CIESC Journal ›› 2025, Vol. 76 ›› Issue (12): 6398-6409.DOI: 10.11949/0438-1157.20250514
• Catalysis, kinetics and reactors • Previous Articles Next Articles
Ming XIA1( ), Shuai HUANG2, Hui SHI3, Congcong NIU4, Debao LI5, Xu QIAO1,6
), Shuai HUANG2, Hui SHI3, Congcong NIU4, Debao LI5, Xu QIAO1,6
Received:2025-05-09
Revised:2025-06-13
Online:2026-01-23
Published:2025-12-31
Contact:
Ming XIA
夏铭1( ), 黄帅2, 石慧3, 牛丛丛4, 李德宝5, 乔旭1,6
), 黄帅2, 石慧3, 牛丛丛4, 李德宝5, 乔旭1,6
通讯作者:
夏铭
作者简介:夏铭(1987—),男,博士,副研究员,Sciengart@163.com, mxia@njtech.edu.cn
基金资助:CLC Number:
Ming XIA, Shuai HUANG, Hui SHI, Congcong NIU, Debao LI, Xu QIAO. The hybrid lumping macroscopic kinetic model for cobalt-based fischer-tropsch synthesis and its application in industrial single-tube simulation[J]. CIESC Journal, 2025, 76(12): 6398-6409.
夏铭, 黄帅, 石慧, 牛丛丛, 李德宝, 乔旭. 钴基费托合成复合集总宏观动力学模型及工业单管模拟[J]. 化工学报, 2025, 76(12): 6398-6409.
Add to citation manager EndNote|Ris|BibTeX
| 时间 | M0 | 反应条件 | 放料间隔/h | 产物生成质量/g | CO 总转化率/ % | CO单程转化率/% | 进料中各组分与 CO的摩尔比 | 单程转化的 CO物质的量/mol | 系数 | 实验数据计算的平均 分子量/(g/mol) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T/K | P/MPa | U | V | V0/L | t | CH4 | C3H8 | C12H26 | C29H60 | H2O | x | xexp | xcalc | R1 | R2 | R3 | R4 | mCO | A | B | C | D | E | |||||||
| 2019.06.02 | 3.914 | 464.05 | 6.07 | 2.121 | 141.346 | 141346 | 8 | 1771.64 | 601.57 | 2948.62 | 2964.91 | 9924 | 79.18 | 31.378 | 30.97 | 2.414 | 0 | 0.295 | 0.205 | 1276.53 | 0.087 | 3.821 | 4.761 | 1.707 | 140.862 | 16 | 40.22 | 158.24 | 443.8 | 18 |
| 2019.06.05 | 4.087 | 467.85 | 6.08 | 2.009 | 137.583 | 137583 | 8 | 2026.62 | 773.86 | 3573.76 | 2482.79 | 10898 | 75.69 | 36.038 | 36.46 | 2.431 | 0 | 0.444 | 0.212 | 1137.498 | 0.111 | 4.616 | 5.335 | 1.443 | 148.139 | 16 | 41.02 | 163.9 | 420.89 | 18 |
| 2019.06.09 | 3.534 | 473.35 | 6.07 | 1.994 | 136.235 | 136235 | 8 | 1871.86 | 684.61 | 4503.84 | 2803.50 | 11687 | 77.48 | 34.439 | 33.94 | 1.921 | 0 | 0.421 | 0.192 | 1333.259 | 0.088 | 4.799 | 7.632 | 1.963 | 183.702 | 16 | 40.36 | 166.96 | 404.16 | 18 |
| 2019.06.10 | 3.303 | 473.45 | 6.06 | 2.102 | 139.371 | 139371 | 8 | 1735.6 | 658.86 | 3798.21 | 3561.24 | 11469 | 74.36 | 30.922 | 30.48 | 1.804 | 0 | 0.312 | 0.187 | 1400.732 | 0.077 | 4.977 | 7.082 | 2.418 | 192.905 | 16 | 40.08 | 162.37 | 445.87 | 18 |
| 2019.06.12 | 3.150 | 473.65 | 6.08 | 1.971 | 139.147 | 139147 | 8 | 1241.75 | 545.73 | 3890.46 | 3719.36 | 11437 | 74.07 | 29.439 | 29.98 | 1.675 | 0 | 0.244 | 0.231 | 1460.689 | 0.053 | 4.422 | 7.346 | 2.580 | 201.711 | 16 | 39.18 | 168.13 | 457.73 | 18 |
| 2019.06.13 | 3.001 | 473.65 | 6.08 | 1.996 | 142.245 | 142245 | 8 | 1273.47 | 561.31 | 3882.04 | 3647.42 | 11252 | 73.37 | 28.011 | 27.96 | 1.598 | 0 | 0.207 | 0.196 | 1552.535 | 0.051 | 4.893 | 7.859 | 2.824 | 208.301 | 16 | 38.23 | 164.6 | 430.31 | 18 |
| 2019.06.21 | 3.085 | 484.05 | 6.07 | 2.076 | 140.504 | 140504 | 8 | 1226.43 | 503.67 | 4221.75 | 4793.27 | 12939 | 83.38 | 30.848 | 32.24 | 1.520 | 0 | 0.307 | 0.258 | 1695.303 | 0.045 | 4.044 | 7.531 | 3.497 | 233.009 | 16 | 40.37 | 181.72 | 444.36 | 18 |
| 2019.06.24 | 3.280 | 488.95 | 6.07 | 2.005 | 132.947 | 132947 | 8 | 1314.00 | 374.07 | 5383.6 | 4094.02 | 13514 | 88.25 | 33.178 | 33.63 | 1.380 | 0 | 0.503 | 0.397 | 1596.877 | 0.051 | 2.873 | 9.407 | 3.000 | 228.896 | 16 | 39.69 | 174.48 | 415.99 | 18 |
| 2019.07.01 | 3.009 | 493.55 | 6.04 | 2.063 | 139.369 | 139369 | 8 | 1167.82 | 620.68 | 5413.03 | 5152.76 | 15192 | 87.66 | 35.540 | 33.23 | 1.310 | 0 | 0.35 | 0.349 | 1812.581 | 0.040 | 5.290 | 10.832 | 3.837 | 280.492 | 16 | 38.99 | 166.08 | 446.34 | 18 |
| 2019.07.08 | 3.062 | 493.85 | 6.05 | 1.97 | 145.156 | 145156 | 8 | 1479.35 | 708.24 | 4875.58 | 5282.83 | 15253 | 85.89 | 35.684 | 37.09 | 1.440 | 0 | 0.372 | 0.25 | 1817.709 | 0.051 | 6.122 | 9.904 | 3.915 | 276.744 | 16 | 37.78 | 160.77 | 440.72 | 18 |
