CIESC Journal ›› 2022, Vol. 73 ›› Issue (10): 4448-4460.DOI: 10.11949/0438-1157.20220857
• Catalysis, kinetics and reactors • Previous Articles Next Articles
Xiang GONG( ), Linsen LI, Zhao JIANG(
), Linsen LI, Zhao JIANG( )
)
Received:2022-06-21
Revised:2022-09-07
Online:2022-11-02
Published:2022-10-05
Contact:
Zhao JIANG
通讯作者:
姜召
作者简介:龚翔(1994—),男,博士研究生,gx2017@stu.xjtu.edu.cn
基金资助:CLC Number:
Xiang GONG, Linsen LI, Zhao JIANG. Employing PdCo/SiO2 catalyst in high activity dehydrogenation reaction of heterocyclic H2 storage carrier[J]. CIESC Journal, 2022, 73(10): 4448-4460.
龚翔, 李林森, 姜召. PdCo/SiO2双金属催化剂用于杂环储氢载体的高效脱氢[J]. 化工学报, 2022, 73(10): 4448-4460.
Add to citation manager EndNote|Ris|BibTeX
| 催化剂 | Pd0 /eV | Pd δ+ /eV | Co0 /eV | Co δ+ /eV | Pd0/Pd δ+ | Co0/Co δ+ | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3d5/2 | 3d3/2 | 3d5/2 | 3d3/2 | 3d3/2 | 3d1/2 | 3d3/2 | 3d1/2 | |||
| Pd/SiO2 | 335.1 | 340.4 | 336.6 | 341.9 | — | — | — | — | 84%/16% | — |
| Co/SiO2 | — | — | — | — | 778.3 | 793.3 | 792.1 | 798.4 | 81%/19% | — |
| Pd3Co1/SiO2 | 335.3 | 340.6 | 336.6 | 341.9 | 777.5 | 792.7 | 783.2 | 798.4 | 82%/18% | 77%/23% |
| Pd1Co1/SiO2 | 335.5 | 340.7 | 336.8 | 342.1 | 777.8 | 793.0 | 783.6 | 798.8 | 85%/15% | 76%/24% |
| Pd1Co3/SiO2 | 335.8 | 341.1 | 337.2 | 342.5 | 778.0 | 793.1 | 783.9 | 799.1 | 81%/19% | 76%/24% |
Table 1 XPS results of bimetal catalysts
| 催化剂 | Pd0 /eV | Pd δ+ /eV | Co0 /eV | Co δ+ /eV | Pd0/Pd δ+ | Co0/Co δ+ | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3d5/2 | 3d3/2 | 3d5/2 | 3d3/2 | 3d3/2 | 3d1/2 | 3d3/2 | 3d1/2 | |||
| Pd/SiO2 | 335.1 | 340.4 | 336.6 | 341.9 | — | — | — | — | 84%/16% | — |
| Co/SiO2 | — | — | — | — | 778.3 | 793.3 | 792.1 | 798.4 | 81%/19% | — |
| Pd3Co1/SiO2 | 335.3 | 340.6 | 336.6 | 341.9 | 777.5 | 792.7 | 783.2 | 798.4 | 82%/18% | 77%/23% |
| Pd1Co1/SiO2 | 335.5 | 340.7 | 336.8 | 342.1 | 777.8 | 793.0 | 783.6 | 798.8 | 85%/15% | 76%/24% |
| Pd1Co3/SiO2 | 335.8 | 341.1 | 337.2 | 342.5 | 778.0 | 793.1 | 783.9 | 799.1 | 81%/19% | 76%/24% |
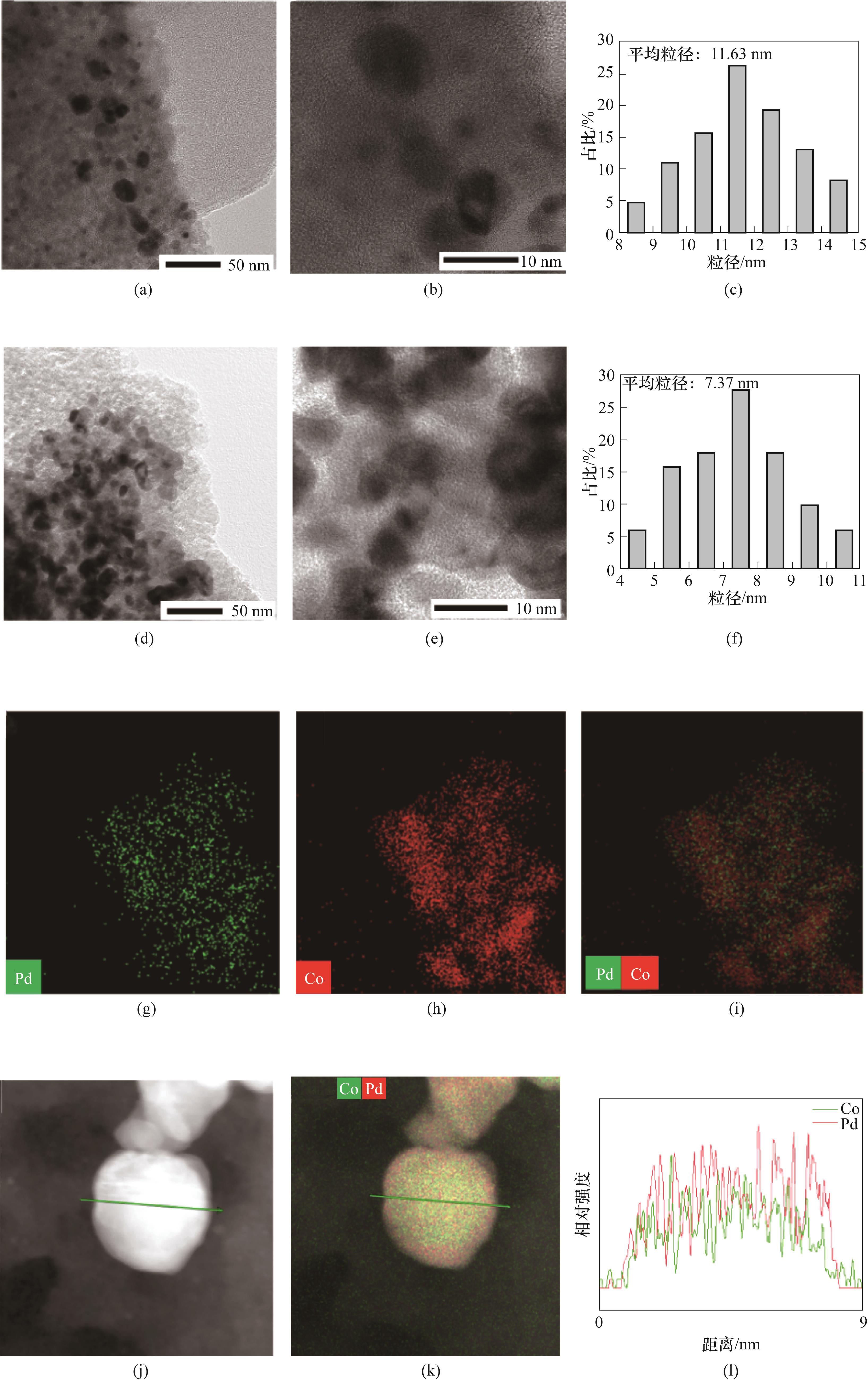
Fig.6 HRTEM images [(a), (b)] and particle size distributions (c) of Pd/SiO2; HRTEM images [(d), (e)], particle size distributions (f), mapping images [(g)—(i)] and line scanning images [(j)—(l)] of Pd1Co3/SiO2 catalyst
| 样品 | 脱氢量/%(质量) | 12H-NECZ转化率/% | 4H-NECZ选择性/% | NECZ选择性/% | ||
|---|---|---|---|---|---|---|
| 1 h | 4 h | 8 h | ||||
| Pd/SiO2 | 2.18 | 4.33 | 5.20 | 100 | 30.82 | 69.18 |
| Pd3Co1/SiO2 | 0.92 | 2.81 | 3.78 | 100 | 66.70 | 14.60 |
| Pd1Co1/SiO2 | 0.89 | 2.51 | 3.17 | 100 | 43.70 | 10.21 |
| Pd1Co3/SiO2 | 2.93 | 4.67 | 5.25 | 100 | 28.34 | 71.66 |
| Pd1Co4/SiO2 | 1.10 | 2.70 | 3.70 | 100 | 36.20 | 9.72 |
| Pd1Co5/SiO2 | 0.73 | 1.87 | 2.47 | 38.68 | 23.27 | 10.09 |
| Co/SiO2 | 0 | 0.07 | 0.12 | 4.10 | 1.05 | 0.0055 |
Table 2 H2 release amount and selectivity data of mentioned catalysts for 12H-NECZ dehydrogenation at 453 K
