CIESC Journal ›› 2022, Vol. 73 ›› Issue (9): 3929-3939.DOI: 10.11949/0438-1157.20220501
• Separation engineering • Previous Articles Next Articles
Chengwei LI1( ), Huayong LUO1(
), Huayong LUO1( ), Mingxuan ZHANG1, Peng LIAO2, Qian FANG1, Hongwei RONG1, Jingyin WANG1
), Mingxuan ZHANG1, Peng LIAO2, Qian FANG1, Hongwei RONG1, Jingyin WANG1
Received:2022-05-04
Revised:2022-06-09
Online:2022-10-09
Published:2022-09-05
Contact:
Huayong LUO
李承威1( ), 骆华勇1(
), 骆华勇1( ), 张铭轩1, 廖鹏2, 方茜1, 荣宏伟1, 王竞茵1
), 张铭轩1, 廖鹏2, 方茜1, 荣宏伟1, 王竞茵1
通讯作者:
骆华勇
作者简介:李承威(1996—),男,硕士研究生,1287377003@qq.com
基金资助:CLC Number:
Chengwei LI, Huayong LUO, Mingxuan ZHANG, Peng LIAO, Qian FANG, Hongwei RONG, Jingyin WANG. Microfludically-generated lanthanum hydroxide cross-linked chitosan microspheres for phosphate removal[J]. CIESC Journal, 2022, 73(9): 3929-3939.
李承威, 骆华勇, 张铭轩, 廖鹏, 方茜, 荣宏伟, 王竞茵. 氢氧化镧交联壳聚糖微球的微流控制备及其除磷性能[J]. 化工学报, 2022, 73(9): 3929-3939.
Add to citation manager EndNote|Ris|BibTeX
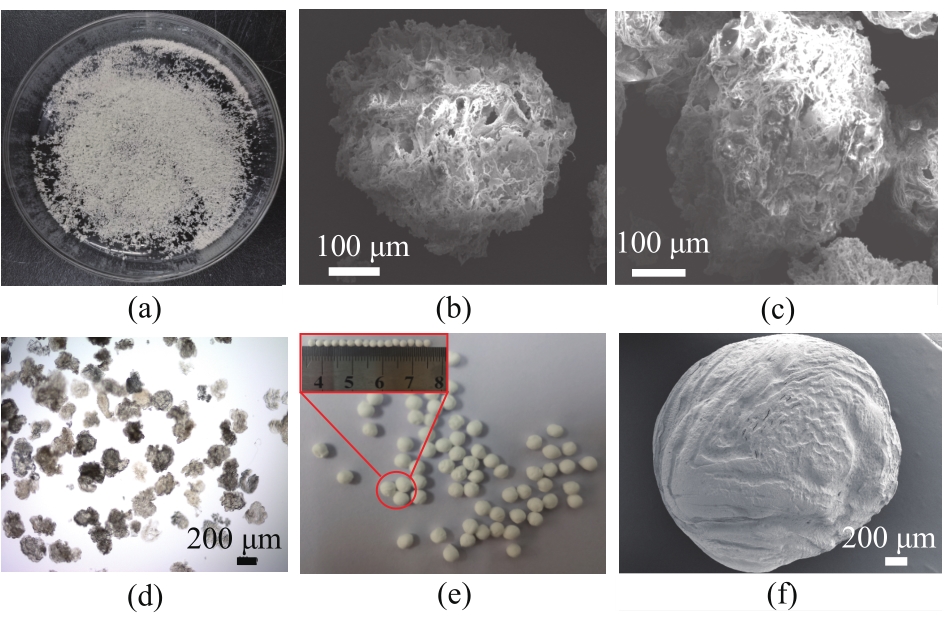
Fig.2 Digital image of freeze-dried La-CS-M (a), SEM images of La-CS-M before (b) and after (c) phosphate adsorption, optical image of La-CS-M (d), digital (e) and SEM (f) images of freeze-dried La-CS
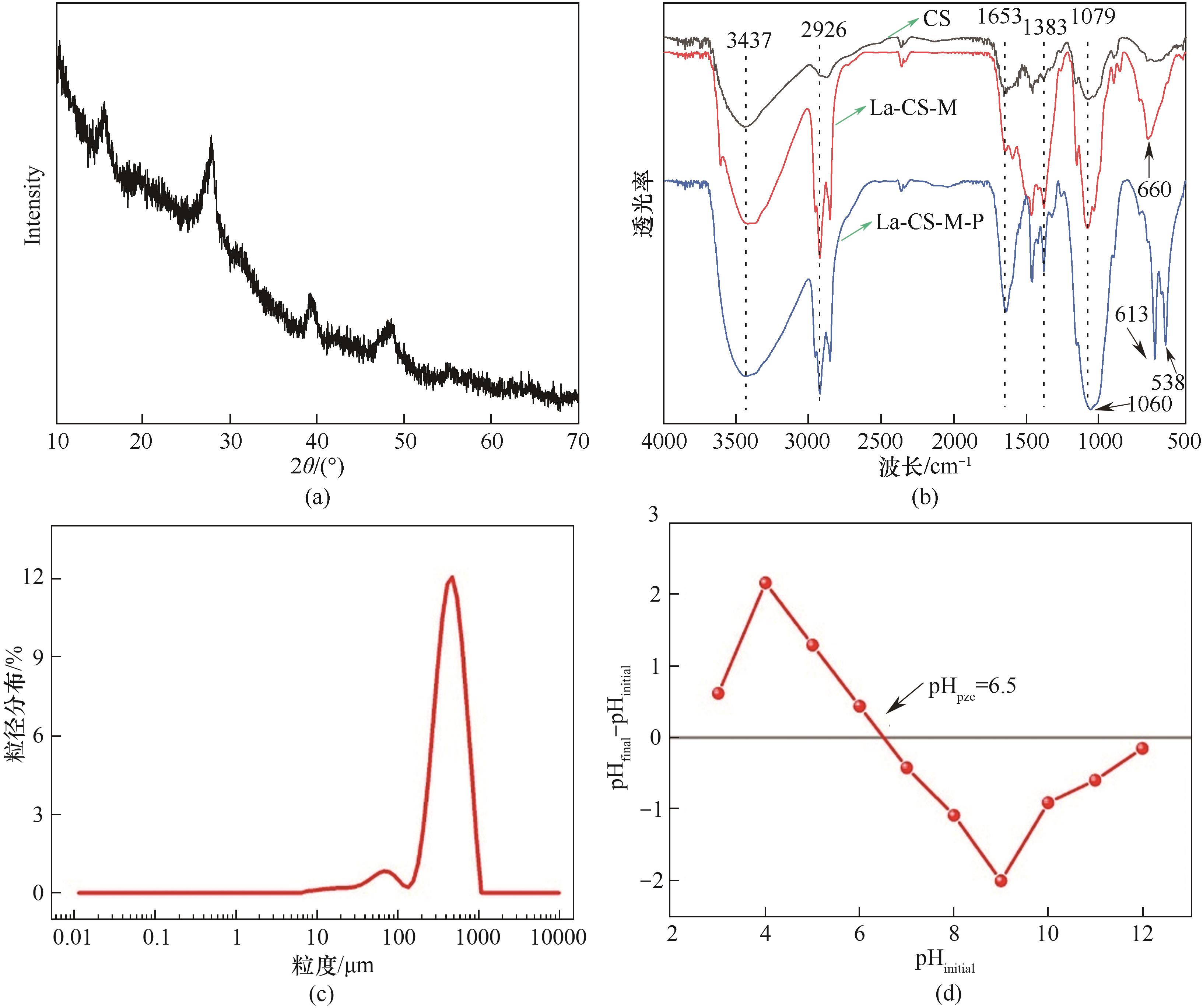
Fig.3 XRD pattern of La-CS-M (a), FTIR spectra of CS and La-CS-M before and after phosphate adsorption (b), particle size distribution of La-CS-M (c) and the pH at point of zero charge (pHpzc) of La-CS-M (d)
| 吸附剂 | 孔隙度/% | 平均孔径/nm | La负载量/%(质量) |
|---|---|---|---|
| La-CS-M | 89.22 | 960.0 | 36.26 |
| La-CS | 87.86 | 473.9 | 37.99 |
Table 1 The pore characteristics and La loading capacities of chitosan microspheres
| 吸附剂 | 孔隙度/% | 平均孔径/nm | La负载量/%(质量) |
|---|---|---|---|
| La-CS-M | 89.22 | 960.0 | 36.26 |
| La-CS | 87.86 | 473.9 | 37.99 |
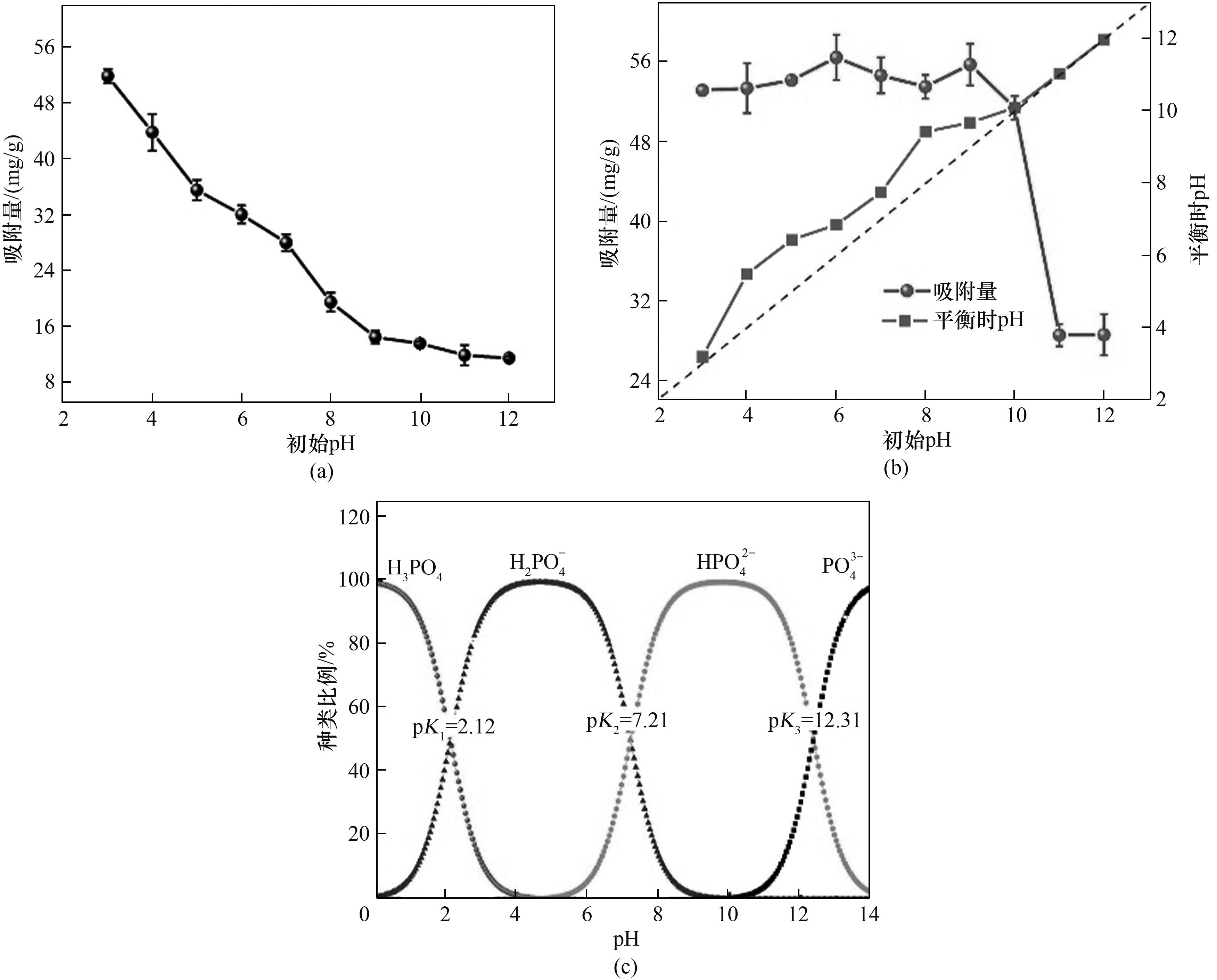
