CIESC Journal ›› 2021, Vol. 72 ›› Issue (8): 4204-4214.DOI: 10.11949/0438-1157.20201680
• Separation engineering • Previous Articles Next Articles
Hu LI1( ),Zisheng ZHANG1,Jiuzhou CHEN2,Haoliang SHI2,Yongjie SHI2,Hong LI1,Xingang LI1,Xin GAO1(
),Zisheng ZHANG1,Jiuzhou CHEN2,Haoliang SHI2,Yongjie SHI2,Hong LI1,Xingang LI1,Xin GAO1( )
)
Received:2020-11-23
Revised:2021-02-08
Online:2021-08-05
Published:2021-08-05
Contact:
Xin GAO
李虎1( ),张自生1,陈久洲2,石好亮2,石永杰2,李洪1,李鑫钢1,高鑫1(
),张自生1,陈久洲2,石好亮2,石永杰2,李洪1,李鑫钢1,高鑫1( )
)
通讯作者:
高鑫
作者简介:李虎(1996—),男,硕士研究生, 基金资助:CLC Number:
Hu LI, Zisheng ZHANG, Jiuzhou CHEN, Haoliang SHI, Yongjie SHI, Hong LI, Xingang LI, Xin GAO. Preparation of novel silver-based deep eutectic solvent and its application in separation of 1-hexene/n-hexane[J]. CIESC Journal, 2021, 72(8): 4204-4214.
李虎, 张自生, 陈久洲, 石好亮, 石永杰, 李洪, 李鑫钢, 高鑫. 新型银基低共熔溶剂制备及其在1-己烯/正己烷分离中的应用[J]. 化工学报, 2021, 72(8): 4204-4214.
Add to citation manager EndNote|Ris|BibTeX
| 影响因素 | 各因素对1-己烯分离性能影响探究实验条件 | ||||
|---|---|---|---|---|---|
| Ag-DES质量/ g | C6质量/ g | 1-己烯进料质量分数/% | Ag+与1-己烯摩尔比 | 温度/℃ | |
| 原料中烯烃浓度 | 11.106 | 4.48 | 10/30/50/70/90 | — | 25 |
| 银离子与烯烃摩尔比 | 11.106 | 5.04 | 50 | 1∶1/1∶1.5/1∶2/1∶2.5/1∶3 | 25 |
| 分离温度 | 11.106 | 5.04 | 50 | 1∶1.5 | 0/10/20/25/30/40 |
| 循环稳定性 | 11.106 | 5.04 | 50 | 1∶1.5 | 25 |
Table 1 The experimental conditions for the separation performance of 1-hexene
| 影响因素 | 各因素对1-己烯分离性能影响探究实验条件 | ||||
|---|---|---|---|---|---|
| Ag-DES质量/ g | C6质量/ g | 1-己烯进料质量分数/% | Ag+与1-己烯摩尔比 | 温度/℃ | |
| 原料中烯烃浓度 | 11.106 | 4.48 | 10/30/50/70/90 | — | 25 |
| 银离子与烯烃摩尔比 | 11.106 | 5.04 | 50 | 1∶1/1∶1.5/1∶2/1∶2.5/1∶3 | 25 |
| 分离温度 | 11.106 | 5.04 | 50 | 1∶1.5 | 0/10/20/25/30/40 |
| 循环稳定性 | 11.106 | 5.04 | 50 | 1∶1.5 | 25 |

Fig.2 Effect of olefin concentration in initial feed on the distribution coefficient and selectivity of 1-hexene to n-hexane(Including 11.106 g Ag-DES and 4.48 g C6 with the mass ratio of 1-hexene in initial feed range 10%—90% at 25℃)
原料中烯烃浓度(质量分数) x0 | 有机相组成(摩尔分数) | 溶剂相组成(摩尔分数) | 分配系数 | 选择性 S1,2 | 1-己烯收率 η1 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| x1 | x2 | x3 | x1 | x2 | x3 | D1 | D2 | |||
| 0.1 | 0.037 | 0.962 | 0.000 | 0.126 | 0.180 | 0.693 | 0.706 | 0.039 | 17.967 | 0.686 |
| 0.3 | 0.128 | 0.871 | 0.002 | 0.311 | 0.140 | 0.550 | 0.614 | 0.040 | 15.183 | 0.696 |
| 0.5 | 0.273 | 0.726 | 0.001 | 0.427 | 0.106 | 0.467 | 0.440 | 0.041 | 10.712 | 0.689 |
| 0.7 | 0.513 | 0.486 | 0.001 | 0.535 | 0.068 | 0.397 | 0.328 | 0.044 | 7.454 | 0.697 |