| 2019.07.12 | 3.049 | 498.95 | 6.05 | 2.044 | 148.188 | 148188 | 8 | 1626.57 | 724.48 | 5405.9 | 6390.77 | 17146 | 86.10 | 37.749 | 37.66 | 1.400 | 0 | 0.362 | 0.287 | 1868.146 | 0.054 | 6.196 | 11.422 | 4.466 | 312.416 | 16 | 38.35 | 155.22 | 469.36 | 18 |
Table 1 Industrial single-tube test data and their conversion to lumped kinetics data
| 时间 | M0 | 反应条件 | 放料间隔/h | 产物生成质量/g | CO 总转化率/ % | CO单程转化率/% | 进料中各组分与 CO的摩尔比 | 单程转化的 CO物质的量/mol | 系数 | 实验数据计算的平均 分子量/(g/mol) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T/K | P/MPa | U | V | V0/L | t | CH4 | C3H8 | C12H26 | C29H60 | H2O | x | xexp | xcalc | R1 | R2 | R3 | R4 | mCO | A | B | C | D | E | |||||||
| 2019.06.02 | 3.914 | 464.05 | 6.07 | 2.121 | 141.346 | 141346 | 8 | 1771.64 | 601.57 | 2948.62 | 2964.91 | 9924 | 79.18 | 31.378 | 30.97 | 2.414 | 0 | 0.295 | 0.205 | 1276.53 | 0.087 | 3.821 | 4.761 | 1.707 | 140.862 | 16 | 40.22 | 158.24 | 443.8 | 18 |
| 2019.06.05 | 4.087 | 467.85 | 6.08 | 2.009 | 137.583 | 137583 | 8 | 2026.62 | 773.86 | 3573.76 | 2482.79 | 10898 | 75.69 | 36.038 | 36.46 | 2.431 | 0 | 0.444 | 0.212 | 1137.498 | 0.111 | 4.616 | 5.335 | 1.443 | 148.139 | 16 | 41.02 | 163.9 | 420.89 | 18 |
| 2019.06.09 | 3.534 | 473.35 | 6.07 | 1.994 | 136.235 | 136235 | 8 | 1871.86 | 684.61 | 4503.84 | 2803.50 | 11687 | 77.48 | 34.439 | 33.94 | 1.921 | 0 | 0.421 | 0.192 | 1333.259 | 0.088 | 4.799 | 7.632 | 1.963 | 183.702 | 16 | 40.36 | 166.96 | 404.16 | 18 |
| 2019.06.10 | 3.303 | 473.45 | 6.06 | 2.102 | 139.371 | 139371 | 8 | 1735.6 | 658.86 | 3798.21 | 3561.24 | 11469 | 74.36 | 30.922 | 30.48 | 1.804 | 0 | 0.312 | 0.187 | 1400.732 | 0.077 | 4.977 | 7.082 | 2.418 | 192.905 | 16 | 40.08 | 162.37 | 445.87 | 18 |
| 2019.06.12 | 3.150 | 473.65 | 6.08 | 1.971 | 139.147 | 139147 | 8 | 1241.75 | 545.73 | 3890.46 | 3719.36 | 11437 | 74.07 | 29.439 | 29.98 | 1.675 | 0 | 0.244 | 0.231 | 1460.689 | 0.053 | 4.422 | 7.346 | 2.580 | 201.711 | 16 | 39.18 | 168.13 | 457.73 | 18 |
| 2019.06.13 | 3.001 | 473.65 | 6.08 | 1.996 | 142.245 | 142245 | 8 | 1273.47 | 561.31 | 3882.04 | 3647.42 | 11252 | 73.37 | 28.011 | 27.96 | 1.598 | 0 | 0.207 | 0.196 | 1552.535 | 0.051 | 4.893 | 7.859 | 2.824 | 208.301 | 16 | 38.23 | 164.6 | 430.31 | 18 |
| 2019.06.21 | 3.085 | 484.05 | 6.07 | 2.076 | 140.504 | 140504 | 8 | 1226.43 | 503.67 | 4221.75 | 4793.27 | 12939 | 83.38 | 30.848 | 32.24 | 1.520 | 0 | 0.307 | 0.258 | 1695.303 | 0.045 | 4.044 | 7.531 | 3.497 | 233.009 | 16 | 40.37 | 181.72 | 444.36 | 18 |
| 2019.06.24 | 3.280 | 488.95 | 6.07 | 2.005 | 132.947 | 132947 | 8 | 1314.00 | 374.07 | 5383.6 | 4094.02 | 13514 | 88.25 | 33.178 | 33.63 | 1.380 | 0 | 0.503 | 0.397 | 1596.877 | 0.051 | 2.873 | 9.407 | 3.000 | 228.896 | 16 | 39.69 | 174.48 | 415.99 | 18 |
| 2019.07.01 | 3.009 | 493.55 | 6.04 | 2.063 | 139.369 | 139369 | 8 | 1167.82 | 620.68 | 5413.03 | 5152.76 | 15192 | 87.66 | 35.540 | 33.23 | 1.310 | 0 | 0.35 | 0.349 | 1812.581 | 0.040 | 5.290 | 10.832 | 3.837 | 280.492 | 16 | 38.99 | 166.08 | 446.34 | 18 |
| 2019.07.08 | 3.062 | 493.85 | 6.05 | 1.97 | 145.156 | 145156 | 8 | 1479.35 | 708.24 | 4875.58 | 5282.83 | 15253 | 85.89 | 35.684 | 37.09 | 1.440 | 0 | 0.372 | 0.25 | 1817.709 | 0.051 | 6.122 | 9.904 | 3.915 | 276.744 | 16 | 37.78 | 160.77 | 440.72 | 18 |
| 2019.07.12 | 3.049 | 498.95 | 6.05 | 2.044 | 148.188 | 148188 | 8 | 1626.57 | 724.48 | 5405.9 | 6390.77 | 17146 | 86.10 | 37.749 | 37.66 | 1.400 | 0 | 0.362 | 0.287 | 1868.146 | 0.054 | 6.196 | 11.422 | 4.466 | 312.416 | 16 | 38.35 | 155.22 | 469.36 | 18 |
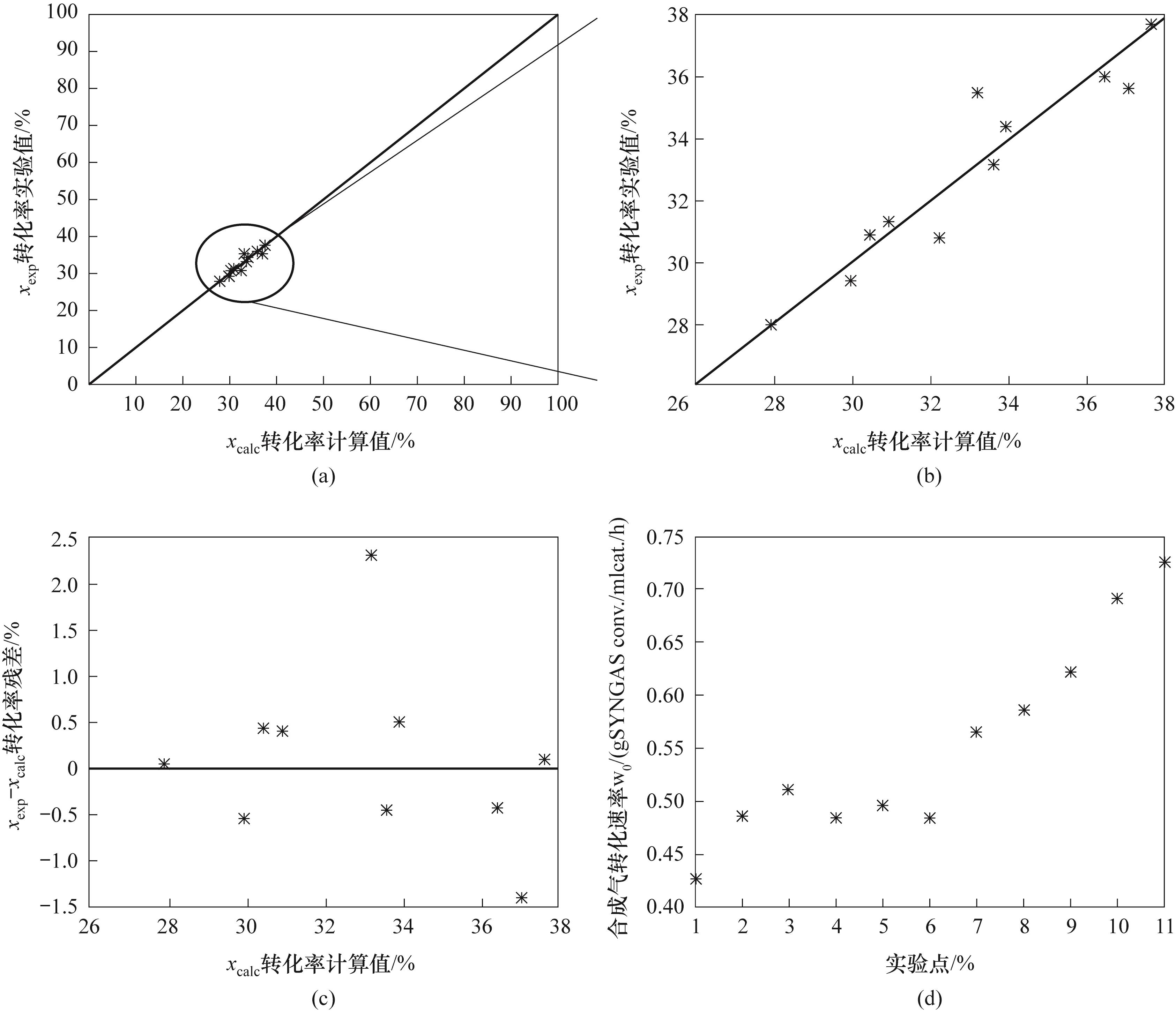
Fig.2 (a),(b) Comparison of calculated conversion and experimental conversion within different ranges; (c) Conversion residual plot (d) Syngas consumption rate
| MP | Mi | F | ρ2 | F0.05 |
