CIESC Journal ›› 2024, Vol. 75 ›› Issue (4): 1333-1354.DOI: 10.11949/0438-1157.20231376
• Reviews and monographs • Previous Articles Next Articles
Wenchao JIANG1,2( ), Zhaochao XU1,2(
), Zhaochao XU1,2( )
)
Received:2023-12-26
Revised:2024-02-18
Online:2024-06-07
Published:2024-04-25
Contact:
Zhaochao XU
通讯作者:
徐兆超
作者简介:江文钞(1994—),男,博士研究生,jiangwc2018@ dicp.ac.cn
基金资助:CLC Number:
Wenchao JIANG, Zhaochao XU. Fluorescent dyes for super-resolution imaging of organelles[J]. CIESC Journal, 2024, 75(4): 1333-1354.
江文钞, 徐兆超. 细胞器超分辨成像荧光染料[J]. 化工学报, 2024, 75(4): 1333-1354.
Add to citation manager EndNote|Ris|BibTeX
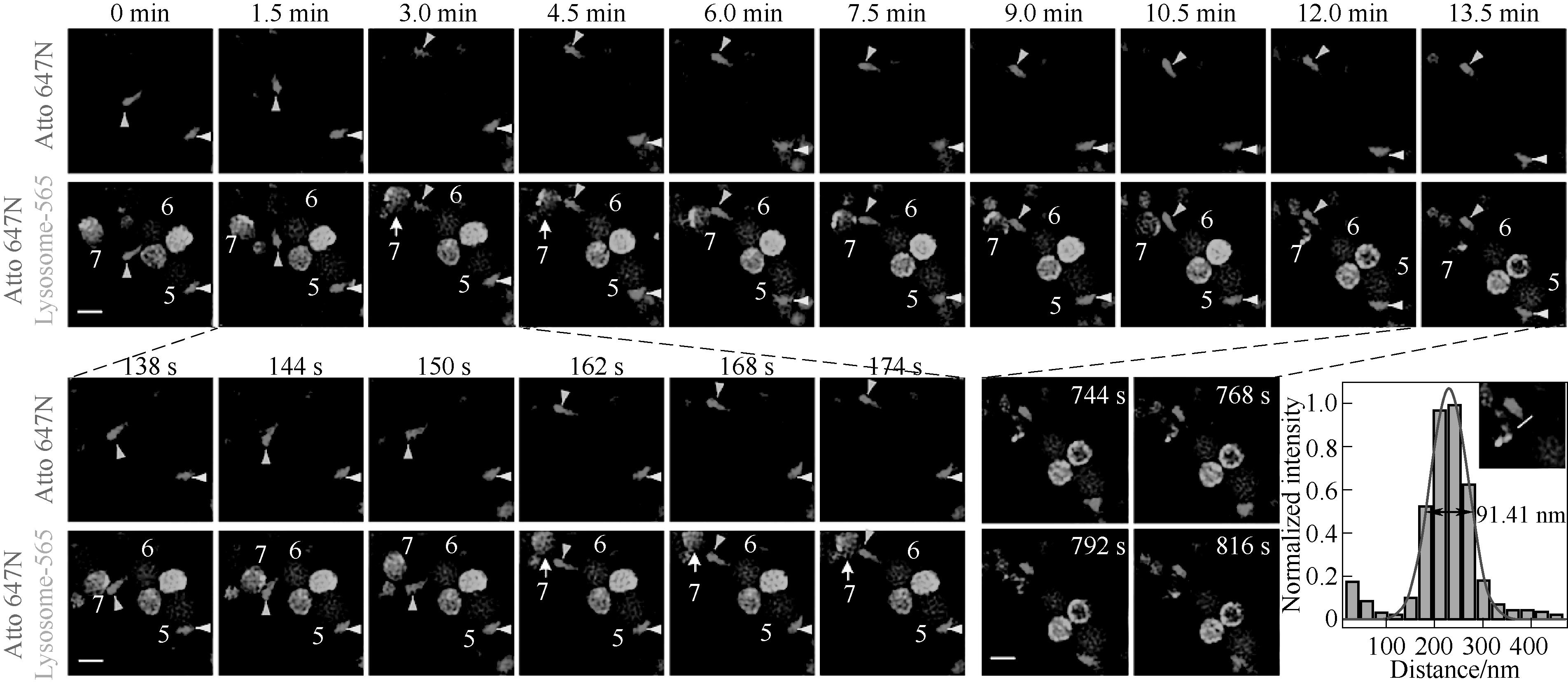
Fig.5 Two-color super-resolution dynamic imaging of lysosomal and mitochondrial dyes by cell-penetrating peptide-targeted lysosomal dyes and commercial mitochondrial dyes[40]
| 70 | Bucevičius J, Gilat T, Lukinavičius G. Far-red switching DNA probes for live cell nanoscopy[J]. Chemical Communications, 2020, 56(94): 14797-14800. |
| 71 | Xu J Q, Sun X J, Kim K, et al. Ultrastructural visualization of chromatin in cancer pathogenesis using a simple small-molecule fluorescent probe[J]. Science Advances, 2022, 8(9): eabm8293. |
| 72 | Liu J J, Gu Q M, Du W, et al. Nucleolar RNA in action: ultrastructure revealed during protein translation through a terpyridyl manganese ( Ⅱ ) complex[J]. Biosensors and Bioelectronics, 2022, 203: 114058. |
| 73 | Qiao Q L, Liu W J, Chi W J, et al. Modulation of dynamic aggregation in fluorogenic SNAP-tag probes for long-term super-resolution imaging[J]. Aggregate, 2023, 4(2): e258. |
| 1 | Jaswal S, Kumar J. Review on fluorescent donor-acceptor conjugated system as molecular probes[J]. Materials Today: Proceedings, 2020, 26: 566-580. |
| 2 | Zeng S, Liu X S, Kafuti Y S, et al. Fluorescent dyes based on rhodamine derivatives for bioimaging and therapeutics: recent progress, challenges, and prospects[J]. Chemical Society Reviews, 2023, 52(16): 5607-5651. |
| 3 | Alander J T, Kaartinen I, Laakso A, et al. A review of indocyanine green fluorescent imaging in surgery[J]. International Journal of Biomedical Imaging, 2012, 2012: 940585. |
| 4 | Fu Q F, Shen S Y, Sun P W, et al. Bioorthogonal chemistry for prodrug activation in vivo [J]. Chemical Society Reviews, 2023, 52(22): 7737-7772. |
| 5 | Scinto S L, Bilodeau D A, Hincapie R, et al. Bioorthogonal chemistry[J]. Nature Reviews Methods Primers, 2021, 1: 30. |
| 6 | Lardon N, Wang L, Tschanz A, et al. Systematic tuning of rhodamine spirocyclization for super-resolution microscopy[J]. Journal of the American Chemical Society, 2021, 143(36): 14592-14600. |
| 7 | Sunbul M, Lackner J, Martin A, et al. Super-resolution RNA imaging using a rhodamine-binding aptamer with fast exchange kinetics[J]. Nature Biotechnology, 2021, 39: 686-690. |
| 8 | Liu X G, Qiao Q L, Tian W M, et al. Aziridinyl fluorophores demonstrate bright fluorescence and superior photostability by effectively inhibiting twisted intramolecular charge transfer[J]. Journal of the American Chemical Society, 2016, 138(22): 6960-6963. |
