CIESC Journal ›› 2024, Vol. 75 ›› Issue (5): 1750-1764.DOI: 10.11949/0438-1157.20240091
• Reviews and monographs • Previous Articles Next Articles
Tingting ZHAO1( ), Lixiang YAN1, Fuli TANG2, Minzhi XIAO1, Ye TAN1, Liubin SONG1(
), Lixiang YAN1, Fuli TANG2, Minzhi XIAO1, Ye TAN1, Liubin SONG1( ), Zhongliang XIAO1(
), Zhongliang XIAO1( ), Lingjun LI3
), Lingjun LI3
Received:2024-01-19
Revised:2024-03-03
Online:2024-06-25
Published:2024-05-25
Contact:
Liubin SONG, Zhongliang XIAO
赵亭亭1( ), 鄢立祥1, 唐福利2, 肖敏之1, 谭烨1, 宋刘斌1(
), 鄢立祥1, 唐福利2, 肖敏之1, 谭烨1, 宋刘斌1( ), 肖忠良1(
), 肖忠良1( ), 李灵均3
), 李灵均3
通讯作者:
宋刘斌,肖忠良
作者简介:赵亭亭(1994—),女,博士,讲师,zhaott_468@163.com
基金资助:CLC Number:
Tingting ZHAO, Lixiang YAN, Fuli TANG, Minzhi XIAO, Ye TAN, Liubin SONG, Zhongliang XIAO, Lingjun LI. Research progress on design strategies and reaction mechanisms of photo-assisted Li-CO2 battery catalysts[J]. CIESC Journal, 2024, 75(5): 1750-1764.
赵亭亭, 鄢立祥, 唐福利, 肖敏之, 谭烨, 宋刘斌, 肖忠良, 李灵均. 光辅助锂-二氧化碳电池催化剂的设计策略与反应机理研究进展[J]. 化工学报, 2024, 75(5): 1750-1764.
Add to citation manager EndNote|Ris|BibTeX

Fig.4 The working mechanism for the light-induced discharging process (a); Band diagram of the In2S3@CNT/SS (b); Photocurrent response to UV light of ICS, In2S3NS/SS, and CNT (c)[40]
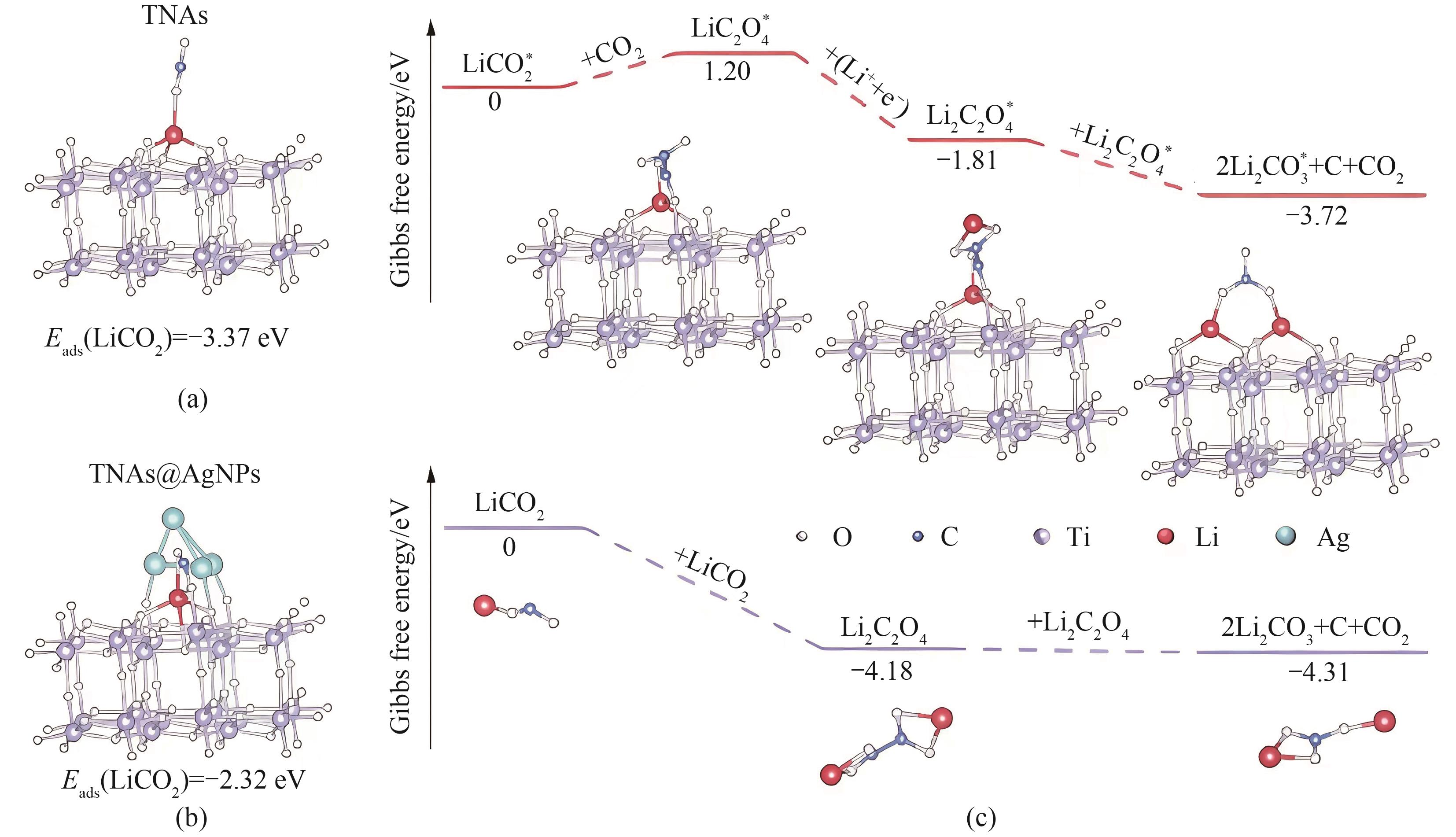
Fig.5 Optimized structures and adsorption energy of intermediate LiCO2 molecules on the TNAs (a); Optimized structures and adsorption energy of intermediate LiCO2 molecules on the TNAs@AgNPs (b); Gibbs free energy of battery surface and solution-mediated reaction pathways (c)[39]

Fig.6 Schematic illustration of different growth mechanisms of Li2CO3 in the Li-CO2 batteries based on TiO2 NAs/CT and RuO2-TiO2 NAs/CT cathodes (a); The optimized structures and the corresponding binding energy of Li2CO3 on TiO2 and RuO2 surfaces (b); The differential charge density Δρ of CO2 or Li2CO3 adsorbed on TiO2 and RuO2 surfaces (c)[38]
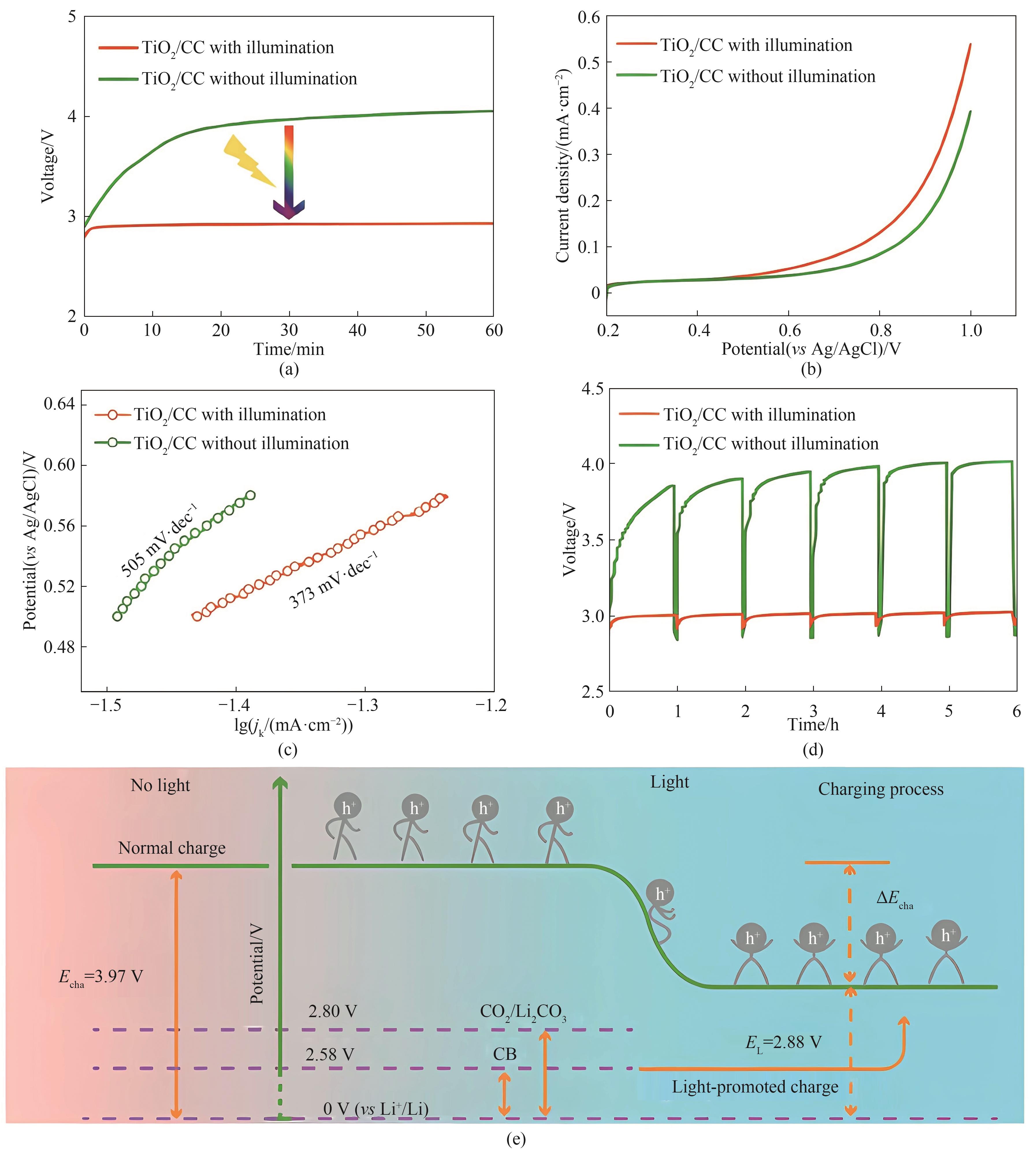
Fig.7 Charge curves of Li-CO2 batteries with TiO2/CC cathode with and without illumination (a); LSV curves measured in the CO2ER region of TiO2/CC with and without illumination in 0.5 mol·L-1 CO2-saturated LiTFSI/DME solution at a scan rate of 5 mV·s-1 (b); Corresponding Tafel curves (c); Galvanostatic intermittent titration technique curves obtained from Li-CO2 battery with TiO2/CC cathode with and without illumination (d); Schematic of energy diagrams for the reduced charge voltage of Li-CO2 battery under illumination (e)[36]
| 光催化剂 | 首次效率/% | 电流密度/(mA·cm-2) | 放电比容量/(mAh·cm-2) | 充电/放电电压平台/V | 循环次数(圈) | 文献 |
|---|---|---|---|---|---|---|
| TiO2/CC | 97.2 | 0.01 | 0.01 | 2.82/2.88 | 30 | [ |
| RuO2/TiO2 | 95.5 | 250 mA·g-1 | 1000 mAh·g-1 | 2.78/2.91 | 238 | [ |
| TiO2@Ag | 87.1 | 0.01 | 0.1 | 2.49/2.86 | 100 | [ |