|---|---|---|---|---|
| 3 | 11 | 341.306 | 0.992 | 34.406 |
Table 2 Statistical test results of the kinetics model
| MP | Mi | F | ρ2 | F0.05 |
|---|---|---|---|---|
| 3 | 11 | 341.306 | 0.992 | 34.406 |
| T/K | P/MPa | GHSV/h-1 | R1 | α1, exp | α1, calc | W1, exp | W1, calc | RD/% |
|---|---|---|---|---|---|---|---|---|
| 464.05 | 6.07 | 3715.27 | 2.414 | 0.5419 | 0.5262 | 0.2138 | 0.2245 | -5.01 |
| 467.85 | 6.08 | 3728.25 | 2.431 | 0.5269 | 0.5260 | 0.2288 | 0.2246 | 1.82 |
| 473.35 | 6.07 | 3789.95 | 1.921 | 0.5604 | 0.5803 | 0.1898 | 0.1762 | 7.17 |
| 473.45 | 6.06 | 3786.64 | 1.804 | 0.5737 | 0.5954 | 0.1779 | 0.1637 | 7.99 |
| 473.65 | 6.08 | 3764.47 | 1.675 | 0.6309 | 0.6153 | 0.1321 | 0.1480 | -12.02 |
| 473.65 | 6.08 | 3774.52 | 1.598 | 0.6260 | 0.6271 | 0.1360 | 0.1391 | -2.25 |
| 484.05 | 6.07 | 3961.53 | 1.52 | 0.6574 | 0.6395 | 0.1141 | 0.1300 | -13.88 |
| 488.95 | 6.07 | 4209.79 | 1.38 | 0.6525 | 0.6613 | 0.1177 | 0.1147 | 2.54 |
| 493.55 | 6.04 | 4193.09 | 1.31 | 0.6885 | 0.6772 | 0.0945 | 0.1042 | -10.26 |
| 493.85 | 6.05 | 4204.55 | 1.44 | 0.6495 | 0.6513 | 0.1198 | 0.1216 | -1.46 |
| 498.95 | 6.05 | 4336.51 | 1.4 | 0.6563 | 0.6579 | 0.1150 | 0.1170 | -1.78 |
Table 3 Calculated values of the carbon chain growth probability factor α1 for methane formation in comparison with the experimental values
| T/K | P/MPa | GHSV/h-1 | R1 | α1, exp | α1, calc | W1, exp | W1, calc | RD/% |
|---|---|---|---|---|---|---|---|---|
| 464.05 | 6.07 | 3715.27 | 2.414 | 0.5419 | 0.5262 | 0.2138 | 0.2245 | -5.01 |
| 467.85 | 6.08 | 3728.25 | 2.431 | 0.5269 | 0.5260 | 0.2288 | 0.2246 | 1.82 |
| 473.35 | 6.07 | 3789.95 | 1.921 | 0.5604 | 0.5803 | 0.1898 | 0.1762 | 7.17 |
| 473.45 | 6.06 | 3786.64 | 1.804 | 0.5737 | 0.5954 | 0.1779 | 0.1637 | 7.99 |
| 473.65 | 6.08 | 3764.47 | 1.675 | 0.6309 | 0.6153 | 0.1321 | 0.1480 | -12.02 |
| 473.65 | 6.08 | 3774.52 | 1.598 | 0.6260 | 0.6271 | 0.1360 | 0.1391 | -2.25 |
| 484.05 | 6.07 | 3961.53 | 1.52 | 0.6574 | 0.6395 | 0.1141 | 0.1300 | -13.88 |
| 488.95 | 6.07 | 4209.79 | 1.38 | 0.6525 | 0.6613 | 0.1177 | 0.1147 | 2.54 |
| 493.55 | 6.04 | 4193.09 | 1.31 | 0.6885 | 0.6772 | 0.0945 | 0.1042 | -10.26 |
| 493.85 | 6.05 | 4204.55 | 1.44 | 0.6495 | 0.6513 | 0.1198 | 0.1216 | -1.46 |
| 498.95 | 6.05 | 4336.51 | 1.4 | 0.6563 | 0.6579 | 0.1150 | 0.1170 | -1.78 |
| T/K | P/MPa | GHSV/h-1 | R1 | α3, exp | α3, calc | W3, exp | W3, calc | RD/% |
|---|---|---|---|---|---|---|---|---|
| 464.05 | 6.07 | 3715.27 | 2.414 | 0.8120 | 0.8052 | 0.0726 | 0.0738 | -1.65 |
| 467.85 | 6.08 | 3728.25 | 2.431 | 0.7868 | 0.7931 | 0.0874 | 0.0808 | 7.56 |
| 473.35 | 6.07 | 3789.95 | 1.921 | 0.8120 | 0.8103 | 0.0694 | 0.0709 | -2.13 |
| 473.45 | 6.06 | 3786.64 | 1.804 | 0.8120 | 0.8141 | 0.0675 | 0.0687 | -1.72 |
| 473.65 | 6.08 | 3764.47 | 1.675 | 0.8281 | 0.8274 | 0.0581 | 0.0612 | -5.38 |
| 473.65 | 6.08 | 3774.52 | 1.598 | 0.8259 | 0.8366 | 0.0599 | 0.0561 | 6.48 |
| 484.05 | 6.07 | 3961.53 | 1.52 | 0.8492 | 0.8309 | 0.0469 | 0.0592 | -26.35 |
| 488.95 | 6.07 | 4209.79 | 1.38 | 0.8754 | 0.8745 | 0.0335 | 0.0361 | -7.80 |
| 493.55 | 6.04 | 4193.09 | 1.31 | 0.8436 | 0.8439 | 0.0502 | 0.0521 | -3.63 |
| 493.85 | 6.05 | 4204.55 | 1.44 | 0.8289 | 0.8349 | 0.0574 | 0.0570 | 0.64 |
| 498.95 | 6.05 | 4336.51 | 1.4 | 0.8400 | 0.8417 | 0.0512 | 0.0533 | -4.02 |
Table 4 Calculated values of the carbon chain growth probability factor α3 generated by C2-5 hydrocarbons (with a total set of C3) in comparison with the experimental values
| T/K | P/MPa | GHSV/h-1 | R1 | α3, exp | α3, calc | W3, exp | W3, calc | RD/% |
|---|---|---|---|---|---|---|---|---|
| 464.05 | 6.07 | 3715.27 | 2.414 | 0.8120 | 0.8052 | 0.0726 | 0.0738 | -1.65 |
| 467.85 | 6.08 | 3728.25 | 2.431 | 0.7868 | 0.7931 | 0.0874 | 0.0808 | 7.56 |
