CIESC Journal ›› 2024, Vol. 75 ›› Issue (12): 4563-4575.DOI: 10.11949/0438-1157.20240743
• Separation engineering • Previous Articles Next Articles
Yuxi WU1,2,3,4( ), Yuanhui TANG1,2,4, Qiang GUO1, Yakai LIN2(
), Yuanhui TANG1,2,4, Qiang GUO1, Yakai LIN2( ), Lixin YU2, Xiaolin WANG2(
), Lixin YU2, Xiaolin WANG2( )
)
Received:2024-07-01
Revised:2024-08-06
Online:2025-01-03
Published:2024-12-25
Contact:
Yakai LIN, Xiaolin WANG
吴雨茜1,2,3,4( ), 唐元晖1,2,4, 郭强1, 林亚凯2(
), 唐元晖1,2,4, 郭强1, 林亚凯2( ), 余立新2, 王晓琳2(
), 余立新2, 王晓琳2( )
)
通讯作者:
林亚凯,王晓琳
作者简介:吴雨茜(1997—),女,硕士研究生,1863138750@163.com
基金资助:CLC Number:
Yuxi WU, Yuanhui TANG, Qiang GUO, Yakai LIN, Lixin YU, Xiaolin WANG. Experimental study and simulation on nanofiltration separation of lithium and magnesium from sulfate desorption solution[J]. CIESC Journal, 2024, 75(12): 4563-4575.
吴雨茜, 唐元晖, 郭强, 林亚凯, 余立新, 王晓琳. 纳滤膜对硫酸型解吸液锂镁分离的实验研究与模拟[J]. 化工学报, 2024, 75(12): 4563-4575.
Add to citation manager EndNote|Ris|BibTeX
| 年份 | 商业化 NF膜型号 | 研究体系 | 主要离子的浓度范围/(mol/L) | 主要结论 | 文献 | |
|---|---|---|---|---|---|---|
| 2006 | DL | 稀释老卤 (Li+、Mg2+、Ca2+、Na+、K+、Cl-、 | Li+:0.052; Mg2+:0.30 | 探究了DL膜分离盐酸型老卤中锂镁的可行性,发现DL膜具有较好的锂镁分离能力,同时能有效分离Cl-和 | [ | |
| 2011 | DK | 模拟卤水 (Li+、Mg2+、Cl-) | Li+:0.014~0.038; Mg2+:0.10~0.20 | 当压力为1.6 MPa时,Li+呈现负截留,且Mg2+的截留率最高,分离因子最高为3.2,说明采用DK膜在中性条件下可以实现高镁锂比盐酸体系模拟卤水的分离 | [ | |
| 2013 | NF90 | 稀释卤水 (Li+、Mg2+、Na+、Cl-) | Li+:0.00087; Mg2+:0.013 | 压力为1.5 MPa时,NF90膜对Mg2+的截留率为100%,对Li+仅为15%,说明NF90膜对于锂镁离子的截留性能与此前的DK体系差异明显 | [ | |
| 2014/2015 | DK、DL、HL、NF270 | 模拟卤水 (Li+、Mg2+、Ca2+、Na+、K+、Cl-) | Li+:0.019~0.046; Mg2+:0.11~0.64 | 对比四种商业化纳滤膜分离盐酸性卤水中锂镁的差异,发现高通量膜(DL、NF270)的分离效果较好;且升高操作压力和降低pH均能够提升NF膜对Mg2+、Li+的选择分离性能,而高镁锂比和升高进料温度不利于分离 | [ | |
| 2014/2019 | DK、NT102、NT103、NT201、NF90、NF270、XN-45 | 模拟卤水 (Li+、Mg2+、Na+、K+、Cl-、 | Li+:0.0016~0.46; Mg2+:0.018~4.9 | NT103、NT201膜对Mg2+的截留性能较高,具有较好的镁锂分离性能;加入一价阳离子Na+、K+和H3BO3均能提升Li+透过率;加入 | [ | |
| 2014/2019/2021 | DK | 稀释老卤/模拟卤水 (Li+、Mg2+、Ca2+、Cl-、 | Li+:0.019~0.18; Mg2+:0.23~2.4 | 镁锂比增加,锂离子截留率更小,但过高镁离子含量会降低镁的截留率;高膜表面切向流速降低浓差极化,有利于镁锂分离 | [ | |
| 2019 | NF90和NF270 | 模拟卤水 (Li+、Mg2+、Cl-) | Li+:0.020; Mg2+:0.029~0.12 | 对比了两种纳滤膜的锂镁分离效果,发现高通量NF270膜更好,原料液中镁锂比下降较多 | [ | |