| Cu2O/CNT | 85 | 100 mA·g-1 | 100 mAh·g-1 | 2.5/4.5 | 50 | [ |
| In2S3@CNT/SS | 98.1 | 0.01 | 0.01 | 3.14/3.2 | 25 | [ |
| CNT@C3N4 | 98.8 | 0.02 | 0.02 | 3.24/3.28 | 100 | [ |
| SiC/RGO | 84.4 | 20 mA·g-1 | 0.01 | 2.77/3.28 | 20 | [ |
| CoPc-Mn-O | 98.5 | 0.02 | — | 3.20/3.25 | 30 | [ |
| Au@TiO2 | 92.4 | 0.01 | 0.01 | 2.95/3.49 | 200 | [ |
| 混合相TiO2 | — | 0.025 | — | 2.2/3.0 | 52 | [ |
Table 1 Structure and performance of photo-assisted Li-CO2 batteries
| 光催化剂 | 首次效率/% | 电流密度/(mA·cm-2) | 放电比容量/(mAh·cm-2) | 充电/放电电压平台/V | 循环次数(圈) | 文献 |
|---|---|---|---|---|---|---|
| TiO2/CC | 97.2 | 0.01 | 0.01 | 2.82/2.88 | 30 | [ |
| RuO2/TiO2 | 95.5 | 250 mA·g-1 | 1000 mAh·g-1 | 2.78/2.91 | 238 | [ |
| TiO2@Ag | 87.1 | 0.01 | 0.1 | 2.49/2.86 | 100 | [ |
| Cu2O/CNT | 85 | 100 mA·g-1 | 100 mAh·g-1 | 2.5/4.5 | 50 | [ |
| In2S3@CNT/SS | 98.1 | 0.01 | 0.01 | 3.14/3.2 | 25 | [ |
| CNT@C3N4 | 98.8 | 0.02 | 0.02 | 3.24/3.28 | 100 | [ |
| SiC/RGO | 84.4 | 20 mA·g-1 | 0.01 | 2.77/3.28 | 20 | [ |
| CoPc-Mn-O | 98.5 | 0.02 | — | 3.20/3.25 | 30 | [ |
| Au@TiO2 | 92.4 | 0.01 | 0.01 | 2.95/3.49 | 200 | [ |
| 混合相TiO2 | — | 0.025 | — | 2.2/3.0 | 52 | [ |

Fig.8 Schematic depiction of the solar-spectrum photothermal effect of plasmonic semiconductor nanomaterials via plasmon-and exciton-based approaches (a); On/off response of the surface temperature increase of Au@TiO2 and TiO2 (b); Distribution of the surface temperature increase on TiO2 (c) and Au@TiO2 (d) spheres under the illumination conditions; I-T curves of Au@TiO2 and TiO2 spheres (e); Distribution of the electric near field under the illumination conditions on TiO2 and Au@TiO2 spheres surface (f); LSV curves for the CDR process of TiO2 and Au@TiO2 spheres with and without illumination (g)[44]
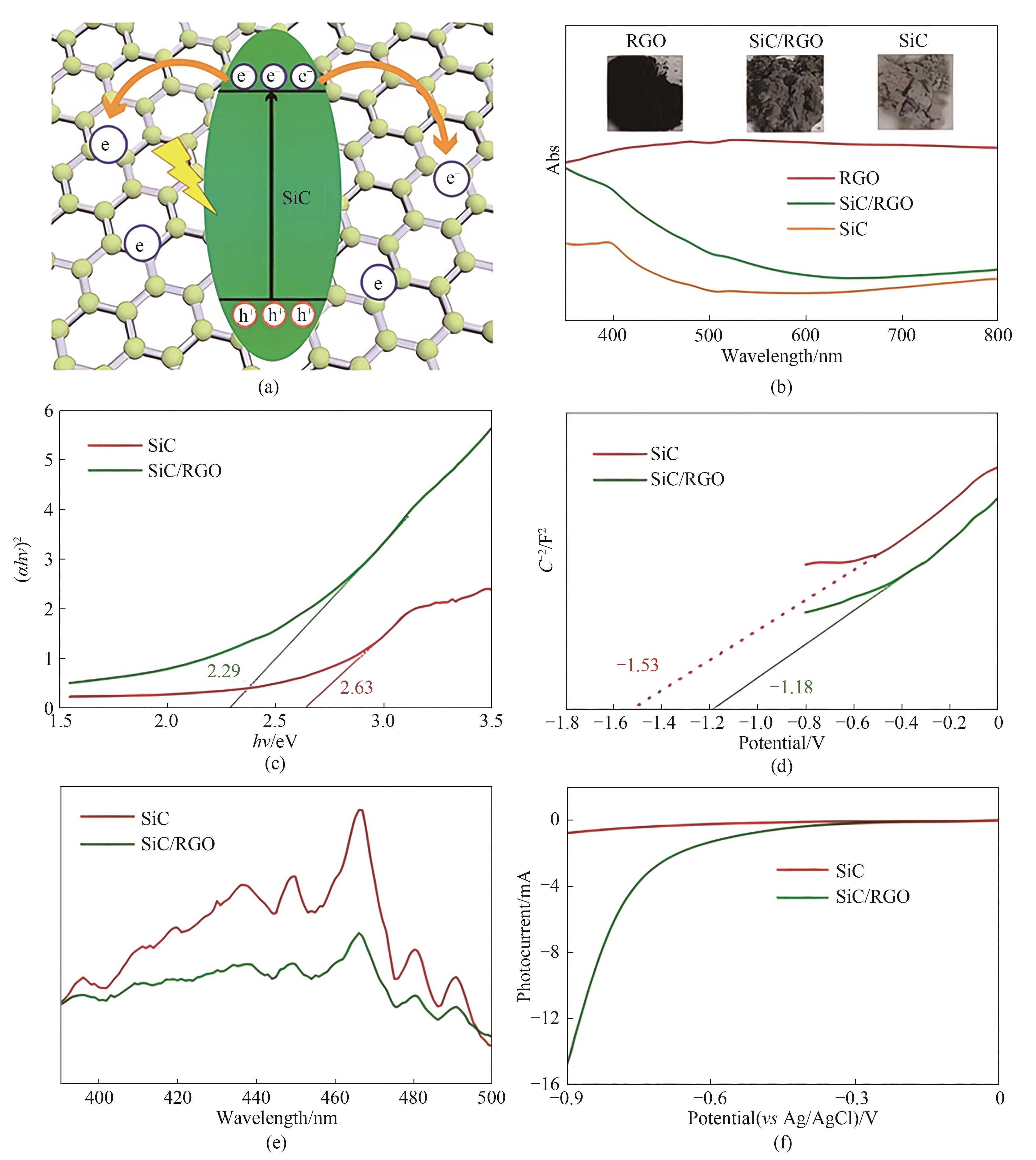
Fig.9 Schematic diagram of the synergistic effect of SiC flakes and RGO (a); UV-Vis diffuse reflectance spectra of RGO, SiC/RGO and pure SiC (b); The Tauc plot (c); Mott-Schottky plots (d); Room-temperature PL spectra of SiC and SiC/RGO samples (e); LSV curves of SiC/RGO and SiC cathodes under illumination (f)[43]

Fig.10 Working mechanism of the dual-field assisted Li-CO2 battery (a); Discharge/charge voltage profiles at the first cycle (b); Cycling performance (c); Rate capability of Li-CO2 batteries with TNAs and TNAs@AgNPs cathodes in light or dark (d); Discharge/charge curves of the TNAs@AgNPs cathode in light at 1.0 mAh·cm-2 and 2.0 mA·cm-2 (e); Comparison of cycling stability (f) and rate capability (g) of the dual-field assisted Li-CO2 battery with some representative Li-CO2 batteries based on the electrocatalysis and photo electrocatalysis mechanisms[39]
| 1 | Armand M, Tarascon J M. Building better batteries[J]. Nature, 2008, 451: 652-657. |