| 9 | Wang C, Chi W J, Qiao Q L, et al. Twisted intramolecular charge transfer (TICT) and twists beyond TICT: from mechanisms to rational designs of bright and sensitive fluorophores[J]. Chemical Society Reviews, 2021, 50(22): 12656-12678. |
| 10 | Wang C, Qiao Q L, Chi W J, et al. Quantitative design of bright fluorophores and AIEgens by the accurate prediction of twisted intramolecular charge transfer (TICT)[J]. Angewandte Chemie International Edition, 2020, 59(25): 10160-10172. |
| 11 | Yadav A, Rao C, Nandi C K. Fluorescent probes for super-resolution microscopy of lysosomes[J]. ACS Omega, 2020, 5(42): 26967-26977. |
| 12 | Jakobs S, Stephan T, Ilgen P, et al. Light microscopy of mitochondria at the nanoscale[J]. Annual Review of Biophysics, 2020, 49: 289-308. |
| 13 | Samanta S, He Y, Sharma A, et al. Fluorescent probes for nanoscopic imaging of mitochondria[J]. Chem, 2019, 5(7): 1697-1726. |
| 14 | Zhao Y Y, Shi W, Li X H, et al. Recent advances in fluorescent probes for lipid droplets[J]. Chemical Communications, 2022, 58(10): 1495-1509. |
| 15 | Stone M B, Shelby S A, Veatch S L. Super-resolution microscopy: shedding light on the cellular plasma membrane[J]. Chemical Reviews, 2017, 117(11): 7457-7477. |
| 16 | Gao P, Pan W, Li N, et al. Fluorescent probes for organelle-targeted bioactive species imaging[J]. Chemical Science, 2019, 10(24): 6035-6071. |
| 17 | Dadina N, Tyson J, Zheng S, et al. Imaging organelle membranes in live cells at the nanoscale with lipid-based fluorescent probes[J]. Current Opinion in Chemical Biology, 2021, 65: 154-162. |
| 18 | Han R C, Li Z H, Fan Y Y, et al. Recent advances in super-resolution fluorescence imaging and its applications in biology[J]. Journal of Genetics and Genomics, 2013, 40(12): 583-595. |
| 19 | Duan X X, Zhang M, Zhang Y H. Organic fluorescent probes for live-cell super-resolution imaging[J]. Frontiers of Optoelectronics, 2023, 16(1): 34. |
| 20 | Nollmann M, Georgieva M. Superresolution microscopy for bioimaging at the nanoscale: from concepts to applications in the nucleus[J]. Research and Reports in Biology, 2015, 2015(6): 157. |
| 21 | Chung K K H, Zhang Z, Kidd P, et al. Fluorogenic DNA-PAINT for faster, low-background super-resolution imaging[J]. Nature Methods, 2022, 19: 554-559. |
| 22 | Zhai R X, Fang B, Lai Y Q, et al. Small-molecule fluorogenic probes for mitochondrial nanoscale imaging[J]. Chemical Society Reviews, 2023, 52(3): 942-972. |
| 23 | Fang H B, Chen Y C, Geng S S, et al. Super-resolution imaging of mitochondrial HClO during cell ferroptosis using a near-infrared fluorescent probe[J]. Analytical Chemistry, 2022, 94(51): 17904-17912. |
| 24 | Li W, Pan W H, Huang M N, et al. Disulfide-reduction-triggered spontaneous photoblinking Cy5 probe for nanoscopic imaging of mitochondrial dynamics in live cells[J]. Analytical Chemistry, 2021, 93(4): 2596-2602. |
| 25 | Wang H W, Fang B, Peng B, et al. Recent advances in chemical biology of mitochondria targeting[J]. Frontiers in Chemistry, 2021, 9: 683220. |
