p高分辨率XPS谱;(d) Mn-Co-S/NF的S 2p高分辨率XPS谱;(e) DFT计算Co3N和PW-Co3N表面上 HzOR的自由能分布[53-55]
Fig.3 (a) S-NiCo LDH LSV curve; High resolution XPS spectra of (b) Co 2p and (c) Mn 2p for Mn-Co-S/NF and Mn Co/NF; (d) S 2p high-resolution XPS spectra of Mn-Co-S/NF; (e) DFT calculation of the free energy distribution of HzOR on the surfaces of Co3N and PW-Co3N[53-55]
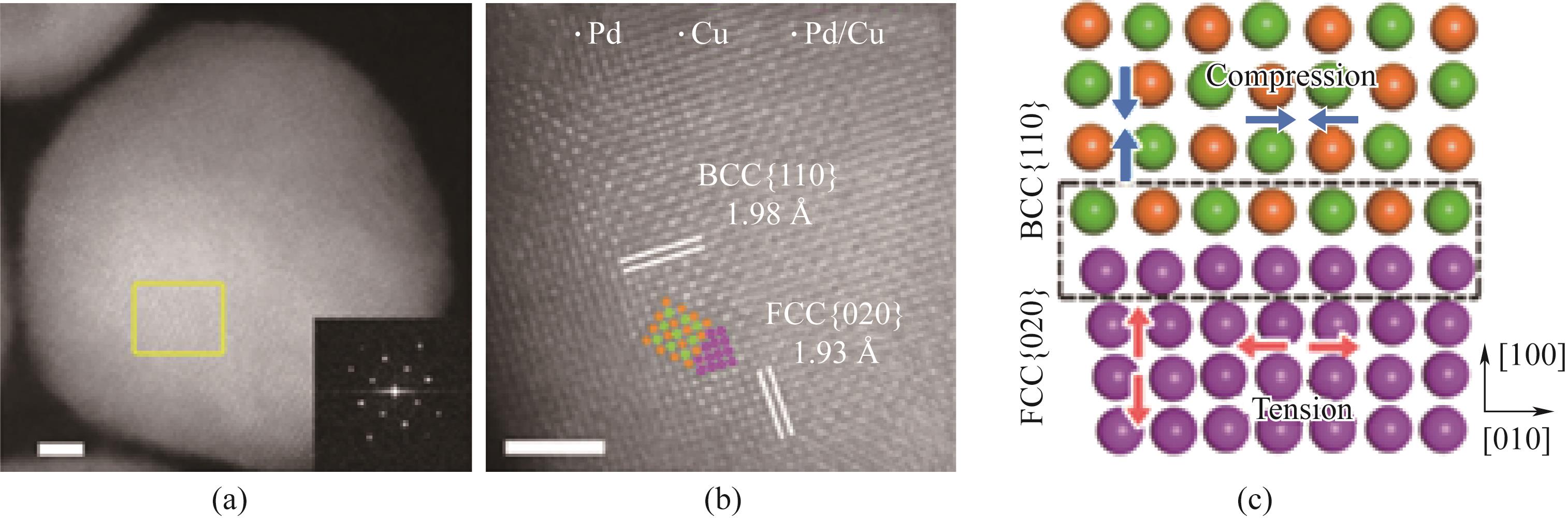
图4 (a)DP-PdCu(DP:双相)沿BCC [001]区轴的像差校正HAADF-STEM图像;(b) 图(a)中所选矩形区域的原子排列;(c)BCC [110]和FCC [020]平面之间界面的模拟原子模型[56]
Fig.4 (a) Aberration corrected HAADF-STEM image of DP PdCu (DP: biphasic) along the BCC [001] region axis; (b) The atomic arrangement of the selected rectangular region in Fig.(a); (c) Simulated atomic model of the interface between BCC [110] and FCC [020] planes[56]
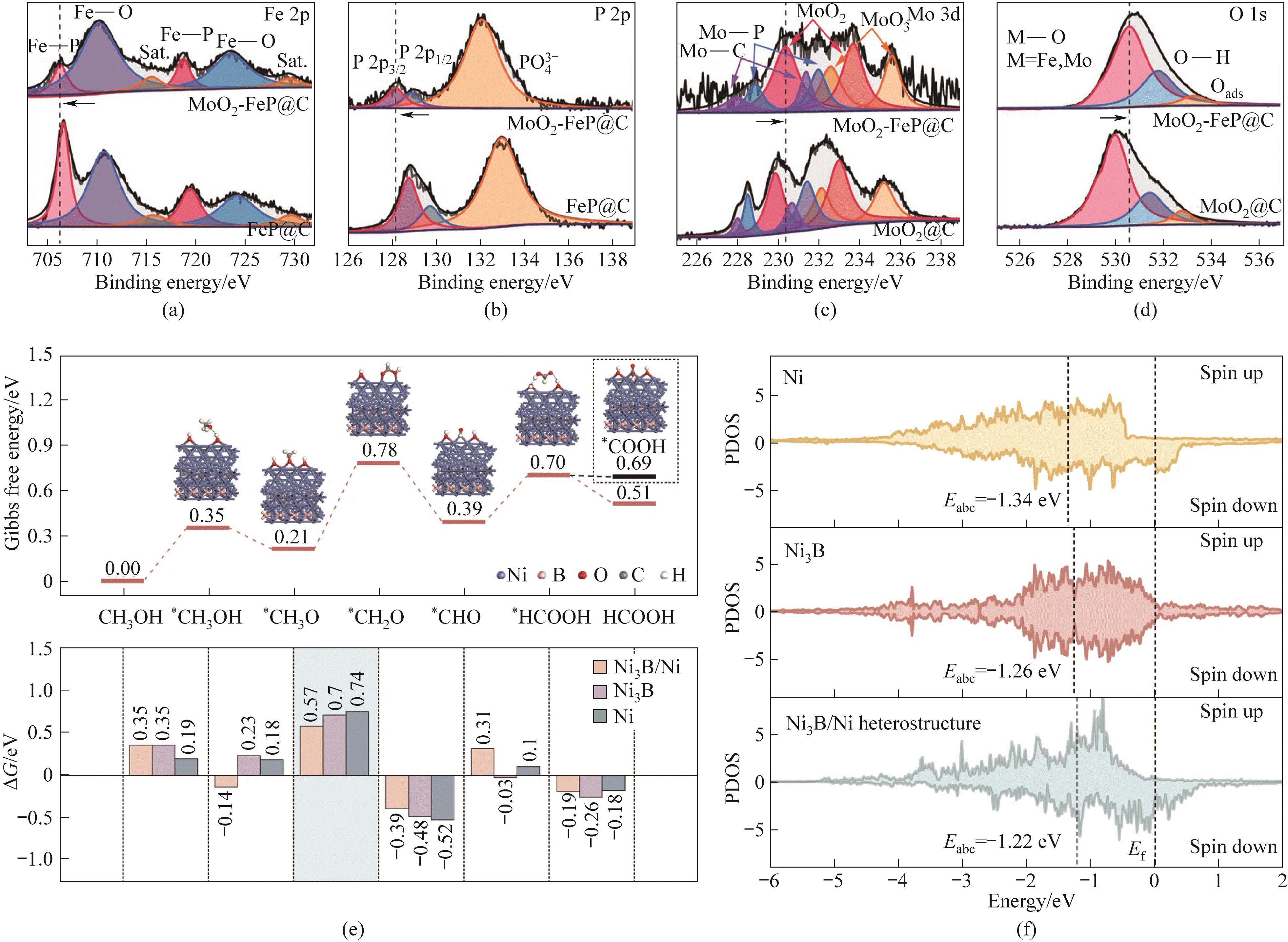
图5 MoO2-FeP@C和FeP@C的(a)Fe 2p、(b)P 2p、(c)Mo 3d、(d)O 1s XPS谱图; (e)Ni3B/Ni异质结构上发生的MOR Gibbs自由能图以及不同表面上台阶之间Gibbs自由能的变化; (f)Ni3B(001)/Ni(111)异质结构、Ni3B和Ni的Ni活性位点的d投影DOS的部分态密度(PDOS)[59,61]
Fig.5 MoO2-FeP@C and FeP@C XPS spectra of (a) Fe 2p, (b) P 2p, (c) Mo 3d, (d) O 1s; (e) MOR Gibbs free energy diagram on Ni3B/Ni heterostructure (top) and Gibbs free energy variation between steps on different surfaces (bottom); (f) Partial density of states (PDOS) of the d-projection DOS of Ni3B (001)/Ni (111) heterostructures and Ni active sites in Ni3B and Ni[59,61]
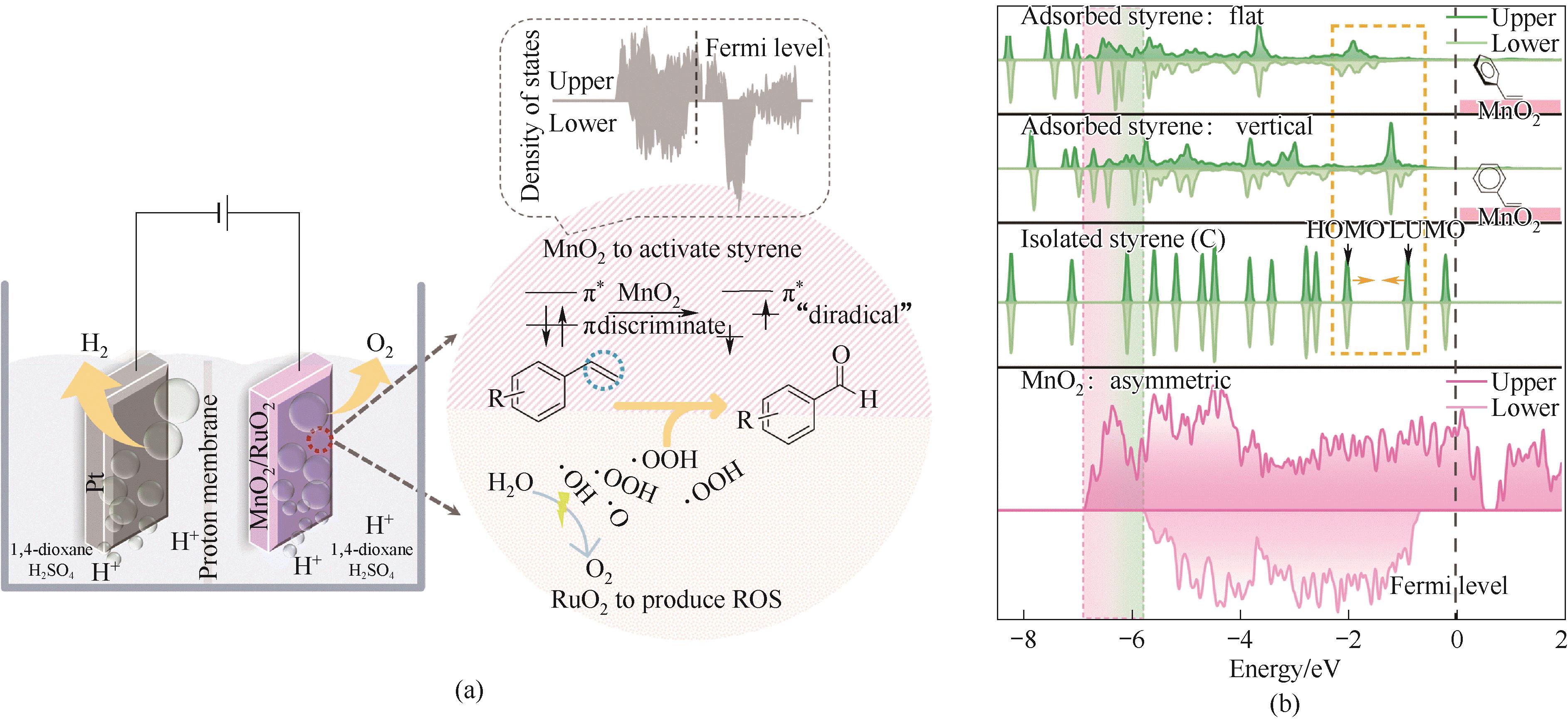
图6 (a)MnO2和RuO2分别用于活化苯乙烯乙烯基和产生ROS示意图;(b)MnO2(131)吸附前后MnO2的总DOS和苯乙烯的部分电子态密度[66]
Fig.6 (a) Schematic diagram of MnO2 and RuO2 used for activating styrene vinyl and generating ROS; (b) The total DOS of MnO2 (131) before and after adsorption, as well as the partial electronic density of states of styrene[66]

图7 (a)加底物50 mmol/L HMF的原位EIS谱图;(b)未加底物50 mmol/L HMF的原位EIS谱图;(c) Vo-Co3O4和Co3O4的电极内氧化电阻(Rp);(d)Vo-Co3O4和Co3O4的电极界面反应电阻(Rct)[70]
Fig.7 (a)In situ EIS spectra with substrate 50 mmol/L HMF added; (b) In situ EIS spectra without 50 mmol/L HMF substrate addition; (c) The electrode internal oxidation resistance (Rp) of Vo-Co3O4 and Co3O4; (d) The electrode interface reaction resistance (Rct) of Vo-Co3O4 and Co3O4
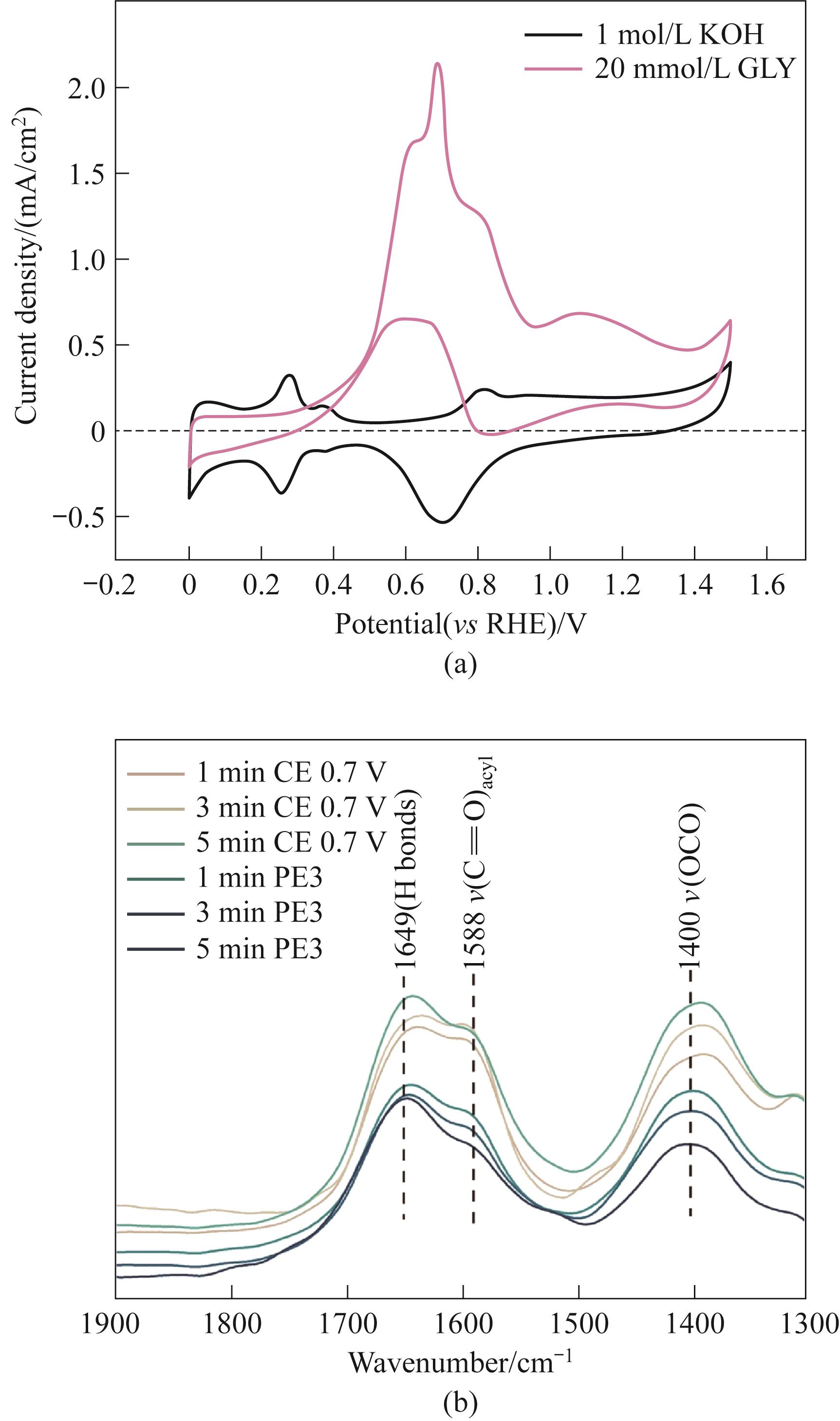
图9 (a)在含有和不含GLY(甘油)的电解液中Pt@G的CV曲线;(b)0.7 V下以不同时间进行PE3(脉冲电解)和CE(恒电位电解)后的Pt@G原位FTIR光谱[74]
Fig.9 (a) CV curves of Pt@G in electrolytes with and without GLY (glycerol); (b) In situ FTIR spectroscopy of Pt@G with PE3 (pulse electrolysis) and CE (constant potential electrolysis) at different time under 0.7 V[74]
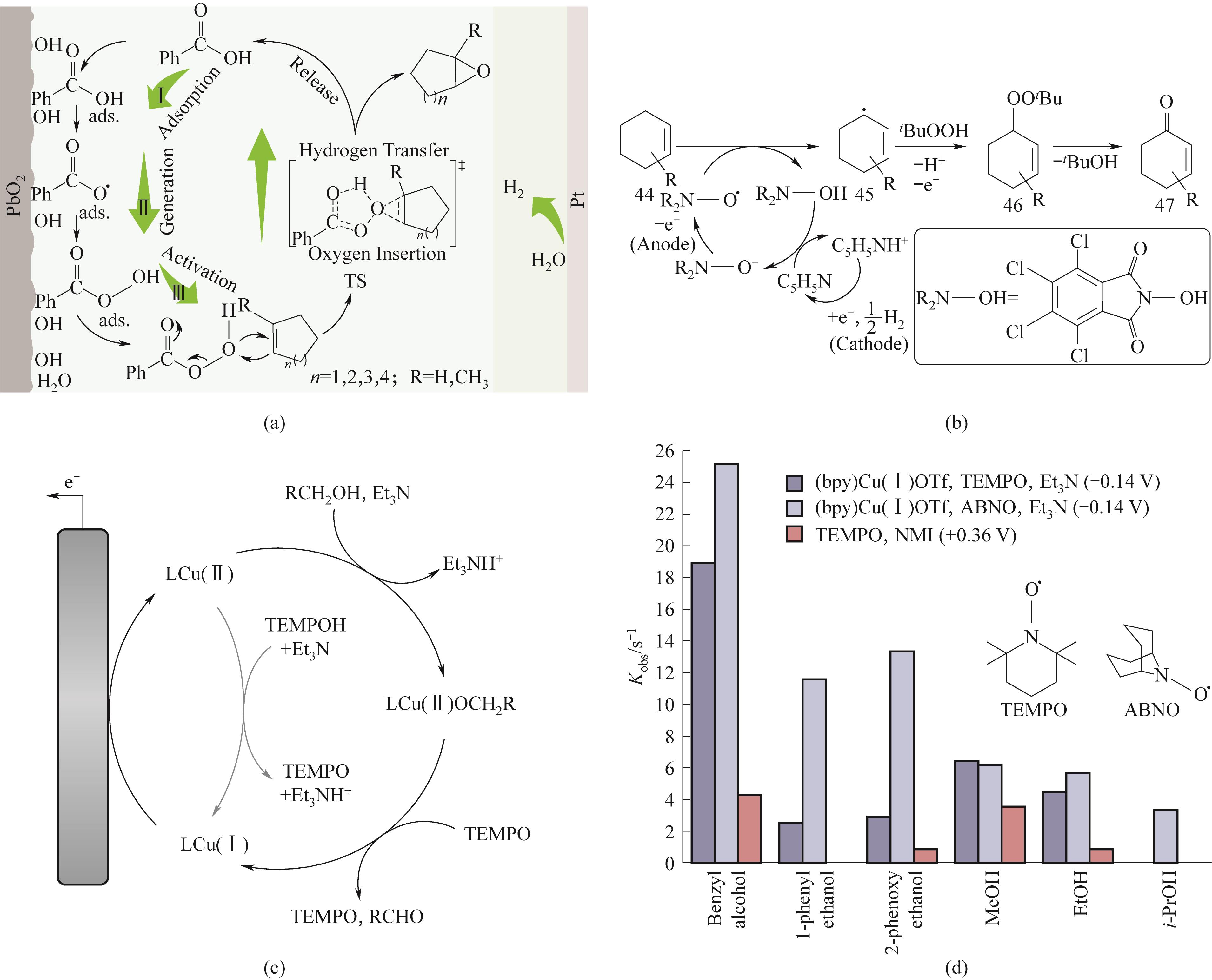
图10 (a)羧酸通过可循环过氧酸介体的方法来实现烯烃可持续环氧化反应的机理示意图;(b)Cl4NHPI作为脱氢剂的反应机理示意图;(c)(bpy)Cu/TEMPO助催化剂系统的反应机理示意图;(d)3种不同催化剂体系与6种不同苄基、脂肪族、伯和仲醇的相对催化活性[80,82-83]
Fig.10 (a)Schematic diagram of the mechanism for achieving sustainable epoxidation of olefins by using carboxylic acid as a recyclable peroxide mediator; (b) Schematic diagram of the reaction mechanism of Cl4NHPI as a dehydrogenation agent; (c) Schematic diagram of the reaction mechanism of (bpy)Cu/TEMPO co-catalyst system; (d) Relative catalytic activity of three different catalyst systems with six different benzyl, aliphatic, primary, and secondary alcohols[80,82-83]