| 样品 | 脱氢量/%(质量) | 12H-NECZ转化率/% | 4H-NECZ选择性/% | NECZ选择性/% | ||
|---|---|---|---|---|---|---|
| 1 h | 4 h | 8 h | ||||
| Pd/SiO2 | 2.18 | 4.33 | 5.20 | 100 | 30.82 | 69.18 |
| Pd3Co1/SiO2 | 0.92 | 2.81 | 3.78 | 100 | 66.70 | 14.60 |
| Pd1Co1/SiO2 | 0.89 | 2.51 | 3.17 | 100 | 43.70 | 10.21 |
| Pd1Co3/SiO2 | 2.93 | 4.67 | 5.25 | 100 | 28.34 | 71.66 |
| Pd1Co4/SiO2 | 1.10 | 2.70 | 3.70 | 100 | 36.20 | 9.72 |
| Pd1Co5/SiO2 | 0.73 | 1.87 | 2.47 | 38.68 | 23.27 | 10.09 |
| Co/SiO2 | 0 | 0.07 | 0.12 | 4.10 | 1.05 | 0.0055 |
| 样品 | k1/min-1 | k2/min-1 | k3/min-1 | R2 |
|---|---|---|---|---|
Pd/SiO2 Pd3Co1/SiO2 Pd1Co1/SiO2 Pd1Co3/SiO2 | 0.0276 0.0104 0.0096 0.0523 | 0.0192 0.0045 0.0026 0.0486 | 0.0031 0.0028 0.0013 0.0034 | 0.979 0.994 0.975 0.991 |
Table 3 Kinetic fitting results of 12H-NECZ sequential dehydrogenation process
| 样品 | k1/min-1 | k2/min-1 | k3/min-1 | R2 |
|---|---|---|---|---|
Pd/SiO2 Pd3Co1/SiO2 Pd1Co1/SiO2 Pd1Co3/SiO2 | 0.0276 0.0104 0.0096 0.0523 | 0.0192 0.0045 0.0026 0.0486 | 0.0031 0.0028 0.0013 0.0034 | 0.979 0.994 0.975 0.991 |
| 表面 | 结合能/eV | |||
|---|---|---|---|---|
| 0H-NECZ | 4H-NECZ | 8H-NECZ | 12H-NECZ | |
| Pd (111) | -2.89 | -2.66 | -2.37 | -2.16 |
| Pd1Co3(111) | -5.44 | -4.99 | -3.98 | -3.68 |
Table 4 DFT calculated binding energies of 12H-NECZ, 8H-NECZ, 4H-NECZ, and NECZ on Pd (111) and Pd1Co3 (111) surfaces
| 表面 | 结合能/eV | |||
|---|---|---|---|---|
| 0H-NECZ | 4H-NECZ | 8H-NECZ | 12H-NECZ | |
| Pd (111) | -2.89 | -2.66 | -2.37 | -2.16 |
| Pd1Co3(111) | -5.44 | -4.99 | -3.98 | -3.68 |
| 反应路径 | ΔG/eV | |
|---|---|---|
| Pd(111) | Pd1Co3(111) | |
12H 8H+2H2 8H+2H2 | 0.96 | 0.85 |
8H 4H+2H2 4H+2H2 | 1.25 | 0.54 |
4H 0H+2H2 0H+2H2 | 1.36 | 1.14 |
Table 5 Change of Gibbs free energies of three elementary reactions from 12H-NECZ dehydrogenation process to NECZ on the Pd (111) and Pd1Co3 (111) surfaces
| 反应路径 | ΔG/eV | |
|---|---|---|
| Pd(111) | Pd1Co3(111) | |
12H 8H+2H2 8H+2H2 | 0.96 | 0.85 |
8H 4H+2H2 4H+2H2 | 1.25 | 0.54 |
4H 0H+2H2 0H+2H2 | 1.36 | 1.14 |
| 1 | Barreto L, Makihira A, Riahi K. The hydrogen economy in the 21st century: a sustainable development scenario[J]. International Journal of Hydrogen Energy, 2003, 28(3): 267-284. |
| 2 | Tarhan C, Cil M A. A study on hydrogen, the clean energy of the future: hydrogen storage methods[J]. Journal of Energy Storage, 2021, 40: 102676. |
| 3 | Schlapbach L, Züttel A. Hydrogen-storage materials for mobile applications[J]. Nature, 2001, 414(6861): 353-358. |
| 4 | Momirlan M, Veziroglu T N. Current status of hydrogen energy[J]. Renewable and Sustainable Energy Reviews, 2002, 6(1/2): 141-179. |
| 5 | Chalk S G, Miller J F. Key challenges and recent progress in batteries, fuel cells, and hydrogen storage for clean energy systems[J]. Journal of Power Sources, 2006, 159(1): 73-80. |
