• •
李佳润1( ), 骆勇名1,2, 韦思辰1, 赵亚娟1(
), 骆勇名1,2, 韦思辰1, 赵亚娟1( ), 何盈盈1(
), 何盈盈1( )
)
收稿日期:2025-10-09
修回日期:2025-11-12
出版日期:2025-12-03
通讯作者:
赵亚娟,何盈盈
作者简介:李佳润(2001—),女,硕士研究生,2606884041@qq.com
基金资助:
Jiarun LI1( ), Yongming LUO1,2, Sichen WEI1, Yajuan ZHAO1(
), Yongming LUO1,2, Sichen WEI1, Yajuan ZHAO1( ), Yingying HE1(
), Yingying HE1( )
)
Received:2025-10-09
Revised:2025-11-12
Online:2025-12-03
Contact:
Yajuan ZHAO, Yingying HE
摘要:
电解水是有潜力的规模化制备氢技术之一,而开发高活性低成本的氧析出反应(OER)和整体水分解催化剂是关键。本研究通过K2FeO4分解与Fe3+刻蚀泡沫镍(NF)制备了具有丰富异质界面的NiFe-LDH/Fe2O3@NF前驱体,经磷化后得到珊瑚状的双金属Ni2P/Fe2P/Fe2O3@NF复合材料。表征结果证实其结构中有丰富的异质界面和微米级通道。OER催化机制研究显示其为耦合的吸附演化机制(AEM)和晶格氧机制(LOM)。DFT计算表明磷化处理优化了活性氧中间体的吸附能,降低了决速步骤的能垒,并使Ni 3d轨道和Fe 3d轨道的态密度重叠程度增强,促进了双金属位点Ni2+®Fe3+之间的电子转移和Ni催化中心的形成,从而提升了材料的OER性能和稳定性。该材料在10和50 mA·cm-2电流密度下的OER过电位仅分别为203和230 mV,且在10和500 mA·cm-2的电流密度下均显示良好的稳定性。该材料同时展现出优异的氢析出反应(HER)活性,构建的全水解系统在1.58 V槽电压下即可驱动10 mA·cm-2电流密度,展现出良好的双催化性能和稳定性。
中图分类号:
李佳润, 骆勇名, 韦思辰, 赵亚娟, 何盈盈. 双金属Ni2P/Fe2P/Fe2O3@NF异质界面材料以耦合机制催化析氧反应并促进整体水分解[J]. 化工学报, DOI: 10.11949/0438-1157.20251098.
Jiarun LI, Yongming LUO, Sichen WEI, Yajuan ZHAO, Yingying HE. Bimetallic Ni2P/Fe2P/Fe2O3@NF heterostructure catalyzes oxygen evolution reaction through coupled mechanism and promotes overall water splitting[J]. CIESC Journal, DOI: 10.11949/0438-1157.20251098.
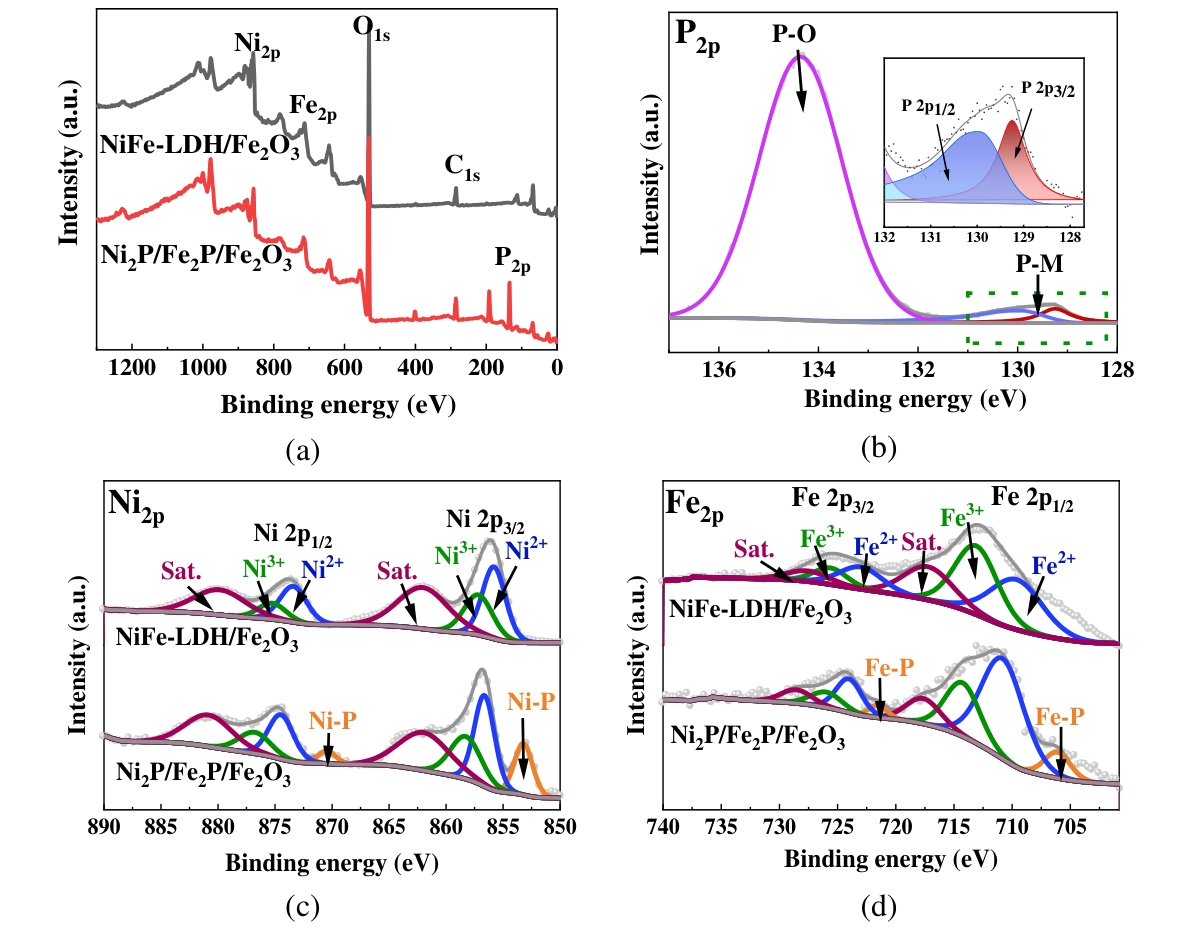
图5 Ni2P/Fe2P/Fe2O3@NF的XPS谱图 (a)总谱;(b)P 2p;(c)Ni 2p;(d)Fe 2p
Fig 5 XPS spectra of Ni2P/Fe2P/Fe2O3@NF (a) Overall spectrum; (b) P 2p; (c) Ni 2P; (d) Fe 2p
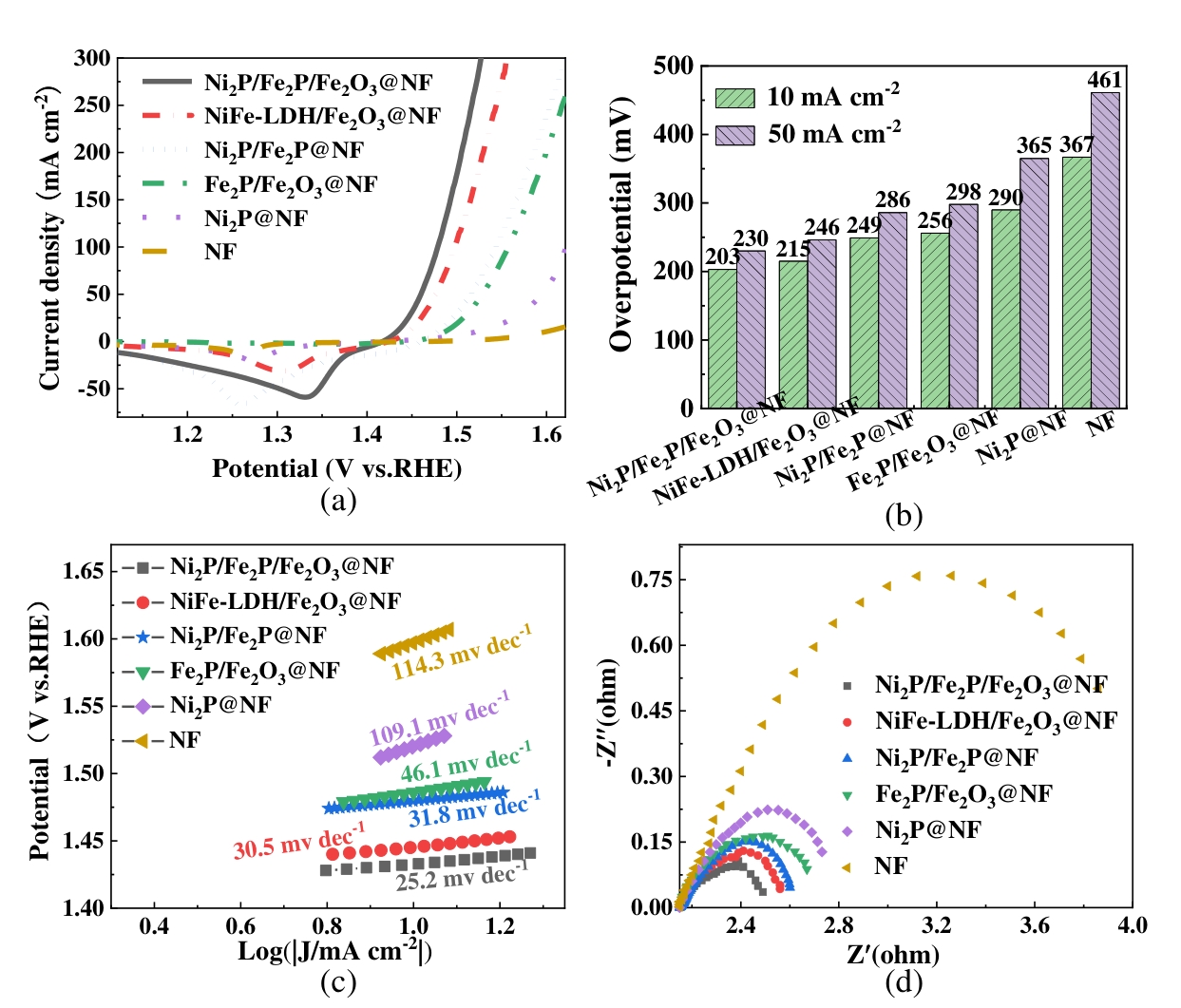
图6 材料的电化学性能测试结果(a)LSV曲线;(b)过电位比较;(c)Tafel曲线;(d)EIS图