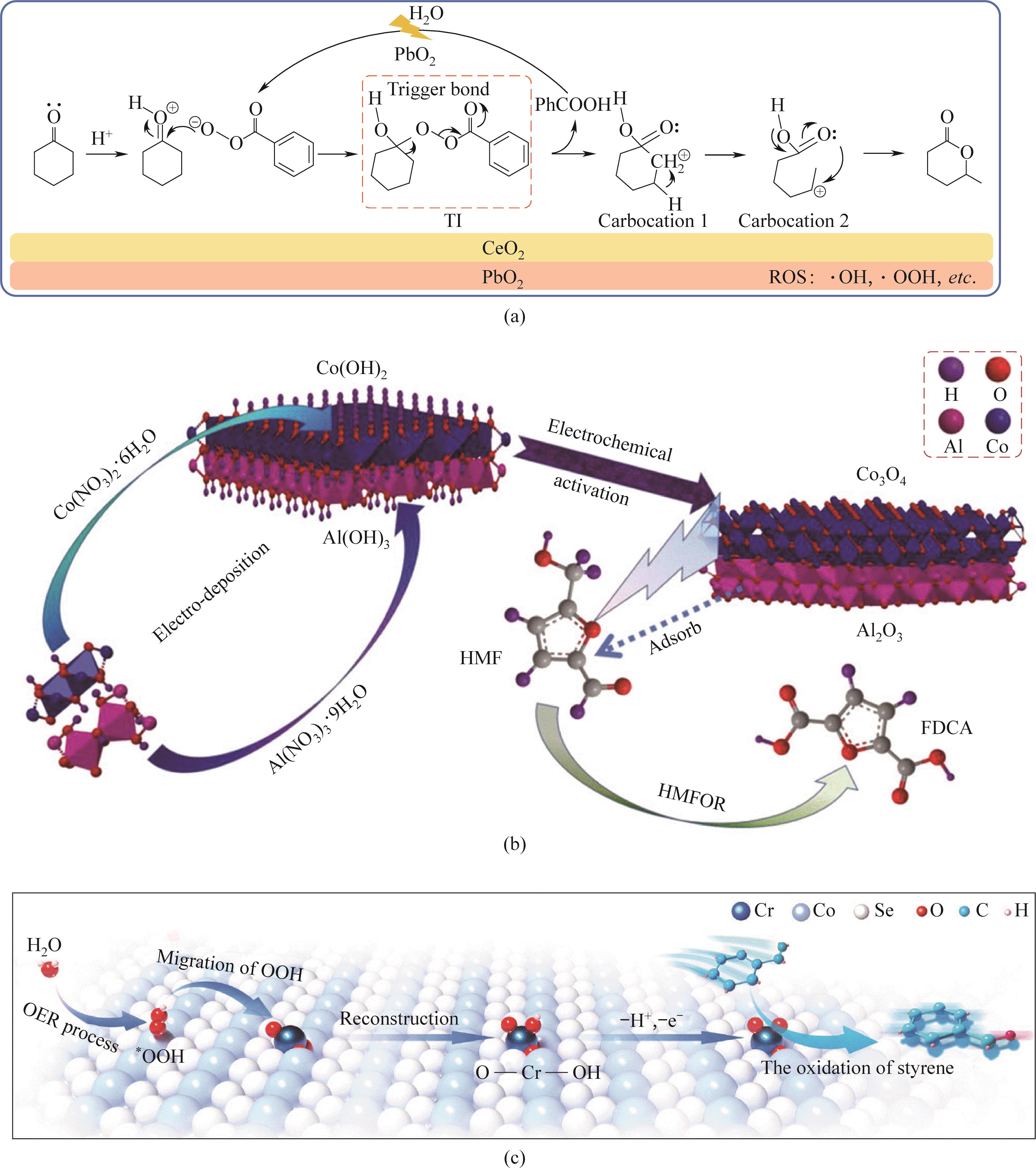
图11 (a)CeO2@PbO2@Ti电极用于Baeyer-Villiger反应的机理示意图;(b)HMFOR的Al(OH)3/Co(OH)2合成及电化学体系示意图;(c)描述串联反应的反应路径的示意图[88-90]
Fig.11 (a)Schematic diagram of the mechanism of Baeyer-Villiger reaction using CeO2@PbO2@Ti electrode; (b) Schematic diagram of Al(OH)3/Co(OH)2 synthesis and electrochemical system of HMFOR; (c) A schematic diagram illustrating the reaction pathway of a series reaction[88-90]
| [1] | Tang W S, Zhang L N, Qiu T Y, et al. Efficient conversion of biomass to formic acid coupled with low energy consumption hydrogen production from water electrolysis[J]. Angewandte Chemie International Edition, 2023, 62(30): e202305843. |
| [2] | Wang J Y, Yang J B, Feng Y, et al. Comparative experimental study of alkaline and proton exchange membrane water electrolysis for green hydrogen production[J]. Applied Energy, 2025, 379: 124936. |
| [3] | Liu M, Yao Z D, Gu J, et al. Issues and opportunities facing hydrolytic hydrogen production materials[J]. Chemical Engineering Journal, 2023, 461: 141918. |
| [4] | Volta A. On the electricity excited by the mere contact of conducting substances of different kinds[J]. Philosophical Transactions of the Royal Society, 1800, 90: 403-431. |
| [5] | Faraday H M. Experimental researches in electricity. First series[J]. Philosophical Transactions of the Royal Society, 1837, 18(121): 125-162. |
| [6] | Faraday M. Siebente reihe von experimental-untersuchungen über elektricität[J]. Annalen der Physik, 1834, 109(31/32/33/34): 481-520. |
| [7] | Kolbe H. Beobachtungen über die oxydirende Wirkung des Sauerstoffs, wenn derselbe mit Hülfe einer elektrischen Säule entwickelt wird[J]. Journal Für Praktische Chemie, 1847, 41(1): 137-139. |
| [8] | Heyrovský J. Electrolysis with a dropping mercury cathode(part Ⅰ): Deposition of alkali and alkaline earth[J]. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science, 1923, 45(266): 303-315. |
| [9] | Baizer M M. Electrolytic reductive coupling[J]. Journal of the Electrochemical Society, 1964, 111(2): 215. |
| [10] | Baizer M M. Recent developments in organic synthesis by electrolysis[J]. Tetrahedron, 1984, 40(6): 935-969. |
| [11] | Simons J H. Production of fluorocarbons(Ⅰ): The generalized procedure and its use with nitrogen compounds[J]. Journal of the Electrochemical Society, 1949, 95: 47-67. |
| [12] | Steckhan E. Organic syntheses with electrochemically regenerable redox systems[C]//Electrochemistry Ⅰ. Berlin, Heidelbery: Springer, 1987: 1-69. |
| [13] | Steckhan E. Indirect electroorganic syntheses: a modern chapter of organic electrochemistry[new synthetic methods(59)][J]. Angewandte Chemie International Edition, 1986, 25(8): 683-701. |
| [14] | Yoshida J I, Murata T, Isoe S. Electrochemical oxidation of organosilicon compounds(Ⅰ): Oxidative cleavage of carbon-silicon bond in allylsilanes and benzylsilanes[J]. Tetrahedron Letters, 1986, 27(29): 3373-3376. |
