CIESC Journal ›› 2023, Vol. 74 ›› Issue (1): 257-275.DOI: 10.11949/0438-1157.20220955
• Reviews and monographs • Previous Articles Next Articles
Guojia YU( ), Dongyu JIN(
), Dongyu JIN( ), Zhiyong ZHOU, Fan ZHANG, Zhongqi REN(
), Zhiyong ZHOU, Fan ZHANG, Zhongqi REN( )
)
Received:2022-07-08
Revised:2022-09-20
Online:2023-03-20
Published:2023-01-05
Contact:
Zhongqi REN
通讯作者:
任钟旗
作者简介:宇国佳(1995—),女,博士研究生,gjyubuct@163.com基金资助:CLC Number:
Guojia YU, Dongyu JIN, Zhiyong ZHOU, Fan ZHANG, Zhongqi REN. Advances in the design, synthesis and application of porous liquids[J]. CIESC Journal, 2023, 74(1): 257-275.
宇国佳, 靳冬玉, 周智勇, 张帆, 任钟旗. 多孔液体的设计合成与应用研究进展[J]. 化工学报, 2023, 74(1): 257-275.
Add to citation manager EndNote|Ris|BibTeX
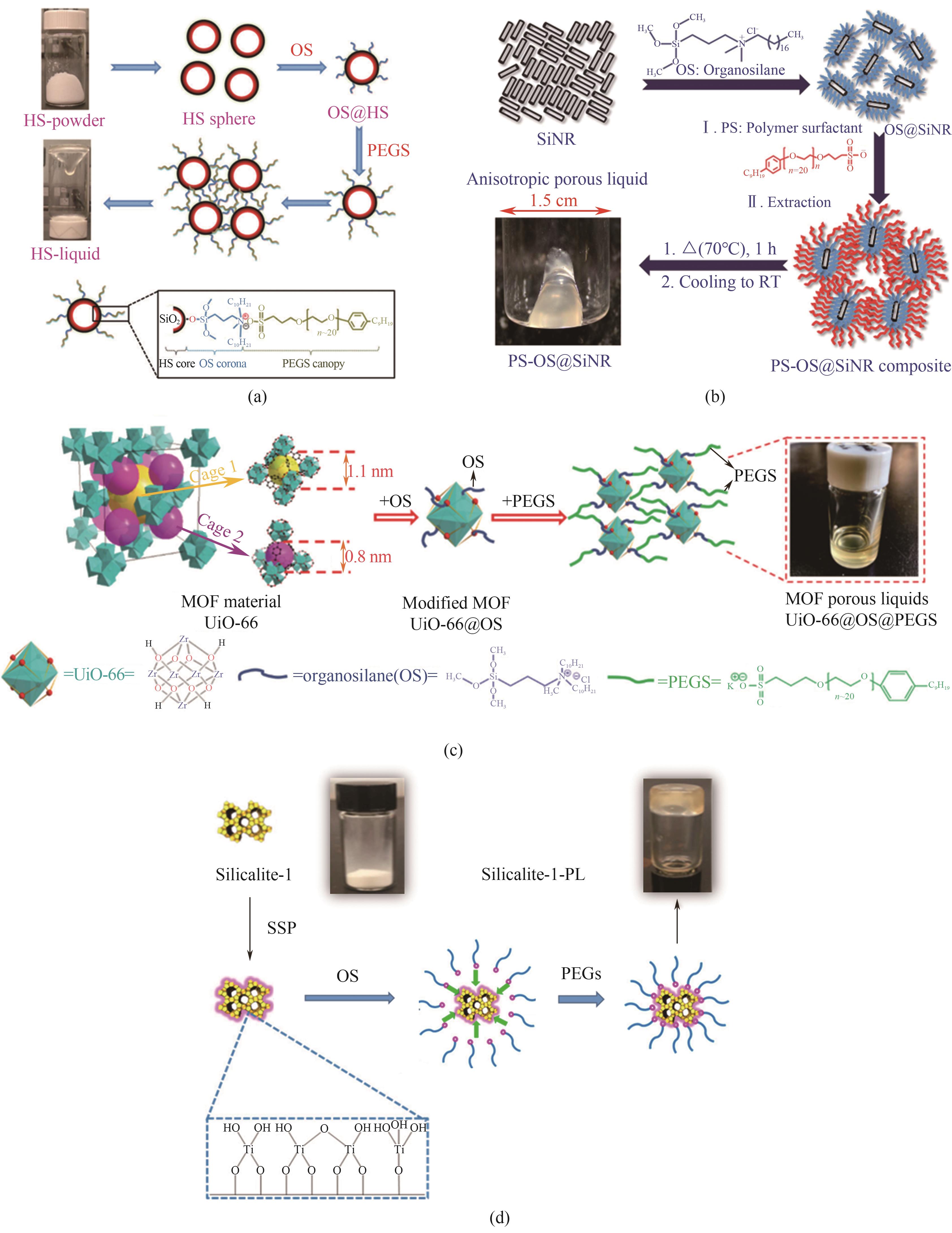
Fig.4 Synthesis schematic diagrams: (a) HS-based PLs by surface engineering method[11]; (b) hollow silica nanorod-based PLs based on different aspect ratio[16]; (c) UiO-66@OS@PEGS PLs[17]; (d) silicalite-1-based PLs based on sol-gel method[18]
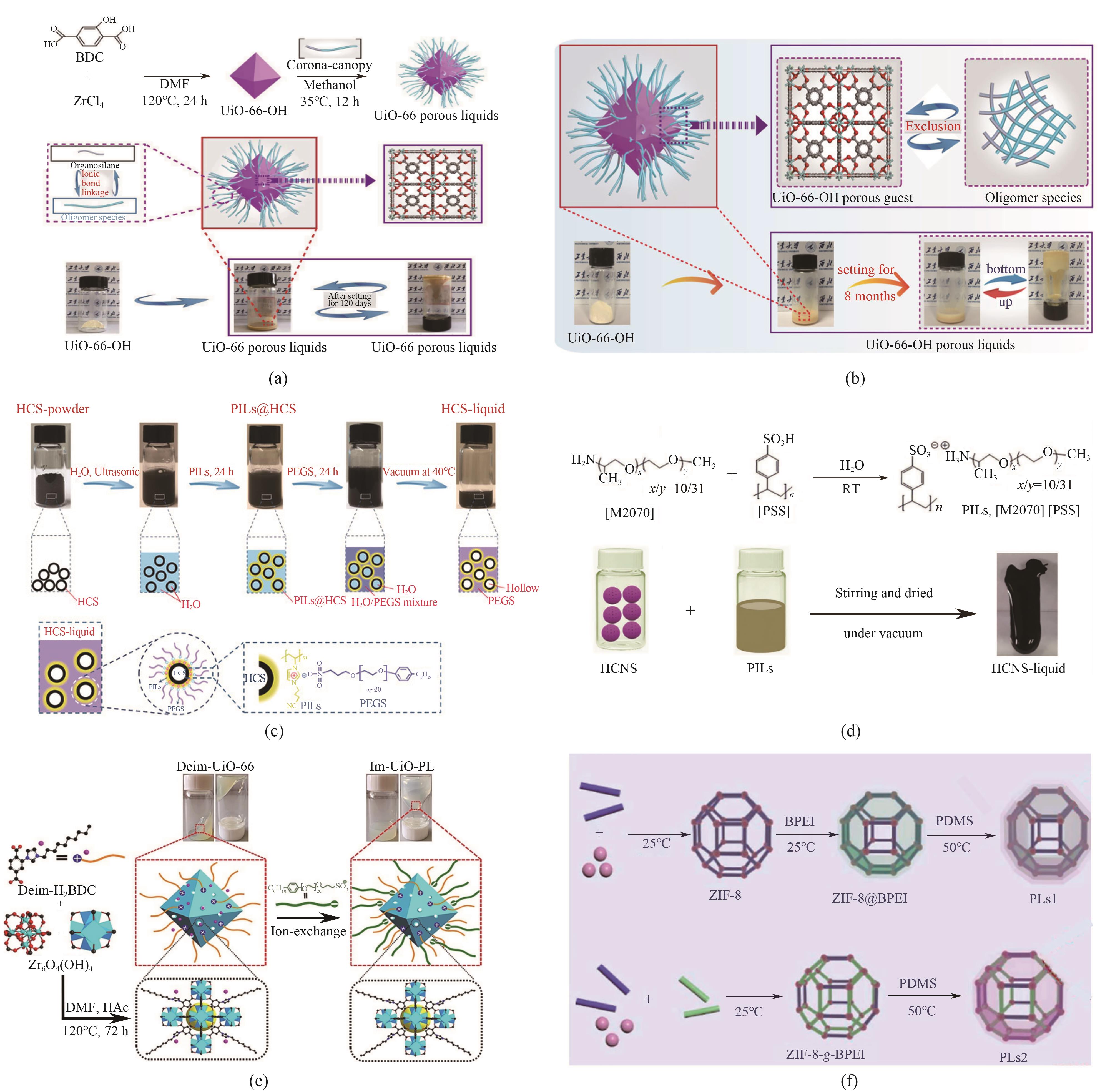
Fig.5 Synthesis schematic diagrams: (a) UiO-66 PLs[19]; (b) UiO-66-OH PLs [20]; (c) HCS-liquid [21]; (d) [M2070][PSS] and HCNS-liquid [22]; (e) Im-UiO-PL [23]; (f) PLs based on ZIF-8@BPEI and ZIF-8-g-BPEI[24]

Fig.6 (a) Synthesis of n-C12[31]; (b) Structure of octahedral hollow cage of n-C12[32]; (c) Synthesis schematic diagram of PL with crown ether[12]; (d) Synthesis schematic diagram of 18-C-6-PL/15-C-5-PL [33]; (e) Synthesis schematic diagram of liquid coordination cages[34]; (f) Plot summarising the results from the porosity screen[36]; (g) Structures of scrambled cages CC33:133-R and CC15-R, and the absorption effect on several gases[37]; (h) Synthesis schematic diagram of MOP-based PLs[38]

Fig.7 (a) Molecular structure of [Bpy][NTf2], crystal structure of ZIF-8 and the Tindal effect of the synthesized ZIF-8-[Bpy][NTf2] colloids[42]; (b) Synthesis schematic diagram of ZIF-8/HKUST-1/Mg-MOF-74 with [P6,6,6,14][NTf2]-based PLs[43]; (c) Synthesis schematic diagram of ZIF-8-based PLs[44]; (d) Synthesis schematic diagram of UiO-66-liquid/[M2070][IPA]-based and the adsorption of gas molecules[45]; (e) Synthesis schematic diagram of MOF-based PLs[14]; (f) Selective separation effect of porous liquid on ethane/ethylene[46]; (g) Synthesis schematic diagram of H-ZSM-5-liquid/[P66614][Br] [51]
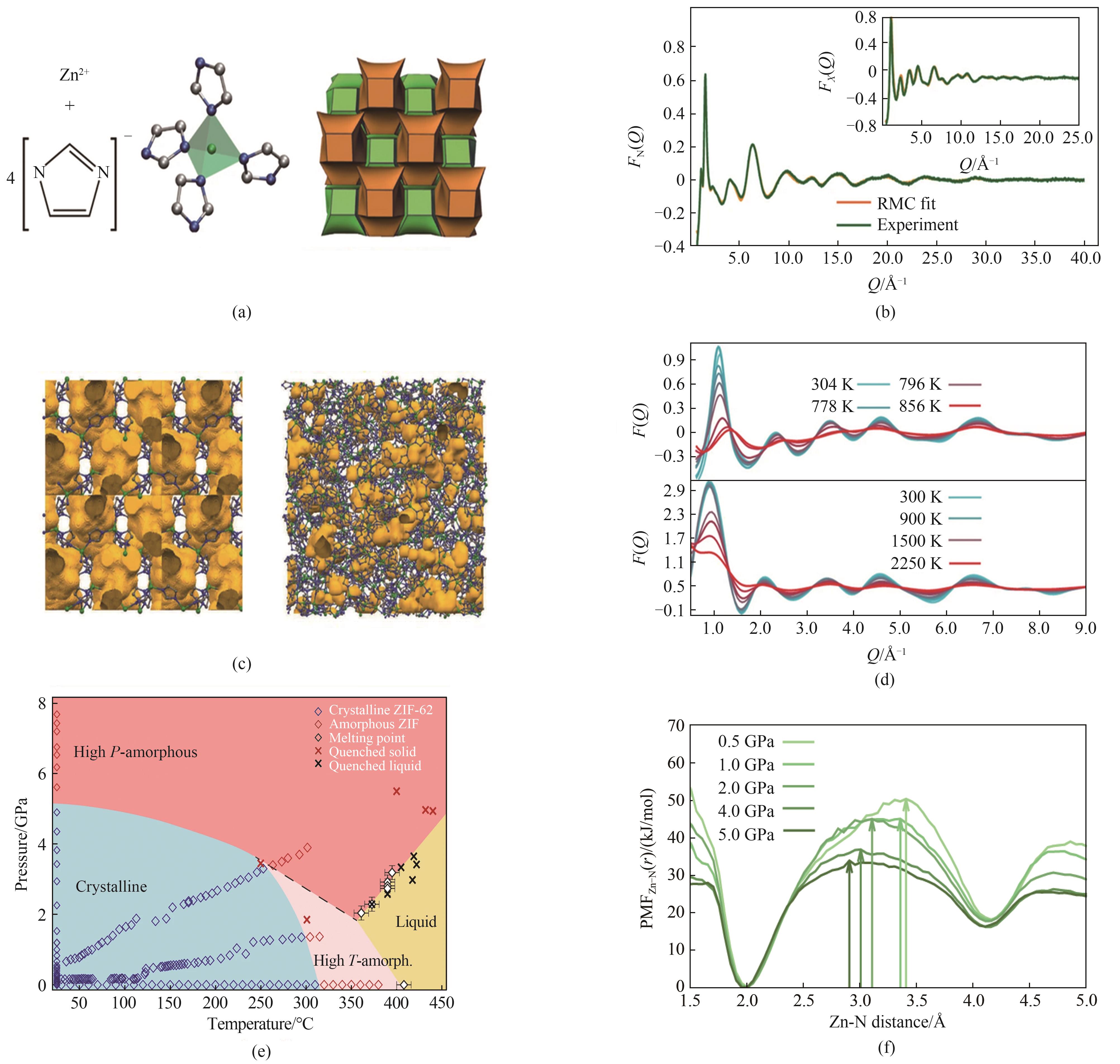
Fig.8 (a) The basic building block of ZIF-4[13]; (b) Experimental neutron structure factor F(Q) data[13]; (c) Crystalline structure of ZIF-4 and atomic configuration of the melt-quenched glass[13]; (d) Experimental and calculated structure factors of ZIF-4 after heating[13]; (e) P-T phase diagram of ZIF-62[52]; (f) Potential of mean force (PMF) for the Zn-N distance of ZIF-62 at pressures between 0.1 GPa and 5.0 GPa, at a temperature of 1200 K[52]