| 2 | Ding X B, Huang Q H, Xiong X H. Research and application of fast-charging graphite anodes for lithium-ion batteries[J]. Chinese Journal of Inorganic Chemistry, 2022, 38(11): 2204057. |
| 3 | Chen C, Zhang J M, Hu B R, et al. Dynamic gel as artificial interphase layer for ultrahigh-rate and large-capacity lithium metal anode[J]. Nature communications, 2023, 14(1): 4018. |
| 4 | Chiang Y M. Building a better battery[J]. MRS Bulletin, 2020, 45(3): 246-247. |
| 5 | Li M, Lu J, Chen Z W, et al. 30 years of lithium-ion batteries[J]. Advanced Materials, 2018: e1800561. |
| 6 | Ding X B, Huang H Y, Huang Q H, et al. Doping sites modulation of T-Nb2O5 to achieve ultrafast lithium storage[J]. Journal of Energy Chemistry, 2023, 77(2): 280-289. |
| 7 | Chen K, Yang D Y, Huang G, et al. Lithium-air batteries: air-electrochemistry and anode stabilization[J]. Accounts of Chemical Research, 2021, 54(3): 632-641. |
| 8 | Ohno S, Zeier W G. Toward practical solid-state lithium-sulfur batteries: challenges and perspectives[J]. Accounts of Materials Research, 2021, 2(10): 869-880. |
| 9 | Zhang L, Wang S, Wang Q, et al. Dendritic solid polymer electrolytes: a new paradigm for high-performance lithium-based batteries[J]. Advanced Materials, 2023, 35(35): e2303355. |
| 10 | Ma L, Yu T W, Tzoganakis E, et al. Fundamental understanding and material challenges in rechargeable nonaqueous Li-O2 batteries: recent progress and perspective[J]. Advanced Energy Materials, 2018, 8(22): 1800348. |
| 11 | Xiao X, Zhang Z J, Yan A J, et al. Upgrading carbon utilization and green energy storage through oxygen-assisted lithium-carbon dioxide batteries[J]. Energy Storage Materials, 2024, 65: 103129. |
| 12 | Chen L, Zhou J W, Wang Y H, et al. Flexible, stretchable, water-/fire-proof fiber-shaped Li-CO2 batteries with high energy density[J]. Advanced Energy Materials, 2023, 13(1): 2202933. |
| 13 | Zhao W T, Yang Y, Deng Q H, et al. Toward an understanding of bimetallic MXene solid-solution in binder-free electrocatalyst cathode for advanced Li-CO2 batteries[J]. Advanced Functional Materials, 2022, 33(5): 1. |
| 14 | Sun X Y, Mu X W, Zheng W, et al. Binuclear Cu complex catalysis enabling Li-CO2 battery with a high discharge voltage above 3.0 V[J]. Nature Communications, 2023, 14: 536. |
| 15 | Li X L, Zhang J X, Qi G C, et al. Vertically aligned N-doped carbon nanotubes arrays as efficient binder-free catalysts for flexible Li-CO2 batteries[J]. Energy Storage Materials, 2021, 35: 148-156. |
| 16 | Zhou L J, Wang H, Zhang K, et al. Fast decomposition of Li2CO3/C actuated by single-atom catalysts for Li-CO2 batteries[J]. Science China Materials, 2021, 64(9): 2139-2147. |
| 17 | Liu B, Sun Y L, Liu L Y, et al. Recent advances in understanding Li-CO2 electrochemistry[J]. Energy & Environmental Science, 2019, 12(3): 887-922. |
