化工学报 ›› 2025, Vol. 76 ›› Issue (10): 5464-5474.DOI: 10.11949/0438-1157.20250215
收稿日期:2025-03-04
修回日期:2025-04-15
出版日期:2025-10-25
发布日期:2025-11-25
通讯作者:
刘振
作者简介:吴宇辉(1999—),男,硕士研究生,aa18760235839@163.com
Yuhui WU1( ), Jialong ZHANG2, Yuanhe HOU2, Zhen LIU1(
), Jialong ZHANG2, Yuanhe HOU2, Zhen LIU1( )
)
Received:2025-03-04
Revised:2025-04-15
Online:2025-10-25
Published:2025-11-25
Contact:
Zhen LIU
摘要:
丙烯酸甲酯(MA)与甲基丙烯酸甲酯(MMA)虽具有相似的单体结构,但在可逆加成-断裂链转移(RAFT)控制的聚合反应中常表现出显著的行为差异。通过对比4种不同链转移剂(CTA),重点揭示了MA与MMA在RAFT聚合中关于分子量控制及多分散指数差异的内在机制。结合聚合动力学实验与密度泛函理论(DFT)计算,重点分析了中间体自由基的断裂倾向(φ)及CTA与增长自由基间的相互作用强度(控制指数C)。实验结果表明,中间体自由基的解离倾向及其与CTA的作用强度是影响聚合速率和分子量分布的关键因素。当φ趋近于1时(如MA/CTA 2-4体系),分子量与理论值吻合良好;而在MMA体系中,由于甲基对Pn·自由基的稳定作用导致R·解离受阻(φ趋近于0),初期分子量显著偏离理论值,后续通过短链再生逐步降低偏离。本研究为RAFT聚合中分子量预测的异常行为提供了实验与理论解释,对RAFT聚合的精确控制具有指导意义。
中图分类号:
吴宇辉, 张家龙, 侯远赫, 刘振. 中间体自由基断裂对丙烯酸甲酯/甲基丙烯酸甲酯RAFT聚合的影响[J]. 化工学报, 2025, 76(10): 5464-5474.
Yuhui WU, Jialong ZHANG, Yuanhe HOU, Zhen LIU. Study of radical intermediate cleavage on RAFT polymerization of methyl acrylate and methyl methacrylate[J]. CIESC Journal, 2025, 76(10): 5464-5474.
| Entry | Monomer | RAFT agent | Conv①/% | Mn,th② | Mn,GPC③ | Ð③ |
|---|---|---|---|---|---|---|
| 1 | MA | CTA1 | 92 | 8171 | 37800 | 1.76 |
| 2 | MA | CTA2 | 98 | 8811 | 9300 | 1.12 |
| 3 | MA | CTA3 | 40 | 3636 | 3400 | 1.12 |
| 4 | MA | CTA4 | 33 | 3086 | 2400 | 1.11 |
| 5 | MMA | CTA1 | 99 | 10134 | 53100 | 2.36 |
| 6 | MMA | CTA2 | 81 | 8455 | 7600 | 1.32 |
| 7 | MMA | CTA3 | 32 | 3425 | 3000 | 1.25 |
| 8 | MMA | CTA4 | 30 | 3250 | 2500 | 1.20 |
表1 不同CTA控制下PMA/PMMA体系的分子量参数比较
Table 1 Comparison of molecular weight for PMA/PMMA regulated by different CTAs
| Entry | Monomer | RAFT agent | Conv①/% | Mn,th② | Mn,GPC③ | Ð③ |
|---|---|---|---|---|---|---|
| 1 | MA | CTA1 | 92 | 8171 | 37800 | 1.76 |
| 2 | MA | CTA2 | 98 | 8811 | 9300 | 1.12 |
| 3 | MA | CTA3 | 40 | 3636 | 3400 | 1.12 |
| 4 | MA | CTA4 | 33 | 3086 | 2400 | 1.11 |
| 5 | MMA | CTA1 | 99 | 10134 | 53100 | 2.36 |
| 6 | MMA | CTA2 | 81 | 8455 | 7600 | 1.32 |
