CIESC Journal ›› 2025, Vol. 76 ›› Issue (5): 2136-2157.DOI: 10.11949/0438-1157.20241222
• Reviews and monographs • Previous Articles Next Articles
Yaqi BA( ), Tao WU, Andi DI, Anhui LU(
), Tao WU, Andi DI, Anhui LU( )
)
Received:2024-10-31
Revised:2024-11-30
Online:2025-06-13
Published:2025-05-25
Contact:
Anhui LU
通讯作者:
陆安慧
作者简介:巴雅琪(1999—),女,博士研究生,bayaqi@mail.dlut.edu.cn
基金资助:CLC Number:
Yaqi BA, Tao WU, Andi DI, Anhui LU. Progress in porous carbons for efficient separation of gaseous light hydrocarbon[J]. CIESC Journal, 2025, 76(5): 2136-2157.
巴雅琪, 吴涛, 邸安頔, 陆安慧. 多孔炭材料用于低碳烃分离的研究进展[J]. 化工学报, 2025, 76(5): 2136-2157.
Add to citation manager EndNote|Ris|BibTeX
| 组分 | 体积分数/% | ||||||
|---|---|---|---|---|---|---|---|
| 天然气 | 沼气 | 炼厂气 | 裂解气 | 油田气 | 煤层气 | 页岩气 | |
| CO2 | 0~10 | 25~65 | 1~2 | — | 1~36 | <5 | 0~10 |
| CH4 | 60~70 | 40~75 | 3~25 | 20~25 | 30~65 | 20~50 | 69~95 |
| C2H4 | — | — | — | 20~25 | — | — | — |
| C2H6 | 5~20 | — | 2~15 | 0~5 | 5~20 | — | 0-2 |
| C3H6 | — | — | — | — | — | — | — |
| C3H8 | 2~15 | — | 2~15 | 0-5 | 2~20 | — | 0~2 |
| H2 | — | — | 50~90 | 10~20 | — | — | 0~1 |
| N2 | 0~25 | — | — | — | 0~5 | 39~70 | 0~30 |
| 其他 | 0~15 | 0~10 | 0~10 | 25~30 | 5~25 | 10~16 | 0~1 |
Table 1 Ratio of CH4 and other gas components in different gas sources [9-15]
| 组分 | 体积分数/% | ||||||
|---|---|---|---|---|---|---|---|
| 天然气 | 沼气 | 炼厂气 | 裂解气 | 油田气 | 煤层气 | 页岩气 | |
| CO2 | 0~10 | 25~65 | 1~2 | — | 1~36 | <5 | 0~10 |
| CH4 | 60~70 | 40~75 | 3~25 | 20~25 | 30~65 | 20~50 | 69~95 |
| C2H4 | — | — | — | 20~25 | — | — | — |
| C2H6 | 5~20 | — | 2~15 | 0~5 | 5~20 | — | 0-2 |
| C3H6 | — | — | — | — | — | — | — |
| C3H8 | 2~15 | — | 2~15 | 0-5 | 2~20 | — | 0~2 |
| H2 | — | — | 50~90 | 10~20 | — | — | 0~1 |
| N2 | 0~25 | — | — | — | 0~5 | 39~70 | 0~30 |
| 其他 | 0~15 | 0~10 | 0~10 | 25~30 | 5~25 | 10~16 | 0~1 |
| 吸附质 | 沸点/K | 动力学直径/nm | 偶极矩/(10-18 esu·cm) | 四极矩/(10-26 esu·cm2) | 极化率/(10-25 cm3) |
|---|---|---|---|---|---|
| CO2 | 194.7 | 0.33 | 0 | 4.3 | 29.1 |
| CH4 | 111.6 | 0.38 | 0 | 0 | 26.0 |
| C2H4 | 169.4 | 0.42 | 0 | 1.5 | 42.5 |
| C2H6 | 184.6 | 0.44 | 0 | 0.65 | 44.3~44.7 |
| C3H6 | 225.5 | 0.46 | 0.366 | — | 62.6 |
| C3H8 | 231.0 | 0.43~0.52 | 0.084 | — | 62.9~63.7 |
Table 2 Table of physical and chemical properties of C1—C3 gas molecules[23]
| 吸附质 | 沸点/K | 动力学直径/nm | 偶极矩/(10-18 esu·cm) | 四极矩/(10-26 esu·cm2) | 极化率/(10-25 cm3) |
|---|---|---|---|---|---|
| CO2 | 194.7 | 0.33 | 0 | 4.3 | 29.1 |
| CH4 | 111.6 | 0.38 | 0 | 0 | 26.0 |
| C2H4 | 169.4 | 0.42 | 0 | 1.5 | 42.5 |
| C2H6 | 184.6 | 0.44 | 0 | 0.65 | 44.3~44.7 |
| C3H6 | 225.5 | 0.46 | 0.366 | — | 62.6 |
| C3H8 | 231.0 | 0.43~0.52 | 0.084 | — | 62.9~63.7 |
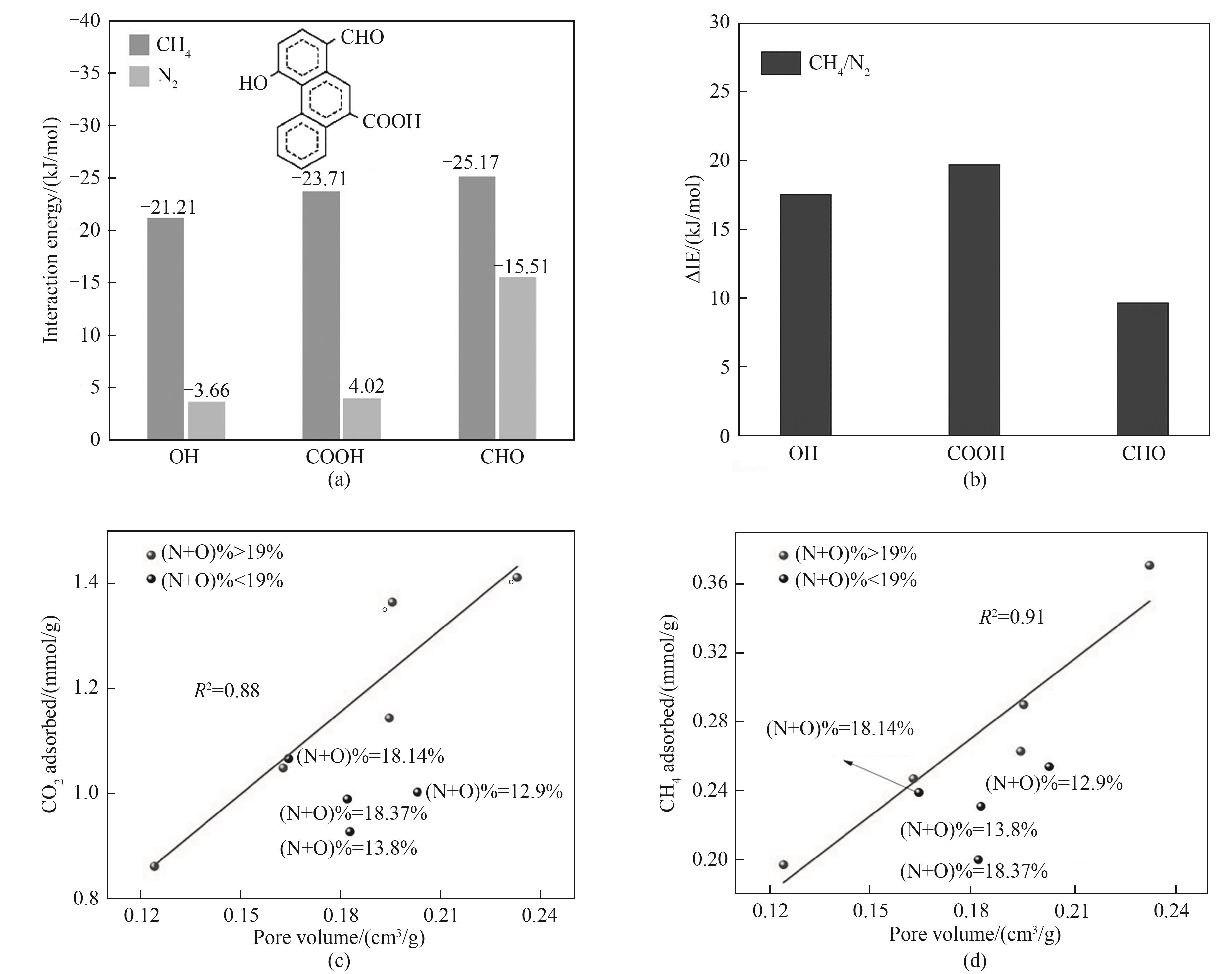
Fig.2 Interaction energy between CH4/N2 molecules and the surface carboxylic/hydroxyl/aldehyde groups of the samples (a); Absolute difference of the interaction energies (ΔIE) between CH4 and N2 on functional groups[27] (b); The relationship of (N+O)-contents with CO2 (c) and CH4 (d) capture capacity at 298 K under 15 kPa and <0.7 nm pore volume[28]
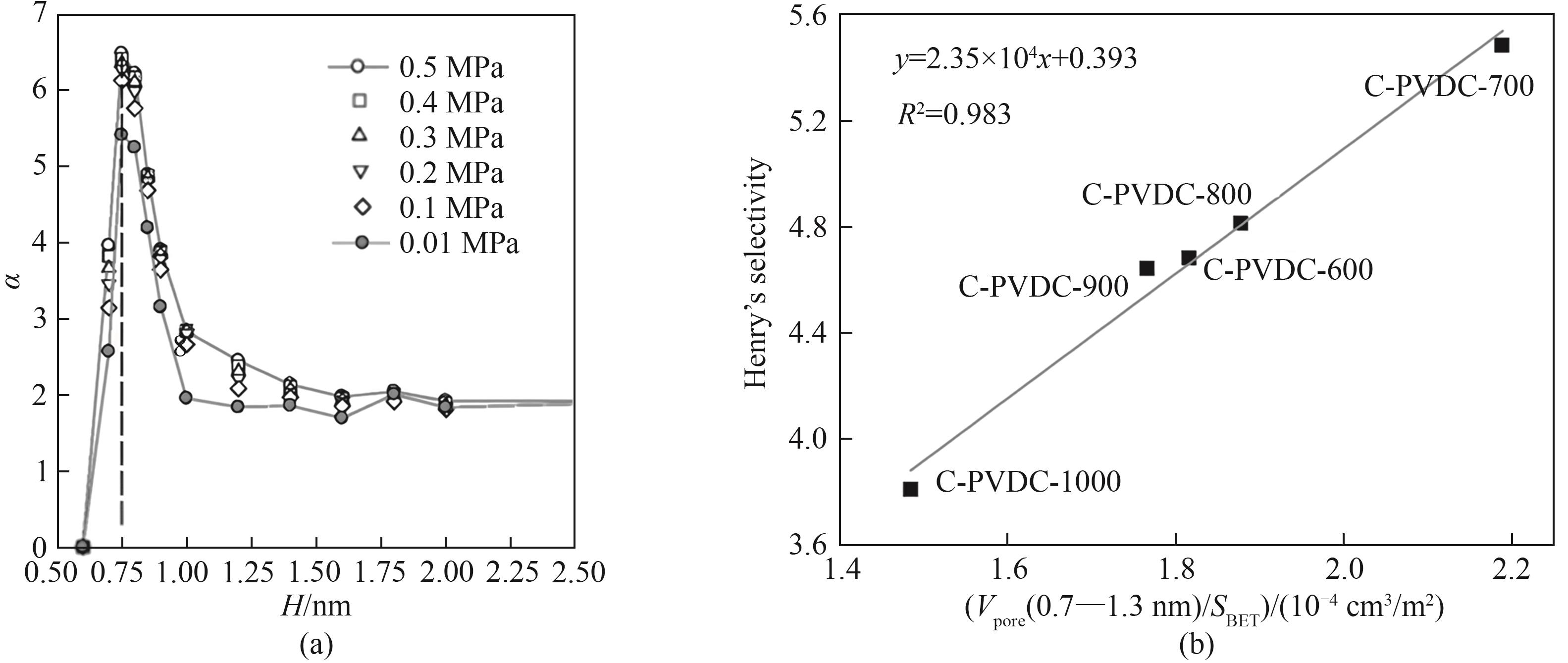
Fig.3 (a) The separation coefficient based on GCMC simulation[37]; (b) Correlation of Henry’s selectivity of CH4 over N2 with pore volume in the range of 0.7—1.3 nm reduced to unit surface area[38]

Fig.4 (a) AFM images of PCNPs and corresponding cross-section analysis for the dashed orange line in the images; (b) Micropore size distribution and micropore volume determined from CO2 adsorption at 273 K; (c) The comparison of kinetic adsorption of CH4 for PCNPs and SCNPs at 2 kPa (298 K); (d) Breakthrough curves of PCNPs, 13X zeolite and CMS for CH4[50]
| 吸附剂 | 比表面积/(m2/g) | 总孔容/(cm3/g) | 微孔孔容/(cm3/g) | 吸附量/(mmol/g) | 选择性 | 文献 | |
|---|---|---|---|---|---|---|---|
| CH4 | N2 | ||||||
| ACK2N1 | 1981 | 0.83 | 0.78 | 3.00① | 0.75① | 7.1② | [ |
| ClCTF-1-650 | 974 | 0.37 | 0.37 | 1.34 | 0.32 | 8.6 | [ |
| PRC-850 | 776 | 0.57 | 0.19 | 1.12 | — | 5.5~9.3③ | [ |
| PS-2-450 | 1089 | 0.55 | 0.42 | 1.13 | 0.22 | 7.6 | [ |
| PZS-900 | 896 | — | — | 1.88 | 0.66 | 4.5 | [ |
| 10%Ni/C | 1367 | — | 0.37 | 1.16 | 0.31 | 5.3⑤ | [ |
| C-PVDC-700 | 1130 | 0.45 | 0.25 | 1.57 | ~0.35 | 5.5⑤ | [ |
| GOC-2 | 1306 | 0.67 | — | 1.82 | — | 5.8 | [ |
| CGUCs-1-8 | 1071 | 0.44 | 0.37 | ~1.3 | ~0.40 | 5.0 | [ |
| AC-KP | 1549 | 0.62 | 0.28 | 1.87 | 0.67 | 6.5② | [ |
| PCNPs | 690 | — | 0.26 | 1.17 | 0.28 | 10.1④ | [ |
Table 3 Summary of the physicochemical properties and CH4/N2 adsorption separation performances of typical porous carbon adsorbents
| 吸附剂 | 比表面积/(m2/g) | 总孔容/(cm3/g) | 微孔孔容/(cm3/g) | 吸附量/(mmol/g) | 选择性 | 文献 | |
|---|---|---|---|---|---|---|---|
| CH4 | N2 | ||||||
| ACK2N1 | 1981 | 0.83 | 0.78 | 3.00① | 0.75① | 7.1② | [ |
| ClCTF-1-650 | 974 | 0.37 | 0.37 | 1.34 | 0.32 | 8.6 | [ |
| PRC-850 | 776 | 0.57 | 0.19 | 1.12 | — | 5.5~9.3③ | [ |
| PS-2-450 | 1089 | 0.55 | 0.42 | 1.13 | 0.22 | 7.6 | [ |