Table 1 Summary of research work on the separation of lithium and magnesium from brines of salt lakes by commercial NF membranes
| 年份 | 商业化 NF膜型号 | 研究体系 | 主要离子的浓度范围/(mol/L) | 主要结论 | 文献 | |
|---|---|---|---|---|---|---|
| 2006 | DL | 稀释老卤 (Li+、Mg2+、Ca2+、Na+、K+、Cl-、 | Li+:0.052; Mg2+:0.30 | 探究了DL膜分离盐酸型老卤中锂镁的可行性,发现DL膜具有较好的锂镁分离能力,同时能有效分离Cl-和 | [ | |
| 2011 | DK | 模拟卤水 (Li+、Mg2+、Cl-) | Li+:0.014~0.038; Mg2+:0.10~0.20 | 当压力为1.6 MPa时,Li+呈现负截留,且Mg2+的截留率最高,分离因子最高为3.2,说明采用DK膜在中性条件下可以实现高镁锂比盐酸体系模拟卤水的分离 | [ | |
| 2013 | NF90 | 稀释卤水 (Li+、Mg2+、Na+、Cl-) | Li+:0.00087; Mg2+:0.013 | 压力为1.5 MPa时,NF90膜对Mg2+的截留率为100%,对Li+仅为15%,说明NF90膜对于锂镁离子的截留性能与此前的DK体系差异明显 | [ | |
| 2014/2015 | DK、DL、HL、NF270 | 模拟卤水 (Li+、Mg2+、Ca2+、Na+、K+、Cl-) | Li+:0.019~0.046; Mg2+:0.11~0.64 | 对比四种商业化纳滤膜分离盐酸性卤水中锂镁的差异,发现高通量膜(DL、NF270)的分离效果较好;且升高操作压力和降低pH均能够提升NF膜对Mg2+、Li+的选择分离性能,而高镁锂比和升高进料温度不利于分离 | [ | |
| 2014/2019 | DK、NT102、NT103、NT201、NF90、NF270、XN-45 | 模拟卤水 (Li+、Mg2+、Na+、K+、Cl-、 | Li+:0.0016~0.46; Mg2+:0.018~4.9 | NT103、NT201膜对Mg2+的截留性能较高,具有较好的镁锂分离性能;加入一价阳离子Na+、K+和H3BO3均能提升Li+透过率;加入 | [ | |
| 2014/2019/2021 | DK | 稀释老卤/模拟卤水 (Li+、Mg2+、Ca2+、Cl-、 | Li+:0.019~0.18; Mg2+:0.23~2.4 | 镁锂比增加,锂离子截留率更小,但过高镁离子含量会降低镁的截留率;高膜表面切向流速降低浓差极化,有利于镁锂分离 | [ | |
| 2019 | NF90和NF270 | 模拟卤水 (Li+、Mg2+、Cl-) | Li+:0.020; Mg2+:0.029~0.12 | 对比了两种纳滤膜的锂镁分离效果,发现高通量NF270膜更好,原料液中镁锂比下降较多 | [ | |
| 解吸液编号 | Li+/ (mol/L) | Na+/ (mol/L) | K+/(mol/L) | Mg2+/(mol/L) | Ca2+/ (mol/L) | pH |
|---|---|---|---|---|---|---|
| 1 | 0.25 | 0.067 | 0.016 | 0.023 | 0.0014 | 1.20 |
| 2 | 0.18 | 0.072 | 0.022 | 0.032 | 0.0065 | 2.46 |
| 3 | 0.21 | 0.034 | 0.0026 | 0.008 | 0.0016 | 1.42 |
Table 2 The main components of the desorption solution
| 解吸液编号 | Li+/ (mol/L) | Na+/ (mol/L) | K+/(mol/L) | Mg2+/(mol/L) | Ca2+/ (mol/L) | pH |
|---|---|---|---|---|---|---|
| 1 | 0.25 | 0.067 | 0.016 | 0.023 | 0.0014 | 1.20 |
| 2 | 0.18 | 0.072 | 0.022 | 0.032 | 0.0065 | 2.46 |
| 3 | 0.21 | 0.034 | 0.0026 | 0.008 | 0.0016 | 1.42 |
| 原料液编号 | 原料液中离子浓度/(mol/L) | pH | ||||
|---|---|---|---|---|---|---|
| Li+ | Mg2+ | Ca2+ | Na+ | K+ | ||
| 1~4 | 0.19 | 0 | 0 | 0 | 0 | 7.7、4.6、3.1、1.7 |
