• •
邢梦可( ), 郑涛, 回天力(
), 郑涛, 回天力( ), 孟祥海, 张睿, 刘海燕, 刘植昌, 徐春明
), 孟祥海, 张睿, 刘海燕, 刘植昌, 徐春明
收稿日期:2025-09-25
修回日期:2025-11-26
出版日期:2025-12-04
通讯作者:
回天力
作者简介:邢梦可(1997—),女,博士研究生,15830194737@163.com
基金资助:
Mengke XING( ), Tao ZHENG, Tianli HUI(
), Tao ZHENG, Tianli HUI( ), Xianghai MENG, Rui ZHANG, Haiyan LIU, Zhichang LIU, Chunming XU
), Xianghai MENG, Rui ZHANG, Haiyan LIU, Zhichang LIU, Chunming XU
Received:2025-09-25
Revised:2025-11-26
Online:2025-12-04
Contact:
Tianli HUI
摘要:
制备了不同Zn掺杂量的PdO/Zn X Zr1-X O2催化剂,考察了氧空位和Lewis酸位点对CO2与苯酚合成碳酸二苯酯(DPC)反应性能的影响。XRD、Raman、EPR和XPS分析表明,适量Zn掺杂可形成锌锆固溶体,有效调节催化剂的氧空位浓度,增强CO2的吸附与活化。NH3-TPD和Py-IR研究结果显示,Zn掺杂和PdO负载增加了催化剂的Lewis酸位点,促进了苯酚的吸附与活化;氧空位和Lewis酸位点协同作用促进了DPC的高效合成。与Zn0.2Zr0.8O2和PdO/ZrO2相比,PdO/Zn0.2Zr0.8O2催化剂表现出最优性能,苯酚转化率达53.1%,DPC选择性达83.5%。原位FTIR分析表明,氧空位促进CO2活化生成双齿碳酸盐物种;Lewis酸位点促进苯酚O-H键断裂生成苯氧基物种,据此提出了氧空位与Lewis酸位点催化CO2和苯酚合成DPC的协同机理。
中图分类号:
邢梦可, 郑涛, 回天力, 孟祥海, 张睿, 刘海燕, 刘植昌, 徐春明. 富氧空位锌掺杂PdO/ZrO2催化CO2和苯酚合成碳酸二苯酯[J]. 化工学报, DOI: 10.11949/0438-1157.20251070.
Mengke XING, Tao ZHENG, Tianli HUI, Xianghai MENG, Rui ZHANG, Haiyan LIU, Zhichang LIU, Chunming XU. Zn-doped PdO/ZrO2 with rich oxygen vacancies for the synthesis of diphenyl carbonate from CO2 and phenol[J]. CIESC Journal, DOI: 10.11949/0438-1157.20251070.
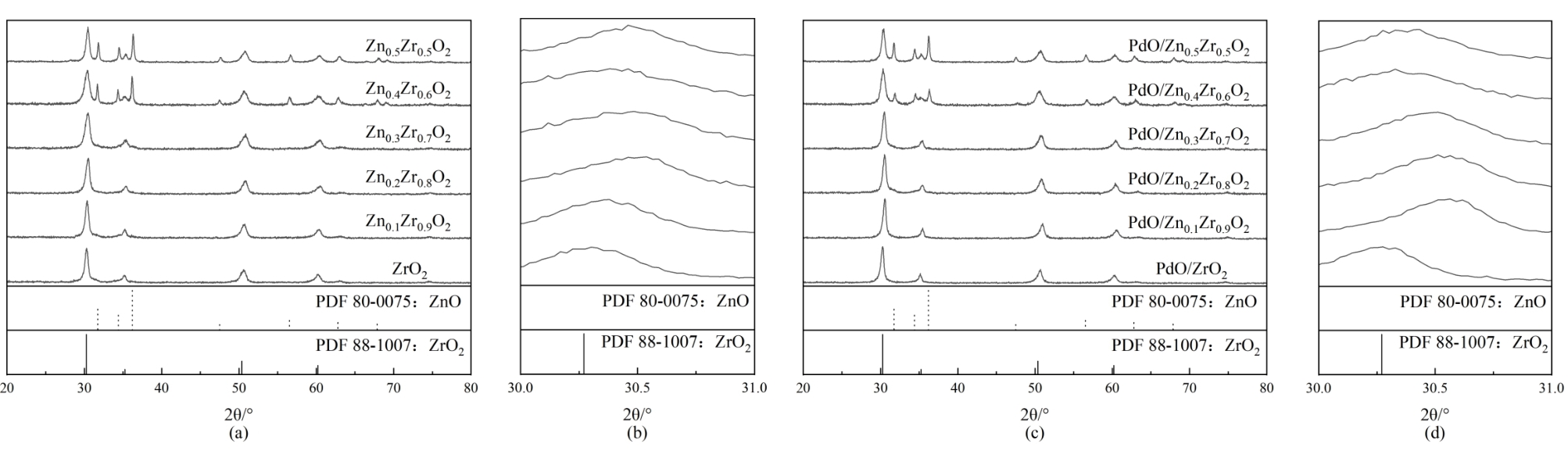
图1 (a, b) Zn X Zr1-X O2催化剂XRD谱图及其局部放大图;(c, d) PdO/Zn X Zr1-X O2催化剂XRD谱图及其局部放大图
Fig. 1 (a, b) XRD pattern and enlarge XRD pattern of Zn X Zr1-X O2 catalysts; (c, d) XRD pattern and enlarge XRD pattern of PdO/Zn X Zr1-X O2 catalysts
| Zn掺杂量 | 2θ/° | Zn X Zr1-X O2(011)晶面/nm | 晶格参数/nm | ||
|---|---|---|---|---|---|
| a | b | c | |||
| - | 30.3 | 0.295 | 3.610 | 3.610 | 5.150 |
| 0.1 | 30.4 | 0.289 | 3.602 | 3.602 | 5.106 |
| 0.2 | 30.6 | 0.285 | 3.593 | 3.599 | 5.088 |
| 0.3 | 30.5 | 0.287 | 3.599 | 3.599 | 5.091 |
| 0.4 | 30.3 | 0.290 | 3.605 | 3.605 | 5.125 |
| 0.5 | 30.3 | 0.292 | 3.608 | 3.608 | 5.142 |
表1 不同Zn掺杂量Zn X Zr1-X O2催化剂的晶面间距和晶格参数
Table 1 Interplanar spacing and lattice parameters of ZnZrO2(011) in Zn X Zr1-XO2 catalysts with different Zn doping content
| Zn掺杂量 | 2θ/° | Zn X Zr1-X O2(011)晶面/nm | 晶格参数/nm | ||
|---|---|---|---|---|---|