Fig.6 The electrochemical performance of the catalysts (a) LSV curves; (b) Comparison of overpotentials; (c) The Tafel curves; (d) Nyquist diagrams of EIS
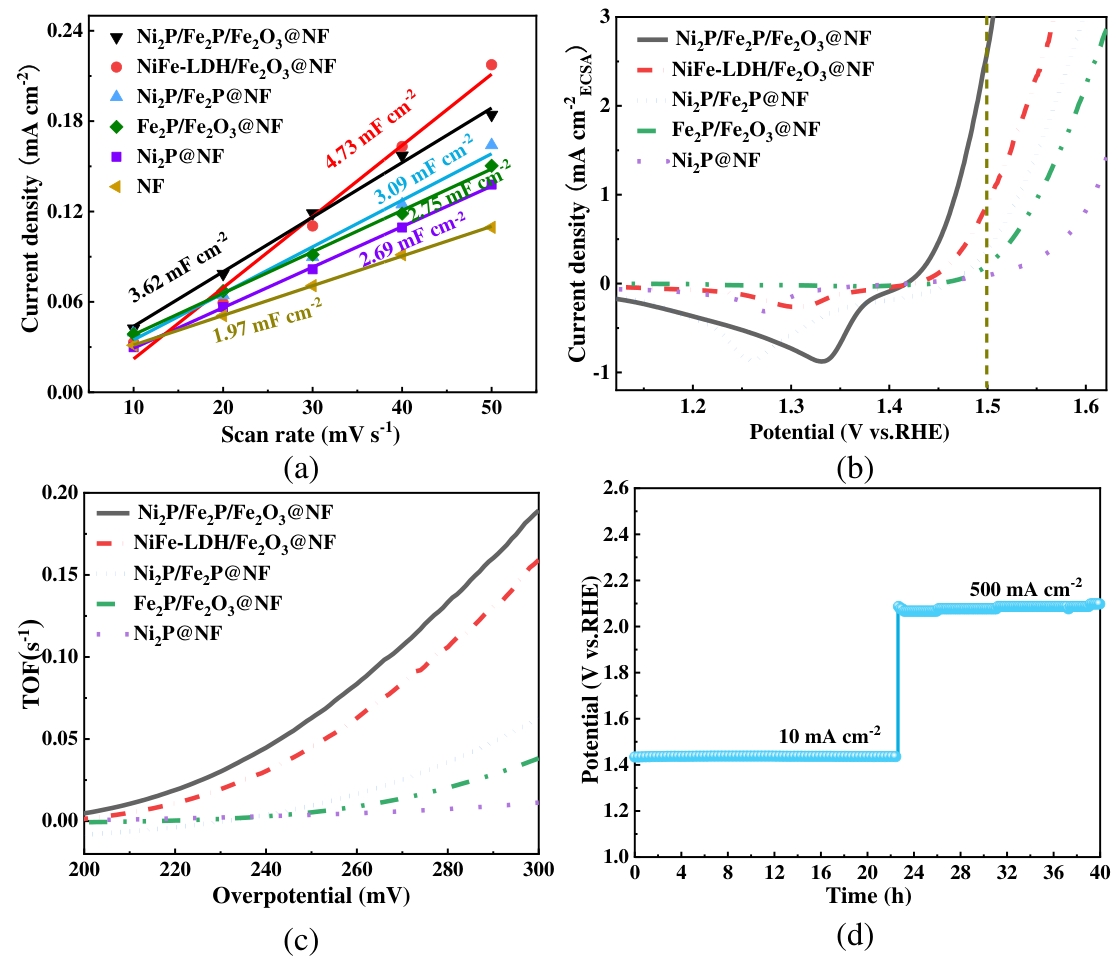
图7(a) 0.86 V电压时的电流密度与扫速的线性关系及相应的Cdl值;(b)ECSA归一化处理的LSV曲线;(c)转换频率;(d)Ni2P/Fe2P/Fe2O3@NF的稳定性测试
Fig.7 (a) The linear relationships between current density and scan rate at 0.86 V voltage and the corresponding Cdl values; (b) The LSV curves normalized by ECSA; (c) TOF curves; (d)Stability test of Ni2P/Fe2P/Fe2O3@NF
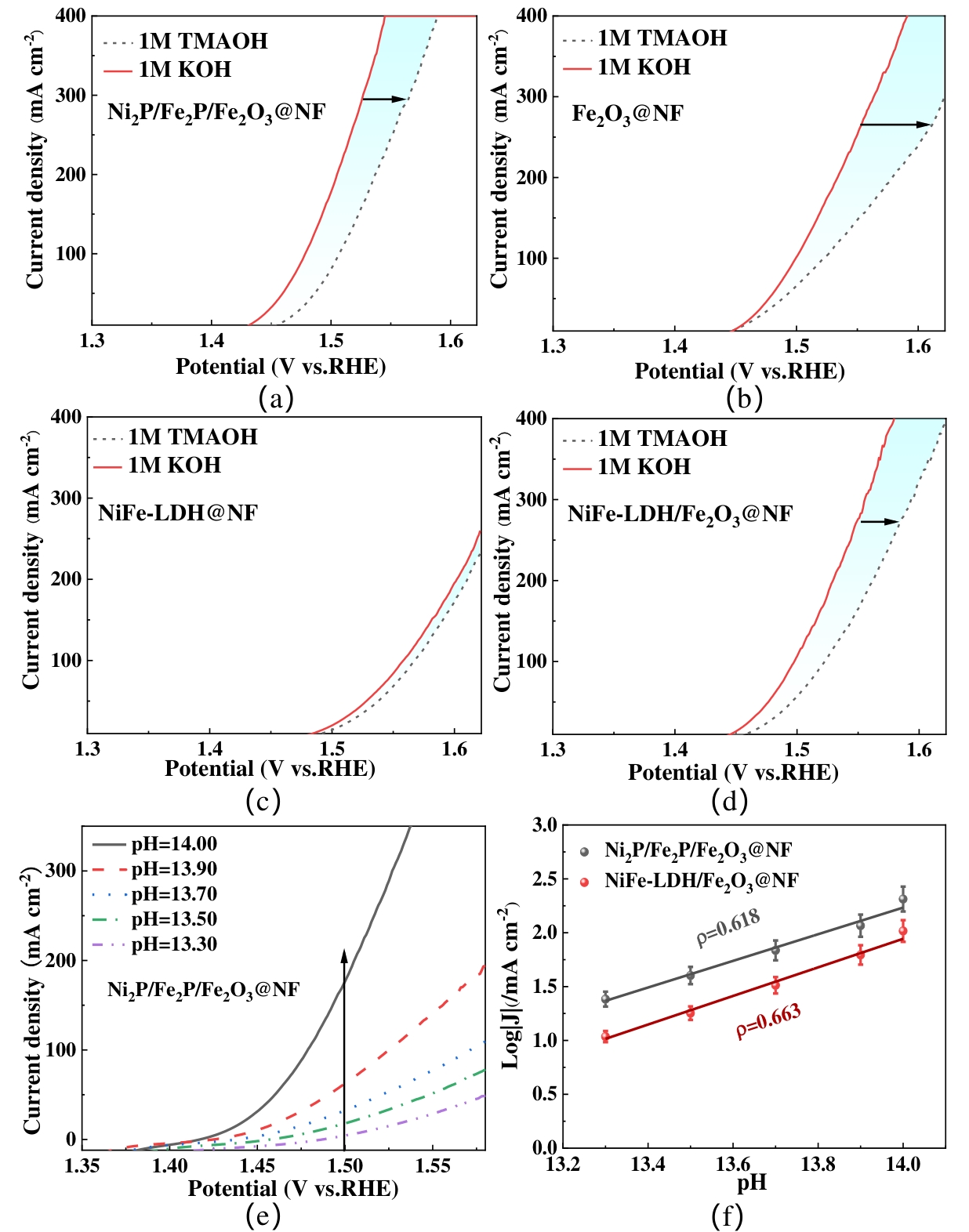
图8 (a~d) 1.0 M KOH和1.0 M TMAOH中的LSV曲线;(e)电解质溶液的pH对LSV曲线的影响;(f)1.5 V电位下的质子反应阶数ρ
Fig.8 (a~d) LSV curves in 1.0 M KOH and 1.0 M TMAOH; (e) Effect of pH values of the electrolyte on LSV curves; (f) Protonic reaction order ρ at 1.5 V

图10 Ni2P/Fe2P/Fe2O3@NF材料测试前后 XPS谱图(a)总谱;(b)P 2p谱;(c)Ni 2p;(d)Fe 2p
Fig.10 XPS spectra of Ni2P/Fe2P/Fe2O3@NF before and after test (a) Overall spectrum; (b) P 2p; (c) Ni 2p; (d) Fe 2p
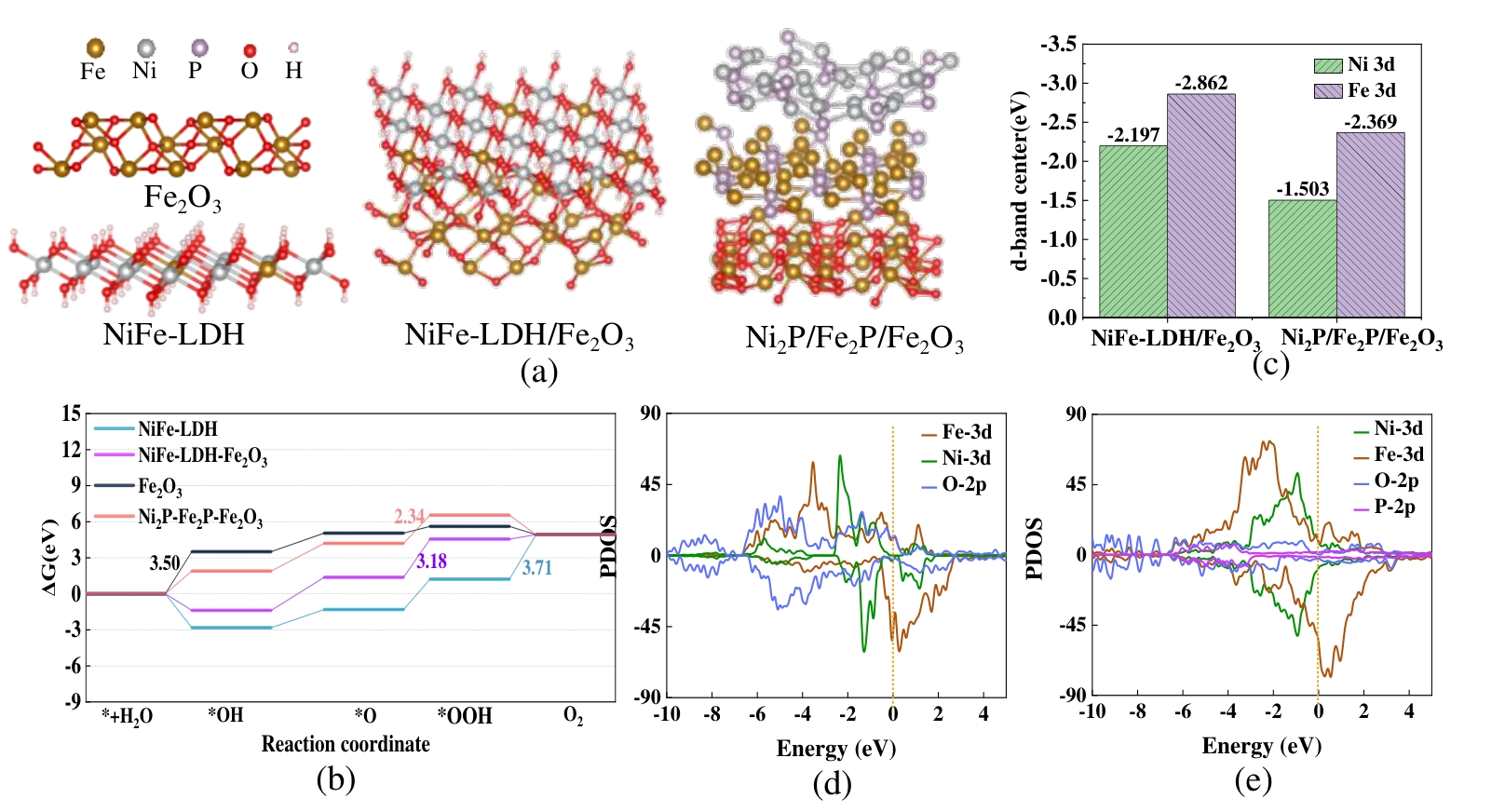
图11 DFT计算 (a) 四种材料的模拟模型;(b) OER过程的自由能台阶图;(c) Ni、Fe的d带中心;模拟态密度PDOS:(d) NiFe-LDH/Fe2O3,(c) Ni2P/Fe2P/Fe2O3
Figure 11 DFT calculations: (a) Simulation models of four catalysts; (b) Free energy diagram for the OER process; (c) The d-band centers of Ni and Fe; Simulated projected density of states(PDOS): (d) NiFe-LDH/Fe2O3; (e) Ni2P/Fe2P/Fe2O3
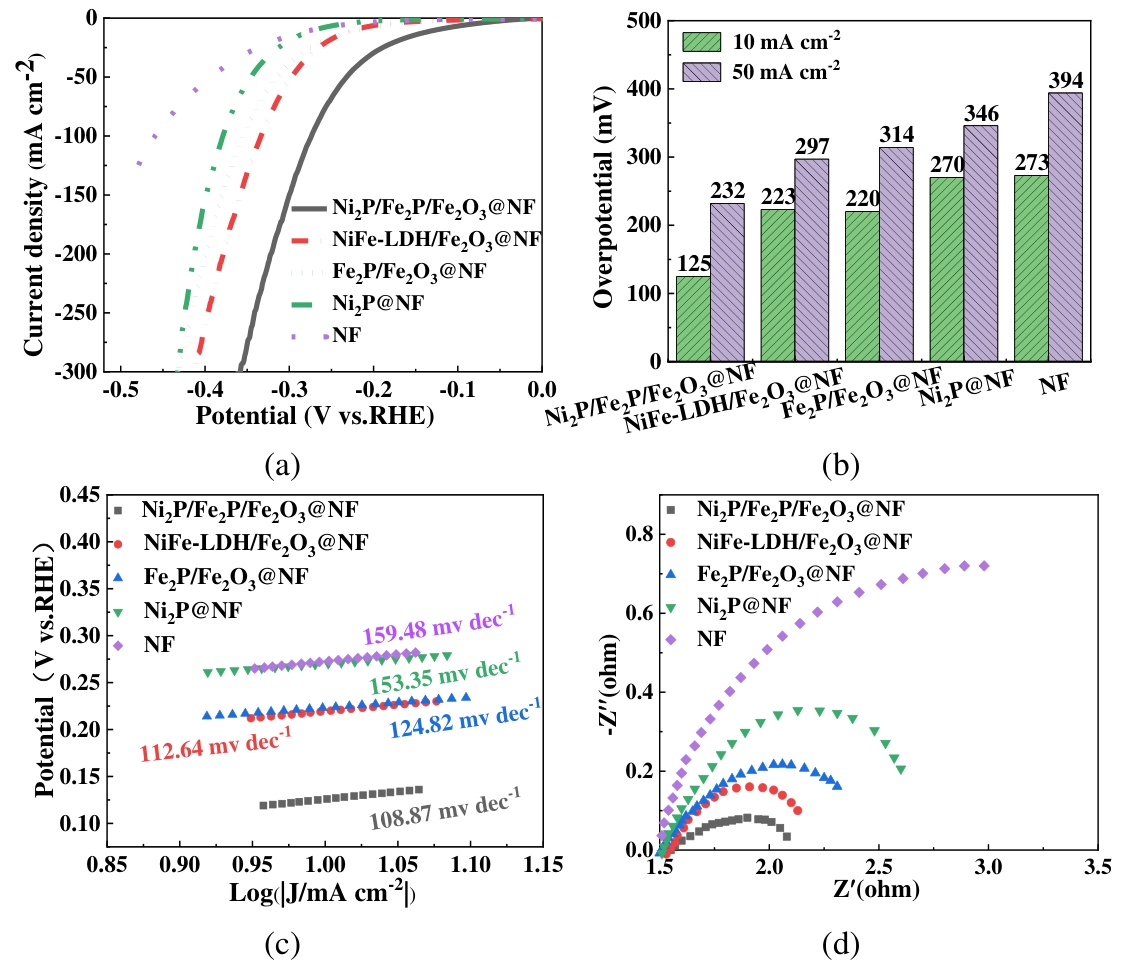
图12 HER催化性能的电化学测试注:(a)LSV曲线;(b)过电位对比;(c)Tafel斜率;(d)奈奎斯特图
Fig.12 Electrochemical test for HER performance(a) LSV curves; (b) Comparison of overpotentials; (c) Tafel slopes; (d) Nyquist diagram

图13 DFT计算(a)HER过程中NiFe-LDH/Fe2O3和Ni2P/Fe2P/Fe2O3的各阶段模型;(b)HER过程的自由能台阶图