| 5~8 | 0 | 0.093 | 0 | 0 | 0 | 7.7、4.6、3.1、1.7 |
| 9~12 | 0.14 | 0.023 | 0 | 0 | 0 | 7.7、4.6、3.1、1.7 |
| 13 | 0.019 | 0.084 | 0 | 0 | 0 | 1.7 |
| 14 | 0.046 | 0.070 | 0 | 0 | 0 | 1.7 |
| 15 | 0.14 | 0.023 | 0 | 0 | 0 | 1.7 |
| 16 | 0.17 | 0.0093 | 0 | 0 | 0 | 1.7 |
| 17 | 0.25 | 0.023 | 0.0014 | 0.067 | 0.016 | 1.7 |
Table 3 The concentrations of the feed solutions
| 原料液编号 | 原料液中离子浓度/(mol/L) | pH | ||||
|---|---|---|---|---|---|---|
| Li+ | Mg2+ | Ca2+ | Na+ | K+ | ||
| 1~4 | 0.19 | 0 | 0 | 0 | 0 | 7.7、4.6、3.1、1.7 |
| 5~8 | 0 | 0.093 | 0 | 0 | 0 | 7.7、4.6、3.1、1.7 |
| 9~12 | 0.14 | 0.023 | 0 | 0 | 0 | 7.7、4.6、3.1、1.7 |
| 13 | 0.019 | 0.084 | 0 | 0 | 0 | 1.7 |
| 14 | 0.046 | 0.070 | 0 | 0 | 0 | 1.7 |
| 15 | 0.14 | 0.023 | 0 | 0 | 0 | 1.7 |
| 16 | 0.17 | 0.0093 | 0 | 0 | 0 | 1.7 |
| 17 | 0.25 | 0.023 | 0.0014 | 0.067 | 0.016 | 1.7 |
| 离子 | Stokes半径/nm | 扩散系数/(10-9 m2/s) |
|---|---|---|
| Li+ | 0.238 | 1.03 |
| K+ | 0.125 | 1.96 |
| Na+ | 0.184 | 1.33 |
| Mg2+ | 0.347 | 0.706 |
| Ca2+ | 0.310 | 0.79 |
| 0.230 | 1.065 |
Table 4 Stokes radius and diffusion coefficients of various ions considered in this study[22-23]
| 离子 | Stokes半径/nm | 扩散系数/(10-9 m2/s) |
|---|---|---|
| Li+ | 0.238 | 1.03 |
| K+ | 0.125 | 1.96 |
| Na+ | 0.184 | 1.33 |
| Mg2+ | 0.347 | 0.706 |
| Ca2+ | 0.310 | 0.79 |
| 0.230 | 1.065 |
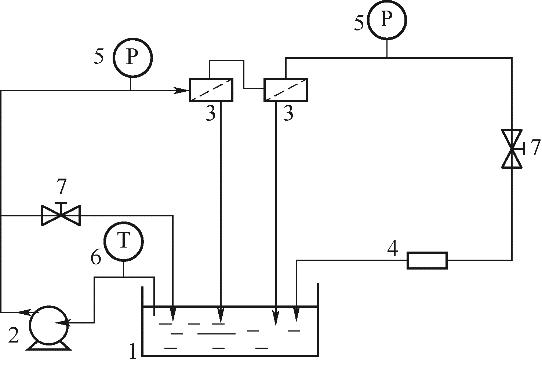
Fig.2 Schematic diagram for the cross-flow NF equipment adopted in the experiments1—raw material tank; 2—plunger pump; 3—membrane; 4—flowmeter; 5—pressure gauge; 6—thermometer; 7—valve
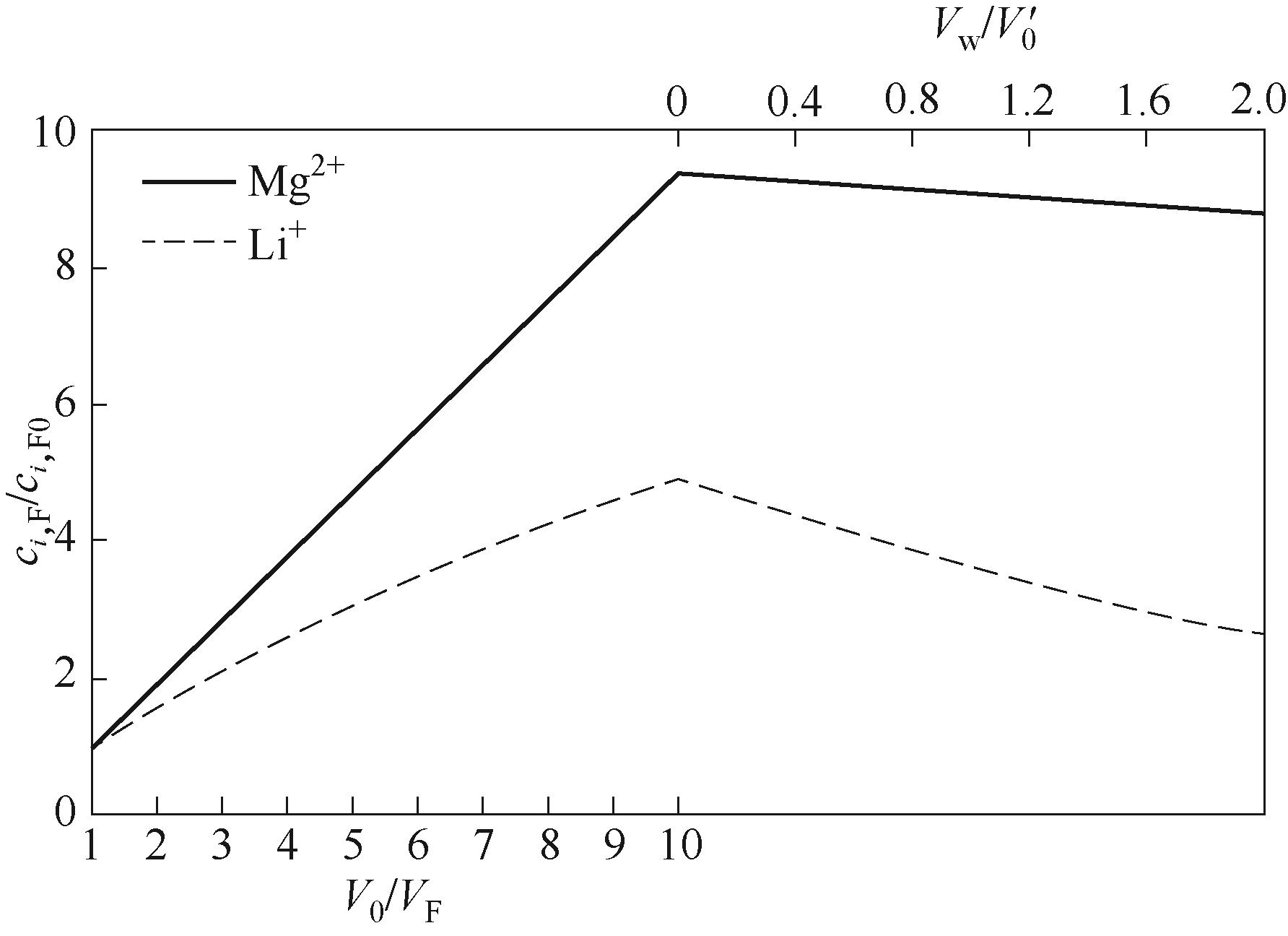
Fig.8 Relationship between the concentration ratio of Mg2+and Li+ in the feed solution during preconcentration-continuous constant volume percolation with the concentration factor (V0/VF ) and the water consumption (Vw/V0′) during the percolation process
| 渗滤过程 | 水消耗量 | 料液 | 透过液 | Li+回收率/% | ||||
|---|---|---|---|---|---|---|---|---|
| 体积 | c(Mg2+)/ (mol/L) | c(Li+)/ (mol/L) | 体积 | c(Mg2+)/ (mol/L) | c(Li+)/ (mol/L) | |||
| 初始 | — | V0 | 0.023 | 0.25 | — | — | — | — |
| 预浓缩后 | 0 | (1/10)V0 | 0.21 | 1.22 | (9/10)V0 | 0.0017 | 0.14 | 50 |