| a | b | c | |||
| - | 30.3 | 0.295 | 3.610 | 3.610 | 5.150 |
| 0.1 | 30.4 | 0.289 | 3.602 | 3.602 | 5.106 |
| 0.2 | 30.6 | 0.285 | 3.593 | 3.599 | 5.088 |
| 0.3 | 30.5 | 0.287 | 3.599 | 3.599 | 5.091 |
| 0.4 | 30.3 | 0.290 | 3.605 | 3.605 | 5.125 |
| 0.5 | 30.3 | 0.292 | 3.608 | 3.608 | 5.142 |
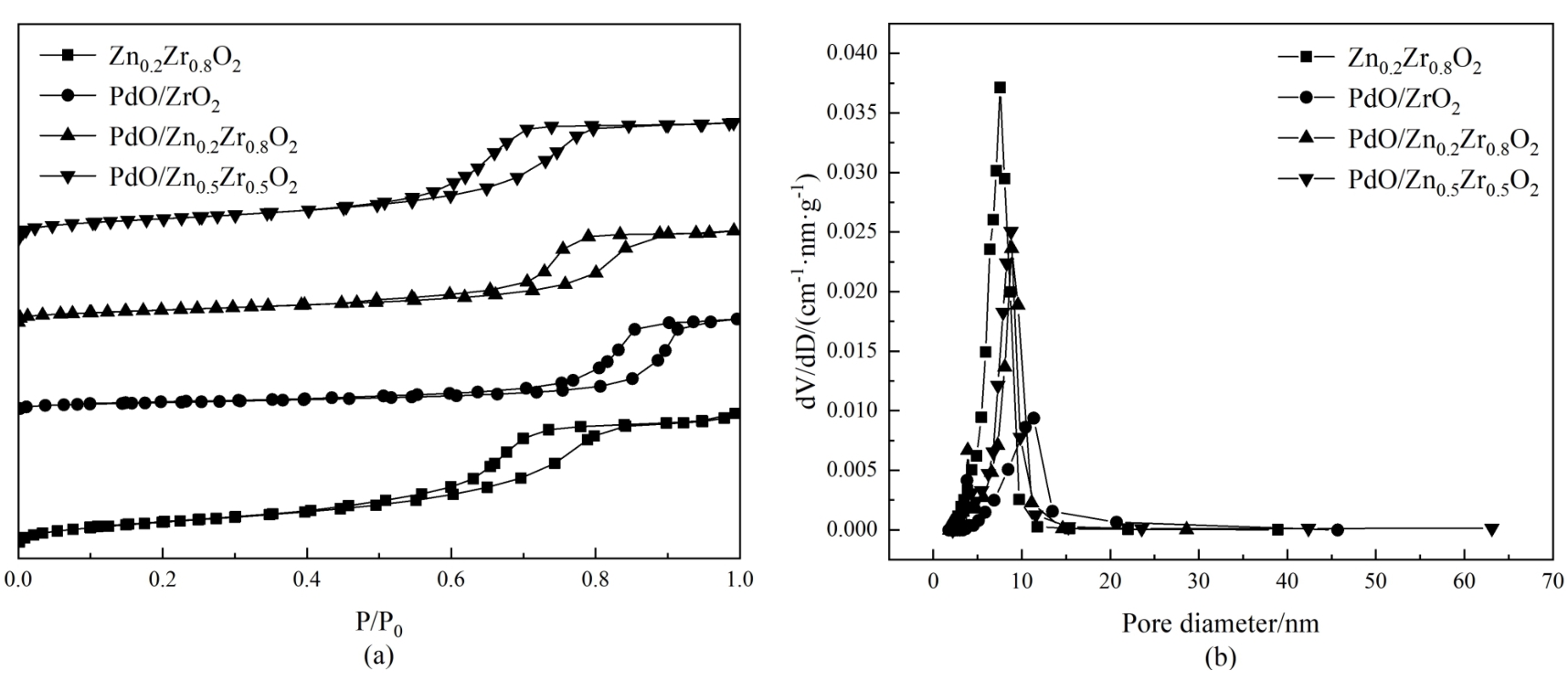
图2 PdO/Zn X Zr1-X O2催化剂的(a)N2吸附-脱附等温线和(b)孔径分布曲线
Fig. 2 (a) N2 adsorption-desorption isotherms and (b) pore size distribution curves of the PdO/Zn X Zr1-X O2 catalysts
| 催化剂 | 比表面积①/(m2·g-1) | 孔容②/(cm3·g-1) | 平均孔径/nm |
|---|---|---|---|
| Zn0.2Zr0.8O2 | 45 | 0.102 | 5 |
| PdO/ZrO2 | 28 | 0.0.70 | 8 |
| PdO/Zn0.2Zr0.8O2 | 39 | 0.076 | 6 |
| PdO/Zn0.5Zr0.5O2 | 34 | 0.069 | 6 |
表2 催化剂的理化性质
Table 2 Physical and chemical properties of catalysts
| 催化剂 | 比表面积①/(m2·g-1) | 孔容②/(cm3·g-1) | 平均孔径/nm |
|---|---|---|---|
| Zn0.2Zr0.8O2 | 45 | 0.102 | 5 |
| PdO/ZrO2 | 28 | 0.0.70 | 8 |
| PdO/Zn0.2Zr0.8O2 | 39 | 0.076 | 6 |
| PdO/Zn0.5Zr0.5O2 | 34 | 0.069 | 6 |
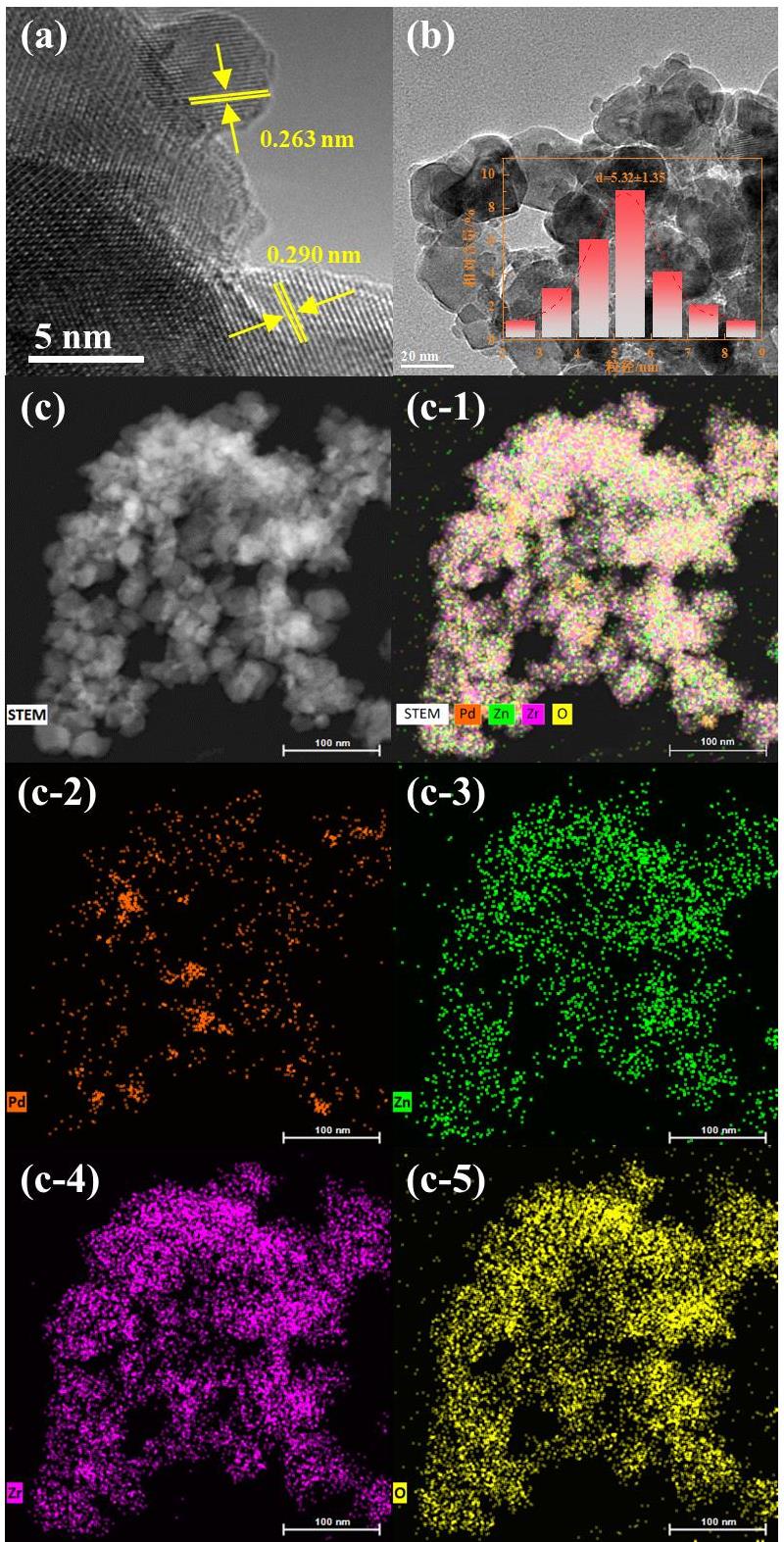
图3 (a) PdO/Zn0.2Zr0.8O2催化剂HRTEM图像;(b) PdO的粒径分布图;(c) PdO/Zn0.2Zr0.8O2对应的相应元素(Zr、Zn和O)映射图
Fig. 3 (a) HRTEM image of the PdO/Zn0.2Zr0.8O2 catalyst; (b) PdO particle size distribution; (c) elemental mapping of corresponding elements (Zr, Zn, and O) for the PdO/Zn0.2Zr0.8O2 catalyst
| 催化剂 | n(Zn)/n(Zr) | [Oβ/(Oα+Oβ+Oγ)]/% | |
|---|---|---|---|
| XPS | ICP | ||
| PdO/Zn0.5Zr0.5O2 | 1.18 | 1.05 | 27.7 |
| PdO/Zn0.2Zr0.8O2 | 0.24 | 0.23 | 36.0 |
| PdO/ZrO2 | - | - | 23.1 |
表3 催化剂中Zn/Zr摩尔比和表面缺氧区(Oβ)浓度
Table 3 Zn/Zr molar ratio and surface oxygen defect (Oβ) concentration in the catalysts
| 催化剂 | n(Zn)/n(Zr) | [Oβ/(Oα+Oβ+Oγ)]/% | |
|---|---|---|---|
| XPS | ICP | ||
| PdO/Zn0.5Zr0.5O2 | 1.18 | 1.05 | 27.7 |
| PdO/Zn0.2Zr0.8O2 | 0.24 | 0.23 | 36.0 |
| PdO/ZrO2 | - | - | 23.1 |
| 催化剂 | 弱酸位性点/mmol·g-1 | 中强酸性位点/mmol·g-1 | 总酸位点/mmol·g-1 | 酸密度 /μmol·m-2 |
|---|---|---|---|---|
| Zn0.2Zr0.8O2 | 0.27 | 0.24 | 0.51 | 11.33 |
| PdO/ZrO2 | 0.09 | 0.23 | 0.32 | 11.43 |
| PdO/Zn0.2Zr0.8O2 | 0.31 | 0.48 | 0.79 | 20.26 |
表4 PdO/Zn X Zr1-X O2催化剂表面酸性质
Table 4 Surface acidic properties of PdO/Zn X Zr1-X O2 catalysts