Fig.9 (a) Distances of nanoparticles in a model system with different diameters of silica nanoparticles and 8600 water molecules[57]; (b) Molecular dynamics simulation of the hydrogen bond between [P66614][Br] and acid sites on the H-ZSM-5[100] surface[51]; (c) Equilibrium configuration of the UiO-66/PDMS4k (blue) complex system and the density of PDMS4k (red and blue lines) and UiO-66 atoms (green line) versus the equilibrium configuration of the complex system in Z-coordinate[14]; (d) Gradient equivalence surface of ABS-1CO2[59]; (e) CO2 adsorption sites of silica-based porous ionic liquid (HSL1)[60]; (f) PMF diagram of the accumulated number of gas molecules as a function of the radial distance from the center of the cage, and the gas molecules in and near the cage[61]; (g) Optimal structures of CO2-containing POCs[62]; (h) Spatial distribution functions of CO2 and N2 (CH4) in PLs with a 1∶12 ratio of cage to solvent at 298.15 K and 60 bar (1 bar=105 Pa)[63]
| 名称 | CO2吸收量 | 测试条件 | 文献 |
|---|---|---|---|
| hollow silica PLs | 0.9 mmol/g | 298 K,10 bar | [ |
| HCS-liquid | 0.57 mmol/g | 298 K,10 bar | [ |
| HCNS-liquid | 4.66%(质量) | 298 K,10 bar | [ |
| 15-C-5-PL | 0.375 mmol/g | 298 K,10 bar | [ |
| 18-C-6-PL | 0.429 mmol/g | 298 K,5 bar | [ |
| COF-300-PLs | 0.78 mol/g | 273 K,0.03 bar | [ |
| POCs/[BPy][NTf2] | 104.30 µmol/g | 298 K,1 bar | [ |
| POCs/hexachloropropene | 55 μmmol/g | 298 K,10 bar | [ |
| PoLi-Bc | 5.5 cm3/g | 273 K,1 bar | [ |
| Im-UiO-PL | 5.93 mmol/g | 298 K,9 bar | [ |
| UiO-66-liquid/[M2070][IPA] | 1.66 mmol/g | 298 K,10 bar | [ |
| UiO-66-liquid-M2070 | 0.86 mmol/g | 298 K,30 bar | [ |
| UiO-66@OS@PEGS | 28 mg/g | 298 K,10 bar | [ |
| UiO-66-PL | 30.8 cm3/g | 273 K,10 bar | [ |
| PL1M2070 | 2.393 mmol/g | 298 K,2 MPa | [ |
| PLs-5% | 6.9 mg/g | 313 K | [ |
| ZIF-8 PLs | 3.43 cm3/g | 298 K,1 bar | [ |
| H-ZSM-5 PLs | 0.46 mmol/g | 298 K,10 bar | [ |
| ZIF-67-PLs-10 | 9.542 mmol/g | 298 K,1 bar | [ |
| ZIF-8 PLs | 1.2 mmol/g | 303 K,5 bar | [ |
| ZIF-8/[DBU-PEG][NTf2] | 1.54 mmol/g | 298 K,10 bar | [ |
| PAF-1/Genosorb PLs | 0.72 mmol/g | 298 K,5 bar | [ |
| Al(fum)(OH)/PDMS PLs | 0.95 mmol/g | 298 K,5 bar | [ |
Table 1 Effect of different types of porous liquids on CO2 absorption
| 名称 | CO2吸收量 | 测试条件 | 文献 |
|---|---|---|---|
| hollow silica PLs | 0.9 mmol/g | 298 K,10 bar | [ |
| HCS-liquid | 0.57 mmol/g | 298 K,10 bar | [ |
| HCNS-liquid | 4.66%(质量) | 298 K,10 bar | [ |
| 15-C-5-PL | 0.375 mmol/g | 298 K,10 bar | [ |
| 18-C-6-PL | 0.429 mmol/g | 298 K,5 bar | [ |
| COF-300-PLs | 0.78 mol/g | 273 K,0.03 bar | [ |
| POCs/[BPy][NTf2] | 104.30 µmol/g | 298 K,1 bar | [ |
| POCs/hexachloropropene | 55 μmmol/g | 298 K,10 bar | [ |
| PoLi-Bc | 5.5 cm3/g | 273 K,1 bar | [ |
| Im-UiO-PL | 5.93 mmol/g | 298 K,9 bar | [ |
| UiO-66-liquid/[M2070][IPA] | 1.66 mmol/g | 298 K,10 bar | [ |
| UiO-66-liquid-M2070 | 0.86 mmol/g | 298 K,30 bar | [ |
| UiO-66@OS@PEGS | 28 mg/g | 298 K,10 bar | [ |
| UiO-66-PL | 30.8 cm3/g | 273 K,10 bar | [ |
| PL1M2070 | 2.393 mmol/g | 298 K,2 MPa | [ |
| PLs-5% | 6.9 mg/g | 313 K | [ |
| ZIF-8 PLs | 3.43 cm3/g | 298 K,1 bar | [ |
| H-ZSM-5 PLs | 0.46 mmol/g | 298 K,10 bar | [ |