| 连续恒容渗滤后 | 1/5V0 | (1/10)V0 | 0.20 | 0.66 | (11/10)V0 | 0.0025 | 0.17 | 75 |
Table 5 Variations of water consumption, volume,concentration of feed and permeate and the yield of lithium during the whole concentrationinfiltration process
| 渗滤过程 | 水消耗量 | 料液 | 透过液 | Li+回收率/% | ||||
|---|---|---|---|---|---|---|---|---|
| 体积 | c(Mg2+)/ (mol/L) | c(Li+)/ (mol/L) | 体积 | c(Mg2+)/ (mol/L) | c(Li+)/ (mol/L) | |||
| 初始 | — | V0 | 0.023 | 0.25 | — | — | — | — |
| 预浓缩后 | 0 | (1/10)V0 | 0.21 | 1.22 | (9/10)V0 | 0.0017 | 0.14 | 50 |
| 连续恒容渗滤后 | 1/5V0 | (1/10)V0 | 0.20 | 0.66 | (11/10)V0 | 0.0025 | 0.17 | 75 |
| 1 | Alessia A, Alessandro B, Maria V G, et al. Challenges for sustainable lithium supply: a critical review[J]. Journal of Cleaner Production, 2021, 300: 126954. |
| 2 | 杨卉芃, 柳林, 丁国峰. 全球锂矿资源现状及发展趋势[J]. 矿产保护与利用, 2019(5): 26-40. |
| Yang H P, Liu L, Ding G F. Present situation and development trend of lithium resources in the world[J]. Conservation and Utilization of Mineral Resources, 2019(5): 26-40. | |
| 3 | Wang X L, Tsuru T, Togoh M, et al. Evaluation of pore structure and electrical properties of nanofiltration membranes[J]. Journal of Chemical Engineering of Japan, 1995, 28(2): 186-192. |
| 4 | Fridman-Bishop N, Nir O, Lahav O, et al. Predicting the rejection of major seawater ions by spiral-wound nanofiltration membranes[J]. Environmental Science & Technology, 2015, 49(14): 8631-8638. |
| 5 | 周久龙, 树银雪. 我国盐湖卤水提锂产业化现状及发展建议[J]. 化工矿物与加工, 2023, 52(1): 57-62. |
| Zhou J L, Shu Y X. Industrialization status and development proposal of lithium extraction from salt lake brine in China[J]. Industrial Minerals & Processing, 2023, 52(1): 57-62. | |
| 6 | 乜贞, 伍倩, 丁涛, 等. 中国盐湖卤水提锂产业化技术研究进展[J]. 无机盐工业, 2022, 54(10): 1-12. |
| Nie Z, Wu Q, Ding T, et al. Research progress on industrialization technology of lithium extraction from salt lake brine in China[J]. Inorganic Chemicals Industry, 2022, 54(10): 1-12. | |
| 7 | 靳佳奇, 李岩, 林森. 盐湖卤水吸附提锂技术研究进展[J]. 化学工程, 2023, 51(5): 20-25. |
| Jin J Q, Li Y, Lin S. Research progress of lithium extraction from salt lake brine by adsorption[J]. Chemical Engineering (China), 2023, 51(5): 20-25. | |