| 0.9 | 0.829 | 0.170 | 0.001 | 0.617 | 0.036 | 0.347 | 0.256 | 0.074 | 3.477 | 0.731 |
Table 2 Phase equilibrium data of 1-hexene(1)-n-hexane(2)-Ag-DES(3) in the ternary biphasic system at 298.15 K
原料中烯烃浓度(质量分数) x0 | 有机相组成(摩尔分数) | 溶剂相组成(摩尔分数) | 分配系数 | 选择性 S1,2 | 1-己烯收率 η1 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| x1 | x2 | x3 | x1 | x2 | x3 | D1 | D2 | |||
| 0.1 | 0.037 | 0.962 | 0.000 | 0.126 | 0.180 | 0.693 | 0.706 | 0.039 | 17.967 | 0.686 |
| 0.3 | 0.128 | 0.871 | 0.002 | 0.311 | 0.140 | 0.550 | 0.614 | 0.040 | 15.183 | 0.696 |
| 0.5 | 0.273 | 0.726 | 0.001 | 0.427 | 0.106 | 0.467 | 0.440 | 0.041 | 10.712 | 0.689 |
| 0.7 | 0.513 | 0.486 | 0.001 | 0.535 | 0.068 | 0.397 | 0.328 | 0.044 | 7.454 | 0.697 |
| 0.9 | 0.829 | 0.170 | 0.001 | 0.617 | 0.036 | 0.347 | 0.256 | 0.074 | 3.477 | 0.731 |
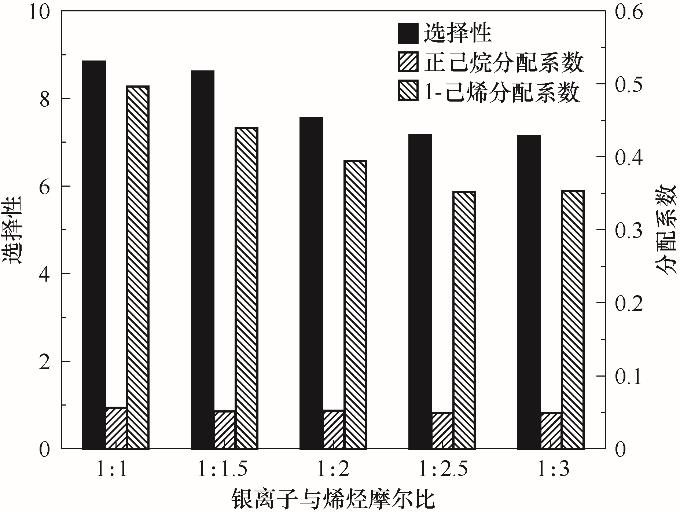
Fig.4 Effect of molar ratio of silver ion to olefin on the distribution coefficient and selectivity of 1-hexene to n-hexane (Including 11.106 g Ag-DES and C6 mixture with 50% olefin and the molar ratio of Ag-DES to 1-hexene ranging from 1∶1 to 1∶3 at 25℃)
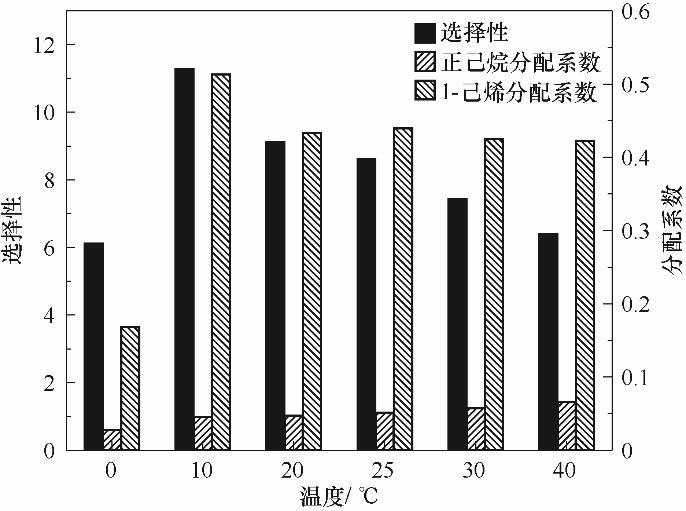
Fig.5 Effect of temperature on the distribution coefficient and selectivity of 1-hexene to n-hexane(Including 11.106 g Ag-DES and 5.04 g C6 mixture with 50% olefin and the operating temperature ranging from 0 to 40℃)
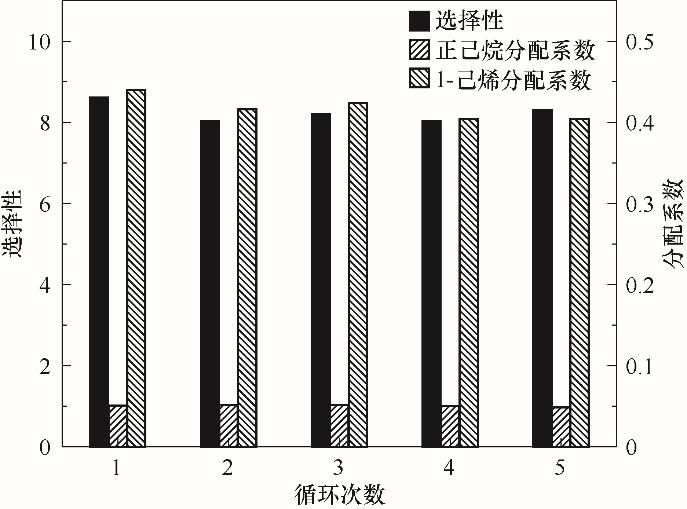
Fig.6 Effect of recycle times on the distribution coefficient and selectivity of 1-hexene to n-hexane(Including 11.106 g Ag-DES and 5.04 g C6 mixture with 50% olefin at 25℃)
| E | Ag-DES-1-己烯 | Ag-DES-正己烷 |
|---|---|---|
| EA/(kJ/mol) | -5431268.05 | -5431268.05 |
| EB/(kJ/mol) | -619411.70 | -622637.68 |
| EAB/(kJ/mol) | -6050813.59 | -6053982.20 |
| EBSSE/(kJ/mol) | 3.09 | 4.22×10-4 |
| EcintAB/(kJ/mol) | -130.75 | -76.47 |
| ?EcintAB/(kJ/mol) | 54.28 | |
Table 3 The interaction energies of the complexes Ag-DES(A)-1-hexene/n-hexane(B) at M062X/def2tzvp level
| E | Ag-DES-1-己烯 | Ag-DES-正己烷 |
|---|---|---|
| EA/(kJ/mol) | -5431268.05 | -5431268.05 |
| EB/(kJ/mol) | -619411.70 | -622637.68 |
| EAB/(kJ/mol) | -6050813.59 | -6053982.20 |
| EBSSE/(kJ/mol) | 3.09 | 4.22×10-4 |
| EcintAB/(kJ/mol) | -130.75 | -76.47 |
| ?EcintAB/(kJ/mol) | 54.28 | |
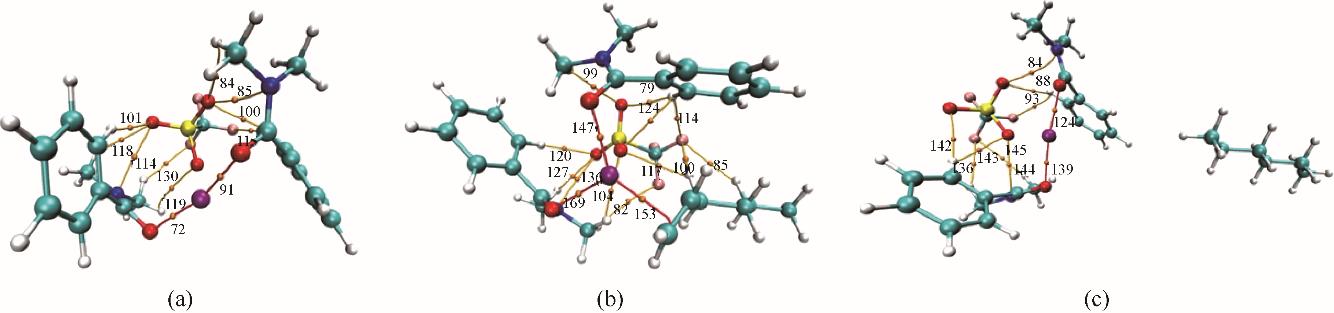
Fig.10 The AIM analysis for Ag-DES(a), Ag-DES-1-hexene(b), Ag-DES-n-hexane (c)The BCPs are marked as orange dots, the coordination covalent bonds are linked by red lines and the hydrogen bonds are linked by orange lines