| 26 | Yang Z G, Xiong J, Zhang W, et al. A reversibly intramolecular cyclization Cy5 optical probe for stochastic optical reconstruction microscopy in live cell mitochondria[J]. Acta Chimica Sinica, 2020, 78(2): 130. |
| 27 | Zhang J, Samanta S, Wang J L, et al. Study on a novel probe for stimulated emission depletion super-resolution imaging of mitochondria[J]. Acta Physica Sinica, 2020, 69(16): 168702. |
| 28 | Wang C G, Taki M, Sato Y, et al. A photostable fluorescent marker for the superresolution live imaging of the dynamic structure of the mitochondrial cristae[J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(32): 15817-15822. |
| 29 | Zheng S, Dadina N, Mozumdar D, et al. Long-term super-resolution inner mitochondrial membrane imaging with a lipid probe[J]. Nature Chemical Biology, 2024, 20: 83-92. |
| 30 | Ren W, Ge X C, Li M Q, et al. Visualization of mitochondrial cristae and mtDNA evolvement and interactions with super-resolution microscopy[J]. bioRxiv, 2022. DOI: 10.1101/2022.12.26.521907 . |
| 31 | Yang X S, Yang Z G, Wu Z Y, et al. Mitochondrial dynamics quantitatively revealed by STED nanoscopy with an enhanced squaraine variant probe[J]. Nature Communications, 2020, 11: 3699. |
| 32 | Kompa J, Bruins J, Glogger M, et al. Exchangeable HaloTag ligands for super-resolution fluorescence microscopy[J]. Journal of the American Chemical Society, 2023, 145(5): 3075-3083. |
| 33 | Holtmannspötter M, Wienbeuker E, Dellmann T, et al. Reversible live-cell labeling with retro-engineered HaloTags enables long-term high- and super-resolution imaging[J]. Angewandte Chemie International Edition, 2023, 62(18): e202219050. |
| 34 | Fan M T, An H Y, Wang C F, et al. STED imaging the dynamics of lysosomes by dually fluorogenic Si-rhodamine[J]. Chemistry, 2021, 27(37): 9620-9626. |
| 35 | Liu L Y, Fang H B, Chen Q X, et al. Multiple-color platinum complex with super-large stokes shift for super-resolution imaging of autolysosome escape[J]. Angewandte Chemie International Edition, 2020, 59(43): 19229-19236. |
| 36 | Lv Z, Man Z W, Cui H T, et al. Red AIE luminogens with tunable organelle specific anchoring for live cell dynamic super resolution imaging[J]. Advanced Functional Materials, 2021, 31(10): 2009329. |
| 37 | Wang H, Fang G Q, Chen H M, et al. Lysosome-targeted biosensor for the super-resolution imaging of lysosome-mitochondrion interaction[J]. Frontiers in Pharmacology, 2022, 13: 865173. |
| 38 | Ye Z W, Zheng Y, Peng X J, et al. Surpassing the background barrier for multidimensional single-molecule localization super-resolution imaging: a case of lysosome-exclusively turn-on probe[J]. Analytical Chemistry, 2022, 94(22): 7990-7995. |
| 39 | Pan D, Hu Z, Qiu F W, et al. A general strategy for developing cell-permeable photo-modulatable organic fluorescent probes for live-cell super-resolution imaging[J]. Nature Communications, 2014, 5: 5573. |
| 40 | Han Y B, Li M H, Qiu F W, et al. Cell-permeable organic fluorescent probes for live-cell long-term super-resolution imaging reveal lysosome-mitochondrion interactions[J]. Nature Communications, 2017, 8: 1307. |