| ZIF-67-PLs-10 | 9.542 mmol/g | 298 K,1 bar | [ |
| ZIF-8 PLs | 1.2 mmol/g | 303 K,5 bar | [ |
| ZIF-8/[DBU-PEG][NTf2] | 1.54 mmol/g | 298 K,10 bar | [ |
| PAF-1/Genosorb PLs | 0.72 mmol/g | 298 K,5 bar | [ |
| Al(fum)(OH)/PDMS PLs | 0.95 mmol/g | 298 K,5 bar | [ |
| 1 | O’Reilly N, Giri N, James S L. Porous liquids[J]. Chemistry-A European Journal, 2007, 13(11): 3020-3025. |
| 2 | Bennett T D, Coudert F X, James S L, et al. The changing state of porous materials[J]. Nature Materials, 2021, 20(9): 1179-1187. |
| 3 | Liu H, Liu B, Lin L C, et al. A hybrid absorption-adsorption method to efficiently capture carbon[J]. Nature Communications, 2014, 5(1): 1-7. |
| 4 | Knebel A, Bavykina A, Datta S J, et al. Solution processable metal-organic frameworks for mixed matrix membranes using porous liquids[J]. Nature Materials, 2020, 19(12): 1346-1353. |
| 5 | Wang D C, Xin Y Y, Yao D D, et al. Shining light on porous liquids: from fundamentals to syntheses, applications and future challenges[J]. Advanced Functional Materials, 2022, 32(1): 2104162. |
| 6 | 王德超, 辛洋洋, 李晓倩, 等. 多孔液体在气体捕集与分离领域的应用[J]. 化学进展, 2021, 33(10): 1874-1886. |
| Wang D C, Xin Y Y, Li X Q, et al. Porous liquids and their applications in gas capture and separation[J]. Progress in Chemistry, 2021, 33(10): 1874-1886. | |
| 7 | 李晓倩, 张靖, 苏芳芳, 等. 多孔离子液体的构筑及应用[J]. 化学进展, 2022, 80: 848-860. |
| Li X Q, Zhang J, Su F F, et al. Construction and application of porous ionic liquids[J]. Progress in Chemistry, 2022, 80: 848-860. | |
| 8 | Robbins T A, Knobler C B, Bellew D R, et al. A highly adaptive and strongly binding hemicarcerand[J]. Journal of the American Chemical Society, 1994, 116(1): 111-122. |
| 9 | Hsu S C N, Ramesh M, Espenson J H, et al. Membership rules for a molecular box: the admission process and protection provided to guest molecules[J]. Angewandte Chemie, 2003, 115(23): 2767-2770. |
| 10 | Bourlinos A B, Chowdhury S R, Jiang D D, et al. Weakly solvated PEG-functionalized silica nanoparticles with liquid-like behavior[J]. Journal of Materials Science, 2005, 40(18): 5095-5097. |
| 11 | Zhang J S, Chai S H, Qiao Z A, et al. Porous liquids: a promising class of media for gas separation[J]. Angewandte Chemie, 2015, 127(3): 946-950. |
| 12 | Giri N, Del Popolo M G, Melaugh G, et al. Liquids with permanent porosity[J]. Nature, 2015, 527(7577): 216-220. |
| 13 | Gaillac R, Pullumbi P, Beyer K A, et al. Liquid Metal-organic frameworks[J]. Nature Materials, 2017, 16(11): 1149-1154. |
| 14 | He S F, Chen L H, Cui J, et al. General way to construct micro-and mesoporous metal-organic framework-based porous liquids[J]. Journal of the American Chemical Society, 2019, 141(50): 19708-19714. |
| 15 | Erdosy D P, Wenny M B, Cho J, et al. Microporous water with high gas solubilities[J]. Nature, 2022, 608(7924): 712-718. |
| 16 | Kumar R, Dhasaiyan P, Naveenkumar P M, et al. A solvent-free porous liquid comprising hollow nanorod-polymer surfactant conjugates[J]. Nanoscale Advances, 2019, 1(10): 4067-4075. |