| 类型 | BCP | 原子标签 | ρ(BCP) | ?2ρ(BCP) | H(BCP) | H(BCP)/ρ(BCP) |
|---|---|---|---|---|---|---|
| 共价作用 | 72 | Ag9-O44 C43 C43 | 5.52×10-2 | 2.73×10-1 | -4.87×10-3 | -8.82×10-2 |
| 91 | Ag9-O22 C21 C21 | 5.15×10-2 | 2.48×10-1 | -4.47×10-3 | -8.69×10-2 | |
| 氢键 | 119 | C24—H27…O5 | 7.98×10-3 | 3.13×10-2 | 1.44×10-3 | 1.81×10-1 |
| 130 | C24—H26…F7 | 3.67×10-3 | 1.48×10-2 | 8.45×10-4 | 2.30×10-1 | |
| 101 | C12—H18…O3 | 1.22×10-2 | 4.72×10-2 | 2.27×10-3 | 1.87×10-1 | |
| 118 | C28—H29…O3 | 1.01×10-2 | 4.08×10-2 | 1.87×10-3 | 1.86×10-1 | |
| 114 | C21—N23…O3 | 7.50×10-3 | 3.13×10-2 | 1.52×10-3 | 2.03×10-1 | |
| 84 | C46—H48…O4 | 7.34×10-3 | 3.19×10-2 | 1.58×10-3 | 2.16×10-1 | |
| 85 | C43—N45…O4 | 9.86×10-3 | 4.14×10-2 | 1.80×10-3 | 1.83×10-1 | |
| 99 | C34—H40…O4 | 9.88×10-3 | 3.63×10-2 | 1.67×10-3 | 1.69×10-1 | |
| 115 | C34—H40…F6 | 7.00×10-3 | 3.05×10-2 | 1.68×10-3 | 2.40×10-1 |
Table 4 Topological parameters of BCPs in Ag-DES
| 类型 | BCP | 原子标签 | ρ(BCP) | ?2ρ(BCP) | H(BCP) | H(BCP)/ρ(BCP) |
|---|---|---|---|---|---|---|
| 共价作用 | 72 | Ag9-O44 C43 C43 | 5.52×10-2 | 2.73×10-1 | -4.87×10-3 | -8.82×10-2 |
| 91 | Ag9-O22 C21 C21 | 5.15×10-2 | 2.48×10-1 | -4.47×10-3 | -8.69×10-2 | |
| 氢键 | 119 | C24—H27…O5 | 7.98×10-3 | 3.13×10-2 | 1.44×10-3 | 1.81×10-1 |
| 130 | C24—H26…F7 | 3.67×10-3 | 1.48×10-2 | 8.45×10-4 | 2.30×10-1 | |
| 101 | C12—H18…O3 | 1.22×10-2 | 4.72×10-2 | 2.27×10-3 | 1.87×10-1 | |
| 118 | C28—H29…O3 | 1.01×10-2 | 4.08×10-2 | 1.87×10-3 | 1.86×10-1 | |
| 114 | C21—N23…O3 | 7.50×10-3 | 3.13×10-2 | 1.52×10-3 | 2.03×10-1 | |
| 84 | C46—H48…O4 | 7.34×10-3 | 3.19×10-2 | 1.58×10-3 | 2.16×10-1 | |
| 85 | C43—N45…O4 | 9.86×10-3 | 4.14×10-2 | 1.80×10-3 | 1.83×10-1 | |
| 99 | C34—H40…O4 | 9.88×10-3 | 3.63×10-2 | 1.67×10-3 | 1.69×10-1 | |
| 115 | C34—H40…F6 | 7.00×10-3 | 3.05×10-2 | 1.68×10-3 | 2.40×10-1 |
| 类型 | BCP | 原子标签 | ρ(BCP) | ?2ρ(BCP) | H(BCP) | H(BCP)/ρ(BCP) |
|---|---|---|---|---|---|---|
| 共价作用 | 153 | Ag9-C69 C67 C67 | 4.30×10-2 | 1.35×10-1 | -4.83×10-3 | -1.13×10-1 |
| 147 | Ag9-O44 C43 C43 | 4.60×10-2 | 1.73×10-1 | -2.55×10-3 | -5.55×10-2 | |
| 169 | Ag9-O22 C21 C21 | 4.41×10-2 | 2.11×10-1 | -2.90×10-3 | -6.58×10-2 | |
| 氢键 | 104 | C24—H27…O5 | 6.80×10-3 | 2.43×10-2 | 1.15×10-3 | 1.69×10-1 |
| 124 | C34—H40…O5 | 7.08×10-3 | 2.57×10-2 | 1.21×10-3 | 1.72×10-1 | |
| 117 | C64—H66…O5 | 7.04×10-3 | 2.48×10-2 | 1.15×10-3 | 1.63×10-1 | |
| 120 | C12—H18…O3 | 6.47×10-3 | 2.23×10-2 | 1.07×10-3 | 1.66×10-1 | |
| 127 | C28—H29…O3 | 1.06×10-2 | 4.29×10-2 | 1.96×10-3 | 1.85×10-1 | |