| PZS-900 | 896 | — | — | 1.88 | 0.66 | 4.5 | [ |
| 10%Ni/C | 1367 | — | 0.37 | 1.16 | 0.31 | 5.3⑤ | [ |
| C-PVDC-700 | 1130 | 0.45 | 0.25 | 1.57 | ~0.35 | 5.5⑤ | [ |
| GOC-2 | 1306 | 0.67 | — | 1.82 | — | 5.8 | [ |
| CGUCs-1-8 | 1071 | 0.44 | 0.37 | ~1.3 | ~0.40 | 5.0 | [ |
| AC-KP | 1549 | 0.62 | 0.28 | 1.87 | 0.67 | 6.5② | [ |
| PCNPs | 690 | — | 0.26 | 1.17 | 0.28 | 10.1④ | [ |

Fig.5 (a) Micropore size distributions of the activated carbon, obtained by fitting CO2 adsorption data at 0℃; (b) Methane adsorption isotherms at 25℃ on the activated carbons. The lines represent the fitting of the virial equation[64]
| 吸附剂 | 比表面积/(m2/g) | 总孔容/(cm3/g) | 微孔孔容/(cm3/g) | 吸附量/(mmol/g) | 选择性 | 文献 | |
|---|---|---|---|---|---|---|---|
| CO2 | CH4 | ||||||
| SA-1-700 | 1406 | 0.72 | 0.60 | 4.20 | 1.53 | 8.0① | [ |
| NAC-4 | 1593 | 0.76 | 0.21 | 3.33 | 0.57 | 5.8 | [ |
| GL | 310 | 0.13 | 0.11 | 1.62② | 0.45② | 54.0② | [ |
| PS | 790 | 0.33 | 0.26 | 3.63② | 1.38② | 15.0② | [ |
| NPC-725 | 314 | 0.21 | 0.14 | 3.22 | 1.02 | 24.3① | [ |
| Glc-Cs | 3153 | 2.06 | 0.30 | 22.40③ | 10.00③ | 27.0③ | [ |
Table 4 Summary of the physicochemical properties and CO2/CH4 adsorption separation performances of typical porous carbon adsorbents
| 吸附剂 | 比表面积/(m2/g) | 总孔容/(cm3/g) | 微孔孔容/(cm3/g) | 吸附量/(mmol/g) | 选择性 | 文献 | |
|---|---|---|---|---|---|---|---|
| CO2 | CH4 | ||||||
| SA-1-700 | 1406 | 0.72 | 0.60 | 4.20 | 1.53 | 8.0① | [ |
| NAC-4 | 1593 | 0.76 | 0.21 | 3.33 | 0.57 | 5.8 | [ |
| GL | 310 | 0.13 | 0.11 | 1.62② | 0.45② | 54.0② | [ |
| PS | 790 | 0.33 | 0.26 | 3.63② | 1.38② | 15.0② | [ |
| NPC-725 | 314 | 0.21 | 0.14 | 3.22 | 1.02 | 24.3① | [ |
| Glc-Cs | 3153 | 2.06 | 0.30 | 22.40③ | 10.00③ | 27.0③ | [ |
| 吸附剂 | 比表面积/ (m2/g) | 总孔容/ (cm3/g) | 微孔孔容/ (cm3/g) | 吸附量/(mmol/g) | 选择性 | 文献 | |||
|---|---|---|---|---|---|---|---|---|---|
| CH4 | C2H6 | C3H8 | C2H6/CH4 | C3H8/CH4 | |||||
| A-AC-4 | 3131 | 1.92 | — | 1.18 | 6.59 | 11.76 | 15.1① | 88.8① | [ |
| SMC3 | 1999 | 0.81 | 0.69 | 1.00 | 5.28 | 8.39 | 27.1① | 146.0① | [ |
| C-PVDC-800 | 1211 | 0.45 | 0.45 | 5.22 | 5.19 | 74.9 | 3387.2 | [ | |
| UC800 | 3839 | 2.3 | 1.13 | 1.26 | 7.19 | 12.02 | 9.1 | 41.8 | [ |
| FCP-1-KC | 800 | 0.4 | 0.36 | 1.70 | 5.10 | 5.30 | 71.0② | 386.0② | [ |
| NPC-Li | 3579 | 1.49 | — | 0.48 | 7.67 | 3.55 | 8.0 | 336.0 | [ |
Table 5 Summary of the physicochemical properties and C2-3H x /CH4 adsorption separation performances of typical porous carbon adsorbents
| 吸附剂 | 比表面积/ (m2/g) | 总孔容/ (cm3/g) | 微孔孔容/ (cm3/g) | 吸附量/(mmol/g) | 选择性 | 文献 | |||
|---|---|---|---|---|---|---|---|---|---|
| CH4 | C2H6 | C3H8 | C2H6/CH4 | C3H8/CH4 | |||||
| A-AC-4 | 3131 | 1.92 | — | 1.18 | 6.59 | 11.76 | 15.1① | 88.8① | [ |
| SMC3 | 1999 | 0.81 | 0.69 | 1.00 | 5.28 | 8.39 | 27.1① | 146.0① | [ |
| C-PVDC-800 | 1211 | 0.45 | 0.45 | 5.22 | 5.19 | 74.9 | 3387.2 | [ | |
| UC800 | 3839 | 2.3 | 1.13 | 1.26 | 7.19 | 12.02 | 9.1 | 41.8 | [ |
| FCP-1-KC | 800 | 0.4 | 0.36 | 1.70 | 5.10 | 5.30 | 71.0② | 386.0② | [ |
| NPC-Li | 3579 | 1.49 | — | 0.48 | 7.67 | 3.55 | 8.0 | 336.0 | [ |
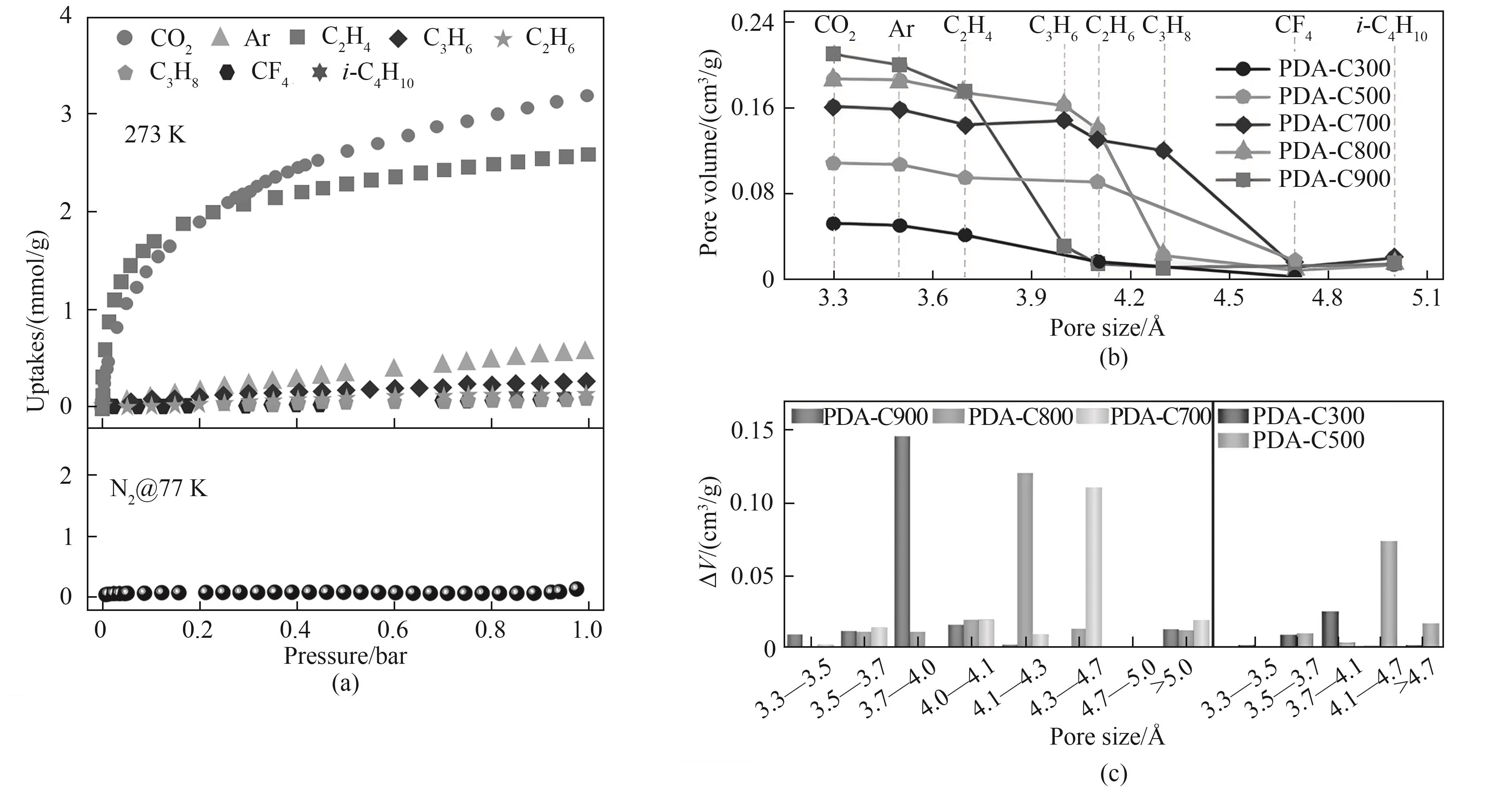
Fig.7 (a) Sorption equilibrium isotherms of various gas probes with minimum molecular dimensions ranging from 0.33 nm (CO2) to 0.5 nm (i-C4H10) at 273 K and N2 at 77 K on PDA-C900 materials; (b) The pore volumes of PDA-C x calculated from different probe gases based on the Dubinin-Astakhov equation (the data points from left to right were calculated from probing gases of CO2, Ar, C2H4, C3H6, C2H6, C3H8, CF4, and i-C4H10 for PDA-C x . While C3H6, C3H8, and i-C4H10 with high polarizability were excluded for heteroatom-rich PDA-C300 and PDA-C500 due to stronger host-guest interaction); (c) The pore size distributions of PDA-C x (the differential pore volume (∆V) of y-axis was obtained from probes with successive sizes. VAr was subtracted from VCO2, VC2H4 from VAr, and so on)[80]
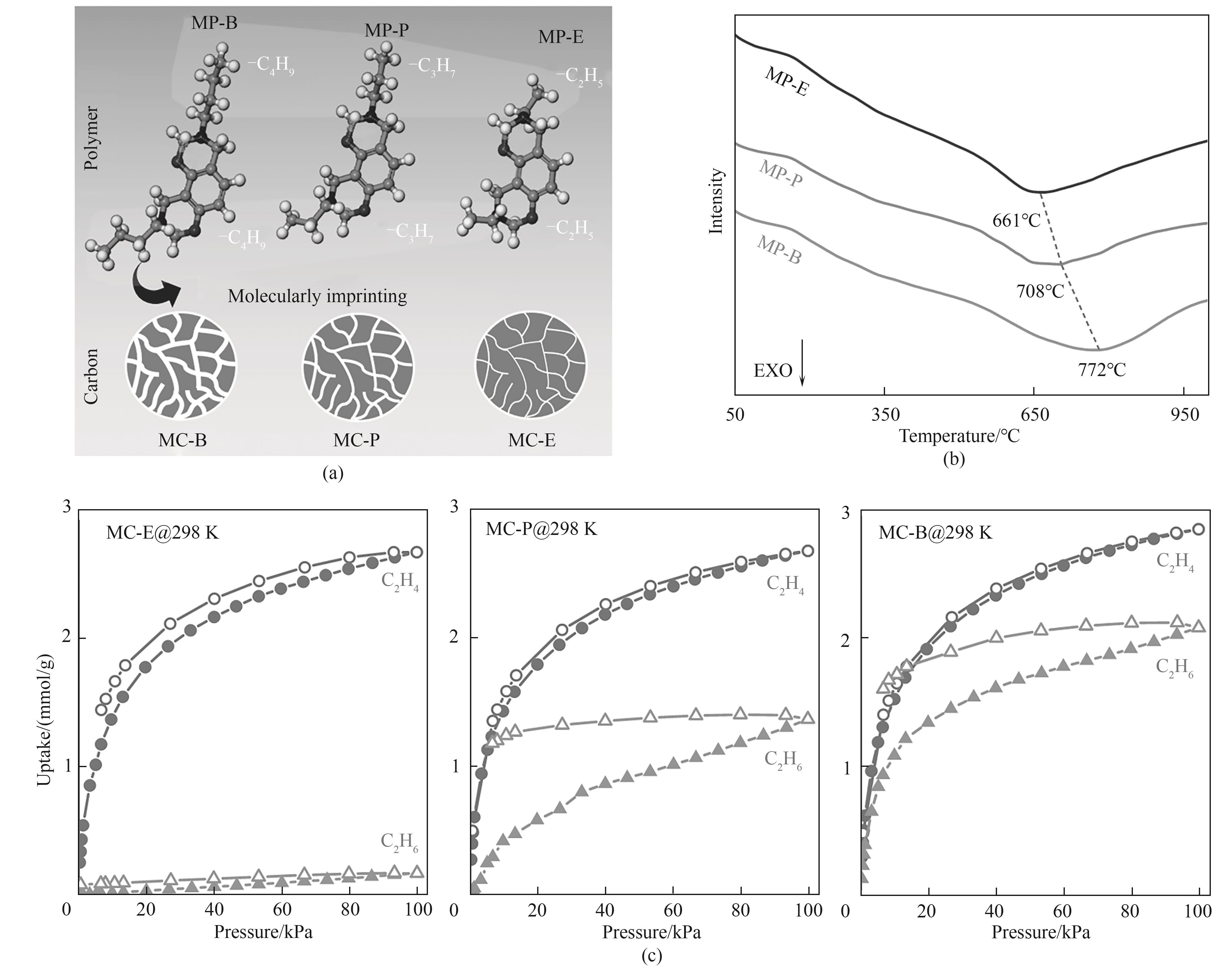
Fig.8 (a) Structure schematic of the three types of polymer monomers with the incorporated amines with different carbon chains; (b) The DSC curves of MP-B, MP-P, and MP-E in argon atmosphere; (c) C2H4/C2H6 adsorption-desorption isotherms for MC-E (left), MC-P (medium), and MC-B (right) at 298 K[83]

Fig.9 Schematic structures and schwarzite models of FAU-ZTC (a), EMT-ZTC (b), and beta-ZTC (c); Adsorption isotherms of ethane and ethylene by adsorbents at 303 K: FAU-ZTC (d), EMT-ZTC (e), beta-ZTC (f)[89]
| 名称 | 比表面积/ (m2/g) | 总孔容/ (cm3/g) | 微孔孔容/ (cm3/g) | 吸附量/(mmol/g) | 扩散速率/s-1 | 选择性 | 文献 | ||
|---|---|---|---|---|---|---|---|---|---|
| C2H4 | C2H6 | C2H4 | C2H6 | ||||||
| PDA-C900 | 400① | — | 0.21① | 2.25 | 0.091 | 4.13 × 10-3 | — | 24.7 | [ |
| CNC-700 | 317① | — | 0.16① | 1.44 | 0.093 | — | — | 15.2 | [ |
| d-CMS-3 | — | — | — | 1.52 | 0.14 | 2.0 × 10-3 | 1 × 10-5 | 10.9 | [ |
| MC-E | 317 | — | — | 2.66 | 0.17 | — | — | 15.6 | [ |
| CMK-3 | 1182 | 1.19 | — | 2.90② | 3.10② | — | — | 0.9 | [ |