| 17 | Zhao X R, An S H, Dai J L, et al. Transforming surface-modified metal organic framework powder into room temperature porous liquids via an electrical balance strategy[J]. New Journal of Chemistry, 2020, 44(29): 12715-12722. |
| 18 | Liu Y T, Bai Y, Tian T. Preparation of porous liquid based on silicalite-1[J]. Materials, 2019, 12(23): 3984. |
| 19 | Wang D C, Xin Y Y, Li X Q, et al. Transforming metal-organic frameworks into porous liquids via a covalent linkage strategy for CO2 capture[J]. ACS Applied Materials & Interfaces, 2021, 13(2): 2600-2609. |
| 20 | Wang D C, Xin Y Y, Li X Q, et al. A universal approach to turn UiO-66 into type 1 porous liquids via post-synthetic modification with corona-canopy species for CO2 capture[J]. Chemical Engineering Journal, 2021, 416: 127625. |
| 21 | Li P P, Schott J A, Zhang J S, et al. Electrostatic-assisted liquefaction of porous carbons[J]. Angewandte Chemie, 2017, 56(47): 14958-14962. |
| 22 | Li P P, Wang D C, Zhang L, et al. An in situ coupling strategy toward porous carbon liquid with permanent porosity[J]. Small, 2021, 17(10): 2006687. |
| 23 | Zou Y H, Huang Y B, Si D H, et al. Porous metal-organic framework liquids for enhanced CO2 adsorption and catalytic conversion[J]. Angewandte Chemie, 2021, 60(38): 20915-20920. |
| 24 | Li X Q, Yao D D, Wang D C, et al. Amino-functionalized ZIFs-based porous liquids with low viscosity for efficient low-pressure CO2 capture and CO2/N2 separation[J]. Chemical Engineering Journal, 2022, 429: 132296. |
| 25 | Liu J, Thallapally P K, McGrail B P, et al. Progress in adsorption-based CO2 capture by metal-organic frameworks[J]. Chemical Society Reviews, 2012, 41(6): 2308-2322. |
| 26 | Masoomi M Y, Morsali A, Dhakshinamoorthy A, et al. Mixed‐metal MOFs: unique opportunities in metal-organic framework (MOF) functionality and design[J]. Angewandte Chemie, 2019, 58(43): 15188-15205. |
| 27 | Tozawa T, Jones J T A, Swamy S I, et al. Porous organic cages[J]. Nature Materials, 2009, 8(12): 973-978. |
| 28 | Hasell T, Cooper A I. Porous organic cages: soluble, modular and molecular pores[J]. Nature Reviews Materials, 2016, 1(9): 1-14. |
| 29 | Geng K Y, He T, Liu R Y, et al. Covalent organic frameworks: design, synthesis, and functions[J]. Chemical Reviews, 2020, 120(16): 8814-8933. |
| 30 | Feng X, Ding X S, Jiang D L. Covalent organic frameworks[J]. Chemical Society Reviews, 2012, 41(18): 6010-6022. |
| 31 | Giri N, Davidson C E, Melaugh G, et al. Alkylated organic cages: from porous crystals to neat liquids[J]. Chemical Science, 2012, 3(6): 2153-2157. |
| 32 | Melaugh G, Giri N, Davidson C E, et al. Designing and understanding permanent microporosity in liquids[J]. Physical Chemistry Chemical Physics, 2014, 16(20): 9422-9431. |
| 33 | Jie K C, Onishi N, Schott J A, et al. Transforming porous organic cages into porous ionic liquids via a supramolecular complexation strategy[J]. Angewandte Chemie, 2020, 59(6): 2268-2272. |