| 6 | 童海航, 石德智, 刘嘉宇, 等. 金属纳米颗粒辅助木质纤维素暗发酵生物制氢的研究进展[J]. 化工学报, 2022, 73(4): 1417-1435. |
| Tong H H, Shi D Z, Liu J Y, et al. Research progress on dark fermentative bio-hydrogen production from lignocellulose assisted by metal nanoparticles[J]. CIESC Journal, 2022, 73(4): 1417-1435. | |
| 7 | 陈晨, 王明明, 王志刚, 等. 镍基非对称中空纤维膜用于乙醇自热重整制氢[J]. 化工学报, 2021, 72(S1): 482-493. |
| Chen C, Wang M M, Wang Z G, et al. Hydrogen production by ethanol autothermal reforming using nickel-based asymmetric hollow fiber membranes[J]. CIESC Journal, 2021, 72(S1): 482-493. | |
| 8 | Jiang Z, Pan Q, Xu J, et al. Current situation and prospect of hydrogen storage technology with new organic liquid[J]. International Journal of Hydrogen Energy, 2014, 39(30): 17442-17451. |
| 9 | Zheng J Y, Liu X X, Xu P, et al. Development of high pressure gaseous hydrogen storage technologies[J]. International Journal of Hydrogen Energy, 2012, 37(1): 1048-1057. |
| 10 | Zhang M, Lv H, Kang H R, et al. A literature review of failure prediction and analysis methods for composite high-pressure hydrogen storage tanks[J]. International Journal of Hydrogen Energy, 2019, 44(47): 25777-25799. |
| 11 | Peschka W, Carpetis C. Cryogenic hydrogen storage and refueling for automobiles[J]. International Journal of Hydrogen Energy, 1980, 5(6): 619-625. |
| 12 | Sadaghiani M S, Mehrpooya M. Introducing and energy analysis of a novel cryogenic hydrogen liquefaction process configuration[J]. International Journal of Hydrogen Energy, 2017, 42(9): 6033-6050. |
| 13 | Dillon A C, Jones K M, Bekkedahl T A, et al. Storage of hydrogen in single-walled carbon nanotubes[J]. Nature, 1997, 386(6623): 377-379. |
| 14 | He Q F, Zeng L P, Han L H, et al. Electrochemical hydrogen-storage capacity of graphene can achieve a carbon-hydrogen atomic ratio of 1∶1[J]. Science China Chemistry, 2022, 65(2): 318-321. |
| 15 | Wang D, Wang Y Q, Huang Z N, et al. Design optimization and sensitivity analysis of the radiation mini-channel metal hydride reactor[J]. Energy, 2019, 173: 443-456. |
| 16 | Zhang X L, Wang K, Zhang X, et al. Synthesis process and catalytic activity of Nb2O5 hollow spheres for reversible hydrogen storage of MgH2 [J]. International Journal of Energy Research, 2021, 45(2): 3129-3141. |
| 17 | Schüth F, Bogdanovi B, Felderhoff M. Light metal hydrides and complex hydrides for hydrogen storage[J]. Chemical Communications, 2004, 36(20): 2249-2258. |
| 18 | Shevlin S A, Kerkeni B, Guo Z X. Dehydrogenation mechanisms and thermodynamics of MNH2BH3 (M = Li, Na) metal amidoboranes as predicted from first principles[J]. Physical Chemistry Chemical Physics, 2011, 13(17): 7649. |
| 19 | Gao C, Qi X B, Zhang Z W, et al. Fabrication of monodisperse precursor gel microspheres for hollow glass microspheres by combining the sol-microemulsion-gel process with a T-shaped microfluidic device[J]. International Journal of Hydrogen Energy, 2011, 36(16): 9758-9766. |