| 7 | MMA | CTA3 | 32 | 3425 | 3000 | 1.25 |
| 8 | MMA | CTA4 | 30 | 3250 | 2500 | 1.20 |
| Entry | Monomer | RAFT agent | ΔG-α①/(kJ·mol-1) | ΔGβ②/(kJ·mol-1) | ΔGpre-equilibrium③/(kJ·mol-1) | φ④ | C⑤ |
|---|---|---|---|---|---|---|---|
| 1 | MA | CTA 1 | 44.9 | 42.0 | 10.8 | 0.7384 | 9.81×10-4 |
| 2 | MA | CTA 2 | 57.6 | 44.6 | -32.4 | 0.9902 | 6.88×10 |
| 3 | MA | CTA 3 | 80.4 | 62.9 | -25.8 | 0.9980 | 4.46×102 |
| 4 | MA | CTA 4 | 82.4 | 62.6 | -30.1 | 0.9991 | 1.38×103 |
| 5 | MMA | CTA 1 | 31.8 | 50.5 | 40.3 | 0.0013 | 5.08×10-3 |
| 6 | MMA | CTA 2 | 46.4 | 61.0 | 2.9 | 0.0117 | 4.49×10 |
| 7 | MMA | CTA 3 | 58.0 | 73.8 | 0.7 | 0.0058 | 2.64×102 |
| 8 | MMA | CTA 4 | 56.1 | 74.1 | 4.8 | 0.0004 | 7.20×102 |
表2 CTA1~CTA4的MA和MMA预平衡阶段物种的相对Gibbs自由能
Table 2 Relative Gibbs free energies of species in pre-equilibrium stage of MA and MMA with CTA1—CTA4
| Entry | Monomer | RAFT agent | ΔG-α①/(kJ·mol-1) | ΔGβ②/(kJ·mol-1) | ΔGpre-equilibrium③/(kJ·mol-1) | φ④ | C⑤ |
|---|---|---|---|---|---|---|---|
| 1 | MA | CTA 1 | 44.9 | 42.0 | 10.8 | 0.7384 | 9.81×10-4 |
| 2 | MA | CTA 2 | 57.6 | 44.6 | -32.4 | 0.9902 | 6.88×10 |
| 3 | MA | CTA 3 | 80.4 | 62.9 | -25.8 | 0.9980 | 4.46×102 |
| 4 | MA | CTA 4 | 82.4 | 62.6 | -30.1 | 0.9991 | 1.38×103 |
| 5 | MMA | CTA 1 | 31.8 | 50.5 | 40.3 | 0.0013 | 5.08×10-3 |
| 6 | MMA | CTA 2 | 46.4 | 61.0 | 2.9 | 0.0117 | 4.49×10 |
| 7 | MMA | CTA 3 | 58.0 | 73.8 | 0.7 | 0.0058 | 2.64×102 |
| 8 | MMA | CTA 4 | 56.1 | 74.1 | 4.8 | 0.0004 | 7.20×102 |
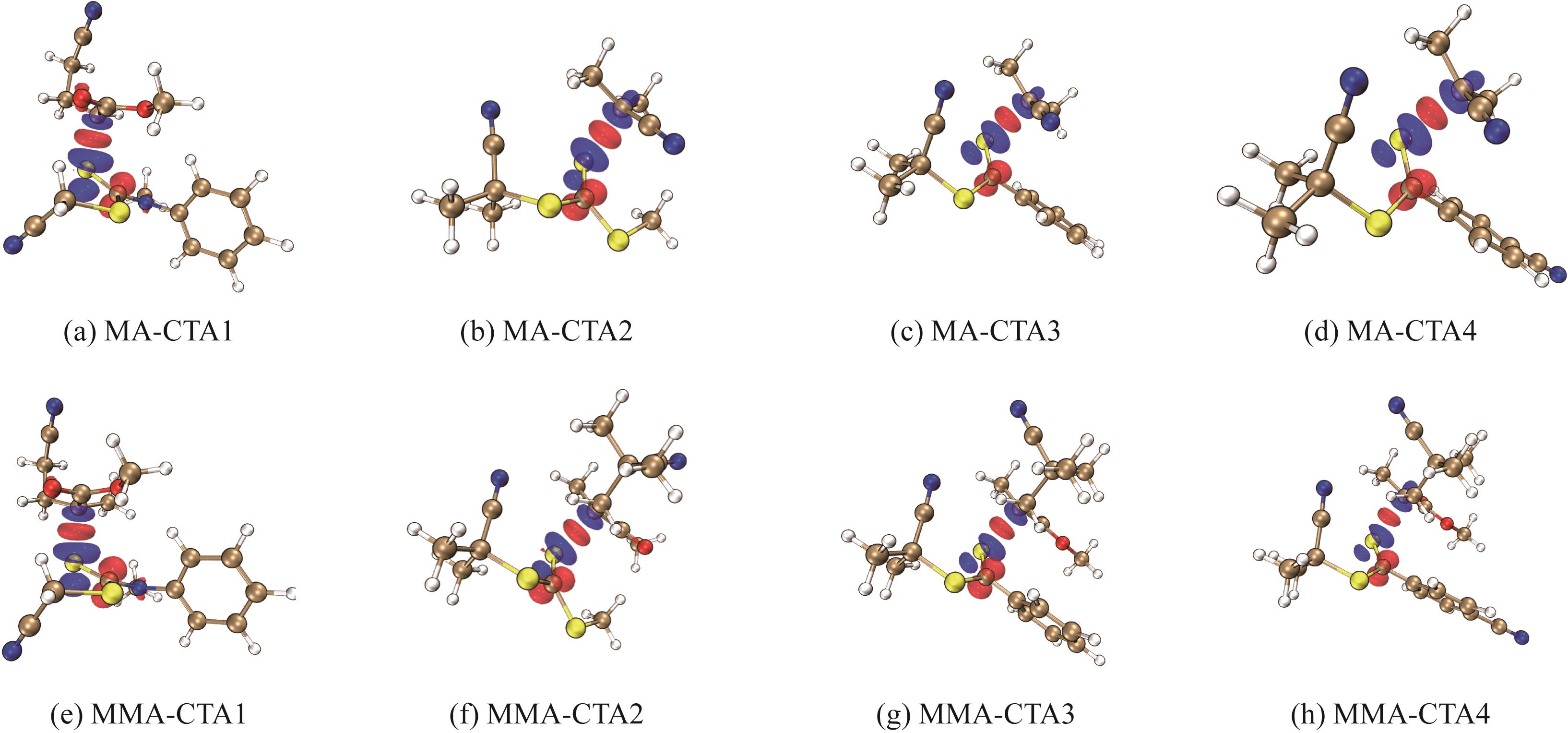
图5 ETS-NOCV分析中主要的轨道相互作用(过渡态结构分为两个片段:CTA和攻击自由基。蓝色区域显示电子密度降低,红色区域显示电子密度增加)
Fig.5 Primary orbital interactions in ETS-NOCV analysis(Transition state structure is divided into two fragments: CTA and attacking radical. Blue areas show decreased electron density while red areas show increased electron density)
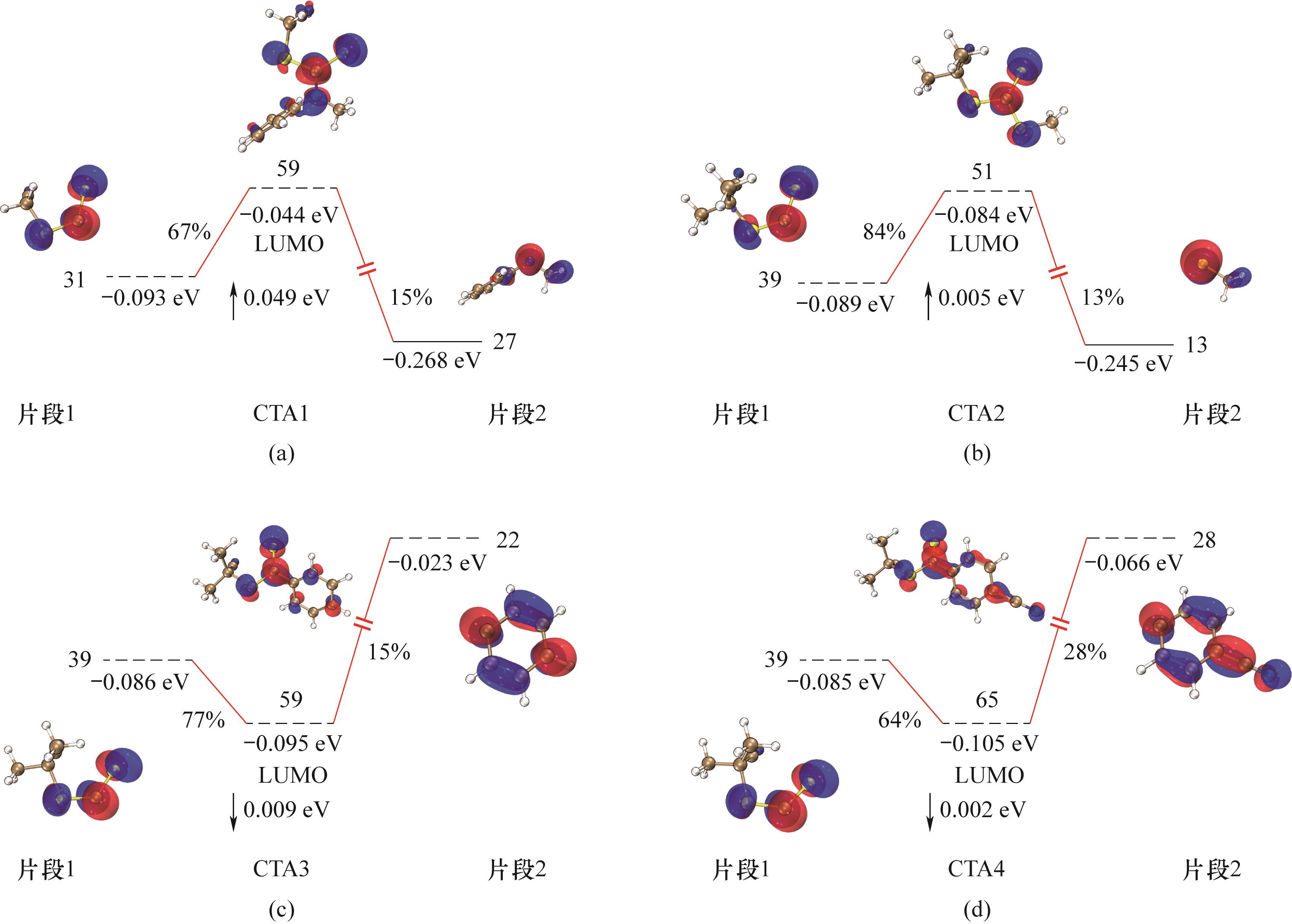
图7 CTA的轨道相互作用(CTA分为两个片段:片段1包括R基团和SC—S结构;片段2为Z基团。蓝色标签表示轨道序号,红色百分比标签表示片段轨道对复合物中相应轨道的贡献。对复合物轨道分量贡献小于10%的片段轨道没有显示。实线代表占据轨道,虚线代表未占据轨道)