Fig.4 Effect of pH on phosphate adsorption onto La-CS (a), effect of pH on phosphate adsorption onto La-CS-M (b), and species of phosphate in different pH (c)
| 模型 | 参数 | 数值 |
|---|---|---|
| 准一级动力学模型 | qe/(mg/g) | 50.95 |
| k1/min-1 | 6.75×10-3 | |
| R2 | 0.923 | |
| 准二级动力学模型 | qe/(mg/g) | 57.43 |
| k2/(g/(mg·min)) | 1.52×10-4 | |
| R2 | 0.969 | |
| 颗粒内扩散模型 | k1d/(mg/(g·min0.5)) | 1.89 |
| C1 | 4.82 | |
| R2 | 0.989 | |
| k2d/(mg/(g·min0.5)) | 0.57 | |
| C2 | 33.97 | |
| R2 | 0.918 | |
| Langmuir模型 | qmax/(mg/g) | 56.48 |
| kL/(L/mg) | 0.40 | |
| R2 | 0.980 | |
| Freundlich模型 | kF/(mg/g)(L/mg)1/n | 23.15 |
| n | 4.11 | |
| R2 | 0.962 |
Table 2 Kinetic and isotherm modeling parameters for phosphate adsorption onto La-CS-M
| 模型 | 参数 | 数值 |
|---|---|---|
| 准一级动力学模型 | qe/(mg/g) | 50.95 |
| k1/min-1 | 6.75×10-3 | |
| R2 | 0.923 | |
| 准二级动力学模型 | qe/(mg/g) | 57.43 |
| k2/(g/(mg·min)) | 1.52×10-4 | |
| R2 | 0.969 | |
| 颗粒内扩散模型 | k1d/(mg/(g·min0.5)) | 1.89 |
| C1 | 4.82 | |
| R2 | 0.989 | |
| k2d/(mg/(g·min0.5)) | 0.57 | |
| C2 | 33.97 | |
| R2 | 0.918 | |
| Langmuir模型 | qmax/(mg/g) | 56.48 |
| kL/(L/mg) | 0.40 | |
| R2 | 0.980 | |
| Freundlich模型 | kF/(mg/g)(L/mg)1/n | 23.15 |
| n | 4.11 | |
| R2 | 0.962 |
| 吸附剂 | 磷酸盐浓度/(mg/L) | pH | 温度/℃ | qmax/(mg/g) | 文献 |
|---|---|---|---|---|---|
| La-CS-M | 5~50 | 6.0±0.1 | 25 | 56.48 | 本研究 |
| 包埋滑石粉的海藻酸镧凝胶 | 2.5~50.0 | 4 | 25 | 16.034 | [ |
| 水合氧化镧改性的硅藻土 | 10~100 | 5.60 | 25 | 58.70 | [ |
| 氢氧化镧改性介孔稻壳生物炭 | 5~100 | 6.6 | 25 | 41.22~45.62 | [ |
| MgFe2O4-生物炭基海藻酸镧珠 | 5~50 | 5.3±0.3 | 25±1 | 27.68 | [ |
| 包埋氢氧化镧的聚乙烯醇/海藻酸铝凝胶球 | 0~50 | 4.0 | 25 | 7.86 | [ |
| 掺杂氢氧化镧的活性炭纤维 | 10~70 | — | 室温 | 15.3 | [ |
Table 3 Comparison of maximum phosphate adsorption capacity (qmax) based on Langmuir model between La-CS-Mand other La-loaded adsorbents
| 吸附剂 | 磷酸盐浓度/(mg/L) | pH | 温度/℃ | qmax/(mg/g) | 文献 |