| 20 | Sotoodeh F, Smith K J. An overview of the kinetics and catalysis of hydrogen storage on organic liquids[J]. The Canadian Journal of Chemical Engineering, 2013, 91(9): 1477-1490. |
| 21 | Kariya N, Fukuoka A, Ichikawa M. Efficient evolution of hydrogen from liquid cycloalkanes over Pt-containing catalysts supported on active carbons under “wet-dry multiphase conditions”[J]. Applied Catalysis A: General, 2002, 233(1/2): 91-102. |
| 22 | Dong Y, Yang M, Yang Z H, et al. Catalytic hydrogenation and dehydrogenation of N-ethylindole as a new heteroaromatic liquid organic hydrogen carrier[J]. International Journal of Hydrogen Energy, 2015, 40(34): 10918-10922. |
| 23 | Cacciola G, Giordano N, Restuccia G. Cyclohexane as a liquid phase carrier in hydrogen storage and transport[J]. International Journal of Hydrogen Energy, 1984, 9(5): 411-419. |
| 24 | Shi L J, Liu X J, Tuo Y X, et al. Graphene-CNT composite as catalyst support for microwave-assisted hydrogen releasing from liquid organic hydride[J]. International Journal of Hydrogen Energy, 2017, 42(27): 17403-17413. |
| 25 | 齐随涛, 李迎迎, 岳佳琪, 等. 活性炭负载Pt-Ni双金属催化剂上十氢化萘脱氢[J]. 催化学报, 2014, 35(11): 1833-1839. |
| Qi S T, Li Y Y, Yue J Q, et al. Hydrogenation kinetics of N-ethylindole on a supported Ru catalyst [J]. Chinese Journal of Catalysis, 2014, 35(11): 1833-1839. | |
| 26 | Dong Y, Yang M, Zhu T, et al. Hydrogenation kinetics of N-ethylindole on a supported Ru catalyst[J]. Energy Technology, 2018, 6(3): 558-562. |
| 27 | Yang M, Dong Y, Fei S X, et al. A comparative study of catalytic dehydrogenation of perhydro-N-ethylcarbazole over noble metal catalysts[J]. International Journal of Hydrogen Energy, 2014, 39(33): 18976-18983. |
| 28 | Jiang Z, Gong X, Wang B, et al. A experimental study on the dehydrogenation performance of dodecahydro-N-ethylcarbazole on M/TiO2 catalysts[J]. International Journal of Hydrogen Energy, 2019, 44(5): 2951-2959. |
| 29 | Wang B, Chang T Y, Jiang Z, et al. Catalytic dehydrogenation study of dodecahydro-N-ethylcarbazole by noble metal supported on reduced graphene oxide[J]. International Journal of Hydrogen Energy, 2018, 43(15): 7317-7325. |
| 30 | Jorschick H, Geißelbrecht M, Eßl M, et al. Benzyltoluene/dibenzyltoluene-based mixtures as suitable liquid organic hydrogen carrier systems for low temperature applications[J]. International Journal of Hydrogen Energy, 2020, 45(29): 14897-14906. |
| 31 | Shi L B, Zhou Y M, Qi S T, et al. Pt catalysts supported on H2 and O2 plasma-treated Al2O3 for hydrogenation and dehydrogenation of the liquid organic hydrogen carrier pair dibenzyltoluene and perhydrodibenzyltoluene[J]. ACS Catalysis, 2020, 10(18): 10661-10671. |
| 32 | Pez G P, Scott A R, Cooper A C, et al. Hydrogen storage by reversible hydrogenation of pi-conjugated substrates: US7351395[P]. 2008-04-01. |