| 18 | Lin J F, Ding J N, Wang H Z, et al. Boosting energy efficiency and stability of Li-CO2 batteries via synergy between Ru atom clusters and single-atom Ru-N4 sites in the electrocatalyst cathode[J]. Advanced Materials, 2022, 34(17): e2200559. |
| 19 | Xiao Y, Hu S L, Miao Y, et al. Recent progress in hot spot regulated strategies for catalysts applied in Li-CO2 batteries[J]. Small, 2024, 20(1): e2305009. |
| 20 | Lu B Y, Min Z W, Xiao X, et al. Recycled tandem catalysts promising ultralow overpotential Li-CO2 batteries[J]. Advanced Materials, 2024, 36(1): e2309264. |
| 21 | Chourasia A K, Shavez M, Naik K M, et al. Candle soot nanoparticles versus multiwalled carbon nanotubes as a high-performance cathode catalyst for Li-CO2Mars batteries for Mars exploration[J]. ACS Applied Energy Materials, 2023, 6(1): 378-386. |
| 22 | Zhou J W, Cheng J L, Wang B, et al. Flexible metal-gas batteries: a potential option for next-generation power accessories for wearable electronics[J]. Energy & Environmental Science, 2020, 13(7): 1933-1970. |
| 23 | Gowda S R, Brunet A, Wallraff G M, et al. Implications of CO2 contamination in rechargeable nonaqueous Li-O2 batteries[J]. The Journal of Physical Chemistry Letters, 2013, 4(2): 276-279. |
| 24 | Zhao Z W, Huang J, Peng Z Q. Achilles’ heel of lithium-air batteries: lithium carbonate[J]. Angewandte Chemie International Edition, 2018, 57(15): 3874-3886. |
| 25 | Yang S X, He P, Zhou H S. Exploring the electrochemical reaction mechanism of carbonate oxidation in Li-air/CO2 battery through tracing missing oxygen[J]. Energy & Environmental Science, 2016, 9(5): 1650-1654. |
| 26 | Li J X, Zhang K, Wang B J, et al. Light-assisted metal-air batteries: progress, challenges, and perspectives[J]. Angewandte Chemie International Edition, 2022, 61(51): e202213026. |
| 27 | Savunthari K V, Chen C H, Chen Y R, et al. Effective Ru/CNT cathode for rechargeable solid-state Li-CO2 batteries[J]. ACS Applied Materials & Interfaces, 2021, 13(37): 44266-44273. |
| 28 | Guo C, Zhang F L, Han X, et al. Intrinsic descriptor guided noble metal cathode design for Li-CO2 battery[J]. Advanced Materials, 2023, 35(33): e2302325. |
| 29 | Wang Y F, Ji G J, Song L N, et al. A highly reversible lithium–carbon dioxide battery based on soluble oxalate[J]. ACS Energy Letters, 2023, 8(2): 1026-1034. |
| 30 | Fan L, Shen H M, Ji D X, et al. Biaxially compressive strain in Ni/Ru core/shell nanoplates boosts Li-CO2 batteries[J]. Advanced Materials, 2022, 34(30): e2204134. |
| 31 | Zhou J W, Wang T S, Chen L, et al. Boosting the reaction kinetics in aprotic lithium-carbon dioxide batteries with unconventional phase metal nanomaterials[J]. Proceedings of the National Academy of Sciences of the United States of America, 2022, 119(40): e2204666119. |