|---|---|---|---|---|---|
| La-CS-M | 5~50 | 6.0±0.1 | 25 | 56.48 | 本研究 |
| 包埋滑石粉的海藻酸镧凝胶 | 2.5~50.0 | 4 | 25 | 16.034 | [ |
| 水合氧化镧改性的硅藻土 | 10~100 | 5.60 | 25 | 58.70 | [ |
| 氢氧化镧改性介孔稻壳生物炭 | 5~100 | 6.6 | 25 | 41.22~45.62 | [ |
| MgFe2O4-生物炭基海藻酸镧珠 | 5~50 | 5.3±0.3 | 25±1 | 27.68 | [ |
| 包埋氢氧化镧的聚乙烯醇/海藻酸铝凝胶球 | 0~50 | 4.0 | 25 | 7.86 | [ |
| 掺杂氢氧化镧的活性炭纤维 | 10~70 | — | 室温 | 15.3 | [ |
| 1 | Zhi Y, Zhang C, Hjorth R, et al. Emerging lanthanum (Ⅲ)-containing materials for phosphate removal from water: a review towards future developments[J]. Environment International, 2020, 145: 106-115. |
| 2 | Razanajatovo M R, Gao W, Song Y, et al. Selective adsorption of phosphate in water using lanthanum-based nanomaterials: a critical review[J]. Chinese Chemical Letters, 2021, 32(9): 2637-2647. |
| 3 | Zhao Y, Guo L, Shen W, et al. Function integrated chitosan-based beads with throughout sorption sites and inherent diffusion network for efficient phosphate removal[J]. Carbohydrate Polymers, 2020, 230: 115639. |
| 4 | Xu W, Zheng W, Wang F, et al. Using iron ion-loaded aminated polyacrylonitrile fiber to efficiently remove wastewater phosphate[J]. Chemical Engineering Journal, 2021, 403: 126349. |
| 5 | Wang B, Zhang W, Li L, et al. Novel talc encapsulated lanthanum alginate hydrogel for efficient phosphate adsorption and fixation[J]. Chemosphere, 2020, 256: 127124. |
| 6 | Xie J, Wang Z, Lu S, et al. Removal and recovery of phosphate from water by lanthanum hydroxide materials[J]. Chemical Engineering Journal, 2014, 254: 163-170. |
| 7 | 罗元, 谢坤, 张克强, 等. 镧(La)改性吸附材料脱除水体磷酸盐研究进展[J]. 化工进展, 2019, 38(11): 5005-5014. |
| Luo Y, Xie K, Zhang K Q, et al. Research progress on removal phosphate in aqueous solution by lanthanum modified adsorption materials[J]. Chemical Industry and Engineering Progress, 2019, 38(11): 5005-5014. | |