| 33 | Stark K, Emel'yanenko V N, Zhabina A A, et al. Liquid organic hydrogen carriers: thermophysical and thermochemical studies of carbazole partly and fully hydrogenated derivatives[J]. Industrial & Engineering Chemistry Research, 2015, 54(32): 7953-7966. |
| 34 | Emel'yanenko V N, Varfolomeev M A, Verevkin S P, et al. Hydrogen storage: thermochemical studies of N-alkylcarbazoles and their derivatives as a potential liquid organic hydrogen carriers[J]. The Journal of Physical Chemistry C, 2015, 119(47): 26381-26389 |
| 35 | Wan C, An Y, Xu G H, et al. Study of catalytic hydrogenation of N-ethylcarbazole over ruthenium catalyst[J]. International Journal of Hydrogen Energy, 2012, 37(17): 13092-13096. |
| 36 | Morawa Eblagon K, Tam K, Yu K M K, et al. Study of catalytic sites on ruthenium for hydrogenation of N-ethylcarbazole: implications of hydrogen storage via reversible catalytic hydrogenation[J]. The Journal of Physical Chemistry C, 2010, 114(21): 9720-9730. |
| 37 | Fei S, Han B, Li L, et al. A study on the catalytic hydrogenation of N-ethylcarbazole on the mesoporous Pd/MoO3 catalyst[J]. International Journal of Hydrogen Energy, 2017, 42(41): 25942-25950. |
| 38 | Ye X F, An Y, Xu G H. Kinetics of 9-ethylcarbazole hydrogenation over Raney-Ni catalyst for hydrogen storage[J]. Journal of Alloys and Compounds, 2011, 509(1): 152-156. |
| 39 | Yu H E, Yang X, Jiang X J, et al. LaNi5.5 particles for reversible hydrogen storage in N-ethylcarbazole[J]. Nano Energy, 2021, 80: 105476. |
| 40 | Yang X, Wu Y M, Yu H E, et al. A YH3 promoted palladium catalyst for reversible hydrogen storage of N-ethylcarbazole[J]. International Journal of Hydrogen Energy, 2020, 45(58): 33657-33662. |
| 41 | Gong X, Jiang Z, Fang T. Enhancing selectivity and reducing cost for dehydrogenation of dodecahydro-N-ethylcarbazole by supporting platinum on titanium dioxide[J]. International Journal of Hydrogen Energy, 2020, 45(11): 6838-6847. |
| 42 | Jiang Z, Gong X, Guo S, et al. Engineering PdCu and PdNi bimetallic catalysts with adjustable alloying degree for the dehydrogenation reaction of dodecahydro-N-ethylcarbazole[J]. International Journal of Hydrogen Energy, 2021, 46(2): 2376-2389. |
| 43 | Yang M, Dong Y, Cheng H S. Hydrogenation kinetics of N-ethylcarbaozle as a heteroaromatic liquid organic hydrogen carrier [J]. Advanced Materials Research, 2014, 953/954: 981-984. |
| 44 | Wang B, Chen Y T, Chang T Y, et al. Facet-dependent catalytic activities of Pd/rGO: exploring dehydrogenation mechanism of dodecahydro-N-ethylcarbazole[J]. Applied Catalysis B: Environmental, 2020, 266: 118658. |
| 45 | Wang B, Chang T Y, Gong X, et al. One-pot synthesis of Au/Pd core/shell nanoparticles supported on reduced graphene oxide with enhanced dehydrogenation performance for dodecahydro-N-ethylcarbazole[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(1): 1760-1768. |