| 催化剂 | 弱酸位性点/mmol·g-1 | 中强酸性位点/mmol·g-1 | 总酸位点/mmol·g-1 | 酸密度 /μmol·m-2 |
|---|---|---|---|---|
| Zn0.2Zr0.8O2 | 0.27 | 0.24 | 0.51 | 11.33 |
| PdO/ZrO2 | 0.09 | 0.23 | 0.32 | 11.43 |
| PdO/Zn0.2Zr0.8O2 | 0.31 | 0.48 | 0.79 | 20.26 |
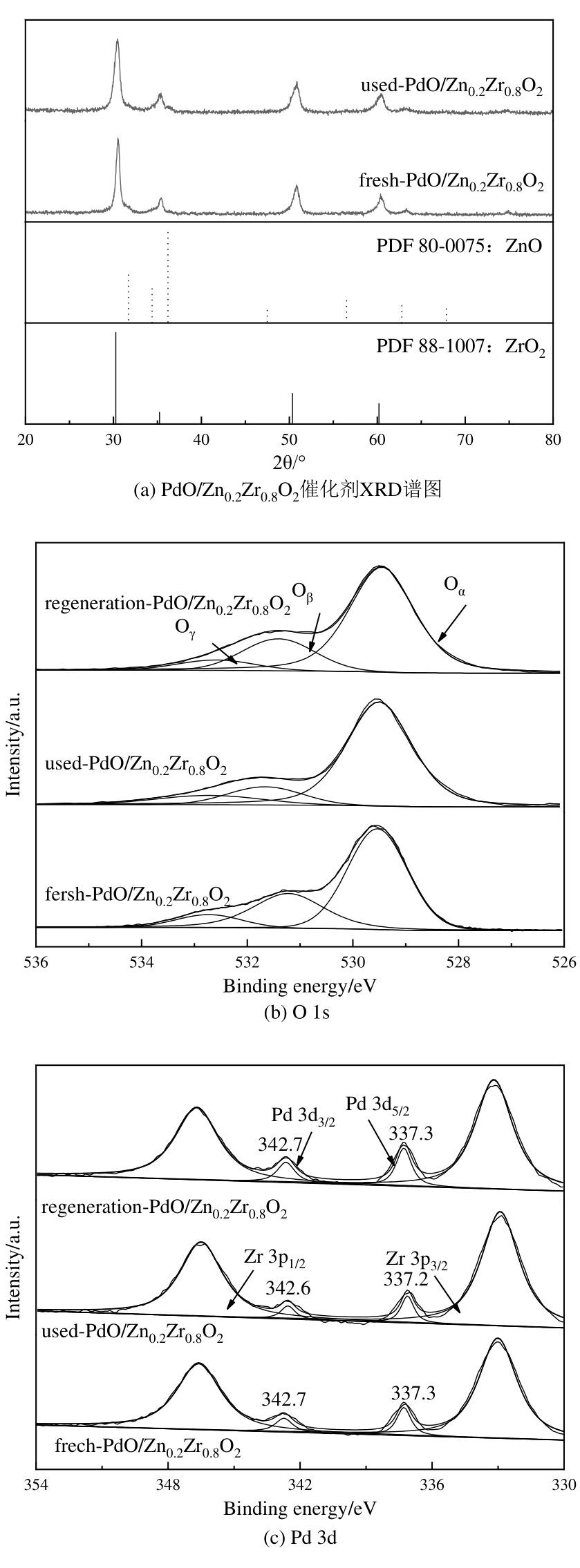
图12 使用前后及再生PdO/Zn0.2Zr0.8O2催化剂的结构表征(a) XRD谱图;(b) O 1s和(c) Pd 3d高分辨XPS谱图
Fig. 12 Structural characterization of the fresh, used and regeneration PdO/Zn0.2Zr0.8O2 catalysts (a) XRD patterns; High-resolution XPS spectra of (b) O 1s and (c) Pd 3d
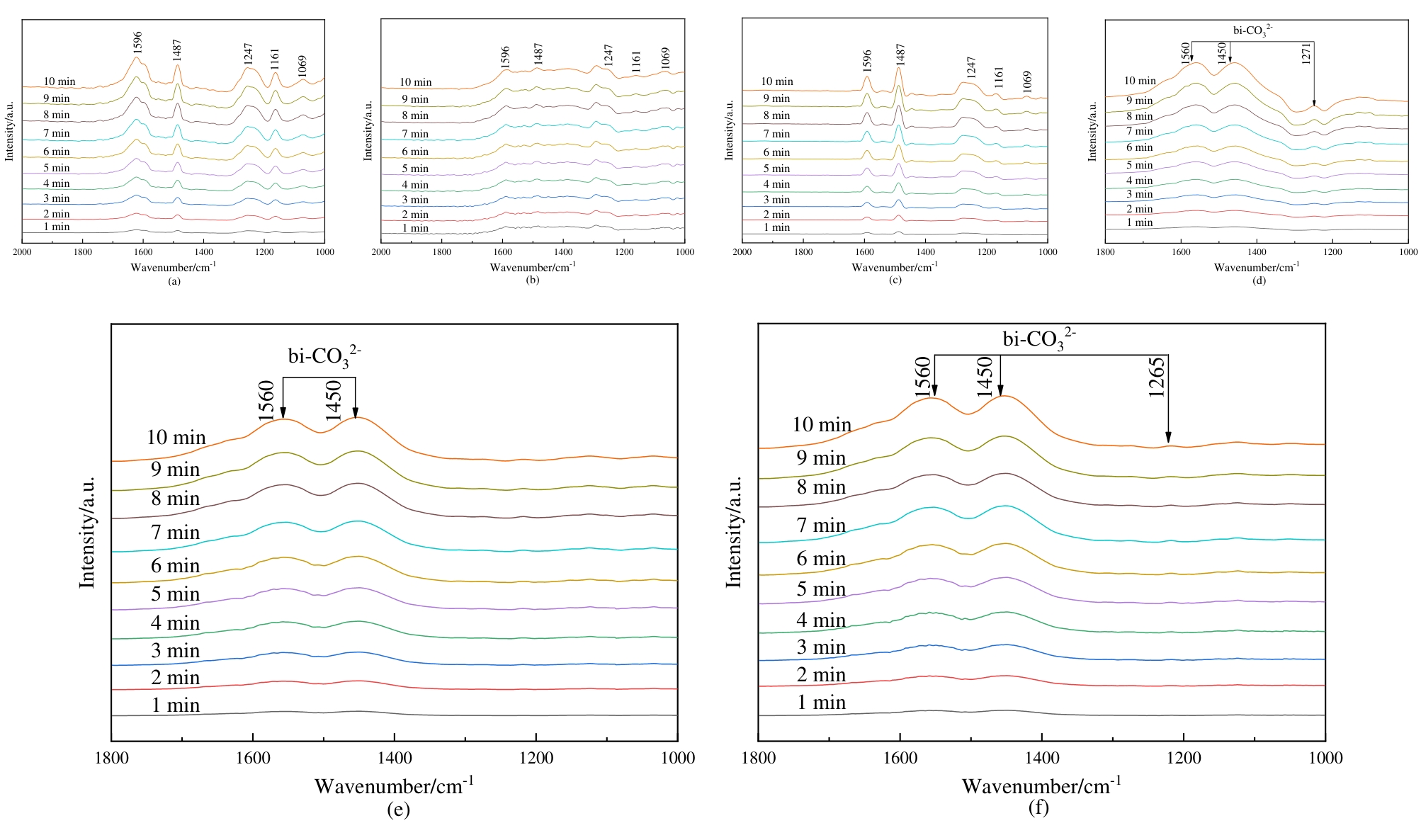
图13 催化剂表面苯酚吸附原位红外光谱图(a) PdO/Zn0.2Zr0.8O2, (b)PdO/ZrO2和(c) Zn0.2Zr0.8O2; CO2吸附原位红外光谱图(d) PdO/Zn0.2Zr0.8O2, (e)PdO/ZrO2 and (f) Zn0.2Zr0.8O2
Fig. 13 In situ FTIR spectra of phenol adsorption on catalyst surfaces: (a) PdO/Zn0.2Zr0.8O2, (b)PdO/ZrO2 and (c) Zn0.2Zr0.8O2; In situ FTIR spectra of CO2 adsorption on catalyst surfaces: (d) PdO/Zn0.2Zr0.8O2, (e) PdO/ZrO2 and (f) Zn0.2Zr0.8O2
| [1] | 安志杭, 李冰豪, 童德, 等. 熔融酯交换法绿色制备聚碳酸酯及抗菌改性[J]. 工程塑料应用, 2024, 52(6): 27-33. |
| An Z H, Li B H, Tong D, et al. Green preparation of polycarbonate with melt transesterification method and its antibacterial modification[J]. Engineering Plastics Application, 2024, 52(6): 27-33. | |
| [2] | 刘勇, 刘坚. 非光气法催化合成碳酸二苯酯研究进展[J]. 化工进展, 2013, 32(11): 2614-2620. |
| Liu Y, Liu J. Progress in the catalytic synthesis of diphenyl carbonate via non-phosgene routes[J]. Chemical Industry and Engineering Progress, 2013, 32(11): 2614-2620. | |
| [3] | Li Y W, Wang M, Liu X W, et al. Catalytic transformation of PET and CO2 into high-value chemicals[J]. Angewandte Chemie International Edition, 2022, 61(10): e202117205. |
| [4] | Kang K H, Jun J O, Han S J, et al. Direct synthesis of diphenyl carbonate from phenol and carbon dioxide over Ti-salen-based catalysts[J]. Journal of Nanoscience and Nanotechnology, 2015, 15(10): 8353-8358. |
| [5] | Fan G Z, Zhao H T, Duan Z X, et al. A novel method to synthesize diphenyl carbonate from carbon dioxide and phenol in the presence of methanol[J]. Catalysis Science & Technology, 2011, 1(7): 1138-1141. |