| [15] | Hammer B, Norskov J K. Why gold is the noblest of all the metals[J]. Nature, 1995, 376(6537): 238-240. |
| [16] | Grabowski G, Lewkowski J, Skowroński R. The electrochemical oxidation of 5-hydroxymethylfurfural with the nickel oxide/hydroxide electrode[J]. Electrochimica Acta, 1991, 36(13): 1995. |
| [17] | Gandeepan P, Finger L H, Meyer T H, et al. 3d metallaelectrocatalysis for resource economical syntheses[J]. Chemical Society Reviews, 2020, 49(13): 4254-4272. |
| [18] | Zhu C J, Ang N W J, Meyer T H, et al. Organic electrochemistry: molecular syntheses with potential[J]. ACS Central Science, 2021, 7(3): 415-431. |
| [19] | Jiao K J, Xing Y K, Yang Q L, et al. Site-selective C—H functionalization via synergistic use of electrochemistry and transition metal catalysis[J]. Accounts of Chemical Research, 2020, 53(2): 300-310. |
| [20] | Yuan Y, Lei A W. Electrochemical oxidative cross-coupling with hydrogen evolution reactions[J]. Accounts of Chemical Research, 2019, 52(12): 3309-3324. |
| [21] | Xia T, Yang J R, Ren Q H, et al. Promoting alcohols electrooxidation coupled with hydrogen production via asymmetric pulse potential strategy[J]. Angewandte Chemie International Edition, 2025, 64(9): e202420992. |
| [22] | 谢文富, 邵明飞, 段雪. 电解水制氢之提效降本[J]. 石油炼制与化工, 2021, 52(10): 18-24. |
| Xie W F, Shao M F, Duan X. Boosting efficiency and reducing cost of hydrogen production from electrochemical water splittinng[J]. Petroleum Processing and Petrochemicals, 2021, 52(10): 18-24. | |
| [23] | 夏天, 栗振华, 邵明飞, 等. 电解水制氢耦合有机物氧化研究进展[J]. 石油炼制与化工, 2024, 55(1): 42-51. |
| Xia T, Li Z H, Shao M F, et al. Research progress in electrocatalytic organic oxidation coupled with hydrogen production[J]. Petroleum Processing and Petrochemicals, 2024, 55(1): 42-51. | |
| [24] | Li J, Duan H H. Recent progress in energy-saving hydrogen production by coupling with value-added anodic reactions[J]. Chem, 2024, 10(10): 3008-3039. |
| [25] | Li J C, Ma Y Q, Mu X G, et al. Recent advances and perspectives on coupled water electrolysis for energy-saving hydrogen production[J]. Advanced Science, 2025, 12(7): e2411964. |
| [26] | Barlocco I, Cipriano L A, Di Liberto G, et al. Does the oxygen evolution reaction follow the classical OH*, O*, OOH* path on single atom catalysts?[J]. Journal of Catalysis, 2023, 417: 351-359. |
| [27] | Lee T H, Lee S A, Park H, et al. Understanding the enhancement of the catalytic properties of goethite by transition metal doping: critical role of O* formation energy relative to OH* and OOH*[J]. ACS Applied Energy Materials, 2020, 3(2): 1634-1643. |
| [28] | Xu X M, Zhang Y M, Chen Y, et al. Revealing *OOH key intermediates and regulating H2O2 photoactivation by surface relaxation of Fenton-like catalysts[J]. Proceedings of the National Academy of Sciences of the United States of America, 2022, 119(36): e2205562119. |
| [29] | Fan L, Wang D, Ma K, et al. Recent advances in hydrogen production from hybrid water electrolysis through alternative oxidation reactions[J]. ChemCatChem, 2024, 16(5): e202301332. |