| 32 | Chen L, Zhou J W, Zhang J X, et al. Copper indium sulfide enables Li-CO2 batteries with boosted reaction kinetics and cycling stability[J]. Energy & Environmental Materials, 2023, 6(5): 12415. |
| 33 | Zhang Z, Yang C, Wu S S, et al. Exploiting synergistic effect by integrating ruthenium-copper nanoparticles highly co-dispersed on graphene as efficient air cathodes for Li-CO2 batteries[J]. Advanced Energy Materials, 2019, 9(8): 1802805. |
| 34 | Li J X, Zhang K, Zhao Y, et al. High-efficiency and stable Li-CO2 battery enabled by carbon nanotube/carbon nitride heterostructured photocathode[J]. Angewandte Chemie International Edition, 2022, 61(4): e202114612. |
| 35 | Wang J H, Li S, Chen Y F, et al. Phthalocyanine based metal-organic framework ultrathin nanosheet for efficient photocathode toward light-assisted Li-CO2 battery[J]. Advanced Functional Materials, 2022, 32(49): 2210259. |
| 36 | Wang X X, Guan D H, Li F, et al. A renewable light-promoted flexible LiCO2 battery with ultrahigh energy efficiency of 97.9%[J]. Small, 2021, 17(26): e2100642. |
| 37 | Zhu T, Wang S, Yu Z Q, et al. High-performance Li-CO2 battery based on carbon-free porous Ru@QNFs cathode[J]. Small, 2023, 19(33): e2301498. |
| 38 | Wang C Z, Shang Y, Lu Y C, et al. Photoinduced homogeneous RuO2 nanoparticles on TiO2 nanowire arrays: a high-performance cathode toward flexible Li-CO2 batteries[J]. Journal of Power Sources, 2020, 475: 228703. |
| 39 | Zhang K, Li J X, Zhai W J, et al. Boosting cycling stability and rate capability of Li-CO2 batteries via synergistic photoelectric effect and plasmonic interaction[J]. Angewandte Chemie International Edition, 2022, 61(17): e202201718. |
| 40 | Guan D H, Wang X X, Li M L, et al. Light/electricity energy conversion and storage for a hierarchical porous In2S3@CNT/SS cathode towards a flexible Li-CO2 battery[J]. Angewandte Chemie International Edition, 2020, 59(44): 19518-19524. |
| 41 | Ma Y, Wang X L, Jia Y S, et al. Titanium dioxide-based nanomaterials for photocatalytic fuel generations[J]. Chemical Reviews, 2014, 114(19): 9987-10043. |
| 42 | Jena A, Hsieh H C, Thoka S, et al. Curtailing the overpotential of Li-CO2 batteries with shape-controlled Cu2O as cathode: effect of illuminating the cathode[J]. ChemSusChem, 2020, 13(10): 2719-2725. |
| 43 | Li Z, Li M L, Wang X X, et al. In situ fabricated photo-electro-catalytic hybrid cathode for light-assisted lithium-CO2 batteries[J]. Journal of Materials Chemistry A, 2020, 8(29): 14799-14806. |
| 44 | Guan D H, Wang X X, Li F, et al. All-solid-state photo-assisted Li-CO2 battery working at an ultra-wide operation temperature[J]. ACS Nano, 2022, 16(8): 12364-12376. |
| 45 | Long L Z, Ding Y Y, Liang N N, et al. A carbon-free and free-standing cathode from mixed-phase TiO2 for photo-assisted Li-CO2 battery[J]. Small, 2023, 19(27): e2300519. |