| [6] | Fan G Z, Wang Z G, Zou B, et al. Synthesis of diphenyl carbonate from compressed carbon dioxide and phenol without use of organic solvent[J]. Fuel Processing Technology, 2011, 92(5): 1052-1055. |
| [7] | Wang S L, Jiang N, Peng J L, et al. Efficient synthesis of diphenyl carbonate from CO2, phenol, and carbon tetrachloride under mild conditions[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(38): 12689-12697. |
| [8] | Fan G Z, Luo S S, Wu Q, et al. ZnBr2 supported on silica-coated magnetic nanoparticles of Fe3O4 for conversion of CO2 to diphenyl carbonate[J]. RSC Advances, 2015, 5(70): 56478-56485. |
| [9] | Su K, Li Z, Cheng B, et al. Catalytic performance of metal oxide modified SiMCM-41 catalysts in diphenyl carbonate synthesis[J]. Kinetics and Catalysis, 2021, 51(3): 359-363. |
| [10] | 柏冬, 路文学, 王振华, 等. 无模板剂介孔锆基固体碱的制备及其催化合成甘油碳酸酯性能[J]. 石油学报(石油加工), 2020, 36(1): 54-62. |
| Bai D, Lu W X, Wang Z H, et al. Preparation of mesoporous Zr-based solid bases without templates for the synthesis of glycerol carbonate[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2020, 36(1): 54-62. | |
| [11] | 陈红萍, 梁英华, 郑小满, 等. CO2和甲醇直接合成碳酸二甲酯的Fe-Zr-O催化剂制备和性能研究[J]. 高校化学工程学报, 2014, 28(4): 745-751. |
| Chen H P, Liang Y H, Zheng X M, et al. Preparation and catalytic performance of Fe-Zr-O catalysts for direct synthesis of dimethyl carbonate using CO2 and methanol[J]. Journal of Chemical Engineering of Chinese Universities, 2014, 28(4): 745-751. | |
| [12] | 郑谦, 官修帅, 靳山彪, 等. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| Zheng Q, Guan X S, Jin S B, et al. Photothermal catalysis synthesis of DMC from CO2 and methanol over Ce0.25Zr0.75O2 solid solution[J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 319-327. | |
| [13] | Wang J J, Li G N, Li Z L, et al. A highly selective and stable ZnO-ZrO2 solid solution catalyst for CO2 hydrogenation to methanol[J]. Science Advances, 2017, 3(10): e1701290. |
| [14] | Zhang X B, Yu X, Mendes R G, et al. Highly dispersed ZnO sites in a ZnO/ZrO2 catalyst promote carbon dioxide-to-methanol conversion[J]. Angewandte Chemie International Edition, 2025, 64(4): e202416899. |