| 8 | Wen X M, Ma P H, Zhu C L, et al. Preliminary study on recovering lithium chloride from lithium-containing waters by nanofiltration[J]. Separation and Purification Technology, 2006, 49(3): 230-236. |
| 9 | Yang G, Shi H, Liu W Q, et al. Investigation of Mg2+/Li+ separation by nanofiltration[J]. Chinese Journal of Chemical Engineering, 2011, 19(4): 586-591. |
| 10 | Somrani A, Hamzaoui A H, Pontie M. Study on lithium separation from salt lake brines by nanofiltration (NF) and low pressure reverse osmosis (LPRO)[J]. Desalination, 2013, 317: 184-192. |
| 11 | 计超. 纳滤分离镁锂过程研究[D]. 上海: 华东理工大学, 2014. |
| Ji C. Study on separation of magnesium and lithium by nanofiltration[D]. Shanghai: East China University of Science and Technology, 2014. | |
| 12 | Sun S Y, Cai L J, Nie X Y, et al. Separation of magnesium and lithium from brine using a desal nanofiltration membrane[J]. Journal of Water Process Engineering, 2015, 7: 210-217. |
| 13 | Bi Q Y, Zhang Z Q, Zhao C Y, et al. Study on the recovery of lithium from high Mg2+/Li+ ratio brine by nanofiltration[J]. Water Science and Technology, 2014, 70(10): 1690-1694. |
| 14 | 成琪. 纳滤膜法用于硫酸镁亚型盐湖卤水锂的分离与富集[D]. 西宁: 青海大学, 2019. |
| Cheng Q. Separation and enrichment of lithium in salt lake brine of magnesium sulfate subtype by nanofiltration membrane method[D]. Xining: Qinghai University, 2019. | |
| 15 | 康为清, 时历杰, 赵有璟, 等. 纳滤法用于盐湖卤水镁锂分离的初步实验[J]. 无机盐工业, 2014, 46(12): 22-24. |
| Kang W Q, Shi L J, Zhao Y J, et al. Preliminary test of separation of Mg2+/Li+ in salt lake brine by nanofiltration[J]. Inorganic Chemicals Industry, 2014, 46(12): 22-24. | |
| 16 | Li Y, Zhao Y J, Wang H Y, et al. The application of nanofiltration membrane for recovering lithium from salt lake brine[J]. Desalination, 2019, 468: 114081. |
| 17 | 李燕, 王敏, 赵有璟, 等. 纳滤膜对高镁锂比盐湖卤水镁锂分离性能研究[J]. 化工学报, 2021, 72(6): 3130-3139. |
| Li Y, Wang M, Zhao Y J, et al. Study on separation of magnesium and lithium from salt lake brine with high magnesium-to-lithium mass ratio by nanofiltration membrane[J]. CIESC Journal, 2021, 72(6): 3130-3139. | |