| [30] | Garlyyev B, Xue S, Fichtner J, et al. Prospects of value-added chemicals and hydrogen via electrolysis[J]. ChemSusChem, 2020, 13(10): 2513-2521. |
| [31] | Deng C, Toe C Y, Li X, et al. Earth-abundant metal-based electrocatalysts promoted anodic reaction in hybrid water electrolysis for efficient hydrogen production: recent progress and perspectives[J]. Advanced Energy Materials, 2022, 12(25): 2201047. |
| [32] | Vadivel N, Murthy A P. Recent developments in membrane-free hybrid water electrolysis for low-cost hydrogen production along with value-added products[J]. Small, 2024, 20(52): e2407845. |
| [33] | Wei Z D, Huang X, Duan H H, et al. Electrochemical synthesis in company with hydrogen production via renewable energy: opportunities and challenges[J]. Chinese Journal of Catalysis, 2024, 58: 1-6. |
| [34] | 向阳, 黄寻, 魏子栋. 电催化有机合成反应的活性和选择性调控研究进展[J]. 化工进展, 2023, 42(8): 4005-4014. |
| Xiang Y, Huang X, Wei Z D. Recent progresses in the activity and selectivity improvement of electrocatalytic organic synthesis[J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4005-4014. | |
| [35] | Ren J T, Chen L, Wang H Y, et al. Water electrolysis for hydrogen production: from hybrid systems to self-powered/catalyzed devices[J]. Energy & Environmental Science, 2024, 17(1): 49-113. |
| [36] | Cheng J, Xiang Y, Huang X, et al. Reducing energy costs during hydrogen production from water electrolysis by coupling small molecule oxidation: from molecular catalysis to industrial exploration[J]. Precision Chemistry, 2024, 2(9): 447-470. |
| [37] | Zhang J F, Shen Y, Wu Z L, et al. Efficient alkaline-free electrooxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid using electrochemically-charged Ni x Co1- x (OH)2 as a redox mediator[J]. Angewandte Chemie International Edition, 2025, 64(13): e202423109. |
| [38] | Wang D, Lu X Y, Xu H Y, et al. Selective synthesis of formyl-2-furancarboxylic acid via enhanced adsorption of 5-hydroxymethylfurfural on composite catalysts[J]. ChemCatChem, 2024, 16(18): e202400449. |
| [39] | Li Y H, Alorku K, Shen C, et al. In-situ redispersion of Ni@C catalyst boosts 5-hydroxymethylfurfural electrooxidation by increasing Ni4+ sites[J]. Applied Catalysis B: Environment and Energy, 2024, 357: 124250. |
| [40] | Pan X, Sun L Z, Zhou Z Y, et al. Positive electronic nickel active site enhances N—H/C—H bonds breaking for electrooxidation of amines to nitriles coupling with hydrogen production[J]. Advanced Energy Materials, 2024, 14(23): 2400374. |
| [41] | Chen L, Yin Z H, Cui J Y, et al. Unlocking lattice oxygen on selenide-derived NiCoOOH for amine electrooxidation and efficient hydrogen production[J]. Journal of the American Chemical Society, 2024, 146(39): 27090-27099. |
| [42] | Sun L Z, Pan X, Xie Y N, et al. Accelerated dynamic reconstruction in metal-organic frameworks with ligand defects for selective electrooxidation of amines to azos coupling with hydrogen production[J]. Angewandte Chemie International Edition, 2024, 63(21): e202402176. |