| 8CuCl/CMK-3 | 303 | 0.37 | — | 3.60② | 1.30② | — | — | 2.8 | |
| CuCl(8.0)/AC | 224 | 0.38 | — | 2.60② | 0.70② | — | — | 3.7 | [ |
| 50CPDA@A-ACs | 1799 | 0.72 | — | 6.30 | 7.10 | — | — | 3.0③ | [ |
| MGA-750-3 | 3189 | 1.48 | 0.93 | 5.70 | 7.00 | — | — | 4.2③ | [ |
| C-PDA-3 | 3160 | 1.51 | — | 6.57 | 5.10 | — | — | 1.9④ | [ |
| beta-ZTC | 3200 | — | — | 7.30② | 4.90② | — | — | 1.7⑤ | [ |
| EMT-ZTC | 2980 | — | — | 5.90② | 4.10② | — | — | 1.6⑤ | |
| FAU-ZTC | 2700 | — | — | 5.00② | 4.00② | — | — | 1.5⑤ | |
Table 6 Summary of the physicochemical properties and C2H4/C2H6 adsorption separation performances of typical porous carbon adsorbents
| 名称 | 比表面积/ (m2/g) | 总孔容/ (cm3/g) | 微孔孔容/ (cm3/g) | 吸附量/(mmol/g) | 扩散速率/s-1 | 选择性 | 文献 | ||
|---|---|---|---|---|---|---|---|---|---|
| C2H4 | C2H6 | C2H4 | C2H6 | ||||||
| PDA-C900 | 400① | — | 0.21① | 2.25 | 0.091 | 4.13 × 10-3 | — | 24.7 | [ |
| CNC-700 | 317① | — | 0.16① | 1.44 | 0.093 | — | — | 15.2 | [ |
| d-CMS-3 | — | — | — | 1.52 | 0.14 | 2.0 × 10-3 | 1 × 10-5 | 10.9 | [ |
| MC-E | 317 | — | — | 2.66 | 0.17 | — | — | 15.6 | [ |
| CMK-3 | 1182 | 1.19 | — | 2.90② | 3.10② | — | — | 0.9 | [ |
| 8CuCl/CMK-3 | 303 | 0.37 | — | 3.60② | 1.30② | — | — | 2.8 | |
| CuCl(8.0)/AC | 224 | 0.38 | — | 2.60② | 0.70② | — | — | 3.7 | [ |
| 50CPDA@A-ACs | 1799 | 0.72 | — | 6.30 | 7.10 | — | — | 3.0③ | [ |
| MGA-750-3 | 3189 | 1.48 | 0.93 | 5.70 | 7.00 | — | — | 4.2③ | [ |
| C-PDA-3 | 3160 | 1.51 | — | 6.57 | 5.10 | — | — | 1.9④ | [ |
| beta-ZTC | 3200 | — | — | 7.30② | 4.90② | — | — | 1.7⑤ | [ |
| EMT-ZTC | 2980 | — | — | 5.90② | 4.10② | — | — | 1.6⑤ | |
| FAU-ZTC | 2700 | — | — | 5.00② | 4.00② | — | — | 1.5⑤ | |

Fig.10 (a) Schematic preparation procedures for the wood frame structured carbons with sieving layer, WSCS; (b) SEM images of wood frame, polybenzoxazine polymer coated wood, PBZ@W, and wood frame structured carbons with sieving layer, WSCS; (c) Breakthrough curves of WSC, WSCS, and PDC for equimolar binary mixture C3H6/C3H8 with a flow rate of 2 ml/min at 298 K and 100 kPa (t1 is the breakthrough point of C3H8; t2 is the breakthrough point of C3H6)[91]
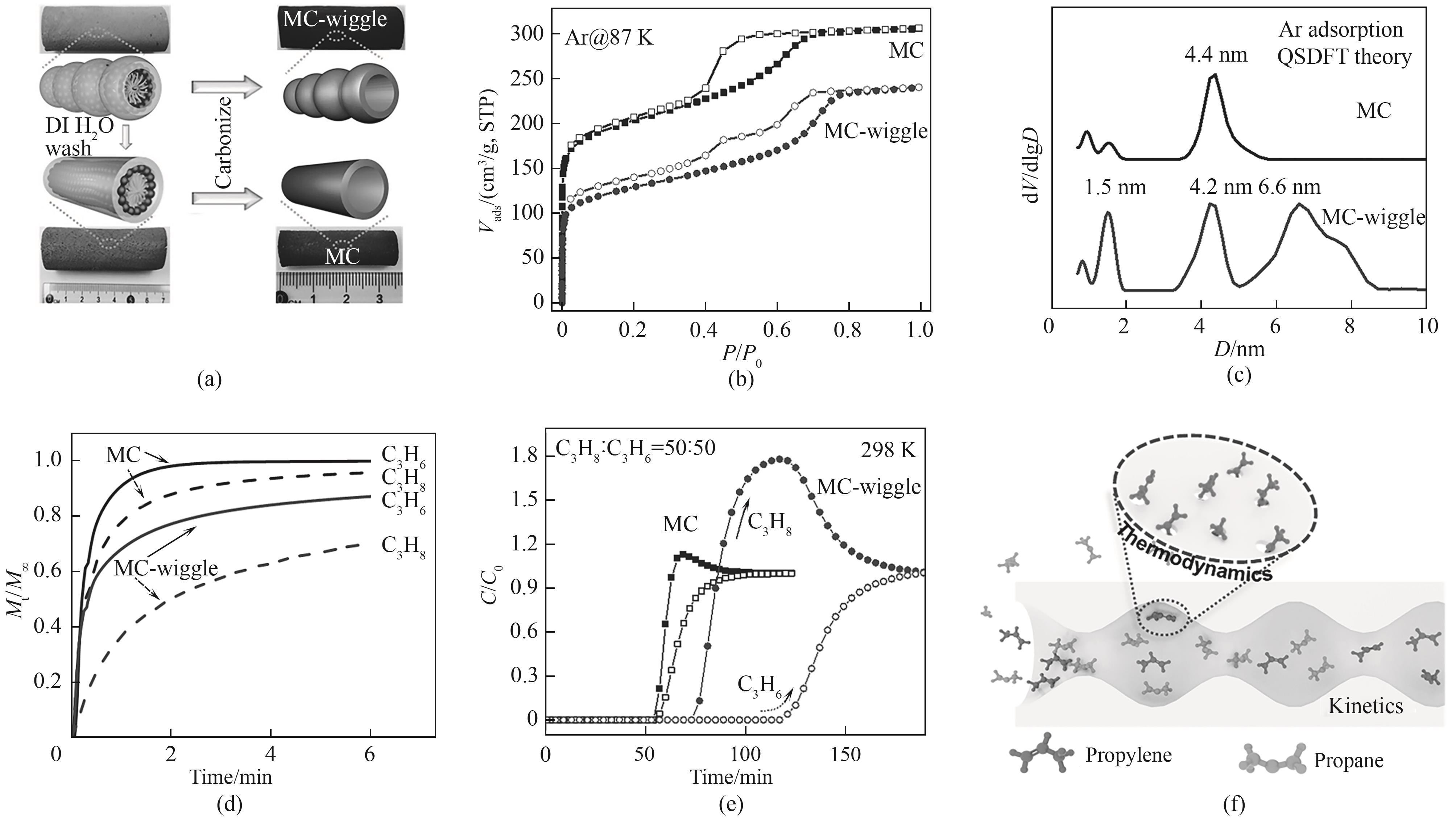
Fig.11 (a) Synthesis schematic of MC-wiggle; (b) Ar adsorption isotherms at 87 K and (c) corresponding pore size distributions; (d) Time-resolved adsorption profiles for C3H6/C3H8 at 298 K; (e) Breakthrough curves for gas feed of equimolar binary mixture of C3H6/C3H8 with a flow rate of 2 ml/min under ambient conditions; (f) Illustration of the proposed selective adsorption of C3H6/C3H8 in wiggling mesopores[98]
| 吸附剂 | 比表面积/ (m2/g) | 总孔容/ (cm3/g) | 微孔孔容/ (cm3/g) | 吸附量/(mmol/g) | 扩散速率/s-1 | 选择性 | 文献 | ||
|---|---|---|---|---|---|---|---|---|---|
| C3H6 | C3H8 | C3H6 | C3H8 | ||||||
| PDA-C800 | 375① | 0.19 | 1.98 | 0.054 | 7.57×10-3 | 36.7 | [ | ||
| SCMS-0.2-800 | 0.24 | 2.54 | 0.08 | 31.8 | [ | ||||
| SCMS-1-800 | 2.22 | 0.02 | 111.0 | ||||||
| WSCS | 19② | 0.18 | 1.72 | 0.17 | 2.68×10-3 | 6.26×10-5 | 10.1 | [ | |
| Carbon-0.2 | 680 | 0.18 | 2.14 | 0.54 | 5.2×10-3 | 1.1×10-4 | 4.0 | [ | |
| SUC-900 | 458 | 0.23 | 2.0 | 0.055 | 36.4 | [ | |||
| CMS-850 | 382 | 0.13 | 1.53③ | 0.66 | 2.3 | [ | |||
| CNP-3 | 3.03 | 1.86 | 9.6×10-3 | 8.8×10-4 | 1.6 | [ | |||
| SCS-800 | 0.18 | 2.0 | 0.25 | 1.34×10-4 | 1.18×10-6 | 8.0 | [ | ||
| MCC-micro | 479② | 0.22② | 0.16② | 2.4 | 1.1 | 1.26×10-2 | 2.76×10-5 | 2.2 | [ |
| MC-wiggle | 413② | 2.6 | 1.5 | 4.2×10-3 | 2.1×10-4 | 1.7 | [ | ||
| C-CDMOF-2-700 | 371① | 0.19① | 1.97 | 0.13 | 3.15×10-3④ | 15.1 | [ | ||
| MFF_8 | 1.4 | 2.9 | 1.2×10-1 | 1.4×10-1 | 2.1 | [ | |||
Table 7 Summary of the physicochemical properties and C3H6/C3H8 adsorption separation performances of typical porous carbon adsorbents
| 吸附剂 | 比表面积/ (m2/g) | 总孔容/ (cm3/g) | 微孔孔容/ (cm3/g) | 吸附量/(mmol/g) | 扩散速率/s-1 | 选择性 | 文献 | ||
|---|---|---|---|---|---|---|---|---|---|
| C3H6 | C3H8 | C3H6 | C3H8 | ||||||
| PDA-C800 | 375① | 0.19 | 1.98 | 0.054 | 7.57×10-3 | 36.7 | [ | ||
| SCMS-0.2-800 | 0.24 | 2.54 | 0.08 | 31.8 | [ | ||||
| SCMS-1-800 | 2.22 | 0.02 | 111.0 | ||||||
| WSCS | 19② | 0.18 | 1.72 | 0.17 | 2.68×10-3 | 6.26×10-5 | 10.1 | [ | |
| Carbon-0.2 | 680 | 0.18 | 2.14 | 0.54 | 5.2×10-3 | 1.1×10-4 | 4.0 | [ | |
| SUC-900 | 458 | 0.23 | 2.0 | 0.055 | 36.4 | [ | |||
| CMS-850 | 382 | 0.13 | 1.53③ | 0.66 | 2.3 | [ | |||
| CNP-3 | 3.03 | 1.86 | 9.6×10-3 | 8.8×10-4 | 1.6 | [ | |||
| SCS-800 | 0.18 | 2.0 | 0.25 | 1.34×10-4 | 1.18×10-6 | 8.0 | [ | ||
| MCC-micro | 479② | 0.22② | 0.16② | 2.4 | 1.1 | 1.26×10-2 | 2.76×10-5 | 2.2 | [ |
| MC-wiggle | 413② | 2.6 | 1.5 | 4.2×10-3 | 2.1×10-4 | 1.7 | [ | ||
| C-CDMOF-2-700 | 371① | 0.19① | 1.97 | 0.13 | 3.15×10-3④ | 15.1 | [ | ||
| MFF_8 | 1.4 | 2.9 | 1.2×10-1 | 1.4×10-1 | 2.1 | [ | |||
| 1 | Fahim M A, Al-Sahhaf T A, Elkilani A. Fundamentals of Petroleum Refining[M]. UK: Elsevier, 2009. |
| 2 | Pines H. The Chemistry of Catalytic Hydrocarbon Conversions[M]. New York: Academic Press, 1981. |