| 130 | C21—N23…O3 | 7.21×10-3 | 2.77×10-2 | 1.36×10-3 | 1.88×10-1 | |
| 99 | C46—H49…O4 | 1.05×10-2 | 3.96×10-2 | 1.93×10-3 | 1.84×10-1 | |
| 79 | C34—H40…O4 | 1.03×10-2 | 4.30×10-2 | 1.95×10-3 | 1.89×10-1 | |
| 82 | C24—H26…F7 | 2.52×10-3 | 1.06×10-2 | 6.74×10-4 | 2.68×10-1 | |
| 114 | C34—H40…F6 | 6.24×10-3 | 2.65×10-2 | 1.47×10-3 | 2.35×10-1 | |
| 100 | C64—H66…F6 | 6.13×10-3 | 2.35×10-2 | 1.24×10-3 | 2.02×10-1 | |
| 85 | C61—H63…F6 | 4.38×10-3 | 1.69×10-2 | 9.23×10-4 | 2.11×10-1 |
Table 5 Topological parameters of BCPs in Ag-DES-1- hexene
| 类型 | BCP | 原子标签 | ρ(BCP) | ?2ρ(BCP) | H(BCP) | H(BCP)/ρ(BCP) |
|---|---|---|---|---|---|---|
| 共价作用 | 153 | Ag9-C69 C67 C67 | 4.30×10-2 | 1.35×10-1 | -4.83×10-3 | -1.13×10-1 |
| 147 | Ag9-O44 C43 C43 | 4.60×10-2 | 1.73×10-1 | -2.55×10-3 | -5.55×10-2 | |
| 169 | Ag9-O22 C21 C21 | 4.41×10-2 | 2.11×10-1 | -2.90×10-3 | -6.58×10-2 | |
| 氢键 | 104 | C24—H27…O5 | 6.80×10-3 | 2.43×10-2 | 1.15×10-3 | 1.69×10-1 |
| 124 | C34—H40…O5 | 7.08×10-3 | 2.57×10-2 | 1.21×10-3 | 1.72×10-1 | |
| 117 | C64—H66…O5 | 7.04×10-3 | 2.48×10-2 | 1.15×10-3 | 1.63×10-1 | |
| 120 | C12—H18…O3 | 6.47×10-3 | 2.23×10-2 | 1.07×10-3 | 1.66×10-1 | |
| 127 | C28—H29…O3 | 1.06×10-2 | 4.29×10-2 | 1.96×10-3 | 1.85×10-1 | |
| 130 | C21—N23…O3 | 7.21×10-3 | 2.77×10-2 | 1.36×10-3 | 1.88×10-1 | |
| 99 | C46—H49…O4 | 1.05×10-2 | 3.96×10-2 | 1.93×10-3 | 1.84×10-1 | |
| 79 | C34—H40…O4 | 1.03×10-2 | 4.30×10-2 | 1.95×10-3 | 1.89×10-1 | |
| 82 | C24—H26…F7 | 2.52×10-3 | 1.06×10-2 | 6.74×10-4 | 2.68×10-1 | |
| 114 | C34—H40…F6 | 6.24×10-3 | 2.65×10-2 | 1.47×10-3 | 2.35×10-1 | |
| 100 | C64—H66…F6 | 6.13×10-3 | 2.35×10-2 | 1.24×10-3 | 2.02×10-1 | |
| 85 | C61—H63…F6 | 4.38×10-3 | 1.69×10-2 | 9.23×10-4 | 2.11×10-1 |
| 类型 | BCP | 原子标签 | ρ(BCP) | ?2ρ(BCP) | H(BCP) | H(BCP)/ρ(BCP) |
|---|---|---|---|---|---|---|
| 共价作用 | 124 | Ag9-O44 C43 C43 | 5.38×10-2 | 2.63×10-1 | -4.84×10-3 | -9.01×10-2 |
| 139 | Ag9-O22 C1 C1 | 5.23×10-2 | 2.55×10-1 | -4.23×10-3 | -8.09×10-2 | |
| 氢键 | 143 | C12—H18…O5 | 7.32×10-3 | 2.70×10-2 | 1.26×10-3 | 1.72×10-1 |
| 144 | C21—N23…O5 | 1.06×10-2 | 4.49×10-2 | 1.84×10-3 | 1.72×10-1 | |
| 136 | C28—H29…F7 | 2.24×10-3 | 8.75×10-3 | 5.07×10-4 | 2.26×10-1 | |
| 145 | C24—H26…F7 | 3.43×10-3 | 1.36×10-2 | 7.70×10-4 | 2.25×10-1 | |
| 142 | C12—H18…O3 | 8.03×10-3 | 3.02×10-2 | 1.56×10-3 | 1.94×10-1 | |
| 84 | C43—N45…O4 | 9.56×10-3 | 3.93×10-2 | 1.76×10-3 | 1.84×10-1 | |
| 88 | C34—H40…O4 | 9.70×10-3 | 3.62×10-2 | 1.63×10-3 | 1.68×10-1 | |