| 8 | Qiu H, Liang C, Yu J, et al. Preferable phosphate sequestration by nano-La (Ⅲ)(hydr)oxides modified wheat straw with excellent properties in regeneration[J]. Chemical Engineering Journal, 2017, 315: 345-354. |
| 9 | Dong S, Wang Y, Zhao Y, et al. La3+/La(OH)3 loaded magnetic cationic hydrogel composites for phosphate removal: effect of lanthanum species and mechanistic study[J]. Water Research, 2017, 126: 433-441. |
| 10 | Zhou A, Zhu C, Chen W, et al. Phosphorus recovery from water by lanthanum hydroxide embedded interpenetrating network poly (vinyl alcohol)/sodium alginate hydrogel beads[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2018, 554: 237-244. |
| 11 | Zhang Y, Pan B, Shan C, et al. Enhanced phosphate removal by nanosized hydrated La(Ⅲ) oxide confined in cross-linked polystyrene networks[J]. Environmental Science & Technology, 2016, 50(3): 1447-1454. |
| 12 | Wang B, Bai Z, Jiang H, et al. Selective heavy metal removal and water purification by microfluidically-generated chitosan microspheres: characteristics, modeling and application[J]. Journal of Hazardous Materials, 2019, 364: 192-205. |
| 13 | Dong S, Ji Q, Wang Y, et al. Enhanced phosphate removal using zirconium hydroxide encapsulated in quaternized cellulose[J]. Journal of Environmental Sciences, 2020, 89: 102-112. |
| 14 | Xu J H, Zhao H, Lan W J, et al. A novel microfluidic approach for monodispersed chitosan microspheres with controllable structures[J]. Advanced Healthcare Materials, 2012, 1, 106-111. |
| 15 | 肖艾. 基于微流控技术一步制备磁性聚乙烯醇微球及其在介入栓塞治疗中的应用[D]. 武汉: 华中科技大学, 2016. |
| Xiao A. Microfluidic one-step preparation of magnetic poly(vinyl alcohol) microspheres and their applications in interventional embolization therapy[D]. Wuhan: Huazhong University of Science & Technology, 2016. | |