| 3 | 王松汉. 乙烯工艺与技术[M]. 北京: 中国石化出版社, 2012. |
| Wang S H. Ethylene Process and Technology[M]. Beijing: China Petrochemical Press, 2012. | |
| 4 | Malek A, Farooq S. Hydrogen purification from refinery fuel gas by pressure swing adsorption[J]. AIChE Journal, 1998, 44(9): 1985-1992. |
| 5 | Qi G G, Fu L L, Giannelis E P. Sponges with covalently tethered amines for high-efficiency carbon capture[J]. Nature Communications, 2014, 5: 5796. |
| 6 | Ko D. Optimization of vacuum pressure swing adsorption processes to sequester carbon dioxide from coalbed methane[J]. Industrial & Engineering Chemistry Research, 2016, 55(33): 8967-8978. |
| 7 | Yaghi O M, Li G M, Li H L. Selective binding and removal of guests in a microporous metal-organic framework[J]. Nature, 1995, 378: 703-706. |
| 8 | Noro S I, Kitagawa S, Kondo M, et al. A new, methane adsorbent, porous coordination polymer [{CuSiF6(4,4'-bipyridine)2} n ][J]. Angewandte Chemie International Edition, 2000, 39(12): 2081-2084. |
| 9 | Ahmed A, Babarao R, Huang R H, et al. Porous aromatic frameworks impregnated with lithiated fullerenes for natural gas purification[J]. The Journal of Physical Chemistry C, 2015, 119(17): 9347-9354. |
| 10 | 王永飞, 华贲, 李亚军. 炼厂干气的综合利用研究[J]. 现代化工, 2008, 28(2): 69-71, 74. |
| Wang Y F, Hua B, Li Y J. Study on comprehensive utilization of refinery dry gas[J]. Modern Chemical Industry, 2008, 28(2): 69-71, 74. | |
| 11 | Sadrameli S M. Thermal/catalytic cracking of hydrocarbons for the production of olefins: a state-of-the-art review (Ⅰ): Thermal cracking review[J]. Fuel, 2015, 140: 102-115. |
| 12 | Wang Y Y, Chen J F, Pang X Q, et al. Faulting controls on oil and gas composition in the Yingmai 2 Oilfield, Tarim Basin, NW China[J]. Organic Geochemistry, 2018, 123: 48-66. |
| 13 | Zhang J H, Li L T, Qin Q. Effects of micropore structure of activated carbons on the CH4/N2 adsorption separation and the enrichment of coal-bed methane[J]. Clean Energy, 2021, 5(2): 329-338. |
| 14 | Yang X, Li Z Y, Zhang C Z, et al. Practical separation performance evaluation of coal mine methane upgrading with carbon molecular sieves[J]. Chemical Engineering Journal, 2019, 367: 295-303. |
| 15 | Dang W, Zhang J C, Tang X, et al. Investigation of gas content of organic-rich shale: a case study from Lower Permian shale in southern North China Basin, Central China[J]. Geoscience Frontiers, 2018, 9(2): 559-575. |
| 16 | Shi J T, Jia Y R, Zhang L L, et al. The generalized method for estimating reserves of shale gas and coalbed methane reservoirs based on material balance equation[J]. Petroleum Science, 2022, 19(6): 2867-2878. |
| 17 | Wang Y S, Zhang X J, Ba Y Q, et al. Recent advances in carbon-based adsorbents for adsorptive separation of light hydrocarbons[J]. Research, 2022, 2022: 9780864. |
| 18 | Zhang J C, Si L L, Chen J G, et al. Stimulation techniques of coalbed methane reservoirs[J]. Geofluids, 2020, 2020: 5152646. |
| 19 | Lan W J, Wang H X, Liu Q H, et al. Investigation on the microwave heating technology for coalbed methane recovery[J]. Energy, 2021, 237: 121450. |
| 20 | Yang Z X, Hussain M Z, Marín P, et al. Enrichment of low concentration methane: an overview of ventilation air methane[J]. Journal of Materials Chemistry A, 2022, 10(12): 6397-6413. |
| 21 | Zhong D L, Wang W C, Zou Z L, et al. Investigation on methane recovery from low-concentration coal mine gas by tetra-n-butyl ammonium chloride semiclathrate hydrate formation[J]. Applied Energy, 2018, 227: 686-693. |
| 22 | Yang J F, Bai H H, Shang H, et al. Experimental and simulation study on efficient CH4/N2 separation by pressure swing adsorption on silicalite-1 pellets[J]. Chemical Engineering Journal, 2020, 388: 124222. |
| 23 | Li J R, Kuppler R J, Zhou H C. Selective gas adsorption and separation in metal-organic frameworks[J]. Chemical Society Reviews, 2009, 38(5): 1477-1504. |
| 24 | Feng Y Y, Yang W, Wang N, et al. Effect of nitrogen-containing groups on methane adsorption behaviors of carbon spheres[J]. Journal of Analytical and Applied Pyrolysis, 2014, 107: 204-210. |
| 25 | Zhang L, Dong Y G, Zhang D, et al. Facile preparation of nitrogen-doped microporous carbon from potassium citrate/urea for effective CH4 separation and uptake[J]. Fuel, 2023, 351: 128915. |
| 26 | Yao K X, Chen Y L, Lu Y, et al. Ultramicroporous carbon with extremely narrow pore distribution and very high nitrogen doping for efficient methane mixture gases upgrading[J]. Carbon, 2017, 122: 258-265. |
| 27 | Tang R L, Dai Q B, Liang W W, et al. Synthesis of novel particle rice-based carbon materials and its excellent CH4/N2 adsorption selectivity for methane enrichment from low-rank natural gas[J]. Chemical Engineering Journal, 2020, 384: 123388. |
| 28 | Liu F, Zhang Y, Zhang P X, et al. Facile preparation of N and O-rich porous carbon from palm sheath for highly selective separation of CO2/CH4/N2 gas-mixture[J]. Chemical Engineering Journal, 2020, 399: 125812. |