| 93 | C34—H40…F6 | 6.11×10-3 | 2.68×10-2 | 1.50×10-3 | 2.46×10-1 |
Table 6 Topological parameters of BCPs in Ag-DES- n-hexane
| 类型 | BCP | 原子标签 | ρ(BCP) | ?2ρ(BCP) | H(BCP) | H(BCP)/ρ(BCP) |
|---|---|---|---|---|---|---|
| 共价作用 | 124 | Ag9-O44 C43 C43 | 5.38×10-2 | 2.63×10-1 | -4.84×10-3 | -9.01×10-2 |
| 139 | Ag9-O22 C1 C1 | 5.23×10-2 | 2.55×10-1 | -4.23×10-3 | -8.09×10-2 | |
| 氢键 | 143 | C12—H18…O5 | 7.32×10-3 | 2.70×10-2 | 1.26×10-3 | 1.72×10-1 |
| 144 | C21—N23…O5 | 1.06×10-2 | 4.49×10-2 | 1.84×10-3 | 1.72×10-1 | |
| 136 | C28—H29…F7 | 2.24×10-3 | 8.75×10-3 | 5.07×10-4 | 2.26×10-1 | |
| 145 | C24—H26…F7 | 3.43×10-3 | 1.36×10-2 | 7.70×10-4 | 2.25×10-1 | |
| 142 | C12—H18…O3 | 8.03×10-3 | 3.02×10-2 | 1.56×10-3 | 1.94×10-1 | |
| 84 | C43—N45…O4 | 9.56×10-3 | 3.93×10-2 | 1.76×10-3 | 1.84×10-1 | |
| 88 | C34—H40…O4 | 9.70×10-3 | 3.62×10-2 | 1.63×10-3 | 1.68×10-1 | |
| 93 | C34—H40…F6 | 6.11×10-3 | 2.68×10-2 | 1.50×10-3 | 2.46×10-1 |
| 1 | Wentink A E, Kuipers N J M, de Haan A B, et al. Effects of ligand structure on reactive vapor-liquid distribution ratio and selectivity for C6-olefin isomers[J]. Industrial & Engineering Chemistry Research, 2005, 44(24): 9221-9229. |
| 2 | Eagan N M, Moore B M, McClelland D J, et al. Catalytic synthesis of distillate-range ethers and olefins from ethanol through Guerbet coupling and etherification[J]. Green Chemistry, 2019, 21(12): 3300-3318. |
| 3 | Wentink A E, Kuipers N J M, de Haan A B, et al. Olefin isomer separation by reactive extractive distillation: modelling of vapour-liquid equilibria and conceptual design for 1-hexene purification[J]. Chemical Engineering and Processing: Process Intensification, 2007, 46(9): 800-809. |
| 4 | Kuipers N J M, Wentink A E, de Haan A B, et al. Functionalized solvents for olefin isomer purification by reactive extractive distillation[J]. Chemical Engineering Research and Design, 2007, 85(1): 88-99. |
| 5 | 李阳, 屈一新, 王际东. 费托合成油C6馏分中提取烯烃的工艺优化模拟[J]. 北京服装学院学报(自然科学版), 2019, 39(3): 59-65. |
| Li Y, Qu Y X, Wang J D. Process simulation and optimization for separating of C6 olefins from C6-cut of Fischer-Tropsch synthesis oil[J]. Journal of Beijing Institute of Fashion Technology (Natural Science Edition), 2019, 39(3): 59-65. | |
| 6 | Piszczek R, Heins B, Hamilton P, et al. Separating linear alpha olefin involves providing a pre-processed product stream comprising linear alpha olefins to first of series of distillation columns, and element of the series of distillation columns comprising a dividing wall column: WO2020114744-A1[P]. 2020-6-11. |