| 18 | Pramanik B K, Asif M B, Kentish S, et al. Lithium enrichment from a simulated salt lake brine using an integrated nanofiltration-membrane distillation process[J]. Journal of Environmental Chemical Engineering, 2019, 7(5): 103395. |
| 19 | 燕志琴. 高浓一二价盐溶液的纳滤膜分离性能研究[D]. 北京: 清华大学, 2016. |
| Yang Z Q. Research on separation performance of mono- and divalent salt solution with high concentration by nanofiltration membrane[D]. Beijing: Tsinghua University, 2016. | |
| 20 | 徐萍, 钱晓明, 郭昌盛, 等. 用于盐湖卤水镁锂分离的纳滤技术研究进展[J]. 材料导报, 2019, 33(3): 410-417. |
| Xu P, Qian X M, Guo C S, et al. Nanofiltration technology used for separation of magnesium and lithium from salt lake brine: a survey[J]. Materials Reports, 2019, 33(3): 410-417. | |
| 21 | Gao L, Wang H Y, Zhang Y, et al. Nanofiltration membrane characterization and application: extracting lithium in lepidolite leaching solution[J]. Membranes, 2020, 10(8): 178. |
| 22 | King H W. CRC Handbook of Chemistry and Physics[M]. Boca Raton: CRC Press, 2002. |
| 23 | Nightingale E R Jr. Phenomenological theory of ion solvation. Effective radii of hydrated ions[J]. The Journal of Physical Chemistry, 1959, 63(9): 1381-1387. |
| 24 | Mohammad A W, Teow Y H, Ang W L, et al. Nanofiltration membranes review: recent advances and future prospects[J]. Desalination, 2015, 356: 226-254. |
| 25 | Su B W, Duan X J, Dou M W, et al. Charge characteristics of nanofiltration membrane by streaming potential method[J]. Advanced Materials Research, 2011, 396/397/398: 547-551. |
| 26 | 姚红娟. 纳滤膜法染料水溶液的脱盐浓缩及其过程模拟[D]. 北京: 清华大学, 2003. |
| Yao H J. Desalination and concentration of aqueous aqueous solution by nanofiltration membrane and its process simulation[D]. Beijing: Tsinghua University, 2003. | |
| 27 | 计超, 张杰, 张志君, 等. DK纳滤膜对高镁锂比卤水的分离性能研究[J]. 膜科学与技术, 2014, 34(3): 79-85. |
| Ji C, Zhang J, Zhang Z J, et al. Separation properties of magnesium and lithium from brine with high Mg2+/Li+ ratio by DK nanofiltration membrane[J]. Membrane Science and Technology, 2014, 34(3): 79-85. | |
| 28 | Park H M, Takaba H, Lee Y T. Preparation and characterization of TFC NF membrane with improved acid resistance behavior[J]. Journal of Membrane Science, 2020, 616: 118620. |