| 16 | Chen Z, Luo H, Rong H. Development of polyaminated chitosan-zirconium (Ⅳ) complex bead adsorbent for highly efficient removal and recovery of phosphorus in aqueous solutions[J]. International Journal of Biological Macromolecules, 2020, 164: 1183-1193. |
| 17 | 骆华勇, 荣宏伟, 曾学阳, 等. 全互穿网络温敏海藻酸锆凝胶球的磷吸附性能[J].高等学校化学学报, 2018, 39(10): 2289-2297. |
| Luo H Y, Rong H W, Zeng X Y, et al. Performance of phosphorus sorption on thermosensitive zirconium alginate hydrogel beads with full-interpenetrating network[J]. Chemical Journal of Chinese Universities, 2018, 39(10): 2289-2297. | |
| 18 | Luo H, Liu Y, Lu H, et al. Efficient adsorption of tetracycline from aqueous solutions by modified alginate beads after the removal of Cu (Ⅱ) ions[J]. ACS omega, 2021, 6(9): 6240-6251. |
| 19 | 陆瀚兴, 李明, 骆华勇, 等. 水合氧化锆改性污泥生物炭对磷酸盐的吸附特性研究[J]. 水处理技术, 2022, 48(4): 65-70. |
| Lu H X, Li M, Luo H Y, et al. Performance of phosphate adsorption on hydrous zirconium oxide-modified biochars derived from sewage sludge[J]. Technology of Water Treatment, 2022, 48(4): 65-70. | |
| 20 | Li H, Zhao Y, Xiao Z, et al. Analysis on approximate site energy distribution and adsorption behaviors unveils reasons for highly efficient phosphorus removal by a novel sludge-based magnetic gel bead[J]. Chemical Engineering Journal, 2021, 422: 130028. |
| 21 | Bansiwal A, Thakre D, Labhshetwar N, et al. Fluoride removal using lanthanum incorporated chitosan beads[J]. Colloids and Surfaces B: Biointerfaces, 2009, 74(1): 216-224. |
| 22 | Xu Q, Chen Z, Wu Z, et al. Novel lanthanum doped biochars derived from lignocellulosic wastes for efficient phosphate removal and regeneration[J]. Bioresource Technology, 2019, 289: 121600. |
| 23 | 华熠, 潘蒙晓, 丁冬东, 等. 四乙烯五胺功能化纳米高分子材料对Cr(Ⅵ)与磷酸盐共存体系的吸附机理[J]. 中国科学: 化学, 2014, 44(11): 1776-1787. |