| 29 | Wang S M, Wu P C, Fu J W, et al. Heteroatom-doped porous carbon microspheres with ultramicropores for efficient CH4/N2 separation with ultra-high CH4 uptake[J]. Separation and Purification Technology, 2021, 274: 119121. |
| 30 | Li S H, Fu S G, Gong Y Q, et al. New insight into efficient CH4/N2 separation based on Ni-decorated porous carbon functional composites[J]. Fuel, 2023, 347: 128480. |
| 31 | Zheng Y N, Li Q Z, Yuan C C, et al. Influence of temperature on adsorption selectivity: coal-based activated carbon for CH4 enrichment from coal mine methane[J]. Powder Technology, 2019, 347: 42-49. |
| 32 | Liu J Q, Shang H, Yang J F, et al. Novel zeolite/carbon monolith adsorbents for efficient CH4/N2 separation[J]. Chemical Engineering Journal, 2021, 426: 130163. |
| 33 | Steel K M, Koros W J. Investigation of porosity of carbon materials and related effects on gas separation properties[J]. Carbon, 2003, 41(2): 253-266. |
| 34 | Cui X J, Bustin R M, Dipple G. Selective transport of CO2, CH4, and N2 in coals: insights from modeling of experimental gas adsorption data[J]. Fuel, 2004, 83(3): 293-303. |
| 35 | Zhao G F, Bai P, Zhu H M, et al. The modification of activated carbons and the pore structure effect on enrichment of coal-bed methane[J]. Asia-Pacific Journal of Chemical Engineering, 2008, 3(3): 284-291. |
| 36 | Cai Y D, Liu D M, Pan Z J, et al. Pore structure and its impact on CH4 adsorption capacity and flow capability of bituminous and subbituminous coals from Northeast China[J]. Fuel, 2013, 103: 258-268. |
| 37 | Liu C M, Dang Y Y, Zhou Y P, et al. Effect of carbon pore structure on the CH4/N2 separation[J]. Adsorption, 2012, 18(3): 321-325. |
| 38 | Chen F Q, Zhang Z G, Yang Q W, et al. Microporous carbon adsorbents prepared by activating reagent-free pyrolysis for upgrading low-quality natural gas[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(2): |
| 39 | Dong Z, Li B, Shang H, et al. Ultramicroporous carbon granules with narrow pore size distribution for efficient CH4 separation from coal-bed gases[J]. AIChE Journal, 2021, 67(9): e17281. |
| 40 | Zhang P X, Wang J, Fan W, et al. Ultramicroporous carbons with extremely narrow pore size distribution via in situ ionic activation for efficient gas-mixture separation[J]. Chemical Engineering Journal, 2019, 375: 121931. |
| 41 | Liu F X, Lin Q, Fu C B, et al. Alkaline KMnO4 solution pretreat hydrochar to prepare high ultra-micropore volume carbon for CH4 enrichment from low-concentration coalbed methane[J]. Fuel, 2021, 303: 121301. |
| 42 | 马东祝. 煤矿乏风瓦斯变压吸附分离吸附剂的研究[D]. 北京: 北京工业大学, 2013. |
| Ma D Z. Study on adsorbent for pressure swing adsorption separation of coal mine ventilation [D]. Beijing: Beijing University of Technology, 2013. | |
| 43 | Krishna R. Methodologies for evaluation of metal-organic frameworks in separation applications[J]. RSC Advances, 2015, 5(64): 52269-52295. |
| 44 | Zhang J H, Qu S J, Li L T, et al. Preparation of carbon molecular sieves used for CH4/N2 separation[J]. Journal of Chemical & Engineering Data, 2018, 63(5): 1737-1744. |
| 45 | Grande C A, Cavenati S, Da Silva F A, et al. Carbon molecular sieves for hydrocarbon separations by adsorption[J]. Industrial & Engineering Chemistry Research, 2005, 44(18): 7218-7227. |
| 46 | Wang S, Cheng F, Zhang P, et al. Fabrication of high-pore volume carbon nanosheets with uniform arrangement of mesopores[J]. Nano Research, 2017, 10(6): 2106-2116. |
| 47 | Hao G P, Jin Z Y, Sun Q, et al. Porous carbon nanosheets with precisely tunable thickness and selective CO2 adsorption properties[J]. Energy & Environmental Science, 2013, 6(12): 3740-3747. |
| 48 | Guo L P, Li W C, Qiu B, et al. Interfacial assembled preparation of porous carbon composites for selective CO2 capture at elevated temperatures[J]. Journal of Materials Chemistry A, 2019, 7(10): 5402-5408. |
| 49 | Jin Z Y, Xu Y Y, Sun Q, et al. Evidence of microporous carbon nanosheets showing fast kinetics in both gas phase and liquid phase environments[J]. Small, 2015, 11(38): 5151-5156. |
| 50 | Xu S, Li W C, Wang C T, et al. Self-pillared ultramicroporous carbon nanoplates for selective separation of CH4/N2 [J]. Angewandte Chemie International Edition, 2021, 60(12): 6339-6343. |
| 51 | Rainone F, D'Agostino O, Erto A, et al. Biogas upgrading by adsorption onto activated carbon and carbon molecular sieves: experimental and modelling study in binary CO2/CH4 mixture[J]. Journal of Environmental Chemical Engineering, 2021, 9(5): 106256. |
| 52 | Santos M P S, Grande C A, Rodrigues A E. Pressure swing adsorption for biogas upgrading. Effect of recycling streams in pressure swing adsorption design[J]. Industrial & Engineering Chemistry Research, 2011, 50(2): 974-985. |