| 29 | Gao S L, Qin Z X, Wang B F, et al. Lithium recovery from the spent lithium-ion batteries by commercial acid-resistant nanofiltration membranes: a comparative study[J]. Desalination, 2024, 572: 117142. |
| 30 | Luo J Q, Wan Y H. Effects of pH and salt on nanofiltration—a critical review[J]. Journal of Membrane Science, 2013, 438: 18-28. |
| 31 | Jun B M, Cho J, Jang A, et al. Charge characteristics (surface charge vs. zeta potential) of membrane surfaces to assess the salt rejection behavior of nanofiltration membranes[J]. Separation and Purification Technology, 2020, 247: 117026. |
| 32 | Wang D X, Su M, Yu Z Y, et al. Separation performance of a nanofiltration membrane influenced by species and concentration of ions[J]. Desalination, 2005, 175(2): 219-225. |
| 33 | Silva V, Geraldes V, A M Brites Alves, et al. Multi-ionic nanofiltration of highly concentrated salt mixtures in the seawater range[J]. Desalination, 2011, 277(1/2/3): 29-39. |
| [1] | Yangguang LYU, Peipei ZUO, Zhengjin YANG, Tongwen XU. Triazine framework polymer membranes for methanol/n-hexane separation via organic solvent nanofiltration [J]. CIESC Journal, 2023, 74(4): 1598-1606. |
| [2] | Guoli ZHOU, Xiangke HAN, Wenjia WU, Jingtao WANG, Maowa ZHANG, Fengli LI. Construction heterostructure g-C3N4@AM lamellar membrane and its performance of organic solvent nanofiltation [J]. CIESC Journal, 2022, 73(2): 941-950. |
| [3] | Houhu ZHANG, Xiaoli WU, Chongchong CHEN, Jingjing CHEN, Jingtao WANG. Preparation of 2D lamellar CD-MOF membranes for accurate separation of mixed solvents [J]. CIESC Journal, 2022, 73(10): 4539-4550. |
| [4] | LIU Jiawei, HAO Yufeng, SU Yanlei. Biomimetic modification and stability of graphene quantum dots nanofiltration membranes [J]. CIESC Journal, 2021, 72(6): 3390-3398. |
| [5] | LI Yan, WANG Min, ZHAO Youjing, WANG Huaiyou, YANG Hongjun, ZHU Zenghu. Study on separation of magnesium and lithium from salt lake brine with high magnesium-to-lithium mass ratio by nanofiltration membrane [J]. CIESC Journal, 2021, 72(6): 3130-3139. |