| 473.35 | 6.07 | 3789.95 | 1.921 | 0.8120 | 0.8103 | 0.0694 | 0.0709 | -2.13 |
| 473.45 | 6.06 | 3786.64 | 1.804 | 0.8120 | 0.8141 | 0.0675 | 0.0687 | -1.72 |
| 473.65 | 6.08 | 3764.47 | 1.675 | 0.8281 | 0.8274 | 0.0581 | 0.0612 | -5.38 |
| 473.65 | 6.08 | 3774.52 | 1.598 | 0.8259 | 0.8366 | 0.0599 | 0.0561 | 6.48 |
| 484.05 | 6.07 | 3961.53 | 1.52 | 0.8492 | 0.8309 | 0.0469 | 0.0592 | -26.35 |
| 488.95 | 6.07 | 4209.79 | 1.38 | 0.8754 | 0.8745 | 0.0335 | 0.0361 | -7.80 |
| 493.55 | 6.04 | 4193.09 | 1.31 | 0.8436 | 0.8439 | 0.0502 | 0.0521 | -3.63 |
| 493.85 | 6.05 | 4204.55 | 1.44 | 0.8289 | 0.8349 | 0.0574 | 0.0570 | 0.64 |
| 498.95 | 6.05 | 4336.51 | 1.4 | 0.8400 | 0.8417 | 0.0512 | 0.0533 | -4.02 |
| T/K | P/MPa | GHSV/h-1 | R1 | α12, exp | α12, calc | W12, exp | W12, calc | RD/% |
|---|---|---|---|---|---|---|---|---|
| 464.05 | 6.07 | 3715.27 | 2.414 | 0.9143 | 0.9155 | 0.3558 | 0.3840 | -7.91 |
| 467.85 | 6.08 | 3728.25 | 2.431 | 0.9182 | 0.9177 | 0.4035 | 0.3840 | 4.84 |
| 473.35 | 6.07 | 3789.95 | 1.921 | 0.9232 | 0.9187 | 0.4566 | 0.3839 | 15.93 |
| 473.45 | 6.06 | 3786.64 | 1.804 | 0.9171 | 0.9170 | 0.3894 | 0.3840 | 1.39 |
| 473.65 | 6.08 | 3764.47 | 1.675 | 0.9232 | 0.9236 | 0.4140 | 0.3825 | 7.61 |
| 473.65 | 6.08 | 3774.52 | 1.598 | 0.9232 | 0.9244 | 0.4146 | 0.3821 | 7.84 |
| 484.05 | 6.07 | 3961.53 | 1.52 | 0.9174 | 0.9223 | 0.3929 | 0.3830 | 2.52 |
| 488.95 | 6.07 | 4209.79 | 1.38 | 0.9236 | 0.9248 | 0.4822 | 0.3819 | 20.80 |
| 493.55 | 6.04 | 4193.09 | 1.31 | 0.9232 | 0.9168 | 0.4381 | 0.3840 | 12.36 |
| 493.85 | 6.05 | 4204.55 | 1.44 | 0.9175 | 0.9179 | 0.3949 | 0.3839 | 2.78 |
| 498.95 | 6.05 | 4336.51 | 1.4 | 0.9165 | 0.9185 | 0.3821 | 0.3839 | -0.47 |
Table 5 Calculated values of the carbon chain growth probability factor α12 generated by C6-19 hydrocarbons (aggregated as C12) in comparison with the experimental values
| T/K | P/MPa | GHSV/h-1 | R1 | α12, exp | α12, calc | W12, exp | W12, calc | RD/% |
|---|---|---|---|---|---|---|---|---|
| 464.05 | 6.07 | 3715.27 | 2.414 | 0.9143 | 0.9155 | 0.3558 | 0.3840 | -7.91 |
| 467.85 | 6.08 | 3728.25 | 2.431 | 0.9182 | 0.9177 | 0.4035 | 0.3840 | 4.84 |
| 473.35 | 6.07 | 3789.95 | 1.921 | 0.9232 | 0.9187 | 0.4566 | 0.3839 | 15.93 |
| 473.45 | 6.06 | 3786.64 | 1.804 | 0.9171 | 0.9170 | 0.3894 | 0.3840 | 1.39 |
| 473.65 | 6.08 | 3764.47 | 1.675 | 0.9232 | 0.9236 | 0.4140 | 0.3825 | 7.61 |
| 473.65 | 6.08 | 3774.52 | 1.598 | 0.9232 | 0.9244 | 0.4146 | 0.3821 | 7.84 |
| 484.05 | 6.07 | 3961.53 | 1.52 | 0.9174 | 0.9223 | 0.3929 | 0.3830 | 2.52 |
| 488.95 | 6.07 | 4209.79 | 1.38 | 0.9236 | 0.9248 | 0.4822 | 0.3819 | 20.80 |
| 493.55 | 6.04 | 4193.09 | 1.31 | 0.9232 | 0.9168 | 0.4381 | 0.3840 | 12.36 |
| 493.85 | 6.05 | 4204.55 | 1.44 | 0.9175 | 0.9179 | 0.3949 | 0.3839 | 2.78 |
| 498.95 | 6.05 | 4336.51 | 1.4 | 0.9165 | 0.9185 | 0.3821 | 0.3839 | -0.47 |
| T/K | P/MPa | GHSV/h-1 | R1 | α29, exp | α29, calc | W29, exp | W29, exp | RD/% |
|---|---|---|---|---|---|---|---|---|
| 464.05 | 6.07 | 3715.27 | 2.414 | 0.9698 | 0.9650 | 0.3578 | 0.3743 | -4.62 |
| 467.85 | 6.08 | 3728.25 | 2.431 | 0.9676 | 0.9678 | 0.2803 | 0.3734 | -33.22 |
| 473.35 | 6.07 | 3789.95 | 1.921 | 0.9601 | 0.9681 | 0.2842 | 0.3732 | -31.30 |
| 473.45 | 6.06 | 3786.64 | 1.804 | 0.9649 | 0.9673 | 0.3651 | 0.3738 | -2.39 |
| 473.65 | 6.08 | 3764.47 | 1.675 | 0.9707 | 0.9687 | 0.3958 | 0.3726 | 5.86 |
| 473.65 | 6.08 | 3774.52 | 1.598 | 0.9705 | 0.9683 | 0.3895 | 0.3730 | 4.23 |
| 484.05 | 6.07 | 3961.53 | 1.52 | 0.9716 | 0.9703 | 0.4461 | 0.3704 | 16.98 |
| 488.95 | 6.07 | 4209.79 | 1.38 | 0.9649 | 0.9684 | 0.3667 | 0.3729 | -1.71 |
| 493.55 | 6.04 | 4193.09 | 1.31 | 0.9711 | 0.9691 | 0.4171 | 0.3721 | 10.79 |
| 493.85 | 6.05 | 4204.55 | 1.44 | 0.9713 | 0.9703 | 0.4279 | 0.3703 | 13.46 |