| 53 | Yang F Q, Wang J, Liu L, et al. Synthesis of porous carbons with high N-content from shrimp shells for efficient CO2-capture and gas separation[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(11): 15550-15559. |
| 54 | Zhang M, Liu L, He T, et al. Molten salt assisted pyrolysis approach for the synthesis of nitrogen-rich microporous carbon nanosheets and its application as gas capture sorbent[J]. Microporous and Mesoporous Materials, 2020, 300: 110177. |
| 55 | Zhang P X, Zhong Y, Ding J, et al. A new choice of polymer precursor for solvent-free method: preparation of N-enriched porous carbons for highly selective CO2 capture[J]. Chemical Engineering Journal, 2019, 355: 963-973. |
| 56 | Li Y, Liu N, Zhang T, et al. Highly microporous nitrogen-doped carbons from anthracite for effective CO2 capture and CO2/CH4 separation[J]. Energy, 2020, 211: 118561. |
| 57 | Furmaniak S, Kowalczyk P, Terzyk A P, et al. Synergetic effect of carbon nanopore size and surface oxidation on CO2 capture from CO2/CH4 mixtures[J]. Journal of Colloid and Interface Science, 2013, 397: |
| 58 | Su W, Yao L, Ran M, et al. Adsorption properties of N2, CH4, and CO2 on sulfur-doped microporous carbons[J]. Journal of Chemical & Engineering Data, 2018, 63(8): 2914-2920. |
| 59 | Saha D, Orkoulas G, Chen J H, et al. Adsorptive separation of CO2 in sulfur-doped nanoporous carbons: selectivity and breakthrough simulation[J]. Microporous and Mesoporous Materials, 2017, 241: 226-237. |
| 60 | Park J, Attia N F, Jung M, et al. Sustainable nanoporous carbon for CO2, CH4, N2, H2 adsorption and CO2/CH4 and CO2/N2 separation[J]. Energy, 2018, 158: 9-16. |
| 61 | Cheng J, Liu N, Wang Y L, et al. Nitrogen-doped microporous carbon material decorated with metal nanoparticles derived from solid Zn/Co zeolitic imidazolate framework with high selectivity for CO2 separation[J]. Fuel, 2020, 265: 116972. |
| 62 | Kapoor A, Yang R T. Kinetic separation of methane-carbon dioxide mixture by adsorption on molecular sieve carbon[J]. Chemical Engineering Science, 1989, 44(8): 1723-1733. |
| 63 | Rocha L A M, Andreassen K A, Grande C A. Separation of CO2/CH4 using carbon molecular sieve (CMS) at low and high pressure[J]. Chemical Engineering Science, 2017, 164: 148-157. |
| 64 | Mestre A S, Freire C, Pires J, et al. High performance microspherical activated carbons for methane storage and landfill gas or biogas upgrade[J]. Journal of Materials Chemistry A, 2014, 2(37): 15337-15344. |
| 65 | Matranga K R, Myers A L, Glandt E D. Storage of natural gas by adsorption on activated carbon[J]. Chemical Engineering Science, 1992, 47(7): 1569-1579. |
| 66 | Wang X J, Yuan B Q, Zhou X, et al. Novel glucose-based adsorbents (Glc-Cs) with high CO2 capacity and excellent CO2/CH4/N2 adsorption selectivity[J]. Chemical Engineering Journal, 2017, 327: 51-59. |
| 67 | Grancha T, Mon M, Ferrando-Soria J, et al. Tuning the selectivity of light hydrocarbons in natural gas in a family of isoreticular MOFs[J]. Journal of Materials Chemistry A, 2017, 5(22): 11032-11039. |
| 68 | Russell B P, LeVan M D. Coadsorption of organic compounds and water vapor on BPL activated carbon. 3. Ethane, propane, and mixing rules[J]. Industrial & Engineering Chemistry Research, 1997, 36(6): 2380-2389. |
| 69 | Xue D, Chen Z X, Lu C G. Establishment of the comprehensive material balance equation for coalbed methane reservoirs at the gas desorption stage[J]. Fuel, 2022, 326: 124979. |
| 70 | He Y F, Yun J H, Seaton N A. Adsorption equilibrium of binary methane/ethane mixtures in BPL activated carbon: isotherms and calorimetric heats of adsorption[J]. Langmuir, 2004, 20(16): 6668-6678. |
| 71 | Holland C E, Al-Muhtaseb S A, Ritter J A. Adsorption of C1-C7 normal alkanes on BAX activated carbon (1): Potential theory correlation and adsorbent characterization[J]. Industrial & Engineering Chemistry Research, 2001, 40(1): 338-346. |
| 72 | Liang W W, Xiao H Y, Lv D F, et al. Novel asphalt-based carbon adsorbents with super-high adsorption capacity and excellent selectivity for separation for light hydrocarbons[J]. Separation and Purification Technology, 2018, 190: 60-67. |
| 73 | Ke Z F, Xiao H Y, Wen Y J, et al. Adsorption property of starch-based microporous carbon materials with high selectivity and uptake for C1/C2/C3 separation[J]. Industrial & Engineering Chemistry Research, 2021, 60(12): 4668-4676. |
| 74 | Chen F Q, Guo K Q, Huang X L, et al. Extraction of propane and ethane from natural gas on ultramicroporous carbon adsorbent with record selectivity[J]. Science China Materials, 2023, 66(1): 319-326. |
| 75 | Xue Q Z, Li X F, Chang X, et al. S-graphite slit pore: a superior selective adsorbent for light hydrocarbons[J]. Applied Surface Science, 2018, 444: 772-779. |
| 76 | Ma X C, Chen R F, Zhou K, et al. Activated porous carbon with an ultrahigh surface area derived from waste biomass for acetone adsorption, CO2 capture, and light hydrocarbon separation[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(31): 11721-11728. |