Fig.7 Orbital interactions of CTAs(CTA is divided into two fragments: Fragment 1 (R group and SC—S unit) and Fragment 2 (Z group). Blue labels indicate orbital serial numbers, and red percentage labels denote contribution of fragment orbitals to corresponding orbitals in complex. Fragment orbitals contributing less than 10% to orbital components of complex are not displayed. Solid bars represent occupied orbitals, while dashed bars represent unoccupied orbitals)
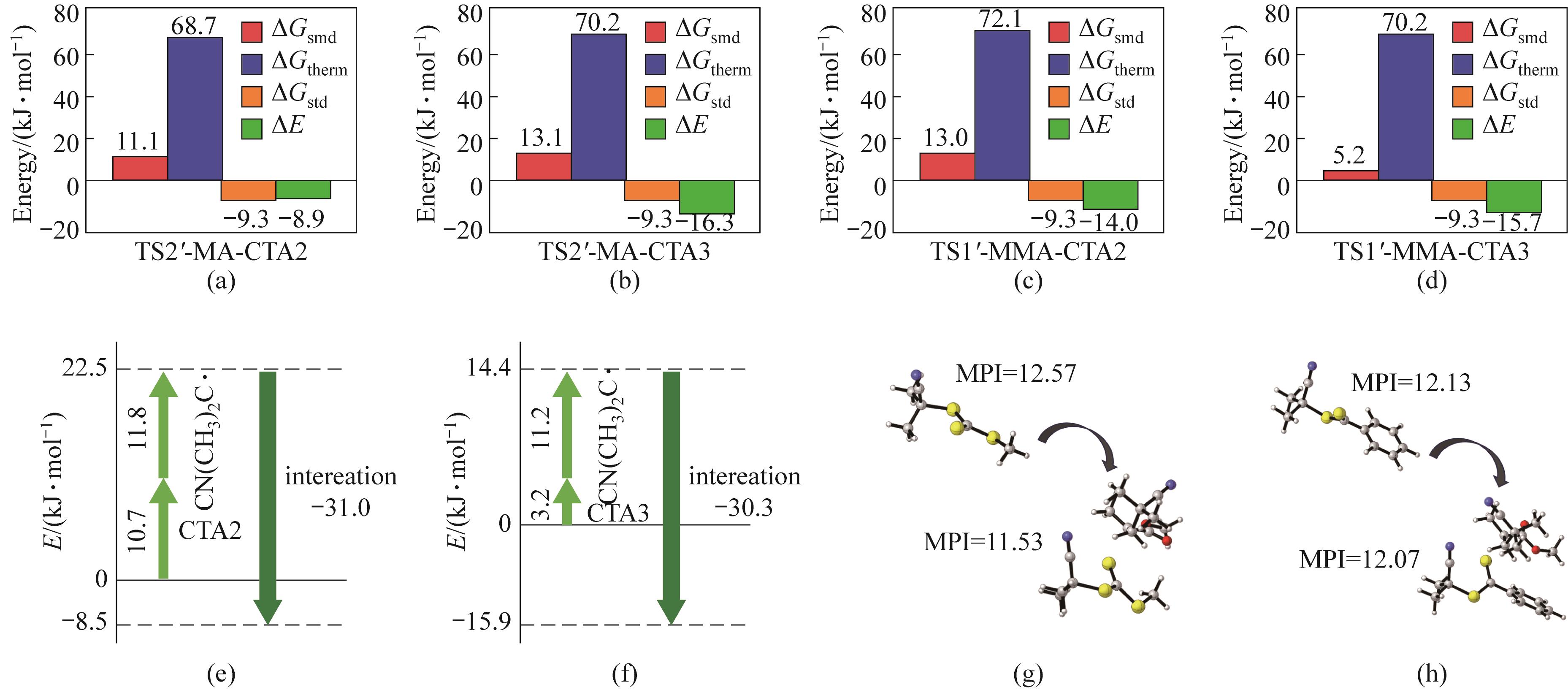
图8 过渡态能量分解:(a)~(d)Gibbs自由能组分分解;(e)、(f)畸变-相互作用能分析;(g)、(h)分子极性指数(MPI)变化
Fig.8 Energy decomposition analysis: (a)—(d) decomposition of relative Gibbs free energy (ΔG) into its contributing components; (e),(f) distortion-interaction analysis for ΔE of TS2′-MA-CTA2 and TS2′-MA-CTA3; (g),(h) MPI variation from CTA to transition state structure
| [1] | Hawker C J, Bosman A W, Harth E. New polymer synthesis by nitroxide mediated living radical polymerizations[J]. Chemical Reviews, 2001, 101(12): 3661-3688. |
| [2] | Matyjaszewski K, Poli R. Comparison of bond dissociation energies of dormant species relevant to degenerative transfer and atom transfer radical polymerization[J]. Macromolecules, 2005, 38(19): 8093-8100. |
| [3] | 罗英武. 复杂聚合物链结构的可控制备与新材料[J]. 化工学报, 2013, 64(2): 415-426. |
| Luo Y W. Controllable preparation of complex polymer chains and novel materials[J]. CIESC Journal, 2013, 64(2): 415-426. | |
| [4] | Destarac M. Industrial development of reversible-deactivation radical polymerization: is the induction period over?[J]. Polymer Chemistry, 2018, 9(40): 4947-4967. |
| [5] | Anastasaki A, Nikolaou V, Nurumbetov G, et al. Cu(0)-mediated living radical polymerization: a versatile tool for materials synthesis[J]. Chemical Reviews, 2016, 116(3): 835-877. |
| [6] | Braunecker W A, Matyjaszewski K. Controlled/living radical polymerization: features, developments, and perspectives[J]. Progress in Polymer Science, 2007, 32(1): 93-146. |