| 498.95 | 6.05 | 4336.51 | 1.4 | 0.9717 | 0.9709 | 0.4517 | 0.3690 | 18.31 |
Table 6 Calculated values of the carbon chain growth probability factor α29 generated by C20+ hydrocarbons (aggregated as C29) in comparison with the experimental values
| T/K | P/MPa | GHSV/h-1 | R1 | α29, exp | α29, calc | W29, exp | W29, exp | RD/% |
|---|---|---|---|---|---|---|---|---|
| 464.05 | 6.07 | 3715.27 | 2.414 | 0.9698 | 0.9650 | 0.3578 | 0.3743 | -4.62 |
| 467.85 | 6.08 | 3728.25 | 2.431 | 0.9676 | 0.9678 | 0.2803 | 0.3734 | -33.22 |
| 473.35 | 6.07 | 3789.95 | 1.921 | 0.9601 | 0.9681 | 0.2842 | 0.3732 | -31.30 |
| 473.45 | 6.06 | 3786.64 | 1.804 | 0.9649 | 0.9673 | 0.3651 | 0.3738 | -2.39 |
| 473.65 | 6.08 | 3764.47 | 1.675 | 0.9707 | 0.9687 | 0.3958 | 0.3726 | 5.86 |
| 473.65 | 6.08 | 3774.52 | 1.598 | 0.9705 | 0.9683 | 0.3895 | 0.3730 | 4.23 |
| 484.05 | 6.07 | 3961.53 | 1.52 | 0.9716 | 0.9703 | 0.4461 | 0.3704 | 16.98 |
| 488.95 | 6.07 | 4209.79 | 1.38 | 0.9649 | 0.9684 | 0.3667 | 0.3729 | -1.71 |
| 493.55 | 6.04 | 4193.09 | 1.31 | 0.9711 | 0.9691 | 0.4171 | 0.3721 | 10.79 |
| 493.85 | 6.05 | 4204.55 | 1.44 | 0.9713 | 0.9703 | 0.4279 | 0.3703 | 13.46 |
| 498.95 | 6.05 | 4336.51 | 1.4 | 0.9717 | 0.9709 | 0.4517 | 0.3690 | 18.31 |
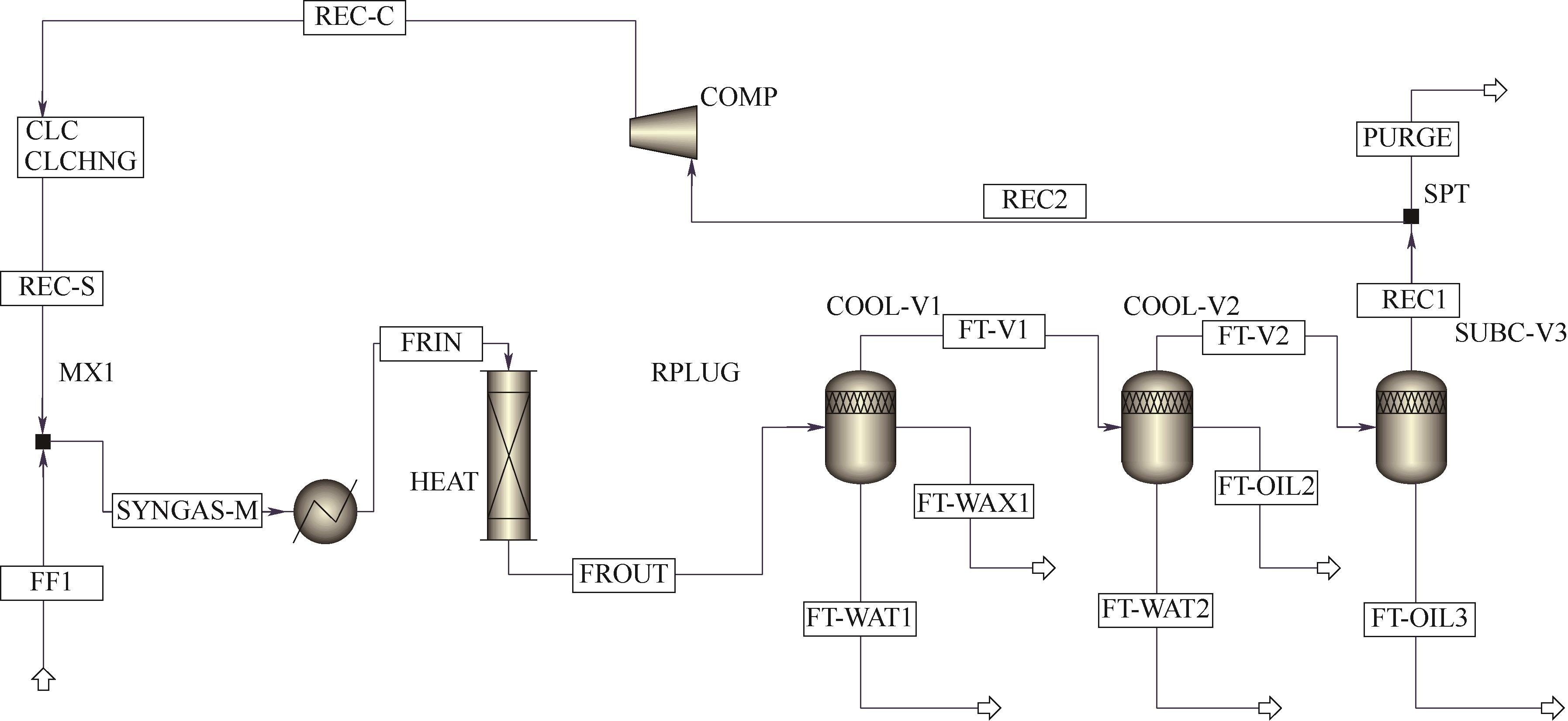
Fig.3 A simplified diagram of the full-process simulation of the industrial single-tube device for cobalt-based Fischer-Tropsch synthesis using Aspen PlusHEAT—feed preheater; RPLUG—fixed-bed reactor; COOL-V1—normal-temperature cooler; COOL-V2—water cooler; CUB-V3—subcooler; COMP—recycle compressor;FF1—fresh feed; REC-S—recycle stream; SYNGAS-M—synthesis gas mixed; FRIN—inlet stream of the reactor; FROUT—outlet stream of the reactor; FT-WAT1—FT synthesized water; FT-WAX1—FT synthesized wax1; FT-V1—vapor stream from the COOL-V1; FT-WAT2—FT synthesized water2; FT-OIL2—FT synthesized oil2; FT-OIL3—FT synthesized oil3; REC1—stream to recycle and purge
| 对比组 | 流股 | 新鲜气 (FF1) | 循环气 (REC-S) | 入器气 (FRIN) | 费托蜡1 (FT-WAX1) | 费托油2 (FT-OIL2) | 费托油3 (FT-OIL3) | 费托水1 (FT-WAT1) | 费托水2 (FT-WAT2) | 弛放气 (PURGE) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 模拟值 | 流量/(kg/h) | 4.052 | 5.507 | 9.558 | 0.763 | 0.067 | 0.003 | 1.077 | 0.273 | 1.868 | |
| SUM = 0.833 | SUM=1.350 | ||||||||||
| 组分 | 摩尔分数 | 摩尔分数 | 摩尔分数 | 质量分数 | 质量分数 | 质量分数 | 质量分数 | 质量分数 | 摩尔分数 | ||
| H2 | 0.6703 | 0.5636 | 0.6138 | 0.0005 | 0.0004 | 0.0004 | 0 | 0 | 0.5636 | ||
| CO | 0.2886 | 0.2245 | 0.2547 | 0.0044 | 0.0050 | 0.0061 | 0 | 0 | 0.2245 | ||
| N2 | 0.0281 | 0.0723 | 0.0515 | 0.0086 | 0.0214 | 0.0418 | 0.0010 | 0.0006 | 0.0723 | ||
| CH4 | 0.0129 | 0.1269 | 0.0732 | 0.0026 | 0.0039 | 0.0055 | 0 | 0 | 0.1269 | ||
| C3H8 | 0 | 0.0114 | 0.0060 | 0.0038 | 0.0131 | 0.0308 | 0 | 0 | 0.0114 | ||
| C12H26 | 0 | 0 | 0 | 0.4719 | 0.9530 | 0.9047 | 0 | 0 | 0 | ||
| C29H60 | 0 | 0 | 0 | 0.4991 | 0.0001 | 0 | 0 | 0 | 0 | ||
| H2O | 0 | 0.0014 | 0.0007 | 0.0092 | 0.0031 | 0.0108 | 0.9989 | 0.9993 | 0.0014 | ||
| 试验值 | 流量/(kg/h) | 3.814 | — | — | SUM = 0.713, 各组分已全分析,不便列出 | SUM = 1.241, 不便列出 | 1.822 | ||||
| 组分 | 摩尔分数 | 摩尔分数 | 摩尔分数 | — | — | — | — | — | 摩尔分数 | ||
| H2 | 0.6728 | 0.5875 | 0.6114 | — | — | — | — | — | 0.5875 | ||
| CO | 0.2990 | 0.2334 | 0.2533 | — | — | — | — | — | 0.2334 | ||
| N2 | 0.018 | 0.0460 | 0.0518 | — | — | — | — | — | 0.0460 | ||
| CH4 | 0.0023 | 0.1060 | 0.0747 | — | — | — | — | — | 0.1060 | ||
| C2H6+ | — | — | — | — | — | — | — | — | — | ||
| H2O | — | — | — | — | — | — | — | — | — | ||
相对 误差/% | (模拟值-试验值)/试验值×100 | 6.24 | — | — | 16.8 | — | — | 8.87 | — | 2.52 | |
Table 7 Comparison of simulated and experimental values of key material data for the entire process of cobalt-based Fischer-Tropsch synthesis industrial single-tube equipment