| 77 | Ma X C, Fang M, Liu B G, et al. Urea-assisted synthesis of biomass-based hierarchical porous carbons for the light hydrocarbons adsorption and separation[J]. Chemical Engineering Journal, 2022, 428: 130985. |
| 78 | Zhang L H, Li W C, Liu H, et al. Thermoregulated phase-transition synthesis of two-dimensional carbon nanoplates rich in sp2 carbon and unimodal ultramicropores for kinetic gas separation[J]. Angewandte Chemie International Edition, 2018, 57(6): 1632-1635. |
| 79 | Ma X C, Liu B G, Wu Q D, et al. Specific Li+ sites in a nanoporous carbon for enhanced light hydrocarbons storage and separation: GCMC and DFT simulations[J]. Fuel, 2021, 288: 119647. |
| 80 | Du S J, Huang J W, Ryder M R, et al. Probing sub-5 Ångstrom micropores in carbon for precise light olefin/paraffin separation[J]. Nature Communications, 2023, 14(1): 1197. |
| 81 | Huang B L, Zhu L, Du Z L, et al. Highly efficient ethylene/ethane separation via molecular sieving using chitosan-derived ultramicroporous carbon[J]. Separation and Purification Technology, 2024, 333: 125862. |
| 82 | Liu R S, Wang M, Li W C, et al. Balancing the kinetic and thermodynamic synergetic effect of doped carbon molecular sieves for selective separation of C2H4/C2H6 [J]. Small, 2024, 20(38): e2401965. |
| 83 | Wang Y S, Li T Y, Song Y H, et al. Stepwise tuning carbon slits at sub-angstrom scale for dynamical separation of hydrogen isotope[J]. Separation and Purification Technology, 2025, 354: 129130. |
| 84 | Jiang W J, Sun L B, Yin Y, et al. Ordered mesoporous carbon CMK-3 modified with Cu(Ⅰ) for selective ethylene/ethane adsorption[J]. Separation Science and Technology, 2013, 48(6): 968-976. |
| 85 | Gao F, Wang Y Q, Wang X, et al. Ethylene/ethane separation by CuCl/AC adsorbent prepared using CuCl2 as a precursor[J]. Adsorption, 2016, 22(7): 1013-1022. |
| 86 | Liang W W, Wu Y, Xiao H Y, et al. Ethane-selective carbon composites CPDA@A-ACs with high uptake and its enhanced ethane/ethylene adsorption selectivity[J]. AIChE Journal, 2018, 64(9): 3390-3399. |
| 87 | Wang X J, Wu Y, Peng J J, et al. Novel glucosamine-based carbon adsorbents with high capacity and its enhanced mechanism of preferential adsorption of C2H6 over C2H4 [J]. Chemical Engineering Journal, 2019, 358: 1114-1125. |
| 88 | Wang X J, Wu Y, Zhou X, et al. Novel C-PDA adsorbents with high uptake and preferential adsorption of ethane over ethylene[J]. Chemical Engineering Science, 2016, 155: 338-347. |
| 89 | Lee S K, Park H, Yoon J W, et al. Microporous 3D graphene-like zeolite-templated carbons for preferential adsorption of ethane[J]. ACS Applied Materials & Interfaces, 2020, 12(25): 28484-28495. |
| 90 | Du S J, Huang J W, Anjum A W, et al. A novel mechanism of controlling ultramicropore size in carbons at sub-angstrom level for molecular sieving of propylene/propane mixtures[J]. Journal of Materials Chemistry A, 2021, 9(42): 23873-23881. |
| 91 | Wang C T, Li W C, Xu S, et al. Wood frame structured carbons with integrated sieving layer for propylene/propane separation[J]. Chemical Engineering Journal, 2023, 477: 146891. |
| 92 | Wang C T, Li W C, Wang M, et al. Wood-structured carbon with reassembled pores showing high propylene adsorption rate for efficient separation of propylene/propane[J]. Separation and Purification Technology, 2025, 354: 128649. |
| 93 | Du S J, Guo M L, Zhou D H, et al. Influence of structural domain evolution in carbon materials on the selective separation of alkenes from alkanes[J]. ACS Sustainable Chemistry & Engineering, 2024, 12(26): 9758-9765. |
| 94 | Liu J Q, Liu Y J, Kayrak Talay D, et al. A new carbon molecular sieve for propylene/propane separations[J]. Carbon, 2015, 85: 201-211. |
| 95 | Xu S, Li W C, Wang C T, et al. Beyond the selectivity-capacity trade-off: ultrathin carbon nanoplates with easily accessible ultramicropores for high-efficiency propylene/propane separation[J]. Nano Letters, 2022, 22(16): 6615-6621. |
| 96 | Guo L P, Liu R S, Qian J H, et al. Surface sieving carbon skins for propylene and propane separation[J]. Nature Chemical Engineering, 2024, 1: 411-420. |
| 97 | Wang Y S, Li T Y, Ba Y Q, et al. “Mortar-and-cobblestone” type carbon pellets with interlinked C3H6-philic domains and mesoporous transport channels for propylene/propane separation[J]. Separation and Purification Technology, 2023, 305: 122436. |
| 98 | Yuan Y F, Wang Y S, Zhang X L, et al. Wiggling mesopores kinetically amplify the adsorptive separation of propylene/propane[J]. Angewandte Chemie International Edition, 2021, 60(35): 19063-19067. |
| 99 | Dang S, Zhu Q L, Xu Q. Nanomaterials derived from metal-organic frameworks[J]. Nature Reviews Materials, 2017, 3: 17075. |
| 100 | 姚显芳, 李映伟. MOFs作为牺牲模板制备纳米多孔炭材料的方法及其应用[J]. 科学通报, 2015, 60(20): 1906-1914. |
| Yao X F, Li Y W. Method for preparing nanoporouss carbon materials using MOFs as sacrificial templates and its applications[J]. Chinese Science Bulletin, 2015, 60(20): 1906-1914. | |