| [7] | Keddie D J, Moad G, Rizzardo E, et al. RAFT agent design and synthesis[J]. Macromolecules, 2012, 45(13): 5321-5342. |
| [8] | Gardiner J, Martinez-Botella I, Kohl T M, et al. 4-halogeno-3,5-dimethyl-1H-pyrazole-1-carbodithioates: versatile reversible addition fragmentation chain transfer agents with broad applicability[J]. Polymer International, 2017, 66(11): 1438-1447. |
| [9] | Chong Y K, Krstina J, Le T P T, et al. Thiocarbonylthio compounds [SC(ph)S-R] in free radical polymerization with reversible addition-fragmentation chain transfer (RAFT polymerization). Role of the free-radical leaving group (R)[J]. Macromolecules, 2003, 36(7): 2256-2272. |
| [10] | Nothling M D, Fu Q, Reyhani A, et al. Progress and perspectives beyond traditional RAFT polymerization[J]. Advanced Science, 2020, 7(20): 2001656. |
| [11] | Kerr A, Moriceau G, Przybyla M A, et al. Bis(trithiocarbonate) disulfides: from chain transfer agent precursors to iniferter control agents in RAFT polymerization[J]. Macromolecules, 2021, 54(14): 6649-6661. |
| [12] | Moad G, Mayadunne R T A, Rizzardo E, et al. Synthesis of novel architectures by radical polymerization with reversible addition fragmentation chain transfer (RAFT polymerization)[J]. Macromolecular Symposia, 2003, 192(1): 1-12. |
| [13] | Zhang W J, Hong C Y, Pan C Y. Efficient fabrication of photosensitive polymeric nano-objects via an ingenious formulation of RAFT dispersion polymerization and their application for drug delivery[J]. Biomacromolecules, 2017, 18(4): 1210-1217. |
| [14] | Harrisson S, Liu X, Ollagnier J N, et al. RAFT polymerization of vinyl esters: synthesis and applications[J]. Polymers, 2014, 6(5): 1437-1488. |
| [15] | Liu J N, Duong H, Whittaker M R, et al. Synthesis of functional core, star polymers via RAFT polymerization for drug delivery applications[J]. Macromolecular Rapid Communications, 2012, 33(9): 760-766. |
| [16] | 郑晋文, 王晓, 安泽胜. RAFT聚合诱导自组装制备不同嵌段序列氧化响应性聚合物囊泡[J]. 高分子学报, 2019, 50(11): 1167-1176. |