| 46 | Wang B, Chang T Y, Jiang Z, et al. Component controlled synthesis of bimetallic PdCu nanoparticles supported on reduced graphene oxide for dehydrogenation of dodecahydro-N-ethylcarbazole[J]. Applied Catalysis B: Environmental, 2019, 251: 261-272. |
| 47 | Sotoodeh F, Huber B J M, Smith K J. Dehydrogenation kinetics and catalysis of organic heteroaromatics for hydrogen storage[J]. International Journal of Hydrogen Energy, 2012, 37(3): 2715-2722. |
| 48 | Sotoodeh F, Smith K J. Kinetics of hydrogen uptake and release from heteroaromatic compounds for hydrogen storage[J]. Industrial & Engineering Chemistry Research, 2010, 49(3): 1018-1026. |
| 49 | Sotoodeh F, Smith K J. Structure sensitivity of dodecahydro-N-ethylcarbazole dehydrogenation over Pd catalysts[J]. Journal of Catalysis, 2011, 279(1): 36-47. |
| 50 | Sotoodeh F, Zhao L, Smith K J. Kinetics of H2 recovery from dodecahydro-N-ethylcarbazole over a supported Pd catalyst[J]. Applied Catalysis A: General, 2009, 362(1/2): 155-162. |
| 51 | Sobota M, Nikiforidis I, Amende M, et al. Dehydrogenation of dodecahydro-N-ethylcarbazole on Pd//Al2O3 model catalysts [J]. Chemistry - A European Journal, 2011, 17(41): 11542-11552. |
| 52 | Amende M, Gleichweit C, Werner K, et al. Model catalytic studies of liquid organic hydrogen carriers: dehydrogenation and decomposition mechanisms of dodecahydro-N-ethylcarbazole on Pt(111)[J]. ACS Catalysis, 2014, 4(2): 657-665. |
| 53 | Yang M, Han C Q, Ni G, et al. Temperature controlled three-stage catalytic dehydrogenation and cycle performance of perhydro-9-ethylcarbazole[J]. International Journal of Hydrogen Energy, 2012, 37(17): 12839-12845. |
| 54 | Wang B, Yan T, Chang T Y, et al. Palladium supported on reduced graphene oxide as a high-performance catalyst for the dehydrogenation of dodecahydro-N-ethylcarbazole[J]. Carbon, 2017, 122: 9-18. |
| 55 | Kustov L M, Tarasov A L, Kirichenko O A. Microwave-activated dehydrogenation of perhydro-N-ethylcarbazol over bimetallic Pd-M/TiO2 catalysts as the second stage of hydrogen storage in liquid substrates[J]. International Journal of Hydrogen Energy, 2017, 42(43): 26723-26729. |
| 56 | Gong X, Guo S Y, Jiang Z, et al. Tuning the alloy degree for Pd-M/Al2O3 (M=Co/ Ni /Cu) bimetallic catalysts to enhance the activity and selectivity of dodecahydro-N-ethylcarbazole dehydrogenation[J]. International Journal of Hydrogen Energy, 2021, 46(68): 33835-33848. |
| 57 | Jiang Z, Guo S Y, Fang T. Enhancing the catalytic activity and selectivity of PdAu/SiO2 bimetallic catalysts for dodecahydro-N-ethylcarbazole dehydrogenation by controlling the particle size and dispersion[J]. ACS Applied Energy Materials, 2019, 2(10): 7233-7243. |
| 58 | Kresse G, Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set[J]. Physical Review B, Condensed Matter, 1996, 54(16): 11169-11186. |
| 59 | Kresse G. Ab initio molecular dynamics for liquid metals[J]. Journal of Non-Crystalline Solids, 1995, 192/193: 222-229. |