| 46 | Zhao Z W, Wang E K, Wang J W, et al. Kinetics of the CO2 reduction reaction in aprotic Li–CO2 batteries: a model study[J]. Journal of Materials Chemistry A, 2021, 9(6): 3290-3296. |
| 47 | Yang C, Guo K K, Yuan D W, et al. Unraveling reaction mechanisms of Mo2C as cathode catalyst in a Li-CO2 battery[J]. Journal of the American Chemical Society, 2020, 142(15): 6983-6990. |
| 48 | Xu S M, Das S K, Archer L A. The Li–CO2 battery: a novel method for CO2 capture and utilization[J]. RSC Advances, 2013, 3(18): 6656-6660. |
| 49 | Wang S, Song H C, Zhu T, et al. An ultralow-charge-overpotential and long-cycle-life solid-state Li-CO2 battery enabled by plasmon-enhanced solar photothermal catalysis[J]. Nano Energy, 2022, 100: 107521. |
| 50 | Pipes R, Bhargav A, Manthiram A. Nanostructured anatase titania as a cathode catalyst for Li-CO2 batteries[J]. ACS Applied Materials & Interfaces, 2018, 10(43): 37119-37124. |
| 51 | Xu Y Y, Gong H, Ren H, et al. Highly efficient Cu-porphyrin-based metal-organic framework nanosheet as cathode for high-rate Li-CO2 battery[J]. Small, 2022, 18(45): e2203917. |
| 52 | Gong H, Wang T, Xue H R, et al. Photo-enhanced lithium oxygen batteries with defective titanium oxide as both photo-anode and air electrode[J]. Energy Storage Materials, 2018, 13: 49-56. |
| 53 | Baek K, Jeon W C, Woo S, et al. Synergistic effect of quinary molten salts and ruthenium catalyst for high-power-density lithium-carbon dioxide cell[J]. Nature Communications, 2020, 11: 456. |
| 54 | Ahmadiparidari A, Warburton R E, Majidi L, et al. A long-cycle-life lithium-CO2 battery with carbon neutrality[J]. Advanced Materials, 2019, 31(40): 1902518. |
| 55 | Guo Q, Zhou C Y, Ma Z B, et al. Fundamentals of TiO2 photocatalysis: concepts, mechanisms, and challenges[J]. Advanced Materials, 2019, 31(50): e1901997. |
| 56 | Hu J Y, Su C B, Li R J, et al. High-performance Li-CO2 batteries enabled by synergistic interaction of iron dopant-modulated catalysts and nitrogen-modified substrates[J]. Journal of Alloys and Compounds, 2024, 976: 173146. |
| 57 | Liu J, Ma N K, Wu W, et al. Recent progress on photocatalytic heterostructures with full solar spectral responses[J]. Chemical Engineering Journal, 2020, 393: 124719. |
| 58 | Low J, Yu J G, Jaroniec M, et al. Heterojunction photocatalysts[J]. Advanced Materials, 2017, 29(20): 1601694. |
| 59 | Jin Y C, Liu Y, Song L, et al. Interfacial engineering in hollow NiS2/FeS2-NSGA heterostructures with efficient catalytic activity for advanced Li-CO2 battery[J]. Chemical Engineering Journal, 2022, 430: 133029. |
| 60 | Sun Z M, Wang D, Lin L, et al. Ultrathin hexagonal boron nitride as a van der Waals’ force initiator activated graphene for engineering efficient non-metal electrocatalysts of Li-CO2 battery[J]. Nano Research, 2022, 15(2): 1171-1177. |