| Zheng J W, Wang X, An Z S. Synthesis of oxidation responsive vesicles with different block sequences via RAFT polymerization-induced self-assembly[J]. Acta Polymerica Sinica, 2019, 50(11): 1167-1176. | |
| [17] | 赵小燕, 单国荣. RAFT聚合制备PMPS-b-PNIPAM嵌段共聚物及温敏性纳米粒子[J]. 化工学报, 2019, 70(10): 4080-4088. |
| Zhao X Y, Shan G R. PMPS-b-PNIPAM copolymers synthesized by RAFT polymerization and their thermo-responsive nanoparticles[J]. CIESC Journal, 2019, 70(10): 4080-4088. | |
| [18] | Liu C, Hong C Y, Pan C Y. Polymerization techniques in polymerization-induced self-assembly (PISA)[J]. Polymer Chemistry, 2020, 11(22): 3673-3689. |
| [19] | Sun H, Kabb C P, Sims M B, et al. Architecture-transformable polymers: reshaping the future of stimuli-responsive polymers[J]. Progress in Polymer Science, 2019, 89: 61-75. |
| [20] | Moad G. RAFT polymerization to form stimuli-responsive polymers[J]. Polymer Chemistry, 2017, 8(1): 177-219. |
| [21] | Moad G, Rizzardo E, Thang S H. RAFT polymerization and some of its applications[J]. Chemistry - An Asian Journal, 2013, 8(8): 1634-1644. |
| [22] | York A W, Kirkland S E, McCormick C L. Advances in the synthesis of amphiphilic block copolymers via RAFT polymerization: stimuli-responsive drug and gene delivery[J]. Advanced Drug Delivery Reviews, 2008, 60(9): 1018-1036. |
| [23] | Penfold N J W, Yeow J, Boyer C, et al. Emerging trends in polymerization-induced self-assembly[J]. ACS Macro Letters, 2019, 8(8): 1029-1054. |
| [24] | Patton D L, Advincula R C. A versatile synthetic route to macromonomers via RAFT polymerization[J]. Macromolecules, 2006, 39(25): 8674-8683. |
| [25] | Nwoko T, Nguyen K, Saha N K, et al. Rate retardation trends in RAFT — an emerging monomer classification tool?[J]. Polymer Chemistry, 2024, 15(11): 1052-1061. |
| [26] | Bereś M A, Zhang B, Junkers T, et al. Kinetic investigation of photoiniferter-RAFT polymerization in continuous flow using inline NMR analysis[J]. Polymer Chemistry, 2024, 15(31): 3166-3175. |