| 34 | Ma L, Haynes C J E, Grommet A B, et al. Coordination cages as permanently porous ionic liquids[J]. Nature Chemistry, 2020, 12(3): 270-275. |
| 35 | Greenaway R L, Holden D, Eden E G B, et al. Understanding gas capacity, guest selectivity, and diffusion in porous liquids[J]. Chemical Science, 2017, 8(4): 2640-2651. |
| 36 | Kearsey R J, Alston B, Briggs M E, et al. Accelerated robotic discovery of type Ⅱ porous liquids[J]. Chemical Science, 2019, 10(41): 9454-9465. |
| 37 | Egleston B D, Luzyanin K V, Brand M C, et al. Controlling gas selectivity in molecular porous liquids by tuning the cage window size[J]. Angewandte Chemie International Edition, 2020, 59(19): 7362-7366. |
| 38 | Deng Z, Ying W, Gong K, et al. Facilitate gas transport through metal-organic polyhedra constructed porous liquid membrane[J]. Small, 2020, 16(11): 1907016. |
| 39 | Kaur G, Kumar H, Singla M. Diverse applications of ionic liquids: a comprehensive review[J]. Journal of Molecular Liquids, 2022,351: 118556. |
| 40 | Ibrahim M H, Hayyan M, Hashim M A, et al. The role of ionic liquids in desulfurization of fuels: a review[J]. Renewable and Sustainable Energy Reviews, 2017, 76: 1534-1549. |
| 41 | Yan Q, Zang H J, Wu C C, et al. Synthesis, characterization and catalytic application of novel ionic liquids based on thiazolium cation[J]. Journal of Molecular Liquids, 2015, 204: 156-161. |
| 42 | Liu S J, Liu J D, Hou X D, et al. Porous liquid: a stable ZIF-8 colloid in ionic liquid with permanent porosity[J]. Langmuir, 2018, 34(12): 3654-3660. |
| 43 | Gomes M C, Pison L, Cervinka C, et al. Porous ionic liquids or liquid metal-organic frameworks? [J]. Angewandte Chemie, 2018, 57(37): 11909-11912. |
| 44 | Shan W, Fulvio P F, Kong L Y, et al. New class of type Ⅲ porous liquids: a promising platform for rational adjustment of gas sorption behavior[J]. ACS Applied Materials & Interfaces, 2018, 10(1): 32-36. |
| 45 | Zhao X M, Yuan Y H, Li P P, et al. A polyether amine modified metal organic framework enhanced the CO2 adsorption capacity of room temperature porous liquids[J]. Chemical Communications, 2019, 55(87): 13179-13182. |
| 46 | Lai B B, Cahir J, Tsang M Y, et al. Type 3 porous liquids for the separation of ethane and ethene[J]. ACS Applied Materials & Interfaces, 2020, 13(1): 932-936. |
| 47 | Hasell T, Schmidtmann M, Cooper A I. Molecular doping of porous organic cages[J]. Journal of the American Chemical Society, 2011, 133(38): 14920-14923. |
| 48 | Briggs M E, Cooper A I. A perspective on the synthesis, purification, and characterization of porous organic cages[J]. Chemistry of Materials, 2017, 29(1): 149-157. |
| 49 | Olsbye U, Svelle S, Bjørgen M, et al. Conversion of methanol to hydrocarbons: how zeolite cavity and pore size controls product selectivity[J]. Angewandte Chemie International Edition, 2012, 51(24): 5810-5831. |
| 50 | Carta M, Malpass-Evans R, Croad M, et al. An efficient polymer molecular sieve for membrane gas separations[J]. Science, 2013, 339(6117): 303-307. |