| [43] | Liu G H, Nie T Q, Wang H J, et al. Size sensitivity of supported palladium species on layered double hydroxides for the electro-oxidation dehydrogenation of hydrazine: from nanoparticles to nanoclusters and single atoms[J]. ACS Catalysis, 2022, 12(17): 10711-10717. |
| [44] | Zhu L B, Huang J, Meng G, et al. Active site recovery and N—N bond breakage during hydrazine oxidation boosting the electrochemical hydrogen production[J]. Nature Communications, 2023, 14(1): 1997. |
| [45] | Zhu Y, Chen Y X, Feng Y F, et al. Constructing Ru—O—TM bridge in NiFe-LDH enables high current hydrazine-assisted H2 production[J]. Advanced Materials, 2024, 36(30): e2401694. |
| [46] | Sun H C, Luo Z E, Chen M P, et al. Manipulating trimetal catalytic activities for efficient urea electrooxidation-coupled hydrogen production at ampere-level current densities[J]. ACS Nano, 2024, 18(52): 35654-35670. |
| [47] | Zhan G M, Hu L F, Li H, et al. Highly selective urea electrooxidation coupled with efficient hydrogen evolution[J]. Nature Communications, 2024, 15(1): 5918. |
| [48] | Xiong H C, Yu P P, Chen K D, et al. Urea synthesis via electrocatalytic oxidative coupling of CO with NH3 on Pt[J]. Nature Catalysis, 2024, 7(7): 785-795. |
| [49] | Yu Y H, Chen Q R, Li J, et al. Progress in the development of heteroatom-doped nickel phosphates for electrocatalytic water splitting[J]. Journal of Colloid and Interface Science, 2022, 607: 1091-1102. |
| [50] | Xiao J H, Duan C P, Song J L, et al. Research progress of single-atomic series catalysts for anodic coupled electrolysis of water and small molecules[J]. Chemical Engineering Journal, 2024, 500: 157208. |
| [51] | Sun L Z, Zhou Z Y, Xie Y N, et al. Surface self-reconstruction of Fe-Ni3S2 electrocatalyst for value-generating nitrile evolution reaction to drive efficient hydrogen production[J]. Advanced Functional Materials, 2023, 33(33): 2301884. |
| [52] | Qin H Y, Ye Y K, Lin G L, et al. Regulating the electrochemical microenvironment of Ni(OH)2 by Cr doping for highly efficient methanol electrooxidation[J]. ACS Catalysis, 2024, 14(21): 16234-16244. |
| [53] | Xiao C Q, Cheng L, Wang Y T, et al. Low full-cell voltage driven high-current-density selective paired formate electrosynthesis[J]. Journal of Materials Chemistry A, 2022, 10(3): 1329-1335. |
| [54] | Fang Y, Dai C F, Liu X Y, et al. Sulfur-doped manganese-cobalt hydroxide with promoted surface reconstruction for glycerol electrooxidation assisted hydrogen production[J]. Nano Energy, 2024, 127: 109754. |
| [55] | Liu Y, Zhang J H, Li Y P, et al. Manipulating dehydrogenation kinetics through dual-doping Co3N electrode enables highly efficient hydrazine oxidation assisting self-powered H2 production[J]. Nature Communications, 2020, 11(1): 1853. |
| [56] | Geng J R, Zhu Z, Ni Y X, et al. Biaxial strained dual-phase palladium-copper bimetal boosts formic acid electrooxidation[J]. Nano Research, 2022, 15(1): 280-284. |