| [27] | Zhang Z B, Zhu X L, Zhu J, et al. Thermal polymerization of methyl (meth)acrylate via reversible addition-fragmentation chain transfer (RAFT) process[J]. Polymer, 2006, 47(20): 6970-6977. |
| [28] | Boner S, Parkatzidis K, de Alwis Watuthanthrige N, et al. RAFT polymerization of renewable monomers with dithiobenzoates: effect of Z-group substituents and reaction conditions[J]. European Polymer Journal, 2024, 205: 112721. |
| [29] | Bradford K G E, Petit L M, Whitfield R, et al. Ubiquitous nature of rate retardation in reversible addition-fragmentation chain transfer polymerization[J]. Journal of the American Chemical Society, 2021, 143(42): 17769-17777. |
| [30] | Perrier S. 50th anniversary perspective: RAFT polymerization — a user guide[J]. Macromolecules, 2017, 50(19): 7433-7447. |
| [31] | Corrigan N, Jung K, Moad G, et al. Reversible-deactivation radical polymerization (controlled/living radical polymerization): from discovery to materials design and applications[J]. Progress in Polymer Science, 2020, 111: 101311. |
| [32] | Matioszek D, Mazières S, Brusylovets O, et al. Experimental and theoretical comparison of addition-fragmentation pathways of diseleno- and dithiocarbamate RAFT agents[J]. Macromolecules, 2019, 52(9): 3376-3386. |
| [33] | Moad C L, Moad G, Rizzardo E, et al. Chain transfer activity of ω-unsaturated methyl methacrylate oligomers[J]. Macromolecules, 1996, 29(24): 7717-7726. |
| [34] | Derboven P, van Steenberge P H M, Reyniers M F, et al. Chain transfer in degenerative RAFT polymerization revisited: a comparative study of literature methods[J]. Macromolecular Theory and Simulations, 2016, 25(2): 104-115. |
| [35] | Moad G, Moad C L. Use of chain length distributions in determining chain transfer constants and termination mechanisms[J]. Macromolecules, 1996, 29(24): 7727-7733. |
| [36] | Frisch M J, Trucks G W, Schlegel H B, et al. Gaussian 09, Revision D.01 [CP]. Wallingford: Gaussian Inc., CT, 2009. |