| 41 | Qiao Q L, Liu W J, Chen J, et al. An acid-regulated self-blinking fluorescent probe for resolving whole-cell lysosomes with long-term nanoscopy[J]. Angewandte Chemie International Edition, 2022, 61(21): e202202961. |
| 42 | Wang C G, Taki M, Kajiwara K, et al. Phosphole-oxide-based fluorescent probe for super-resolution stimulated emission depletion live imaging of the lysosome membrane[J]. ACS Materials Letters, 2020, 2(7): 705-711. |
| 43 | Lesiak L, Dadina N, Zheng S, et al. A bright, photostable, and far-red dye that enables multicolor, time-lapse, and super-resolution imaging of acidic organelles[J]. ACS Central Science, 2023, 10(1): 19-27. |
| 44 | Takakura H, Zhang Y D, Erdmann R S, et al. Long time-lapse nanoscopy with spontaneously blinking membrane probes[J]. Nature Biotechnology, 2017, 35: 773-780. |
| 45 | Thompson A D, Omar M H, Rivera-Molina F, et al. Long-term live-cell STED nanoscopy of primary and cultured cells with the plasma membrane HIDE probe DiI-SiR[J]. Angewandte Chemie International Edition, 2017, 56(35): 10408-10412. |
| 46 | Lorizate M, Terrones O, Nieto-Garai J A, et al. Super-resolution microscopy using a bioorthogonal-based cholesterol probe provides unprecedented capabilities for imaging nanoscale lipid heterogeneity in living cells[J]. Small Methods, 2021, 5(9): e2100430. |
| 47 | Aparin I O, Yan R, Pelletier R, et al. Fluorogenic dimers as bright switchable probes for enhanced super-resolution imaging of cell membranes[J]. Journal of the American Chemical Society, 2022, 144(39): 18043-18053. |
| 48 | Danylchuk D I, Moon S, Xu K, et al. Switchable solvatochromic probes for live-cell super-resolution imaging of plasma membrane organization[J]. Angewandte Chemie International Edition, 2019, 58(42): 14920-14924. |
| 49 | Moon S, Yan R, Kenny S J, et al. Spectrally resolved, functional super-resolution microscopy reveals nanoscale compositional heterogeneity in live-cell membranes[J]. Journal of the American Chemical Society, 2017, 139(32): 10944-10947. |
| 50 | Yan R, Chen K, Xu K. Probing nanoscale diffusional heterogeneities in cellular membranes through multidimensional single-molecule and super-resolution microscopy[J]. Journal of the American Chemical Society, 2020, 142(44): 18866-18873. |
| 51 | Cao M Y, Zhu T, Zhao M Y, et al. Structure rigidification promoted ultrabright solvatochromic fluorescent probes for super-resolution imaging of cytosolic and nuclear lipid droplets[J]. Analytical Chemistry, 2022, 94(30): 10676-10684. |
| 52 | Liu G N, Zheng H L, Zhou R, et al. Ultrabright organic fluorescent probe for quantifying the dynamics of cytosolic/nuclear lipid droplets[J]. Biosensors and Bioelectronics, 2023, 241: 115707. |
| 53 | Connor D O, Byrne A, Berselli G B, et al. Mega-stokes pyrene ceramide conjugates for STED imaging of lipid droplets in live cells[J]. The Analyst, 2019, 144(5): 1608-1621. |
| 54 | Wu M Y, Leung J K, Kam C, et al. A near-infrared AIE probe for super-resolution imaging and nuclear lipid droplet dynamic study[J]. Materials Chemistry Frontiers, 2021, 5(7): 3043-3049. |