| 51 | Li P P, Chen H, Schott J A, et al. Porous liquid zeolites: hydrogen bonding-stabilized H-ZSM-5 in branched ionic liquids[J]. Nanoscale, 2019, 11(4): 1515-1519. |
| 52 | Widmer R N, Lampronti G I, Anzellini S, et al. Pressure promoted low-temperature melting of metal-organic frameworks[J]. Nature Materials, 2019, 18(4): 370-376. |
| 53 | Li X Q, Wang D C, He Z J, et al. Zeolitic imidazolate frameworks-based porous liquids with low viscosity for CO2 and toluene uptakes[J]. Chemical Engineering Journal, 2021, 417: 129239. |
| 54 | Jagiello J, Sterling M, Eliasova P, et al. Structural analysis of IPC zeolites and related materials using positron annihilation spectroscopy and high-resolution argon adsorption[J]. Physical Chemistry Chemical Physics, 2016, 18(22): 15269-15277. |
| 55 | Mow R E, Lipton A S, Shulda S, et al. Colloidal three-dimensional covalent organic frameworks and their application as porous liquids[J]. Journal of Materials Chemistry A, 2020, 8(44): 23455-23462. |
| 56 | Xin Y Y, Wang D C, Yao D D, et al. Post-synthetic modification of UiO-66-OH toward porous liquids for CO2 capture[J]. New Journal of Chemistry, 2022, 46(5): 2189-2197. |
| 57 | Sheng L S, Chen Z Q, Wang Y. Molecular dynamics simulations of stability and fluidity of porous liquids[J]. Applied Surface Science, 2021, 536: 147951. |
| 58 | Sheng L S, Chen Z Q. Molecular dynamics study of dispersion and fluidity of porous liquids with different pore sizes[J]. Journal of Molecular Liquids, 2021, 333: 115890. |
| 59 | Yin J, Zhang J R, Wang C, et al. Theoretical insights into CO2/N2 selectivity of the porous ionic liquids constructed by ion-dipole interactions[J]. Journal of Molecular Liquids, 2021, 344: 117676. |
| 60 | Zhang J R, Lv N X, Chao Y H, et al. The interaction nature between hollow silica-based porous ionic liquids and CO2: a DFT study[J]. Journal of Molecular Graphics and Modelling, 2020, 100: 107694. |
| 61 | Zhang F, Yang F C, Huang J S, et al. Thermodynamics and kinetics of gas storage in porous liquids[J]. The Journal of Physical Chemistry B, 2016, 120(29): 7195-7200. |
| 62 | Yin J, Fu W D, Zhang J R, et al. Unraveling the mechanism of CO2 capture and separation by porous liquids[J]. RSC advances, 2020, 10(70): 42706-42717. |
| 63 | Yin Z J, Chen H Y, Yang L, et al. Investigations of CO2 capture from gas mixtures using porous liquids[J]. Langmuir, 2021, 37(3): 1255-1266. |
| 64 | Bhattacharjee A, Kumar R, Sharma K P. Composite porous liquid for recyclable sequestration, storage and in situ catalytic conversion of carbon dioxide at room temperature[J]. ChemSusChem, 2021, 14(16): 3303-3314. |
| 65 | Zhou Y J, Avila J, Berthet N, et al. Integrated, one-pot carbon capture and utilisation using porous ionic liquids[J]. Chemical Communications, 2021, 57(64): 7922-7925. |
| 66 | Li X Q, Wang D C, He Z J, et al. Dual stimuli-responsive porous ionic liquids with reversible phase transition behavior based on ionic liquid crystals for CO2 and C2H4 adsorption[J]. Journal of Materials Chemistry A, 2022, 10(25): 13333-13344. |