| [15] | Baylon R A L, Sun J M, Kovarik L, et al. Structural identification of ZnxZryOz catalysts for Cascade aldolization and self-deoxygenation reactions[J]. Applied Catalysis B: Environmental, 2018, 234: 337-346. |
| [16] | Li Y H, Ren Y H, Yang G C, et al. Unraveling the role of ZnZrOx morphology and oxygen vacancy in bifunctional catalyst for conversion of syngas into light olefins[J]. Applied Catalysis B: Environment and Energy, 2025, 365: 124945. |
| [17] | Liu B, Li C M, Zhang G Q, et al. Oxygen vacancy promoting dimethyl carbonate synthesis from CO2 and methanol over Zr-doped CeO2 nanorods[J]. ACS Catalysis, 2018, 8(11): 10446-10456. |
| [18] | Cao F X, Xiao Y S, Zhang Z M, et al. Influence of oxygen vacancies of CeO2 on reverse water gas shift reaction[J]. Journal of Catalysis, 2022, 414: 25-32. |
| [19] | Zhou S J, Li S G. Insights into the high activity and methanol selectivity of the Zn/ZrO2 solid solution catalyst for CO2 hydrogenation[J]. The Journal of Physical Chemistry C, 2020, 124(50): 27467-27478. |
| [20] | Huang Z S, Wang Y F, Qi M Y, et al. Interface synergy of exposed oxygen vacancy and Pd lewis acid sites enabling superior cooperative photoredox synthesis[J]. Angewandte Chemie International Edition, 2024, 63(47): e202412707. |
| [21] | 王志苗, 张洪起, 周立超, 等. Ce在负载Pd催化苯酚氧化羰基化合成碳酸二苯酯反应中的作用[J]. 化工学报, 2019, 70(12): 4625-4634, 4920. |
| Wang Z M, Zhang H Q, Zhou L C, et al. Role of Ce in supported Pd catalyst for oxidative carbonylation of phenol to diphenyl carbonate[J]. CIESC Journal, 2019, 70(12): 4625-4634, 4920. | |
| [22] | de Souza P M, Rabelo-Neto R C, Borges L E P, et al. Effect of zirconia morphology on hydrodeoxygenation of phenol over Pd/ZrO2 [J]. ACS Catalysis, 2015, 5(12): 7385-7398. |
| [23] | Yoshida H, Nakajima T, Yazawa Y, et al. Support effect on methane combustion over palladium catalysts[J]. Applied Catalysis B: Environmental, 2007, 71(1/2): 70-79. |
| [24] | Ma K, Zhao S Y, Dou M X, et al. Enhancing the stability of methanol-to-olefins reaction catalyzed by SAPO-34 zeolite in the presence of CO2 and oxygen-vacancy-rich ZnCeZrOx [J]. ACS Catalysis, 2024, 14(2): 594-607. |