| [57] | Chen Y J, Pei J J, Chen Z, et al. Pt atomic layers with tensile strain and rich defects boost ethanol electrooxidation[J]. Nano Letters, 2022, 22(18): 7563-7571. |
| [58] | Feng C, Lv M Y, Shao J X, et al. Lattice strain engineering of Ni2P enables efficient catalytic hydrazine oxidation-assisted hydrogen production[J]. Advanced Materials, 2023, 35(42): 2305598. |
| [59] | Yang G C, Jiao Y Q, Yan H J, et al. Interfacial engineering of MoO2-FeP heterojunction for highly efficient hydrogen evolution coupled with biomass electrooxidation[J]. Advanced Materials, 2020, 32(17): e2000455. |
| [60] | Wu J, Wang K, Yu T Q, et al. Amorphous-crystalline heterostructure: efficient catalyst for biomass oxidation coupled with hydrogen evolution[J]. Journal of Colloid and Interface Science, 2024, 655: 676-684. |
| [61] | Qi Y B, Zhang Y, Yang L, et al. Insights into the activity of nickel boride/nickel heterostructures for efficient methanol electrooxidation[J]. Nature Communications, 2022, 13(1): 4602. |
| [62] | Li Y, Jiao Y Q, Yan H J, et al. Mo-Ni-based heterojunction with fine-customized d-band centers for hydrogen production coupled with benzylamine electrooxidation in low alkaline medium[J]. Angewandte Chemie International Edition, 2023, 62(39): e202306640. |
| [63] | Lu Y X, Liu T Y, Huang Y C, et al. Integrated catalytic sites for highly efficient electrochemical oxidation of the aldehyde and hydroxyl groups in 5-hydroxymethylfurfural[J]. ACS Catalysis, 2022, 12(7): 4242-4251. |
| [64] | Song Y J, Li Z H, Fan K, et al. Ultrathin layered double hydroxides nanosheets array towards efficient electrooxidation of 5-hydroxymethylfurfural coupled with hydrogen generation[J]. Applied Catalysis B: Environmental, 2021, 299: 120669. |
| [65] | Yang C, Shang S S, Li X Y. Oxygen-vacancy-enriched substrate-less SnO x /La-Sb anode for high-performance electrocatalytic oxidation of antibiotics in wastewater[J]. Journal of Hazardous Materials, 2022, 436: 129212. |
| [66] | Luo X X, Tang X X, Ni J T, et al. Electrochemical oxidation of styrene to benzaldehyde by discrimination of spin-paired π electrons[J]. Chemical Science, 2023, 14(7): 1679-1686. |
| [67] | Zeng Z W, Wu S T, Huang X, et al. Electrochemical oxidation of furfural on NiMoP/NF: boosting current density with enhanced adsorption of oxygenates[J]. Small, 2024, 20(4): e2305462. |
| [68] | Jin K, Maalouf J H, Lazouski N, et al. Epoxidation of cyclooctene using water as the oxygen atom source at manganese oxide electrocatalysts[J]. Journal of the American Chemical Society, 2019, 141(15): 6413-6418. |
| [69] | Gao L F, Liu Z B, Ma J L, et al. NiSe@NiO x core-shell nanowires as a non-precious electrocatalyst for upgrading 5-hydroxymethylfurfural into 2,5-furandicarboxylic acid[J]. Applied Catalysis B: Environmental, 2020, 261: 118235. |
| [70] | Lu Y X, Liu T Y, Dong C L, et al. Tailoring competitive adsorption sites by oxygen-vacancy on cobalt oxides to enhance the electrooxidation of biomass[J]. Advanced Materials, 2022, 34(2): e2107185. |