Figure 13 DFT calculations: (a) Simulation models of NiFe-LDH/Fe2O3 and Ni2P/Fe2P/Fe2O3 at each stepduring HER process; (b) Free energy diagram for HER process
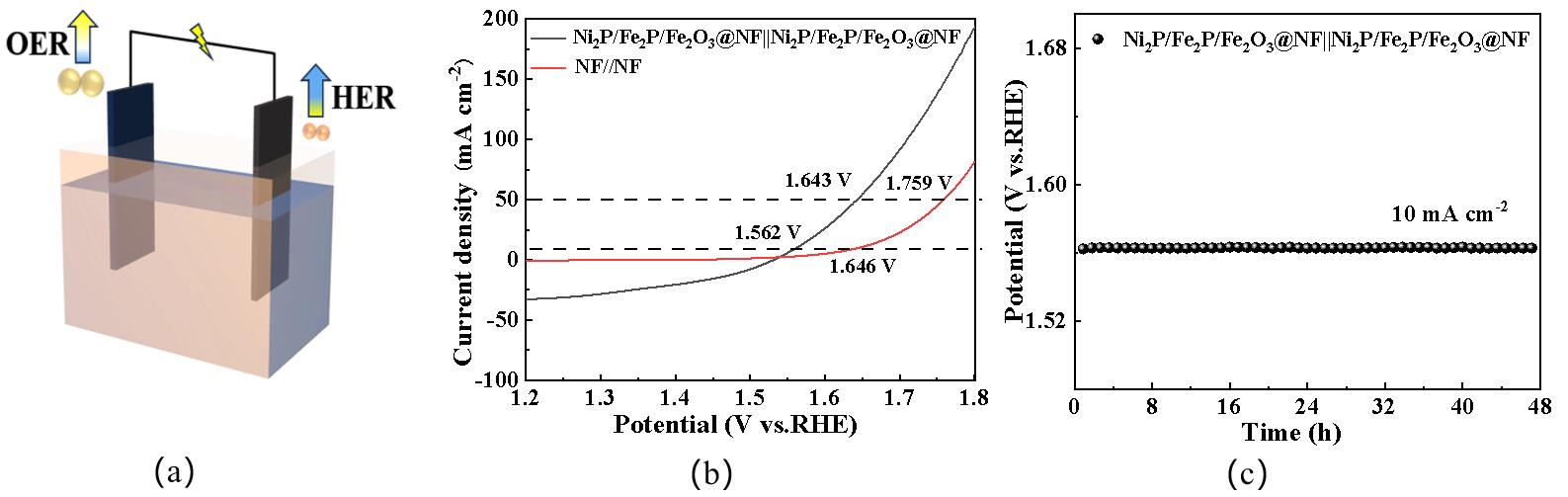
图14(a) 双电极系统电解槽示意图;(b)整体水分解的LSV曲线;(c)稳定性测试
Fig.14 (a) Schematic diagram of the double-electrode system of the electrolytic cell; (b) LSV curves of the overall water splitting test; (c) Stability test
| [1] | 杨森, 薛姿杰, 王彧斐, 等. 基于绿氢的化工低碳转型与研究现状[J]. 化工进展, 2025, 44(6): 3288-3304. |
| Yang S, Xue Z J, Wang Y F, et al. Low carbon transformation and research status of chemical industry based on green hydrogen[J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3288-3304. | |
| [2] | Ehlers J C, Feidenhans'l A A, Therkildsen K T, et al. Affordable green hydrogen from alkaline water electrolysis: key research needs from an industrial perspective[J]. ACS Energy Letters, 2023, 8(3): 1502-1509. |
| [3] | Shih A J, Monteiro M C O, Dattila F, et al. Water electrolysis[J]. Nature Reviews Methods Primers, 2022, 2: 84. |
| [4] | 钟晓航, 许卫, 张文, 等. 碱性水电解制氢中铁杂质的影响研究进展[J]. 化工学报, 2025, 76(2): 519-531. |
| Zhong X H, Xu W, Zhang W, et al. A critical review on the effects of Fe impurity on H2 production via alkaline water electrolysis[J]. CIESC Journal, 2025, 76(2): 519-531. | |
| [5] | Xu Q Q, Huo W, Li S S, et al. Crystal phase determined Fe active sites on Fe2O3 (γ- and α-Fe2O3) yolk-shell microspheres and their phase dependent electrocatalytic oxygen evolution reaction[J]. Applied Surface Science, 2020, 533: 147368. |
| [6] | Dongyang B, Yang W Y, Ye Q R, et al. Modulation of iron-based perovskites for enhanced electrocatalytic oxygen evolution reaction by a multi-method approach[J]. Journal of Alloys and Compounds, 2023, 958: 170368. |