| 60 | Perdew J P, Burke K, Ernzerhof M. Generalized gradient approximation made simple[J]. Physical Review Letters, 1996, 77(18): 3865-3868. |
| 61 | Kresse G, Joubert D. From ultrasoft pseudopotentials to the projector augmented-wave method[J]. Physical Review B, 1999, 59(3): 1758-1775. |
| 62 | Lu S L, Menning C A, Zhu Y X, et al. Correlating benzene hydrogenation activity with binding energies of hydrogen and benzene on co-based bimetallic catalysts[J]. ChemPhysChem, 2009, 10(11): 1763-1765 |
| 63 | Chen S T, Jenkins S V, Tao J, et al. Anisotropic seeded growth of Cu-M (M = Au, Pt, or Pd) bimetallic nanorods with tunable optical and catalytic properties[J]. The Journal of Physical Chemistry C, 2013, 117(17): 8924-8932. |
| 64 | Leppert L, Albuquerque R Q, Küemmel S. Gold-platinum alloys and Vegard's law on the nanoscale[J]. Physical Review B, Condensed Matter, 2012, 86(24): 241401-241403. |
| 65 | Zhang J, Dong Y N, Liu Q X, et al. Hierarchically alloyed Pd-Cu microarchitecture with tunable shapes: morphological engineering, and catalysis for hydrogen evolution reaction of ammonia borane[J]. International Journal of Hydrogen Energy, 2019, 44(57): 30226-30236. |
| 66 | Stefanov P, Todorova S, Naydenov A, et al. On the development of active and stable Pd-Co/γ-Al2O3 catalyst for complete oxidation of methane[J]. Chemical Engineering Journal, 2015, 266: 329-338. |
| 67 | Yuan E X, Wu C, Hou X, et al. Synergistic effects of second metals on performance of (Co, Ag, Cu)-doped Pd/Al2O3 catalysts for 2-ethyl-anthraquinone hydrogenation[J]. Journal of Catalysis, 2017, 347: 79-88. |
| 68 | Shukla A K, Neergat M, Bera P, et al. An XPS study on binary and ternary alloys of transition metals with platinized carbon and its bearing upon oxygen electroreduction in direct methanol fuel cells[J]. Journal of Electroanalytical Chemistry, 2001, 504(1): 111-119. |
| 69 | Aricò A S, Cretì P, Modica E, et al. Investigation of direct methanol fuel cells based on unsupported Pt-Ru anode catalysts with different chemical properties[J]. Electrochimica Acta, 2000, 45(25/26): 4319-4328. |
| 70 | Li Y B, Zhang C B, Ma J Z, et al. High temperature reduction dramatically promotes Pd/TiO2 catalyst for ambient formaldehyde oxidation[J]. Applied Catalysis B: Environmental, 2017, 217: 560-569. |
| 71 | Sotoodeh F, Smith K J. Analysis of H2 release from organic polycyclics over Pd catalysts using DFT[J]. The Journal of Physical Chemistry C, 2013, 117(1): 194-204. |
| 72 | Zhu M Y, Xu L X, Du L, et al. Palladium supported on carbon nanotubes as a high-performance catalyst for the dehydrogenation of dodecahydro-N-ethylcarbazole[J]. Catalysts, 2018, 8(12): 638. |
| [1] | Xin YANG, Wen WANG, Kai XU, Fanhua MA. Simulation analysis of temperature characteristics of the high-pressure hydrogen refueling process [J]. CIESC Journal, 2023, 74(S1): 280-286. |