| 101 | Li B Y, Belmabkhout Y, Zhang Y M, et al. From an equilibrium based MOF adsorbent to a kinetic selective carbon molecular sieve for paraffin/iso-paraffin separation[J]. Chemical Communications, 2016, 52(96): 13897-13900. |
| 102 | Chen F Q, Ding J Q, Guo K Q, et al. CoNi alloy nanoparticles embedded in metal-organic framework-derived carbon for the highly efficient separation of xenon and krypton via a charge-transfer effect[J]. Angewandte Chemie International Edition, 2021, 60(5): 2431-2438. |
| 103 | Chen F Q, Huang X L, Guo K Q, et al. Molecular sieving of propylene from propane in metal-organic framework-derived ultramicroporous carbon adsorbents[J]. ACS Applied Materials & Interfaces, 2022, 14(26): 30443-30453. |
| 104 | Li X Y, Liu J Q, Zhou K, et al. Tuning metal-organic framework (MOF) topology by regulating ligand and secondary building unit (SBU) geometry: structures built on 8-connected M6 (M = Zr, Y) clusters and a flexible tetracarboxylate for propane-selective propane/propylene separation[J]. Journal of the American Chemical Society, 2022, 144(47): 21702-21709. |
| 105 | Gong W, Xie Y, Yamano A, et al. Rational design and reticulation of infinite qbe rod secondary building units into metal-organic frameworks through a global desymmetrization approach for inverse C3H8/C3H6 separation[J]. Angewandte Chemie International Edition, 2024, 63(5): e202318475. |
| 106 | Andrade M, Parnell A J, Bernardo G, et al. Propane selective carbon adsorbents from phenolic resin precursor[J]. Microporous and Mesoporous Materials, 2021, 320: 111071. |
| [1] | Yujie MAO, Xiaofei LU, Xian SUO, Lifeng YANG, Xili CUI, Huabin XING. Advances in research on catalysts for deep removal of trace oxygen in industrial gases [J]. CIESC Journal, 2025, 76(5): 1997-2010. |
| [2] | Renze SHI, Qiuyan DING, Zhenjun YUAN, Jian NA, Jianhua LIU, Shuhu GUO, Xiong ZHAO, Hong LI, Xin GAO. Study on the purification technology of 4N electronic-grade diethoxymethylsilane [J]. CIESC Journal, 2025, 76(5): 2186-2197. |
| [3] | Liao HE, Jun LI, Mengshu GAO, Dongyang LIU, Yuhao ZHANG, Liang ZHAO, Jinsen GAO, Chunming XU. Research progress on aromatic hydrocarbons separation from petroleum hydrocarbons [J]. CIESC Journal, 2025, 76(5): 1909-1926. |
| [4] | Zehai XU, Chao LIU, Guoliang ZHANG. Hydrophobic pervaporation membranes on polymer substrate for solvent recovery [J]. CIESC Journal, 2025, 76(5): 2055-2069. |
| [5] | Zhichao XU, Zhendong YU, Haofeng WU, Peiwen WU, Hongxiang WU, Yanhong CHAO, Wenshuai ZHU, Zhichang LIU, Chunming XU. Preparation of acid-rich 13X molecular sieve and its ultra-deep adsorption removal of mercaptan in biodiesel [J]. CIESC Journal, 2025, 76(5): 2198-2208. |
| [6] | Jiashun LI, Wang LI, Zuzeng QIN, Tongming SU, Xinling XIE, Hongbing JI. Preparation of polyimide-reinforced lignocellulosic nanofibril aerogel and its oil-water separation performance [J]. CIESC Journal, 2025, 76(5): 2169-2185. |
| [7] | Jialang HU, Mingyuan JIANG, Lyuming JIN, Yonggang ZHANG, Peng HU, Hongbing JI. Machine learning-assisted high-throughput computational screening of MOFs and advances in gas separation research [J]. CIESC Journal, 2025, 76(5): 1973-1996. |
| [8] | Dong GU, Xingjian PI, Die ZHANG, Ying ZHANG. Construction and H2/CO2 separation performance evaluation of CAU-1/PI mixed matrix membrane with different nanoparticle sizes [J]. CIESC Journal, 2025, 76(5): 2410-2418. |
| [9] | Ruijie MA, Zixuan HUANG, Xueqian GUAN, Guangjin CHEN, Bei LIU. Efficient ethane and methane separation using ZIF-8/DMPU slurry [J]. CIESC Journal, 2025, 76(5): 2262-2269. |
| [10] | Bingbing GAO, Nuo XU, Yunxiang BAI, Chunfang ZHANG, Yongqiang YANG, Liangliang DONG. Polymeric membranes for helium separation [J]. CIESC Journal, 2025, 76(5): 2119-2135. |
| [11] | Jinyue WANG, Enze XIE, Hanze MA, Sheng YUAN, Guangwei HE, Zhongyi JIANG. Monoatomic layer separation membrane: progress and prospect [J]. CIESC Journal, 2025, 76(5): 1943-1959. |
| [12] | Yanqiu LU, Yang DI, Wenbo SHI, Congcong YIN, Yong WANG. Research progress of smart responsive membranes based on novel porous organic polymers [J]. CIESC Journal, 2025, 76(5): 2101-2118. |
| [13] | Hao QI, Yujie WANG, Shenhui LI, Qi ZOU, Yiqun LIU, Zhiping ZHAO. Molecular simulation study on adsorption and diffusion of C3H6 and C3H8 on Co/Zn-ZIFs [J]. CIESC Journal, 2025, 76(5): 2313-2326. |
| [14] | Chunhui TAO, Yinhui LI, Yu FU, Ran DUAN, Zeyi ZHAO, Yufeng TANG, Gang ZHANG, Heping MA. Selective adsorption and purification of low-concentration Kr gas using various adsorbents [J]. CIESC Journal, 2025, 76(5): 2358-2366. |
| [15] | Haofan ZHAO, Haojie REN, Zongkai LIU, Guanying DONG, Yatao ZHANG. Research progress of MOFs glass membranes in gas separation applications [J]. CIESC Journal, 2025, 76(5): 2042-2054. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||