| [7] | Ren X R, Zhai Y Y, Yang N, et al. Lattice oxygen redox mechanisms in the alkaline oxygen evolution reaction[J]. Advanced Functional Materials, 2024, 34(32): 2401610. |
| [8] | Wetzel A, Morell D, von der Au M, et al. Transpassive metal dissolution vs. oxygen evolution reaction: implication for alloy stability and electrocatalysis[J]. Angewandte Chemie International Edition, 2024, 63(18): e202317058. |
| [9] | Zou S H, Burke M S, Kast M G, et al. Fe(oxy)hydroxide oxygen evolution reaction electrocatalysis: intrinsic activity and the roles of electrical conductivity, substrate, and dissolution[J]. Chemistry of Materials, 2015, 27(23): 8011-8020. |
| [10] | Chung D Y, Lopes P P, Farinazzo Bergamo Dias Martins P, et al. Dynamic stability of active sites in hydr(oxy)oxides for the oxygen evolution reaction[J]. Nature Energy, 2020, 5(3): 222-230. |
| [11] | Wang H, Zhai T T, Wu Y F, et al. High‐valence oxides for high performance oxygen evolution electrocatalysis[J]. Advanced Science, 2023, 10(22): 2301706. |
| [12] | Wang Y H, Li L, Shi J H, et al. Oxygen defect engineering promotes synergy between adsorbate evolution and single lattice oxygen mechanisms of OER in transition metal‐based (oxy)hydroxide[J]. Advanced Science, 2023, 10(32): 2303321. |
| [13] | Dionigi F, Zeng Z H, Sinev I, et al. In-situ structure and catalytic mechanism of NiFe and CoFe layered double hydroxides during oxygen evolution[J]. Nature Communications, 2020, 11: 2522. |
| [14] | 王施媛, 王斌, 杨福胜, 等. 过渡金属基层状双金属氢氧化物作为析氧反应催化剂的优化策略[J/OL]. 应用化工, 2025: 1-7. (2025-08-01). . |