| 对比组 | 流股 | 新鲜气 (FF1) | 循环气 (REC-S) | 入器气 (FRIN) | 费托蜡1 (FT-WAX1) | 费托油2 (FT-OIL2) | 费托油3 (FT-OIL3) | 费托水1 (FT-WAT1) | 费托水2 (FT-WAT2) | 弛放气 (PURGE) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 模拟值 | 流量/(kg/h) | 4.052 | 5.507 | 9.558 | 0.763 | 0.067 | 0.003 | 1.077 | 0.273 | 1.868 | |
| SUM = 0.833 | SUM=1.350 | ||||||||||
| 组分 | 摩尔分数 | 摩尔分数 | 摩尔分数 | 质量分数 | 质量分数 | 质量分数 | 质量分数 | 质量分数 | 摩尔分数 | ||
| H2 | 0.6703 | 0.5636 | 0.6138 | 0.0005 | 0.0004 | 0.0004 | 0 | 0 | 0.5636 | ||
| CO | 0.2886 | 0.2245 | 0.2547 | 0.0044 | 0.0050 | 0.0061 | 0 | 0 | 0.2245 | ||
| N2 | 0.0281 | 0.0723 | 0.0515 | 0.0086 | 0.0214 | 0.0418 | 0.0010 | 0.0006 | 0.0723 | ||
| CH4 | 0.0129 | 0.1269 | 0.0732 | 0.0026 | 0.0039 | 0.0055 | 0 | 0 | 0.1269 | ||
| C3H8 | 0 | 0.0114 | 0.0060 | 0.0038 | 0.0131 | 0.0308 | 0 | 0 | 0.0114 | ||
| C12H26 | 0 | 0 | 0 | 0.4719 | 0.9530 | 0.9047 | 0 | 0 | 0 | ||
| C29H60 | 0 | 0 | 0 | 0.4991 | 0.0001 | 0 | 0 | 0 | 0 | ||
| H2O | 0 | 0.0014 | 0.0007 | 0.0092 | 0.0031 | 0.0108 | 0.9989 | 0.9993 | 0.0014 | ||
| 试验值 | 流量/(kg/h) | 3.814 | — | — | SUM = 0.713, 各组分已全分析,不便列出 | SUM = 1.241, 不便列出 | 1.822 | ||||
| 组分 | 摩尔分数 | 摩尔分数 | 摩尔分数 | — | — | — | — | — | 摩尔分数 | ||
| H2 | 0.6728 | 0.5875 | 0.6114 | — | — | — | — | — | 0.5875 | ||
| CO | 0.2990 | 0.2334 | 0.2533 | — | — | — | — | — | 0.2334 | ||
| N2 | 0.018 | 0.0460 | 0.0518 | — | — | — | — | — | 0.0460 | ||
| CH4 | 0.0023 | 0.1060 | 0.0747 | — | — | — | — | — | 0.1060 | ||
| C2H6+ | — | — | — | — | — | — | — | — | — | ||
| H2O | — | — | — | — | — | — | — | — | — | ||
相对 误差/% | (模拟值-试验值)/试验值×100 | 6.24 | — | — | 16.8 | — | — | 8.87 | — | 2.52 | |
| [1] | Yates I C, Satterfield C N. Intrinsic kinetics of the Fischer-Tropsch synthesis on a cobalt catalyst[J]. Energy and Fuels, 1991, 5(1): 168-173. |
| [2] | Zennaro R, Tagliabue M, Bartholomew C H. Kinetics of Fischer–Tropsch synthesis on titania-supported cobalt[J]. Catalysis Today, 2000, 58(4): 309-319. |
| [3] | Atashi H, Mansouri M, Hosseini S H, et al. Intrinsic kinetics of the Fischer-Tropsch synthesis over an impregnated cobalt-potassium catalyst[J]. Korean Journal of Chemical Engineering, 2012, 29(3): 304-309. |
| [4] | Ma W P, Jacobs G, Sparks D E, et al. Fischer–Tropsch synthesis: support and cobalt cluster size effects on kinetics over Co/Al2O3 and Co/SiO2 catalysts[J]. Fuel, 2011, 90(2): 756-765. |
| [5] | Visconti C G, Tronconi E, Lietti L, et al. Detailed kinetics of the Fischer-Tropsch synthesis on cobalt catalysts based on H-assisted CO activation[J]. Topics in Catalysis, 2011, 54(13): 786. |
| [6] | Kaiser P, Pöhlmann F, Jess A. Intrinsic and effective kinetics of cobalt-catalyzed Fischer-Tropsch synthesis in view of a power-to-liquid process based on renewable energy[J]. Chemical Engineering & Technology, 2014, 37(6): 964-972. |
| [7] | Visconti C G, Lietti L, Tronconi E, et al. Kinetics of low-temperature Fischer-Tropsch synthesis on cobalt catalysts: are both slurry autoclave and tubular packed-bed reactors adequate to collect relevant data at lab-scale?[J]. The Canadian Journal of Chemical Engineering, 2016, 94(4): 685-695. |
| [8] | Ostadi M, Rytter E, Hillestad M. Evaluation of kinetic models for Fischer-Tropsch cobalt catalysts in a plug flow reactor[J]. Chemical Engineering Research and Design, 2016, 114: 236-246. |
| [9] | Keyvanloo K, Lanham S J, Hecker W C. Kinetics of Fischer-Tropsch synthesis on supported cobalt: effect of temperature on CO and H2 partial pressure dependencies[J]. Catalysis Today, 2016, 270: 9-18. |
| [10] | Moazami N, Wyszynski M L, Rahbar K, et al. A comprehensive study of kinetics mechanism of Fischer-Tropsch synthesis over cobalt-based catalyst[J]. Chemical Engineering Science, 2017, 171: 32-60. |
| [11] | Golestan S, Mirzaei A A, Atashi H. Kinetic and mechanistic studies of Fischer-Tropsch synthesis over the nano-structured iron-cobalt-manganese catalyst prepared by hydrothermal procedure[J]. Fuel, 2017, 200: 407-418. |