| 55 | Xu H K, Zhang H H, Liu G, et al. Coumarin-based fluorescent probes for super-resolution and dynamic tracking of lipid droplets[J]. Analytical Chemistry, 2019, 91(1): 977-982. |
| 56 | Zhang C Y, Shao H R, Zhang J, et al. Long-term live-cell lipid droplet-targeted biosensor development for nanoscopic tracking of lipid droplet-mitochondria contact sites[J]. Theranostics, 2021, 11(16): 7767-7778. |
| 57 | Zheng X J, Zhu W C, Ni F, et al. Simultaneous dual-colour tracking lipid droplets and lysosomes dynamics using a fluorescent probe[J]. Chemical Science, 2018, 10(8): 2342-2348. |
| 58 | Zheng X J, Zhu W C, Ni F, et al. A specific bioprobe for super-resolution fluorescence imaging of lipid droplets[J]. Sensors and Actuators B: Chemical, 2018, 255: 3148-3154. |
| 59 | 周日, 王晨光, 卢革宇. 用于细胞脂滴超分辨荧光成像的有机荧光探针研究进展[J]. 中国光学, 2022, 15(6): 1228-1242. |
| Zhou R, Wang C G, Lu G Y. Advances in organic fluorescent probes for super-resolution imaging of cellular lipid droplets[J]. Chinese Optics, 2022, 15(6): 1228-1242. | |
| 60 | Taki M, Kajiwara K, Yamaguchi E, et al. Fused thiophene-S, S-dioxide-based super-photostable fluorescent marker for lipid droplets[J]. ACS Materials Letters, 2021, 3(1): 42-49. |
| 61 | Zhou R, Wang C G, Liang X S, et al. Stimulated emission depletion (STED) super-resolution imaging with an advanced organic fluorescent probe: visualizing the cellular lipid droplets at the unprecedented nanoscale resolution[J]. ACS Materials Letters, 2021, 3(5): 516-524. |
| 62 | Liu G N, Peng G S, Dai J N, et al. STED nanoscopy imaging of cellular lipid droplets employing a superior organic fluorescent probe[J]. Analytical Chemistry, 2021, 93(44): 14784-14791. |
| 63 | Zhou R, Liu G N, Li D, et al. An advanced organic molecular probe for multimodal fluorescence imaging of cellular lipid droplets[J]. Sensors and Actuators B: Chemical, 2023, 387: 133772. |
| 64 | Chen J, Wang C, Liu W J, et al. Stable super-resolution imaging of lipid droplet dynamics through a buffer strategy with a hydrogen-bond sensitive fluorogenic probe[J]. Angewandte Chemie International Edition, 2021, 60(47): 25104-25113. |
| 65 | Bucevičius J, Lukinavičius G, Gerasimaitė R. The use of hoechst dyes for DNA staining and beyond[J]. Chemosensors, 2018, 6(2): 18. |
| 66 | Nakamura A, Takigawa K, Kurishita Y, et al. Hoechst tagging: a modular strategy to design synthetic fluorescent probes for live-cell nucleus imaging[J]. Chemical Communications, 2014, 50(46): 6149-6152. |
| 67 | Lukinavičius G, Blaukopf C, Pershagen E, et al. SiR-Hoechst is a far-red DNA stain for live-cell nanoscopy[J]. Nature Communications, 2015, 6: 8497. |
| 68 | Zhang X D, Ye Z W, Zhang X F, et al. A targetable fluorescent probe for dSTORM super-resolution imaging of live cell nucleus DNA[J]. Chemical Communications, 2019, 55(13): 1951-1954. |
| 69 | Bucevičius J, Keller-Findeisen J, Gilat T, et al. Rhodamine-Hoechst positional isomers for highly efficient staining of heterochromatin[J]. Chemical Science, 2019, 10(7): 1962-1970. |
| [1] | Dongfei LIU, Fan ZHANG, Zheng LIU, Diannan LU. A review of machine learning potentials and their applications to molecular simulation [J]. CIESC Journal, 2024, 75(4): 1241-1255. |