| Wang S Y, Wang B, Yang F S, et al. Enhanced strategies of transition metal-based layered double hydroxides as efficient electrocatalysts for oxygen evolution reaction[J/OL]. Applied Chemical Industry, 2025: 1-7. (2025-08-01). . | |
| [15] | Li J T. Oxygen evolution reaction in energy conversion and storage: design strategies under and beyond the energy scaling relationship[J]. Nano-Micro Letters, 2022, 14(1): 112. |
| [16] | Yoo J S, Rong X, Liu Y S, et al. Role of lattice oxygen participation in understanding trends in the oxygen evolution reaction on perovskites[J]. ACS Catalysis, 2018, 8(5): 4628-4636. |
| [17] | Tang R, Ying M H, Zhang X M, et al. Interfacial heterojunction-engineered Fe2O3/CoFe-layered double hydroxide catalyst for the electrocatalytic oxygen evolution reaction[J]. Energy & Fuels, 2022, 36(19): 11584-11590. |
| [18] | Meena A, Shin G, Cho S, et al. Engineering a synergistic CoMn-LDH/Fe2O3@NF heterostructure for highly efficient oxygen evolution reaction[J]. Ceramics International, 2023, 49(23): 37929-37935. |
| [19] | Xin S S, Tang Y, Jia B H, et al. Coupling adsorbed evolution and lattice oxygen mechanism in Fe-Co(OH)2/Fe2O3 heterostructure for enhanced electrochemical water oxidation[J]. Advanced Functional Materials, 2023, 33(45): 2305243. |
| [20] | Guo C X, Chen Q M, Zhong J Y, et al. Constructing amorphous-crystalline interfaces of nickel-iron phosphides/oxides for oxygen evolution reaction[J]. Industrial & Engineering Chemistry Research, 2023, 62(10): 4356-4363. |