| Hua Y, Pan M X, Ding D D, et al. Adsorption mechanism of tetraethylenepentamine-functionalized nano polymers in Cr(Ⅵ), phosphate co-existing water system[J]. Scientia Sinica Chimica, 2014, 44(11): 1776-1787. | |
| 24 | 许润, 石程好, 唐倩, 等. 氢氧化镧改性介孔稻壳生物炭除磷性能[J]. 环境科学, 2019, 40(4): 1834-1841. |
| Xu R, Shi C H, Tang Q, et al. Phosphate removal using rice husk biochars modified with lanthanum hydroxide[J]. Environmental Science, 2019, 40(4): 1834-1841. | |
| 25 | Wu Y, Li X, Yang Q, et al. Hydrated lanthanum oxide-modified diatomite as highly efficient adsorbent for low-concentration phosphate removal from secondary effluents[J]. Journal of Environmental Management, 2019, 231: 370-379. |
| 26 | Zhang L, Zhou Q, Liu J, et al. Phosphate adsorption on lanthanum hydroxide-doped activated carbon fiber[J]. Chemical Engineering Journal, 2012, 185: 160-167. |
| 27 | Spears B M, Lürling M, Yasseri S, et al. Lake responses following lanthanum-modified bentonite clay (Phoslock®) application: an analysis of water column lanthanum data from 16 case study lakes[J]. Water Research, 2013, 47(15): 5930-5942. |
| 28 | Acelas N Y, Martin B D, López D, et al. Selective removal of phosphate from wastewater using hydrated metal oxides dispersed within anionic exchange media[J]. Chemosphere, 2015, 119: 1353-1360. |
| 29 | Wang L, Wang J, Yan W, et al. MgFe2O4-biochar based lanthanum alginate beads for advanced phosphate removal[J]. Chemical Engineering Journal, 2020, 387: 123305. |
| 30 | Wang B, Hu X, Zhou D, et al. Highly selective and sustainable clean-up of phosphate from aqueous phase by eco-friendly lanthanum cross-linked polyvinyl alcohol/alginate/palygorskite composite hydrogel beads[J]. Journal of Cleaner Production, 2021, 298: 126878. |
| 31 | 宋小宝, 何世颖, 冯彦房, 等. 载镧磁性水热生物炭的制备及其除磷性能[J]. 环境科学, 2020, 41(2): 773-783. |
| Song X B, He S Y, Feng Y F, et al. Fabrication of La-MHTC composites for phosphate removal: adsorption behavior and mechanism[J]. Environmental Science, 2020, 41(2): 773-783. | |