| [2] | Haoqi CHEN, Bohui SHI, Qi PENG, Qi KANG, Shangfei SONG, Haiyuan YAO, Haihong CHEN, Haihao WU, Jing GONG. Phase equilibrium calculation of acid/alcohol hydrocarbon and water system based on stability analysis [J]. CIESC Journal, 2024, 75(3): 789-800. |
| [3] | Shiliang GU, Boren TAN, Quanzhong CHENG, Weijie YAO, Zhipeng DONG, Feng XU, Yong WANG. Numerical simulation of hydraulic characteristics in axial flow pump type mixer [J]. CIESC Journal, 2024, 75(3): 815-822. |
| [4] | Xinzi ZHOU, Zenghui LI, Xianyang MENG, Jiangtao WU. Experimental study on viscosity of high purity air at low temperatures [J]. CIESC Journal, 2024, 75(3): 782-788. |
| [5] | Baiping XU, Ruifeng LIANG, Huiwen YU, Guiqun WU, Shuping XIAO. Simulation of intra-cavity distribution mixing under the action of enhanced triangular rotor of twin-screw extruder [J]. CIESC Journal, 2024, 75(3): 858-866. |
| [6] | Yujiao ZENG, Xin XIAO, Gang YANG, Yibo ZHANG, Guangming ZHENG, Fang LI, Fengling WANG. Surrogate modeling and optimization of wet phosphoric acid production process based on mechanism and data hybrid driven [J]. CIESC Journal, 2024, 75(3): 936-944. |
| [7] | Wenkai CHENG, Jinyu YAN, Jiajun WANG, Lianfang FENG. Research progress of horizontal kneading reactor and its application in polymerization industry [J]. CIESC Journal, 2024, 75(3): 768-781. |
| [8] | Xiaobin ZHAN, Huibin WANG, Yalong JIANG, Tielin SHI. Research on power consumption characteristics of high viscosity fluid mixing in acoustic resonance mixer [J]. CIESC Journal, 2024, 75(2): 531-542. |
| [9] | Yating LI, Zhongdong WANG, Yanpeng DONG, Chunying ZHU, Youguang MA, Taotao FU. Research progress of capillary flow in microchannels and its engineering application [J]. CIESC Journal, 2024, 75(1): 159-170. |
| [10] | Wenqi ZHAO, Yanjun DENG, Chunying ZHU, Taotao FU, Youguang MA. Research progress on nanoparticle stabilizing Pickering emulsion and droplet coalescence dynamics [J]. CIESC Journal, 2024, 75(1): 33-46. |
| [11] | Xin YANG, Wen WANG, Kai XU, Fanhua MA. Simulation analysis of temperature characteristics of the high-pressure hydrogen refueling process [J]. CIESC Journal, 2023, 74(S1): 280-286. |
| [12] | Siyu ZHANG, Yonggao YIN, Pengqi JIA, Wei YE. Study on seasonal thermal energy storage characteristics of double U-shaped buried pipe group [J]. CIESC Journal, 2023, 74(S1): 295-301. |
| [13] | Minghui CHANG, Lin WANG, Jiajia YUAN, Yifei CAO. Study on the cycle performance of salt solution-storage-based heat pump [J]. CIESC Journal, 2023, 74(S1): 329-337. |
| [14] | Mingkun XIAO, Guang YANG, Yonghua HUANG, Jingyi WU. Numerical study on bubble dynamics of liquid oxygen at a submerged orifice [J]. CIESC Journal, 2023, 74(S1): 87-95. |
| [15] | Lisen BI, Bin LIU, Hengxiang HU, Tao ZENG, Zhuorui LI, Jianfei SONG, Hanming WU. Molecular dynamics study on evaporation modes of nanodroplets at rough interfaces [J]. CIESC Journal, 2023, 74(S1): 172-178. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||