| 67 | Shi T, Zheng Y P, Wang T Y, et al. Effect of pore size on the carbon dioxide adsorption behavior of porous liquids based on hollow silica[J]. ChemPhysChem, 2018, 19(1): 130-137. |
| 68 | Kai A T, Egleston B D, Tarzia A, et al. Modular type Ⅲ porous liquids based on porous organic cage microparticles[J]. Advanced Functional Materials, 2021, 31(51): 2106116. |
| 69 | Sheng L S, Chen Z Q, Wang X, et al. Transforming porous silica nanoparticles into porous liquids with different canopy structures for CO2 capture[J]. ACS Omega, 2022, 7(7): 5687-5697. |
| 70 | Avila J, Lepre L F, Santini C C, et al. High-performance porous ionic liquids for low-pressure CO2 capture[J]. Angewandte Chemie, 2021, 60(23): 12876-12882. |
| 71 | Cahir J, Tsang M Y, Lai B B, et al. Type 3 porous liquids based on non-ionic liquid phases—a broad and tailorable platform of selective, fluid gas sorbents[J]. Chemical Science, 2020, 11(8): 2077-2084. |
| 72 | Wang Y, Sun Y W, Bian H, et al. Cyclodextrin porous liquid materials for efficient chiral recognition and separation of nucleosides[J]. ACS Applied Materials & Interfaces, 2020, 12(41): 45916-45928. |
| 73 | Wang Y, Feng Z, Sun Y W, et al. Chiral induction in a novel self-assembled supramolecular system composed of α-cyclodextrin porous liquids, chiral silver nanoparticles and planar conjugated molecules[J]. Soft Matter, 2022, 18(5): 975-982. |
| 74 | Chen L, Reiss P S, Chong S Y, et al. Separation of rare gases and chiral molecules by selective binding in porous organic cages[J]. Nature Materials, 2014, 13(10): 954-960. |
| 75 | Wu J M, Wu X M, Zhao P P, et al. Extraction desulphurization of fuels using ZIF-8-based porous liquid[J]. Fuel, 2021, 300: 121013. |
| 76 | 桂鑫. PL-MCM-41多孔液体材料制备及对模拟油品中硫,氮脱除性能研究[D]. 北京:中国石油大学, 2019. |
| Gui X. Synthesis of PL-MCM-41 porous liquid and study on the performance of desulfurization and denitrification in the simulated oil[D]. Beijing: China University of Petroleum, 2019. | |
| 77 | Wang Z H, Zhao P P, Wu J M, et al. ZIF-8-porous ionic liquids for the extraction of 2, 2, 3, 3-tetrafluoro-1-propanol and water mixture[J]. New Journal of Chemistry, 2021, 45(19): 8557-8562. |
| 78 | Horin I, Shalev O, Cohen Y. Aggregation mode, host-guest chemistry in water, and extraction capability of an uncharged, water-soluble, liquid pillar [5] arene derivative[J]. ChemistryOpen, 2021, 10(11): 1111-1115. |
| 79 | Hemming E B, Masters A F, Maschmeyer T. The encapsulation of metal nanoparticles within porous liquids[J]. Chemical Communications, 2019, 55(75): 11179-11182. |
| 80 | Hemming E B, Masters A F, Maschmeyer T. Exploring opportunities for platinum nanoparticles encapsulated in porous liquids as hydrogenation catalysts[J]. Chemistry-A European Journal, 2020, 26(31): 7059-7064. |
| 81 | Yang N, Lu L J, Zhu L H, et al. Phosphomolybdic acid encapsulated in ZIF-8-based porous ionic liquids for reactive extraction desulfurization of fuels[J]. Inorganic Chemistry Frontiers, 2022, 9(1): 165-178. |