| 32 | Shan S, Wang W, Liu D, et al. Remarkable phosphate removal and recovery from wastewater by magnetically recyclable La2O2CO3/γ-Fe2O3 nanocomposites[J]. Journal of Hazardous Materials, 2020, 397: 122597. |
| [1] | Bingchun SHENG, Jianguo YU, Sen LIN. Study on lithium resource separation from underground brine with high concentration of sodium by aluminum-based lithium adsorbent [J]. CIESC Journal, 2023, 74(8): 3375-3385. |
| [2] | Ruihang ZHANG, Pan CAO, Feng YANG, Kun LI, Peng XIAO, Chun DENG, Bei LIU, Changyu SUN, Guangjin CHEN. Analysis of key parameters affecting product purity of natural gas ethane recovery process via ZIF-8 nanofluid [J]. CIESC Journal, 2023, 74(8): 3386-3393. |
| [3] | Yan GAO, Peng WU, Chao SHANG, Zejun HU, Xiaodong CHEN. Preparation of magnetic agarose microspheres based on a two-fluid nozzle and their protein adsorption properties [J]. CIESC Journal, 2023, 74(8): 3457-3471. |
| [4] | Ji CHEN, Ze HONG, Zhao LEI, Qiang LING, Zhigang ZHAO, Chenhui PENG, Ping CUI. Study on coke dissolution loss reaction and its mechanism based on molecular dynamics simulations [J]. CIESC Journal, 2023, 74(7): 2935-2946. |
| [5] | Wentao WU, Liangyong CHU, Lingjie ZHANG, Weimin TAN, Liming SHEN, Ningzhong BAO. High-efficient preparation of cardanol-based self-healing microcapsules [J]. CIESC Journal, 2023, 74(7): 3103-3115. |
| [6] | Zhilong WANG, Ye YANG, Zhenzhen ZHAO, Tao TIAN, Tong ZHAO, Yahui CUI. Influence of mixing time and sequence on the dispersion properties of the cathode slurry of lithium-ion battery [J]. CIESC Journal, 2023, 74(7): 3127-3138. |
| [7] | Xuanzhi HE, Yongqing HE, Guiye WEN, Feng JIAO. Ferrofluid droplet neck self-similar breakup behavior [J]. CIESC Journal, 2023, 74(7): 2889-2897. |