| 7 | 杨正伟, 孙启文, 张宗森. 萃取精馏脱高温费托合成C6馏分中的含氧化合物[J]. 石油化工, 2016, 45(4): 402-407. |
| Yang Z W, Sun Q W, Zhang Z S. Removing oxygenates from C6 fraction in high-temperature Fisher-Tropsch synthesis products by extractive distillation[J]. Petrochemical Technology, 2016, 45(4): 402-407. | |
| 8 | Yang R H, Gao R M, Qian Z, et al. Batch and fixed bed column selective adsorption of C6, C8 and C10 linear α-olefins from binary liquid olefin/paraffin mixtures onto 5A and 13X microporous molecular sieves[J]. Separation and Purification Technology, 2020, 230: 115884. |
| 9 | Yang R H, Gao R M, Wang Y J, et al. Selective adsorption of C6, C8, and C10 linear α-olefins from binary liquid-phase olefin/paraffin mixtures using zeolite adsorbents: experiment and simulations[J]. Langmuir, 2020, 36(29): 8597-8609. |
| 10 | Safarik D J, Eldridge R B. Olefin/paraffin separations by reactive absorption: a review[J]. Industrial & Engineering Chemistry Research, 1998, 37(7): 2571-2581. |
| 11 | Sun Y L, Bi H R, Dou H Z, et al. A novel copper(I)-based supported ionic liquid membrane with high permeability for ethylene/ethane separation[J]. Industrial & Engineering Chemistry Research, 2017, 56(3): 741-749. |
| 12 | Li R L, Xing H B, Yang Q W, et al. Selective extraction of 1-hexene against n-hexane in ionic liquids with or without silver salt[J]. Industrial & Engineering Chemistry Research, 2012, 51(25): 8588-8597. |
| 13 | Rychlewska K, Kujawski W, Konieczny K. Pervaporative performance of PEBA and PDMS based commercial membranes in thiophene removal from its binary mixtures with hydrocarbons[J]. Fuel Processing Technology, 2017, 165: 9-18. |
| 14 | Smith E L, Abbott A P, Ryder K S. Deep eutectic solvents (DESs) and their applications[J]. Chemical Reviews, 2014, 114(21): 11060-11082. |
| 15 | Esfahani H S, Khoshsima A, Pazuki G. Choline chloride-based deep eutectic solvents as green extractant for the efficient extraction of 1-butanol or 2-butanol from azeotropic n-heptane + butanol mixtures[J]. Journal of Molecular Liquids, 2020, 313: 113524. |
| 16 | Liu F J, Chen W, Mi J X, et al. Thermodynamic and molecular insights into the absorption of H2S, CO2, and CH4 in choline chloride plus urea mixtures[J]. AIChE Journal, 2019, 65(5): e16574. |
| 17 | Ren X Y, Zhu X L, Xu C Y, et al. The electrodeposition of amorphous/nanocrystalline Ni-Cr alloys from ChCl-EG deep eutectic solvent[J]. Journal of The Electrochemical Society, 2020, 167(6): 062502. |