| [12] | Asiaee A, Benjamin K M. A density functional theory based elementary reaction mechanism for early steps of Fischer-Tropsch synthesis over cobalt catalyst. 1. Reaction kinetics[J]. Molecular Catalysis, 2017, 436: 218-227. |
| [13] | Mousavi S, Zamaniyan A, Irani M, et al. Statistical investigation of macro kinetics for iron and cobalt based Fischer-Tropsch synthesis: Mechanistic and kinetic implications[J]. Journal of Natural Gas Science and Engineering, 2016, 34: 1333-1346. |
| [14] | Parnian M J, Taheri Najafabadi A, Mortazavi Y, et al. Ru promoted cobalt catalyst on γ-Al2O3: influence of different catalyst preparation method and Ru loadings on Fischer–Tropsch reaction and kinetics[J]. Applied Surface Science, 2014, 313: 183-195. |
| [15] | Botes F G. Influences of water and syngas partial pressure on the kinetics of a commercial alumina-supported cobalt Fischer-Tropsch catalyst[J]. Industrial and Engineering Chemistry Research, 2009, 48(4): 1859-1865. |
| [16] | Keyser M J, Everson R C, Espinoza R L. Fischer-Tropsch studies with cobalt-manganese oxide catalysts: synthesis performance in a fixed bed reactor[J]. Applied Catalysis A: General, 1998, 171(1): 99-107. |
| [17] | Das T K, Conner W A, Li J L, et al. Fischer–Tropsch synthesis: kinetics and effect of water for a Co/SiO2Catalyst[J]. Energy & Fuels, 2005, 19(4): 1430-1439. |
| [18] | Yang C-H, Massoth F E, Oblad A G. Kinetics of CO + H2 Reaction over Co-Cu-Al2O3 Catalyst. In Hydrocarbon Synthesis from Carbon Monoxide and Hydrogen[M]. American Chemical Society: Washington, 1979: 35-46. |
| [19] | Schulz H. Short history and present trends of Fischer-Tropsch synthesis[J]. Applied Catalysis A: General, 1999, 186(1/2): 3-12. |
| [20] | Vervloet D, Kapteijn F, Nijenhuis J, et al. Fischer-Tropsch reaction-diffusion in a cobalt catalyst particle: aspects of activity and selectivity for a variable chain growth probability[J]. Catalysis Science & Technology, 2012, 2(6): 1221-1233. |
| [21] | 鲁丰乐. 费托合成催化剂反应动力学研究与反应器数学模拟[D]. 上海: 华东理工大学, 2010. |
| Lu F L. Study on reaction kinetics of Fischer-Tropsch synthesis catalyst and mathematical simulation of reactor[D]. Shanghai: East China University of Science and Technology, 2010. | |
| [22] | 鲁丰乐, 张海涛, 马向东, 等. 钴基催化剂费托合成动力学模型[J]. 中国科技论文在线, 2009, 4(9): 632-637. |
| Lu F L, Zhang H T, Ma X D, et al. Kinetics models of Fischer-Tropsch synthesis on the cobalt-based catalyst[J]. Sciencepaper Online, 2009, 4(9): 632-637. | |
| [23] | Lox E S, Froment G F. Kinetics of the Fischer-Tropsch reaction on a precipitated promoted iron catalyst. 1. Experimental procedure and results[J]. Industrial & Engineering Chemistry Research, 1993, 32(1): 61-70. |
| [24] | Lox E S, Froment G F. Kinetics of the Fischer-Tropsch reaction on a precipitated promoted iron catalyst. 2. Kinetic modeling[J]. Industrial & Engineering Chemistry Research, 1993, 32(1): 71-82. |
| [25] | 吉媛媛, 杨继礼, 相宏伟, 等. Fe-Mn工业催化剂F-T合成详细机理动力学研究 I.反应性能及初步反应机理[J]. 燃料化学学报, 1999, 27(S1): 130-137. |
| Ji Y Y, Yang J L, Xiang H W, et al. Study of F-T synthesis detailed mechanism kinetics over Fe-Mn industrial catalyst I. Catalyst Performance and Preliminary Mechanism[J]. Journal of Fuel Chemistry and Technology, 1999, 27(S1): 130-137. | |
| [26] | 马文平, 李永旺, 赵玉龙, 等. 工业Fe-Cu-K催化剂上费托合成反应动力学 ( Ⅱ ) : 模型筛选与参数估值[J]. 化工学报, 1999, 50(2): 167-173. |
| Ma W P, Li Y W, Zhao Y L, et al. Kinetics of Fischer - Tropsch synthesis over Fe - Cu - K catalyst ( Ⅱ ) model discrimination and parameter estimation[J]. Journal of Chemical Industry and Engineering (China), 1999, 50(2): 167-173. | |
| [27] | 马文平, 李永旺, 赵玉龙, 等. 工业Fe-Cu-K催化剂上费托合成反应动力学(Ⅰ): 基于机理的动力学模型[J]. 化工学报, 1999, 50(2): 159-166. |