| [8] | Jie WANG, Xiaolin QIU, Ye ZHAO, Xinyang LIU, Zhongqiang HAN, Yong XU, Wenhan JIANG. Preparation and properties of polyelectrolyte electrostatic deposition modified PHBV antioxidant films [J]. CIESC Journal, 2023, 74(7): 3068-3078. |
| [9] | Feng ZHU, Kailin CHEN, Xiaofeng HUANG, Yinzhu BAO, Wenbin LI, Jiaxin LIU, Weiqiang WU, Wangwei GAO. Performance study of KOH modified carbide slag for removal of carbonyl sulfide [J]. CIESC Journal, 2023, 74(6): 2668-2679. |
| [10] | Lanhe ZHANG, Qingyi LAI, Tiezheng WANG, Xiaozhuo GUAN, Mingshuang ZHANG, Xin CHENG, Xiaohui XU, Yanping JIA. Effect of H2O2 on nitrogen removal and sludge properties in SBR [J]. CIESC Journal, 2023, 74(5): 2186-2196. |
| [11] | Shaoyun CHEN, Dong XU, Long CHEN, Yu ZHANG, Yuanfang ZHANG, Qingliang YOU, Chenglong HU, Jian CHEN. Preparation and adsorption properties of monolayer polyaniline microsphere arrays [J]. CIESC Journal, 2023, 74(5): 2228-2238. |
| [12] | Caihong LIN, Li WANG, Yu WU, Peng LIU, Jiangfeng YANG, Jinping LI. Effect of alkali cations in zeolites on adsorption and separation of CO2/N2O [J]. CIESC Journal, 2023, 74(5): 2013-2021. |
| [13] | Chenxin LI, Yanqiu PAN, Liu HE, Yabin NIU, Lu YU. Carbon membrane model based on carbon microcrystal structure and its gas separation simulation [J]. CIESC Journal, 2023, 74(5): 2057-2066. |
| [14] | Jianhua ZHANG, Mengmeng CHEN, Yawen SUN, Yongzhen PENG. Efficient nitrogen and phosphorus removal from domestic wastewater via simultaneous partial nitritation and phosphorus removal combined Anammox [J]. CIESC Journal, 2023, 74(5): 2147-2156. |
| [15] | Lu DENG, Xiaojie JU, Wenjie ZHANG, Rui XIE, Wei WANG, Zhuang LIU, Dawei PAN, Liangyin CHU. Controllable preparation of radioactive chitosan embolic microspheres by microfluidic method [J]. CIESC Journal, 2023, 74(4): 1781-1794. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||