| [21] | Wang T H, Fu X Z, Wang S Y. Etching oxide overlayers of NiFe phosphide to facilitate surface reconstruction for oxygen evolution reaction[J]. Green Energy & Environment, 2022, 7(3): 365-371. |
| [22] | Wu Z P, Zuo S W, Pei Z H, et al. Operando unveiling the activity origin via preferential structural evolution in Ni-Fe(oxy) phosphides for efficient oxygen evolution[J]. Science Advances, 2025, 11(10): eadu5370. |
| [23] | 李宇明, 徐砚文, 刘红宇, 等. 镍基磷化物的合成及其在电解水制氢中的应用[J]. 化工学报, 2024, 75(12): 4385-4402. |
| Li Y M, Xu Y W, Liu H Y, et al. Synthesis and application of nickel-based phosphide in water electrolysis for hydrogen evolution[J]. CIESC Journal, 2024, 75(12): 4385-4402. | |
| [24] | Wang Z K, Wang S Y, Ma L X, et al. Water-induced formation of Ni2P–Ni12P5 interfaces with superior electrocatalytic activity toward hydrogen evolution reaction[J]. Small, 2021, 17(6): 2006770. |
| [25] | Wang D, Duan C Q, He H, et al. Microwave solvothermal synthesis of Component-Tunable High-Entropy oxides as High-Efficient and stable electrocatalysts for oxygen evolution reaction[J]. Journal of Colloid and Interface Science, 2023, 646: 89-97. |
| [26] | Lei Y Q, Xu T T, Ye S H, et al. Engineering defect-rich Fe-doped NiO coupled Ni cluster nanotube arrays with excellent oxygen evolution activity[J]. Applied Catalysis B: Environmental, 2021, 285: 119809. |
| [27] | Wang L Q, Li J S, Meng Q L, et al. Facilitating active NiOOH formation via Mo doping towards high-efficiency oxygen evolution[J]. Catalysis Science & Technology, 2024, 14(15): 4166-4173. |
| [28] | Zhang J Q, Zhao Y F, Chen C, et al. Tuning the coordination environment in single-atom catalysts to achieve highly efficient oxygen reduction reactions[J]. Journal of the American Chemical Society, 2019, 141(51): 20118-20126. |