| [2] | Congqi HUANG, Yimei WU, Jianye CHEN, Shuangquan SHAO. Simulation study of thermal management system of alkaline water electrolysis device for hydrogen production [J]. CIESC Journal, 2023, 74(S1): 320-328. |
| [3] | Cheng CHENG, Zhongdi DUAN, Haoran SUN, Haitao HU, Hongxiang XUE. Lattice Boltzmann simulation of surface microstructure effect on crystallization fouling [J]. CIESC Journal, 2023, 74(S1): 74-86. |
| [4] | Zehao MI, Er HUA. DFT and COSMO-RS theoretical analysis of SO2 absorption by polyamines type ionic liquids [J]. CIESC Journal, 2023, 74(9): 3681-3696. |
| [5] | Yepin CHENG, Daqing HU, Yisha XU, Huayan LIU, Hanfeng LU, Guokai CUI. Application of ionic liquid-based deep eutectic solvents for CO2 conversion [J]. CIESC Journal, 2023, 74(9): 3640-3653. |
| [6] | Junfeng LU, Huaiyu SUN, Yanlei WANG, Hongyan HE. Molecular understanding of interfacial polarization and its effect on ionic liquid hydrogen bonds [J]. CIESC Journal, 2023, 74(9): 3665-3680. |
| [7] | Ke LI, Jian WEN, Biping XIN. Study on influence mechanism of vacuum multi-layer insulation coupled with vapor-cooled shield on self-pressurization process of liquid hydrogen storage tank [J]. CIESC Journal, 2023, 74(9): 3786-3796. |
| [8] | Linzheng WANG, Yubing LU, Ruizhi ZHANG, Yonghao LUO. Analysis on thermal oxidation characteristics of VOCs based on molecular dynamics simulation [J]. CIESC Journal, 2023, 74(8): 3242-3255. |
| [9] | Feifei YANG, Shixi ZHAO, Wei ZHOU, Zhonghai NI. Sn doped In2O3 catalyst for selective hydrogenation of CO2 to methanol [J]. CIESC Journal, 2023, 74(8): 3366-3374. |
| [10] | Mengmeng ZHANG, Dong YAN, Yongfeng SHEN, Wencui LI. Effect of electrolyte types on the storage behaviors of anions and cations for dual-ion batteries [J]. CIESC Journal, 2023, 74(7): 3116-3126. |
| [11] | Yaxin CHEN, Hang YUAN, Guanzhang LIU, Lei MAO, Chun YANG, Ruifang ZHANG, Guangya ZHANG. Advances in enzyme self-immobilization mediated by protein nanocages [J]. CIESC Journal, 2023, 74(7): 2773-2782. |
| [12] | Xiaoling TANG, Jiarui WANG, Xuanye ZHU, Renchao ZHENG. Biosynthesis of chiral epichlorohydrin by halohydrin dehalogenase based on Pickering emulsion system [J]. CIESC Journal, 2023, 74(7): 2926-2934. |
| [13] | Bin LI, Zhenghu XU, Shuang JIANG, Tianyong ZHANG. Clean and efficient synthesis of accelerator CBS by hydrogen peroxide catalytic oxidation method [J]. CIESC Journal, 2023, 74(7): 2919-2925. |
| [14] | Xiaoyang LIU, Jianliang YU, Yujie HOU, Xingqing YAN, Zhenhua ZHANG, Xianshu LYU. Effect of spiral microchannel on detonation propagation of hydrogen-doped methane [J]. CIESC Journal, 2023, 74(7): 3139-3148. |
| [15] | Ming DONG, Jinliang XU, Guanglin LIU. Molecular dynamics study on heterogeneous characteristics of supercritical water [J]. CIESC Journal, 2023, 74(7): 2836-2847. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||