| [37] | Becke A D. Density-functional thermochemistry(Ⅲ): The role of exact exchange[J]. The Journal of Chemical Physics, 1993, 98(7): 5648-5652. |
| [38] | Lee C, Yang W, Parr R G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density[J]. Physical Review B, 1988, 37(2): 785-789. |
| [39] | Grimme S, Antony J, Ehrlich S, et al. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu[J]. The Journal of Chemical Physics, 2010, 132(15): 154104. |
| [40] | Hehre W J, Ditchfield R, Pople J A. Self-consistent molecular orbital methods(Ⅻ): Further extensions of Gaussian: type basis sets for use in molecular orbital studies of organic molecules[J]. The Journal of Chemical Physics, 1972, 56(5): 2257-2261. |
| [41] | Marenich A V, Cramer C J, Truhlar D G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions[J]. The Journal of Physical Chemistry B, 2009, 113(18): 6378-6396. |
| [42] | Zhao Y, Truhlar D G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals[J]. Theoretical Chemistry Accounts, 2008, 120(1): 215-241. |
| [43] | Pracht P, Bohle F, Grimme S. Automated exploration of the low-energy chemical space with fast quantum chemical methods[J]. Physical Chemistry Chemical Physics, 2020, 22(14): 7169-7192. |
| [44] | Lu T, Chen F W. Multiwfn: a multifunctional wavefunction analyzer[J]. Journal of Computational Chemistry, 2012, 33(5): 580-592. |
| [45] | Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics[J]. Journal of Molecular Graphics, 1996, 14(1): 33-38. |
| [46] | Mitoraj M P, Michalak A, Ziegler T. A combined charge and energy decomposition scheme for bond analysis[J]. Journal of Chemical Theory and Computation, 2009, 5(4): 962-975. |
| [47] | Xiao M, Lu T. Generalized charge decomposition analysis (GCDA) method[J]. Journal of Advances in Physical Chemistry, 2015, 4(4): 111-124. |
| [48] | Bickelhaupt F M, Houk K N. Das distortion/interaction-activation-strain-modell zur analyse von reaktionsgeschwindigkeiten[J]. Angewandte Chemie, 2017, 129(34): 10204-10221. |