| 18 | Wadekar P H, Khose R V, Pethsangave D A, et al. One-pot synthesis of sulfur and nitrogen co-functionalized graphene material using deep eutectic solvents for supercapacitors[J]. Chem Sus Chem, 2019, 12(14): 3326-3335. |
| 19 | Li H, Zhang Z S, Sun G L, et al. Performance and mechanism of the separation of C8α-olefin from F-T synthesis products using novel Ag-DES[J]. AIChE Journal, 2021: e17252. |
| 20 | Wang Y, Thompson J, Zhou J J, et al. Use of water in aiding olefin/paraffin (liquid + liquid) extraction via complexation with a silver bis(trifluoromethylsulfonyl)imide salt[J]. The Journal of Chemical Thermodynamics, 2014, 77: 230-240. |
| 21 | Zhao Y, Truhlar D G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals[J]. Theoretical Chemistry Accounts, 2008, 120(1/2/3): 215-241. |
| 22 | Weigend F, Ahlrichs R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy[J]. Physical Chemistry Chemical Physics, 2005, 7(18): 3297-3305. |
| 23 | Bader R F W. Atoms in molecules[J]. Accounts of Chemical Research, 1985, 18(1): 9-15. |
| 24 | Ahosseini A, Sensenich B, Weatherley L R, et al. Phase equilibrium, volumetric, and interfacial properties of the ionic liquid, 1-hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)amide and 1-octene[J]. Journal of Chemical & Engineering Data, 2010, 55(4): 1611-1617. |
| 25 | Mortaheb H R, Mafi M, Mokhtarani B, et al. Experimental kinetic analysis of ethylene absorption in ionic liquid [Bmim]NO3 with dissolved AgNO3 by a semi-continuous process[J]. Chemical Engineering Journal, 2010, 158(3): 384-392. |
| 26 | Zhu L, Li F F, Zhu J Q, et al. Liquid-liquid equilibria of ternary systems of 1-hexene/hexane and extraction solvents[J]. Chemical Papers, 2016, 70(5): 585-593. |
| 27 | 李如龙. 离子液体在乙烯/乙烷、1-己烯/正己烷分离中的应用基础研究[D]. 杭州: 浙江大学, 2012. |
| Li R L. Applied fundamental research on the separation of ethylene/ethane and 1-hexene/n-hexane by ionic liquid[D]. Hangzhou: Zhejiang University, 2012. | |
| 28 | Wang Y, Hao W Y, Jacquemin J, et al. Enhancing liquid-phase olefin-paraffin separations using novel silver-based ionic liquids[J]. Journal of Chemical & Engineering Data, 2015, 60(1): 28-36. |
| 29 | Sunderrajan S, Freeman B D, Hall C K. Fourier transform infrared spectroscopic characterization of olefin complexation by silver salts in solution[J]. Industrial & Engineering Chemistry Research, 1999, 38(10): 4051-4059. |