| [71] | Han S Y, Wang C H, Shi Y M, et al. Membrane-free selective oxidation of thioethers with water over a nickel phosphide nanocube electrode[J]. Cell Reports Physical Science, 2021, 2(6): 100462. |
| [72] | Wu B J, Li J R, Luo X X, et al. A round-trip journey of electrons: electron catalyzed direct fixation of N2 to azos[J]. Chinese Journal of Catalysis, 2025, 68: 386-393. |
| [73] | Xia Z C, Ma C Y, Fan Y, et al. Vacancy optimized coordination on nickel oxide for selective electrocatalytic oxidation of glycerol[J]. ACS Catalysis, 2024, 14(3): 1930-1938. |
| [74] | Chen W, Zhang L, Xu L T, et al. Pulse potential mediated selectivity for the electrocatalytic oxidation of glycerol to glyceric acid[J]. Nature Communications, 2024, 15(1): 2420. |
| [75] | Boudjelel M, Zhong J, Ballerini L, et al. Electrochemical generation of aryl radicals from organoboron reagents enabled by pulsed electrosynthesis[J]. Angewandte Chemie International Edition, 2024, 63(31): e202406203. |
| [76] | Atkins A P, Chaturvedi A K, Tate J A, et al. Pulsed electrolysis: enhancing primary benzylic C(sp3)—H nucleophilic fluorination[J]. Organic Chemistry Frontiers, 2024, 11(3): 802-808. |
| [77] | Zeng L, Yang Q H, Wang J X, et al. Programmed alternating current optimization of Cu-catalyzed C—H bond transformations[J]. Science, 2024, 385(6705): 216-223. |
| [78] | Wan Q Q, Chen K X, Dong X, et al. Elucidating the underlying reactivities of alternating current electrosynthesis by time-resolved mapping of short-lived reactive intermediates[J]. Angewandte Chemie International Edition, 2023, 62(40): e202306460. |
| [79] | Lee B, Naito H, Nagao D M, et al. Alternating-current electrolysis for the production of phenol from benzene[J]. Angewandte Chemie International Edition, 2012, 51(28): 6961-6965. |
| [80] | Luo X X, Wu B J, Li J R, et al. Benzoic acid: electrode-regenerated molecular catalyst to boost cycloolefin epoxidation[J]. Journal of the American Chemical Society, 2023, 145(37): 20665-20671. |
| [81] | Hoque M A, Jiang T X, Poole D L, et al. Manganese-mediated electrochemical oxidation of thioethers to sulfoxides using water as the source of oxygen atoms[J]. Journal of the American Chemical Society, 2024, 146(31): 21960-21967. |
| [82] | Horn E J, Rosen B R, Chen Y, et al. Scalable and sustainable electrochemical allylic C—H oxidation[J]. Nature, 2016, 533(7601): 77-81. |
| [83] | Badalyan A, Stahl S S. Cooperative electrocatalytic alcohol oxidation with electron-proton-transfer mediators[J]. Nature, 2016, 535(7612): 406-410. |
| [84] | Rafiee M, Alherech M, Karlen S D, et al. Electrochemical aminoxyl-mediated oxidation of primary alcohols in lignin to carboxylic acids: polymer modification and depolymerization[J]. Journal of the American Chemical Society, 2019, 141(38): 15266-15276. |
| [85] | McLoughlin E A, Armstrong K C, Waymouth R M. Electrochemically regenerable hydrogen atom acceptors: mediators in electrocatalytic alcohol oxidation reactions[J]. ACS Catalysis, 2020, 10(19): 11654-11662. |