| [25] | Tada S, Ochiai N, Kinoshita H, et al. Active sites on Zn x Zr1– x O2– x solid solution catalysts for CO2-to-methanol hydrogenation[J]. ACS Catalysis, 2022, 12(13): 7748-7759. |
| [26] | Dev G S, Sharma V, Singh A, et al. Raman spectroscopic study of ZnO/NiO nanocomposites based on spatial correlation model[J]. RSC Advances, 2019, 9(46): 26956-26960. |
| [27] | Chen H, Cui H S, Lv Y, et al. CO2 hydrogenation to methanol over Cu/ZnO/ZrO2 catalysts: Effects of ZnO morphology and oxygen vacancy[J]. Fuel, 2022, 314: 123035. |
| [28] | Ammar A U, Yildirim I D, Aleinawi M H, et al. Multifrequency EPR spectroscopy study of Mn, Fe, and Cu doped nanocrystalline ZnO[J]. Materials Research Bulletin, 2023, 160: 112117. |
| [29] | Drouilly C, Krafft J M, Averseng F, et al. ZnO oxygen vacancies formation and filling followed by in situ photoluminescence and in situ EPR[J]. The Journal of Physical Chemistry C, 2012, 116(40): 21297-21307. |
| [30] | Chen S L, Abdel-Mageed A M, Mochizuki C, et al. Controlling the O-vacancy formation and performance of Au/ZnO catalysts in CO2 reduction to methanol by the ZnO particle size[J]. ACS Catalysis, 2021, 11(15): 9022-9033. |
| [31] | Xue X D, Wang T, Jiang X D, et al. Interaction of hydrogen with defects in ZnO nanoparticles–studied by positron annihilation, Raman and photoluminescence spectroscopy[J]. CrystEngComm, 2014, 16(6): 1207-1216. |
| [32] | Liu M H, Chen Y W, Lin T S, et al. Defective mesocrystal ZnO-supported gold catalysts: facilitating CO oxidation via vacancy defects in ZnO[J]. ACS Catalysis, 2018, 8(8): 6862-6869. |
| [33] | Ling Y, Tan S Q, Wang D L, et al. An experimental and DFT study on enhanced elemental mercury removal performance via cerium chloride modified carbon aerogel: a synergistic effect between chemical adsorption and thermal catalysis[J]. Chemical Engineering Journal, 2021, 425: 127344. |
| [34] | Han S L, Zhao D, Otroshchenko T, et al. Elucidating the nature of active sites and fundamentals for their creation in Zn-containing ZrO2–based catalysts for nonoxidative propane dehydrogenation[J]. ACS Catalysis, 2020, 10(15): 8933-8949. |