| [29] | Huang Z F, Xi S B, Song J J, et al. Tuning of lattice oxygen reactivity and scaling relation to construct better oxygen evolution electrocatalyst[J]. Nature Communications, 2021, 12: 3992. |
| [30] | He X R, Liu M H, Liu F, et al. Oxyanion engineering renewable lattice oxygen mechanism of CoFe oxide for enhanced water oxidation[J]. Advanced Functional Materials, 2025: e05936. |
| [31] | Mei Y J, Feng Y B, Zhang C X, et al. High-entropy alloy with Mo-coordination as efficient electrocatalyst for oxygen evolution reaction[J]. ACS Catalysis, 2022, 12(17): 10808-10817. |
| [32] | Guo X, Li L, Wang S, et al. An in situ formed ZIF-67 derived NiFeCo-P nano-array for accelerating the electrocatalytic oxygen evolution reaction[J]. Energy Advances, 2024, 3(3): 654-663. |
| [33] | Yang C Z, Laberty-Robert C, Batuk D, et al. Phosphate ion functionalization of perovskite surfaces for enhanced oxygen evolution reaction[J]. The Journal of Physical Chemistry Letters, 2017, 8(15): 3466-3472. |
| [34] | Trębala M, Łamacz A. Modern catalytic materials for the oxygen evolution reaction[J]. Molecules, 2025, 30(8): 1656. |
| [35] | Yuan B B, Sun F Z, Li C Q, et al. Formation of Prussian blue analog on Ni foam via in-situ electrodeposition method and conversion into Ni-Fe-mixed phosphates as efficient oxygen evolution electrode[J]. Electrochimica Acta, 2019, 313: 91-98. |
| [36] | Ren J T, Chen L, Wang H Y, et al. Synergistic activation of crystalline Ni2P and amorphous NiMoOx for efficient water splitting at high current densities[J]. ACS Catalysis, 2023, 13(14): 9792-9805. |