| [1] | Weiqi JIN, Yuerong WU, Xia WANG, Li LI, Su QIU, Pan YUAN, Minghe WANG. Progress in infrared imaging detection technology and domestic equipment for industrial gas leakage in chemical industry parks [J]. CIESC Journal, 2023, 74(S1): 32-44. |
| [2] | Maolin DONG, Lidong CHEN, Liulian HUANG, Weibing WU, Hongqi DAI, Huiyang BIAN. Research progress in preparation of lignonanocellulose by acid hydrotropes and their functional applications [J]. CIESC Journal, 2023, 74(6): 2281-2295. |
| [3] | Yulong HUANG, Fan LYU, Junjie QIU, Hua ZHANG, Pinjing HE. Physicochemical properties and VOCs molecular characteristics of liquid digestate from anaerobic digestion of putrescible waste [J]. CIESC Journal, 2023, 74(3): 1275-1285. |
| [4] | Yixiu DONG, Ruzhu WANG. High temperature heat pump: cycle configurations, working fluids and application potentials [J]. CIESC Journal, 2023, 74(1): 133-144. |
| [5] | Duanhui GAO, Weiqiang XIAO, Feng GAO, Qian XIA, Manqiu WANG, Xinbo LU, Xiaoli ZHAN, Qinghua ZHANG. Preparation and application of polyimide-based aerogels [J]. CIESC Journal, 2022, 73(7): 2757-2773. |
| [6] | Qi WANG, Kuo FANG, Conghui HE, Kaijun WANG. Recent development and future challenges of flow-electrode capacitive deionization [J]. CIESC Journal, 2022, 73(3): 975-989. |
| [7] | ZHAO Zhijian,FU Jie,ZHAO Changjian,ZHANG Guojun. Summary and perspective of NSFC-Qinghai Qaidam Saline Lake Joint Research Fund [J]. CIESC Journal, 2021, 72(6): 3188-3193. |
| [8] | WANG Yanqiu,ZHONG Zhaoxiang,XING Weihong. Progress in three-dimensional metal oxide nanomaterials [J]. CIESC Journal, 2021, 72(5): 2339-2353. |
| [9] | ZHANG Lili, LI Yan, GAO Jing. Phase behavior and physicochemical properties of thermoreversible aqueous biphasic systems composed of ionic liquids and deep eutectic solvents [J]. CIESC Journal, 2021, 72(5): 2493-2505. |
| [10] | QI Na, SONG Wei, LIU Liming, WU Jing. Biocatalysis C—C bonding reaction and its application [J]. CIESC Journal, 2021, 72(1): 216-228. |
| [11] | Xiaoling ZHANG,Jianing BAO,Yunjia LI,Lin HUANGFU,Wensong LI,Shiqiu GAO,Guangwen XU,Changming LI,Jian YU. Preparation and industrial application of MnOx particle catalyst for low temperature denitration [J]. CIESC Journal, 2020, 71(11): 5169-5177. |
| [12] | Zhaowen ZENG, Cheng ZHENG, Taoyan MAO, Yuan WEI, Runhui XIAO, Siyu PENG. Progress in research and application of microwave in chemical process [J]. CIESC Journal, 2019, 70(S1): 1-14. |
| [13] | Ping LI, Changming LI, Zhengkang DUAN, Shiqiu GAO, Guangwen XU, Jian YU. Application conditions and kinetics simulation over SCR catalyst for flue gas denitrification under low temperature [J]. CIESC Journal, 2019, 70(8): 2981-2990. |
| [14] | LIN Zhifeng, HU Riming, ZHOU Xiaolong. Research progress of Ni-based catalysts [J]. CIESC Journal, 2017, 68(S1): 26-36. |
| [15] | ZHANG Xiaodong, LI Hongxin, HOU Fulin, YANG Yang, DONG Han, CUI Lifeng. Progress in preparation of MnOx and its application [J]. CIESC Journal, 2017, 68(6): 2249-2257. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||