| [35] | Srivastava M, Bera P, Balaraju J N, et al. FESEM and XPS studies of ZrO2 modified electrodeposited NiCoCrAlY nanocomposite coating subjected to hot corrosion environment[J]. RSC Advances, 2016, 6(110): 109083-109090. |
| [36] | Zao H J, Liu J, Chen G Y, et al. Enhanced conversion of syngas to high-quality diesel fuel over ZrO2 and acidized carbon nanotube bifunctional catalyst[J]. Fuel Processing Technology, 2023, 250: 107920. |
| [37] | Yang Y, Wang G, Zheng P, et al. Carbon deposits during catalytic combustion of toluene on Pd–Pt-based catalysts[J]. Catalysis Science & Technology, 2020, 10(8): 2452-2461. |
| [38] | Xie S H, Deng J G, Zang S M, et al. Au–Pd/3DOM Co3O4: Highly active and stable nanocatalysts for toluene oxidation[J]. Journal of Catalysis, 2015, 322: 38-48. |
| [39] | Wu Y, Chen J J, Hu W, et al. Phase transformation and oxygen vacancies in Pd/ZrO2 for complete methane oxidation under lean conditions[J]. Journal of Catalysis, 2019, 377: 565-576. |
| [40] | Su K M, Li Z H, Cheng B W, et al. The decomposition of CCl4 into diphenyl carbonate over MClχ/SiMCM-41[J]. Catalysis Communications, 2008, 9(7): 1666-1670. |
| [41] | Esteves L M, Brijaldo M H, Oliveira E G, et al. Effect of support on selective 5-hydroxymethylfurfural hydrogenation towards 2, 5-dimethylfuran over copper catalysts[J]. Fuel, 2020, 270: 117524. |
| [42] | Xu L L, Zhao R R, Zhang W P. One-step high-yield production of renewable propene from bioethanol over composite ZnCeOx oxide and HBeta zeolite with balanced Brönsted/Lewis acidity[J]. Applied Catalysis B: Environmental, 2020, 279: 119389. |
| [43] | Manrı́quez M E, López T, Gómez R, et al. Preparation of TiO2–ZrO2 mixed oxides with controlled acid–basic properties[J]. Journal of Molecular Catalysis A: Chemical, 2004, 220(2): 229-237. |
| [44] | 卢思, 陈晓丽, 苏秋成, 等. 原位红外光谱-吡啶吸附法表征分子筛酸性性质的实验方法研究[J]. 光谱学与光谱分析, 2024, 44(9): 2488-2493. |
| Lu S, Chen X L, Su Q C, et al. The study of experimental method on the characterization of acidic properties of zeolites by in situ FTIR-pyridine adsorption[J]. Spectroscopy and Spectral Analysis, 2024, 44(9): 2488-2493. | |