| [1] | 佟丽丽, 陈英, 艾敏华, 舒玉美, 张香文, 邹吉军, 潘伦. ZnO/WO3异质结光催化环烯烃[2+2]环加成制备高能量密度燃料[J]. 化工学报, 2025, 76(9): 4882-4892. |
| [2] | 钱慧慧, 王文婕, 陈文尧, 周兴贵, 张晶, 段学志. 聚丙烯定向转化制芳烃:金属-分子筛协同催化机制[J]. 化工学报, 2025, 76(9): 4838-4849. |
| [3] | 周怀荣, 伊嘉伟, 曹阿波, 郭奥雪, 王东亮, 杨勇, 杨思宇. 共电解耦合CO2间接加氢制甲醇工艺集成设计与性能评价[J]. 化工学报, 2025, 76(9): 4586-4600. |
| [4] | 邹家庆, 张肇钰, 张建国, 张博宇, 刘定胜, 毛庆, 王挺, 李建军. 碱水制氢电解槽极板通道中气泡的生成及演化性质[J]. 化工学报, 2025, 76(9): 4786-4799. |
| [5] | 赵维, 邢文乐, 韩朝旭, 袁兴中, 蒋龙波. g-C3N4基非金属异质结光催化降解水中有机污染物的研究进展[J]. 化工学报, 2025, 76(9): 4752-4769. |
| [6] | 周奕彤, 周明熙, 刘若晨, 叶爽, 黄伟光. 光伏与电网协同驱动氢基直接还原铁炼钢的技术经济分析[J]. 化工学报, 2025, 76(8): 4318-4330. |
| [7] | 范夏雨, 孙建辰, 李可莹, 姚馨雅, 商辉. 机器学习驱动液态有机储氢技术的系统优化[J]. 化工学报, 2025, 76(8): 3805-3821. |
| [8] | 杨宁, 李皓男, LIN Xiao, GEORGIADOU Stella, LIN Wen-Feng. 从塑料废弃物到能源催化剂:塑料衍生碳@CoMoO4复合材料在电解水析氢反应中的应用[J]. 化工学报, 2025, 76(8): 4081-4094. |
| [9] | 吴阿强, 诸葛祥群, 刘通, 王明星, 罗鲲. 纳米普鲁士蓝悬浮电解液对锂氧电池性能的影响[J]. 化工学报, 2025, 76(8): 4310-4317. |
| [10] | 刘建海, 王磊, 鲁朝金, 白志山, 张平雨. 耦合电化学与多相流模型的电解槽性能研究[J]. 化工学报, 2025, 76(8): 3885-3893. |
| [11] | 罗佳欣, 袁艳. 压电材料在固态金属二次电池中的研究进展[J]. 化工学报, 2025, 76(8): 3822-3833. |
| [12] | 巢欣旖, 陈文尧, 张晶, 钱刚, 周兴贵, 段学志. 甲醇和乙酸甲酯一步法制丙酸甲酯催化剂的可控制备与性能调控[J]. 化工学报, 2025, 76(8): 4030-4041. |
| [13] | 王御风, 罗小雪, 范鸿亮, 吴白婧, 李存璞, 魏子栋. 耦合电解水制氢的绿色有机电合成——电极界面调控策略综述[J]. 化工学报, 2025, 76(8): 3753-3771. |
| [14] | 林嘉豪, 付芳忠, 叶昊辉, 胡金, 姚明灿, 范鹤林, 王旭, 王瑞祥, 徐志峰. NdF3含量对NdF3-LiF熔盐局域结构和输运性质的影响[J]. 化工学报, 2025, 76(8): 3834-3841. |
| [15] | 陆学瑞, 周帼彦, 方琦, 俞孟正, 张秀成, 涂善东. 固体氧化物燃料电池外重整器积炭效应数值模拟研究[J]. 化工学报, 2025, 76(7): 3295-3304. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号