| 30 | Okoshi M, Yamada Y, Komaba S, et al. Theoretical analysis of interactions between potassium ions and organic electrolyte solvents: a comparison with lithium, sodium, and magnesium ions[J]. Journal of the Electrochemical Society, 2016, 164(2): A54-A60. |
| 31 | Lipkowski P, Grabowski S J, Robinson T L, et al. Properties of the C—H···H dihydrogen bond: an ab initio and topological analysis[J]. The Journal of Physical Chemistry A, 2004, 108(49): 10865-10872. |
| 32 | Cremer D, Kraka E. Chemical bonds without bonding electron density—does the difference electron-density analysis suffice for a description of the chemical bond?[J]. Angewandte Chemie International Edition in English, 1984, 23(8): 627-628. |
| [1] | Yangguang LYU, Peipei ZUO, Zhengjin YANG, Tongwen XU. Triazine framework polymer membranes for methanol/n-hexane separation via organic solvent nanofiltration [J]. CIESC Journal, 2023, 74(4): 1598-1606. |
| [2] | WANG Ning, HUI Lei, CHEN Mei, LI Wei, ZHOU Qi. POSS-modified supported Ziegler-Natta catalyst and its ethylene /1-hexene copolymerization [J]. CIESC Journal, 2021, 72(4): 2102-2112. |
| [3] | WU Peiwen, XUN Suhang, JIANG Wei, LI Huaming, ZHU Wenshuai. Recent progress on extractive desulfurization of fuel oils through reactions based on ionic liquids as solvents and catalysts [J]. CIESC Journal, 2021, 72(1): 276-291. |
| [4] | LI Deliang, YU Fei, CHANG Zhixian. Reactive extraction of nicotinic acid with trialkylamine in n-octanol [J]. Chin.J.Chem.Eng., 2012, 20(5): 843-848. |
| [5] | ZHANG Luan,ZHU Hongji,BAI Peng. Separation of ethanol-isopropanol binary mixture by azeotropic distillation [J]. Chemical Industry and Engineering Progree, 2012, 31(10): 2187-2190. |
| [6] | WANG Yazhen1,2,3,WANG Libo2,CHEN Jie1,WANG Sihan2,YANG Yulin3 . Design and evaluation of ethylene trimerization catalysts with composite ligands [J]. , 2011, 30(3): 520-. |
| [7] | WANG Libo1,CHEN Jie2,WANG Yazhen2,LI Jie3,WANG Sihan1,ZHANG Baojun1. Advances of Cr(III)based catalysts for 1-hexene production [J]. , 2010, 29(3): 472-. |
| [8] | ZHU Liyang, DUAN Wuhua, XU Jingming, ZHU Yongjun. Kinetics of Reactive Extraction of Nd from Nd2O3 with TBP-HNO3 Complex in Supercritical Carbon Dioxide [J]. , 2009, 17(2): 214-218. |
| [9] | LI Deliang(李德亮), LIU Xiaoqiang(刘小强) and CUI Jiehu(崔节虎). Reactive Extraction of o-Aminophenol Using Trialkylphosphine Oxide [J]. , 2006, 14(1): 46-50. |
| [10] | QIN Wei, LI Zhenyu, WANG Min, DAI Youyuan. Extraction Behavior and Wastewater Treatment of Amino Sulfonic Acid with Alamine 336 [J]. , 2004, 12(1): 137-142. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||