| [86] | Sun Y X, Li X S, Yang M, et al. Highly selective electrocatalytic oxidation of benzyl C—H using water as safe and sustainable oxygen source[J]. Green Chemistry, 2020, 22(21): 7543-7551. |
| [87] | Behera S, Ganguly S, Loha C, et al. Critical role of interface design in acceleration of overall water splitting and hybrid electrolysis process: state of the art and perspectives[J]. Energy & Fuels, 2023, 37(11): 7603-7633. |
| [88] | Luo X X, Wang Y F, Wu B J, et al. A stepwise electrochemical baeyer-villiger oxidation with water as the oxygen source[J]. The Journal of Physical Chemistry Letters, 2024, 15(42): 10435-10441. |
| [89] | Dai H L, Huang Y F, Bai H Y, et al. Adsorption-activation bifunctional center of Al/CO-base catalyst for boosting 5-hydroxymethylfurfural oxidation[J]. Advanced Energy Materials, 2024, 14(43): 2402789. |
| [90] | Dang K, Dong H L, Wang L G, et al. Boosting electrochemical styrene transformation via tandem water oxidation over a single-atom Cr1/CoSe2 catalyst[J]. Advanced Materials, 2022, 34(27): e2200302. |
| [91] | Li Z H, Li X F, Zhou H, et al. Electrocatalytic synthesis of adipic acid coupled with H2 production enhanced by a ligand modification strategy[J]. Nature Communications, 2022, 13(1): 5009. |
| [92] | Li L, Zhang Z Y, Chen H T, et al. Promoting electrocatalytic alcohols oxidation coupled with H2 production via ligand intercalation strategy[J]. Nano Research, 2023, 16(4): 4596-4602. |
| [1] | 周奕彤, 周明熙, 刘若晨, 叶爽, 黄伟光. 光伏与电网协同驱动氢基直接还原铁炼钢的技术经济分析[J]. 化工学报, 2025, 76(8): 4318-4330. |
| [2] | 范夏雨, 孙建辰, 李可莹, 姚馨雅, 商辉. 机器学习驱动液态有机储氢技术的系统优化[J]. 化工学报, 2025, 76(8): 3805-3821. |
| [3] | 杨宁, 李皓男, LIN Xiao, GEORGIADOU Stella, LIN Wen-Feng. 从塑料废弃物到能源催化剂:塑料衍生碳@CoMoO4复合材料在电解水析氢反应中的应用[J]. 化工学报, 2025, 76(8): 4081-4094. |
| [4] | 刘世昌, 李一白, 王靖, 刘永忠. 氢气驱动电化学捕碳系统的模块化设计与优化[J]. 化工学报, 2025, 76(8): 4108-4118. |
| [5] | 刘建海, 王磊, 鲁朝金, 白志山, 张平雨. 耦合电化学与多相流模型的电解槽性能研究[J]. 化工学报, 2025, 76(8): 3885-3893. |
| [6] | 罗佳欣, 袁艳. 压电材料在固态金属二次电池中的研究进展[J]. 化工学报, 2025, 76(8): 3822-3833. |
| [7] | 陈佳祥, 周伟, 张学伟, 王丽杰, 黄玉明, 于洋, 孙苗婷, 李宛静, 袁骏舒, 张宏博, 孟晓晓, 高继慧, 赵广播. 脉冲电压下二维PEMWE模型的制氢特性仿真研究[J]. 化工学报, 2025, 76(7): 3521-3530. |
| [8] | 王珺仪, 夏章讯, 景粉宁, 王素力. 基于重整气的高温聚合物电解质膜燃料电池电化学阻抗谱弛豫时间分布研究[J]. 化工学报, 2025, 76(7): 3509-3520. |
| [9] | 王子恒, 李文怀, 周嵬. 图形电极在固体氧化物燃料电池中的应用[J]. 化工学报, 2025, 76(7): 3153-3171. |
| [10] | 吴天灏, 叶霆威, 林延, 黄振. 生物质化学链气化原位补氢制H2/CO可控合成气[J]. 化工学报, 2025, 76(7): 3498-3508. |
| [11] | 李欣然, 常龙娇, 罗绍华, 李永兵, 杨瑞芬, 侯增磊, 邹杰. Ho掺杂诱导NCM622局域电子重构抑制阳离子混排的改性机制研究[J]. 化工学报, 2025, 76(7): 3733-3741. |
| [12] | 孙国庆, 李海波, 丁志阳, 郭文辉, 徐浩, 赵艳侠. 硅基负极材料的研究进展[J]. 化工学报, 2025, 76(7): 3197-3211. |
| [13] | 高凤凤, 程慧峰, 杨博, 郝晓刚. 电驱动NiFeMn LDH/CNTs/PVDF膜电极选择性提取钨酸根离子[J]. 化工学报, 2025, 76(7): 3350-3360. |
| [14] | 陈培强, 郑群, 姜玉廷, 熊春华, 陈今茂, 王旭东, 黄龙, 阮曼, 徐万里. 电液流量及电流密度对海水激活电池输出特性的影响[J]. 化工学报, 2025, 76(7): 3235-3245. |
| [15] | 吴鹂霄, 燕溪溪, 张素娜, 徐一鸣, 钱佳颖, 乔永民, 王利军. 磷掺杂微晶石墨的制备及其在锂离子电池负极材料中的电化学性能研究[J]. 化工学报, 2025, 76(7): 3615-3625. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号