| 61 | Cheng Z B, Wu Z Y, Tang Y Y, et al. Cationic metal-organic framework derived ruthenium-copper nano-alloys in porous carbon to catalytically boost the cycle life of Li-CO2 batteries[J]. Nanoscale, 2022, 14(40): 15073-15078. |
| 62 | Han J R, Wu H Y, Song R L, et al. Defect-rich porous carbon as a metal-free catalyst for high-performance Li-CO2 batteries[J]. Electrochimica Acta, 2024, 477: 143779. |
| 63 | Qie L, Lin Y, Connell J W, et al. Highly rechargeable lithium-CO2 batteries with a boron- and nitrogen-codoped holey-graphene cathode[J]. Angewandte Chemie International Edition, 2017, 56(24): 6970-6974. |
| 64 | 王禹婷, 杨天怡, 章应辉. 卟啉框架材料在光催化领域的应用[J]. 应用化学, 2020, 37(6): 611-619. |
| Wang Y T, Yang T Y, Zhang Y H. Application of porphyrin-based framework materials on photocatalysis[J]. Chinese Journal of Applied Chemistry, 2020, 37(6): 611-619. | |
| 65 | 焦帅, 杨磊, 武婷婷, 等. 混合盐模板法制备超级电容器用氮掺杂分级多孔碳纳米片[J]. 化工学报, 2021, 72(5): 2869-2877. |
| Jiao S, Yang L, Wu T T, et al. Synthesis of nitrogen doped hierarchically porous carbon nanosheets for supercapacitor by mixed salt template[J]. CIESC Journal, 2021, 72(5): 2869-2877. | |
| 66 | 后振中, 彭龙贵, 李颖, 等. 分级多孔聚吡咯膜的界面自组装合成与电化学电容性[J]. 化工学报, 2018, 69(9): 4121-4128. |
| Hou Z Z, Peng L G, Li Y, et al. Interfacial self-assembly synthesis and electrochemical capacitance of hierarchical porous polypyrrole films[J]. CIESC Journal, 2018, 69(9): 4121-4128. | |
| 67 | Hu C, Tu S C, Tian N, et al. Photocatalysis enhanced by external fields[J]. Angewandte Chemie International Edition, 2021, 60(30): 16309-16328. |
| 68 | Sun Z H, Yin H, Liu K L, et al. Machine learning accelerated calculation and design of electrocatalysts for CO2 reduction[J]. SmartMat, 2022, 3(1): 68-83. |
| 69 | Liu J W, Luo W Z, Wang L, et al. Toward excellence of electrocatalyst design by emerging descriptor-oriented machine learning[J]. Advanced Functional Materials, 2022, 32(17): 2110748. |
| 70 | Németh K, Srajer G. CO2/oxalate cathodes as safe and efficient alternatives in high energy density metal–air type rechargeable batteries[J]. RSC Advances, 2014, 4(4): 1879-1885. |
| 71 | Qiao Y, Yi J, Wu S C, et al. Li-CO2 electrochemistry: a new strategy for CO2 fixation and energy storage[J]. Joule, 2017, 1(2): 359-370. |
| 72 | Su L W, Zhou Z, Qin X, et al. CoCO3 submicrocube/graphene composites with high lithium storage capability[J]. Nano Energy, 2013, 2(2): 276-282. |
| [1] | Guoyi XIAN, Lifang CHEN, Zhiwen QI. DFT-based study of liquid-phase Beckmann rearrangement mechanism of cyclohexanone oxime [J]. CIESC Journal, 2024, 75(1): 302-311. |
| [2] | YE Kai, LIU Xianghua, JIANG Yue, YU Ying, ZHAO Yafei, ZHUANG Ye, ZHENG Jinbao, CHEN Binghui. Combing low-temperature plasma with CeO2/13X for toluene degradation [J]. CIESC Journal, 2021, 72(7): 3706-3715. |