| [6] | RAN Jin,HUANG Qiang,AI Xinyu,WU Yuying,ZHANG Pengpeng,DOU Yan. Construction of Zn-BTC/MoS2 composite two dimensional membranes and performance of organic solvent nanofiltation [J]. CIESC Journal, 2021, 72(4): 2148-2155. |
| [7] | LU Zhibin, XIE Xing, LU Sida, HE Chang, ZHANG Bingjian, CHEN Qinglin. Surrogate model-based optimal design of multi-stage nanofiltration separation system for saline wastewater [J]. CIESC Journal, 2021, 72(3): 1400-1408. |
| [8] | HE Pengpeng, ZHAO Song, MAO Chenyue, WANG Zhi, WANG Jixiao. Research progress of solvent-resistant composite nanofiltration membrane [J]. CIESC Journal, 2021, 72(2): 727-747. |
| [9] | YANG Fengrui, WANG Zhi, YAN Fangzheng, HAN Xianglei, WANG Jixiao. Progress in separation of monovalent/divalent inorganic salt solutions by nanofiltration [J]. CIESC Journal, 2021, 72(2): 799-813. |
| [10] | LIU Ning, CHU Changhui, WANG Qian, SUN Shipeng. Preparation of nanofiltration membrane for separation of mixed monovalent salts [J]. CIESC Journal, 2021, 72(1): 578-588. |
| [11] | Ju WANG, Shufeng NIU, Ying FEI, Hong QI. Fabrication and stability of GO/Al2O3 composite nanofiltration membranes [J]. CIESC Journal, 2020, 71(6): 2795-2803. |
| [12] | Yanqing XU, Wenfei LI, Mengyao WU, Jiangnan SHEN. Preparation of self-assembled graphene oxide / nano TiO2 composite nanofiltration membrane for inkjet printing dye [J]. CIESC Journal, 2020, 71(3): 1352-1361. |
| [13] | Lixue LIU, Shaofeng ZHANG, Changwei ZHAO, Erhu BAOLE, Ling YU, Jun WANG. Preparation of composite nanofiltration membrane with β-cyclodextrin as aqueous monomer and dye rejection properties [J]. CIESC Journal, 2020, 71(2): 889-898. |
| [14] | Yanjun XU, Zehai XU, Qin MENG, Chong SHEN, Rui HOU, Guoliang ZHANG. Preparation and performance of novel rGO/uCN composite nanofiltration membrane [J]. CIESC Journal, 2019, 70(9): 3565-3572. |
| [15] | Yuanhui TANG, Yang HU, Zhiqin YAN, Chunyu LI. Experimental study on nanofiltration separation of high concentrated saline glyphosate solution [J]. CIESC Journal, 2019, 70(7): 2574-2583. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||