| Ma W P, Li Y W, Zhao Y L, et al. Kinetics of Fischer - Tropsch synthesis over Fe - Cu -K catalyst ( Ⅰ ) kinetic model on the basis of mechanism[J]. Journal of Chemical Industry and Engineering (China), 1999, 50(2): 159-166. | |
| [28] | Wang Y N, Ma W P, Lu Y J, et al. Kinetics modelling of Fischer-Tropsch synthesis over an industrial Fe-Cu-K catalyst[J]. Fuel, 2003, 82(2): 195-213. |
| [29] | Yang J, Liu Y, Chang J, et al. Detailed kinetics of Fischer-Tropsch synthesis on an industrial Fe-Mn catalyst[J]. Industrial & Engineering Chemistry Research, 2003, 42(21): 5066-5090. |
| [30] | Niu C C, Guo S P, Xia M, et al. A hybrid kinetics integrating feed-consumption rate and product selectivity models for Fischer-Tropsch synthesis over an industrial cobalt-based catalyst[J]. Chemical Engineering Journal, 2023, 455: 140817. |
| [1] | Ke JIN, Chenguang WANG, Longlong MA, Qi ZHANG. Preparation of core-shell nanomaterials and their application in thermocatalytic hydrogenation of CO/CO2 [J]. CIESC Journal, 2022, 73(3): 990-1007. |
| [2] | Wuyu WANG, Yuzhu SHI, Long YAN, Xinghua ZHANG, Longlong MA, Qi ZHANG. Synthesis of valerate biofuels on supported Co-based bifunctional catalysts [J]. CIESC Journal, 2022, 73(2): 689-698. |
| [3] | TAN Khangwei, XIONG Wenting, FU Jile, CHEN Binghui. Preparation and catalytic performance of Ru-Co/SiC catalysts for the synthesis of heavy hydrocarbons from syngas by Fischer-Tropsch reaction [J]. CIESC Journal, 2021, 72(7): 3648-3657. |
| [4] | Nan SONG, Minjian PAN, Bingxu CHEN, Gang QIAN, Xuezhi DUAN, Xinggui ZHOU. CH4 formation and C—C coupling mechanism on (011) surface of η-Fe2C Fischer-Tropsch catalyst [J]. CIESC Journal, 2019, 70(7): 2540-2547. |
| [5] | ZHANG Jianli, WANG Xu, MA Liping, YU Xufei, MA Qingxiang, FAN Subing, ZHAO Tiansheng. Preparation of modified MgFeMn-HTLcs and catalytic performance in CO hydrogenation [J]. CIESC Journal, 2018, 69(5): 2073-2080. |
| [6] | ZHANG Jun, ZHANG Zhengpai, SU Junjie, FU Donglong, DAI Weiwei, LIU Da, XU Jing, HAN Yifan. Effect of support basicity on iron-based catalysts for Fischer-Tropsch synthesis [J]. CIESC Journal, 2016, 67(2): 549-556. |
| [7] | LIU Yi, LIU Yong, CHEN Jianfeng, ZHANG Yi. Effects of MnOx supports on light olefin synthesis using cobalt catalyst in Fischer-Tropsch reaction [J]. CIESC Journal, 2015, 66(9): 3413-3420. |
| [8] | WANG Yan1,GE Xihui2,ZHANG Minqing1,ZHU Huaigong3,ZHANG Zijian2,WANG Ming1. Separation of n-hydrocarbons from high temperature oil phase products of Fischer-Tropsch synthesis [J]. Chemical Industry and Engineering Progree, 2014, 33(11): 2894-2898. |
| [9] | SUN Qiwen, WU Jianmin, ZHANG Zongsen, PANG Lifeng. Indirect coal liquefaction technology and its research progress [J]. Chemical Industry and Engineering Progree, 2013, 32(01): 1-12. |
| [10] | GUAN Guofeng,WANG Lei,WANG Fengna. Effects of metal oxide promoters on supported cobalt-based Fischer-Tropsch catalysts [J]. Chemical Industry and Engineering Progree, 2012, 31(12): 2595-2602. |
| [11] | WANG Xiangyun. Progress of carbon dioxide removal from the recycle gas of F-T synthesis [J]. , 2011, 30(1): 52-. |
| [12] | JI Yuguo,ZHAO Zhen,YU Changchun,DUAN Aijun,JIANG Guiyuan. Progress of cobalt-based catalysts in Fischer-Tropsch synthesis [J]. , 2007, 26(7): 927-. |
| [13] | WANG Xiangyun. Progress of carbon dioxide removal from the recycle gas of Fischer-Tropsch synthesis [J]. , 2007, 26(12): 1708-. |
| [14] | HOU ,Weifeng, SU ,Hongye, MU ,Shengjing, CHU ,Jian. Multiobjective optimization of the industrial naphtha catalytic reforming process [J]. , 2007, 15(1): 75-80. |
| [15] |
YANG Xiazhen,LIU Huazhang,TANG Haodong,CAI Liping,WU Zaiguo.
Research progress of promoters for Fe,Co-based Fischer-Tropsch synthesis catalysts [J]. , 2006, 25(8): 867-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||