| [3] | ZHU Qianqian, JIN Haibo, GUO Xiaoyan, HE Guangxiang, MA Lei, ZHANG Rongyue, GU Qingyang, YANG Suohe. Study on synthesis of ε-caprolactone with MgO catalysis by Baeyer-Villiger green oxidation of cyclohexanone in H2O2/acetonitrile system [J]. CIESC Journal, 2021, 72(5): 2638-2646. |
| [4] | Gang WANG,Xuezhi DUAN,Weikang YUAN,Xinggui ZHOU. Mechanistic insights into catalytic isomerization of propylene oxide over TS-1 [J]. CIESC Journal, 2021, 72(10): 5150-5158. |
| [5] | Hong ZHANG, Liu TANG. Study on reaction mechanism of p-type dopant Cp2Mg in MOCVD gas phase [J]. CIESC Journal, 2020, 71(7): 3000-3008. |
| [6] | Yan JIN, Qian YANG, Wenbin ZHAO, Baoshan HU. Catalytic reaction system for controllable synthesis of graphene with chemical vapor deposition [J]. CIESC Journal, 2020, 71(6): 2564-2585. |
| [7] | Lufeng WANG, Xin QIAN, Lifang DENG, Haoran YUAN. Recent progress on catalysts about electrochemical synthesis of ammonia from nitrogen [J]. CIESC Journal, 2019, 70(8): 2854-2863. |
| [8] | Yonggang CHENG, Jiao LIU, Zhennan HAN, Lei SHI, Guangwen XU. Transfer dynamics and reaction control mechanism over methanation catalyst particles in transport bed [J]. CIESC Journal, 2019, 70(8): 2876-2887. |
| [9] | Tianshui LIANG, Zongying WANG, Kun GAO, Runwan LI, Zheng WANG, Wei ZHONG, Jun ZHAO. Analysis of fire suppression effectiveness of ultra-fine water mist containing iron compounds additives in cup burner [J]. CIESC Journal, 2019, 70(3): 1236-1242. |
| [10] | ZHANG Yi, LI Jianbo, WANG Quanhai, LU Xiaofeng. Simulation of NOx formation in novel dual circulating fluidized-bed boiler [J]. CIESC Journal, 2018, 69(4): 1703-1713. |
| [11] | LI Shuyan, SUN Lina, SHEN Shujun, CHENG Tianxing, CHENG Shuanghua, CHEN Jiuxi. Reaction of diaryl disulfides with nitroarenes [J]. CIESC Journal, 2017, 68(6): 2394-2398. |
| [12] | ZHANG Hongmei, LIN Feng, REN Mingqi, LI Jinlian, HAO Yulan, WU Hongjun, ZHAO Jingying, ZHAO Liang, HE Yongdian. Free radical models of small molecular alkane pyrolysis [J]. CIESC Journal, 2017, 68(4): 1423-1433. |
| [13] | ZHANG Guangjie, GENG Yanlou, AN Hualiang, ZHAO Xinqiang, WANG Yanji. Synthesis of propylene carbonate from CO2 and 1,2-propylene glycol on fixed-bed reactor and its reaction mechanism catalyzed by Zn(OAc)2/AC [J]. CIESC Journal, 2015, 66(2): 567-575. |
| [14] | WANG Caijun, LU Shaojie, WANG Ping. Sub-grid scale combustion model based on REDIM method and its application [J]. CIESC Journal, 2015, 66(12): 4948-4959. |
| [15] | WANG Rongjie, SHEN Benxian, MA Jian, ZHAO Jigang. Ring-open reaction mechanism of sulfur S8 based on density functional theory [J]. CIESC Journal, 2015, 66(10): 3919-3924. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||