| [49] | Shao Y H, Gan Z T, Epifanovsky E, et al. Advances in molecular quantum chemistry contained in the Q-Chem 4 program package[J]. Molecular Physics, 2015, 113(2): 184-215. |
| [50] | Horn P R, Mao Y Z, Head-Gordon M. Probing non-covalent interactions with a second generation energy decomposition analysis using absolutely localized molecular orbitals[J]. Physical Chemistry Chemical Physics, 2016, 18(33): 23067-23079. |
| [51] | Liu Z Y, Lu T, Chen Q X. Intermolecular interaction characteristics of the all-carboatomic ring, cyclo[18] carbon: focusing on molecular adsorption and stacking[J]. Carbon, 2021, 171: 514-523. |
| [1] | 张圣美, 李明, 张莹, 易茜, 杨依婷, 刘雅莉. 乳化剂和温度对相变微胶囊性能的影响分析[J]. 化工学报, 2025, 76(S1): 444-452. |
| [2] | 娄岚浩, 杨立鹏, 杨晓光. 锂离子电池电化学机理模型参数辨识研究综述[J]. 化工学报, 2025, 76(9): 4369-4382. |
| [3] | 王梦娇, 胡凯学, 孟祥铠, 江锦波, 彭旭东. 碳化硅表面微织构尺寸和面密度对滑动密封面摩擦学性能的影响[J]. 化工学报, 2025, 76(8): 4165-4176. |
| [4] | 王子恒, 李文怀, 周嵬. 图形电极在固体氧化物燃料电池中的应用[J]. 化工学报, 2025, 76(7): 3153-3171. |
| [5] | 孙睿, 王军锋, 许浩洁, 李步发, 徐雅弦. 喷雾冷却技术及其强化传热机制研究进展[J]. 化工学报, 2025, 76(4): 1404-1421. |
| [6] | 高越, 李丁, 高玉苗. 有机污染场地土壤催化氧化修复技术研究[J]. 化工学报, 2025, 76(3): 1297-1304. |
| [7] | 徐芳, 张锐, 崔达, 王擎. ReaxFF-MD揭示木质素热解反应机制的分子动力学研究[J]. 化工学报, 2025, 76(3): 1253-1263. |
| [8] | 徐桂培, 孙倩, 赖洁文, 卢毅锋, 邸会芳, 黄辉, 王振兵. 电化学双电层电容器失效机理的研究进展[J]. 化工学报, 2025, 76(3): 951-962. |
| [9] | 吴雨轩, 常诚, 顾雪萍, 冯连芳, 张才亮. 面向立体异构的丁二烯乳液聚合过程模型化[J]. 化工学报, 2025, 76(2): 879-887. |
| [10] | 张奇, 张睿, 郑涛, 曹欣, 刘植昌, 刘海燕, 徐春明, 张荣, 孟祥海. 基于分子模拟的新型双阳离子质子型离子液体捕集CO2研究[J]. 化工学报, 2025, 76(2): 797-811. |
| [11] | 马学忠, 谢庆祥. 高速接触式机械密封动环外侧面人字槽强化换热机理与冷却性能研究[J]. 化工学报, 2025, 76(10): 5277-5289. |
| [12] | 王燕子, 代佳楠, 马晶, 张腾月, 梁子莉. 稀土元素(RE: Nd、Sm、Eu、Er、Tm)修饰B-TiO2氧空位特性及其催化性能研究[J]. 化工学报, 2025, 76(10): 5162-5175. |
| [13] | 史晓茜, 穆瑞花, 武峥. γ-Al2O3纳米颗粒摩擦催化降解有机污染物性能研究[J]. 化工学报, 2025, 76(10): 5453-5463. |
| [14] | 王元哲, 刘振宇, 闫玉新, 王丝语, 石磊, 刘清雅. 煤直接液化技术换代的化学反应问题[J]. 化工学报, 2025, 76(10): 5522-5532. |
| [15] | 董纪广, 谢绍雷, 时东, 李丽娟, 赵晨宇, 黄雨婕, 石成龙, 许淘善, 曹大伟. 邻羟基苯甲酸正辛酯萃取体系提锂:协萃剂结构变化对萃取性能影响[J]. 化工学报, 2025, 76(10): 5190-5202. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号