| [45] | Xing M K, Hui T L, Zhang R, et al. Diphenyl carbonate synthesis from CO2 over a ZnCeZrOX ternary solid solution: synergistic catalysis using oxygen vacancies and Lewis acid sites[J]. Catalysis Science & Technology, 2025, 15(16): 4872-4884. |
| [46] | 刘速, 孙晓艳, 樊宏飞, 等. 再生温度对FC-28型催化剂再生效果的影响[J]. 工业催化, 2012, 20(12): 45-49. |
| Liu S, Sun X Y, Fan H F, et al. Influence of temperature on regeneration effect of FC-28 catalyst[J]. Industrial Catalysis, 2012, 20(12): 45-49. | |
| [47] | Feng Z D, Tang C Z, Zhang P F, et al. Asymmetric sites on the ZnZrO x catalyst for promoting formate formation and transformation in CO2 hydrogenation[J]. Journal of the American Chemical Society, 2023, 145(23): 12663-12672. |
| [1] | 孙云龙, 徐肖肖, 黄永方, 郭纪超, 陈卫卫. 水平光滑管内CO2流动沸腾的非绝热可视化研究[J]. 化工学报, 2025, 76(S1): 230-236. |
| [2] | 郭纪超, 徐肖肖, 孙云龙. 基于植物工厂中的CO2浓度气流模拟及优化研究[J]. 化工学报, 2025, 76(S1): 237-245. |
| [3] | 孔繁臣, 张硕, 唐明生, 邹慧明, 胡舟航, 田长青. 二氧化碳直线压缩机气体轴承模拟[J]. 化工学报, 2025, 76(S1): 281-288. |
| [4] | 何婷, 张开, 林文胜, 陈利琼, 陈家富. 沼气超临界压力低温脱碳-液化耦合流程研究[J]. 化工学报, 2025, 76(S1): 418-425. |
| [5] | 张建民, 何美贵, 贾万鑫, 赵静, 金万勤. 聚氧化乙烯/冠醚共混膜及其二氧化碳分离性能[J]. 化工学报, 2025, 76(9): 4862-4871. |
| [6] | 王一飞, 李玉星, 欧阳欣, 赵雪峰, 孟岚, 胡其会, 殷布泽, 郭雅琦. 基于裂尖减压特性的CO2管道断裂扩展数值计算[J]. 化工学报, 2025, 76(9): 4683-4693. |
| [7] | 周运桃, 崔丽凤, 张杰, 于富红, 李新刚, 田野. Ga2O3调控CuCeO催化CO2加氢制甲醇的研究[J]. 化工学报, 2025, 76(8): 4042-4051. |
| [8] | 刘沁雯, 叶恒冰, 张逸伟, 朱法华, 钟文琪. 煤与禽类粪便混合燃料的加压富氧燃烧特性研究[J]. 化工学报, 2025, 76(7): 3487-3497. |
| [9] | 丁宏鑫, 干文翔, 赵雍洋, 贾润泽, 康子祺, 赵玉隆, 向勇. X65钢焊接接头在超临界CO2相及富H2O相中的腐蚀机理研究[J]. 化工学报, 2025, 76(7): 3426-3435. |
| [10] | 董泽明, 娄聚伟, 王楠, 陈良奇, 王江峰, 赵攀. 含余热回收的超临界压缩二氧化碳储能系统热力学特性研究[J]. 化工学报, 2025, 76(7): 3477-3486. |
| [11] | 范振宁, 梁海宁, 房茂立, 赫一凡, 于帅, 闫兴清, 安佳然, 乔帆帆, 喻健良. CO2管道不同相态节流放空特性研究与对比[J]. 化工学报, 2025, 76(7): 3742-3751. |
| [12] | 卢丽丽, 李晨, 陈柳云, 谢新玲, 罗轩, 苏通明, 秦祖赠, 纪红兵. BiOBr的形貌调控及其光催化CO2还原性能的研究[J]. 化工学报, 2025, 76(6): 2687-2700. |
| [13] | 陈建兵, 常昊, 高明, 邢兵, 张磊, 刘奇磊. 基于反应模板与分子动力学的胺基相变吸收剂分相预测方法[J]. 化工学报, 2025, 76(5): 2387-2396. |
| [14] | 赵俊德, 周爱国, 陈彦霖, 郑家乐, 葛天舒. 吸附法CO2直接空气捕集技术能耗现状[J]. 化工学报, 2025, 76(4): 1375-1390. |
| [15] | 张赵雪, 李正宇, 崔文慧, 王倩, 王志平, 龚领会. 基于液氖液氮梯级蓄冷的液氢储能中冷能回收利用研究[J]. 化工学报, 2025, 76(4): 1731-1741. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号