CIESC Journal ›› 2025, Vol. 76 ›› Issue (9): 4770-4785.DOI: 10.11949/0438-1157.20250218
• Thermodynamics • Previous Articles Next Articles
Sifan WANG( ), Yifan LI, Jiangbo CHEN, Huan ZHOU(
), Yifan LI, Jiangbo CHEN, Huan ZHOU( )
)
Received:2025-03-05
Revised:2025-05-06
Online:2025-10-23
Published:2025-09-25
Contact:
Huan ZHOU
通讯作者:
周桓
作者简介:王偲凡(1999—),女,硕士研究生,952874405@qq.com
基金资助:CLC Number:
Sifan WANG, Yifan LI, Jiangbo CHEN, Huan ZHOU. Thermodynamics and phase diagram modeling of carbonate-type brines Li+, Na+, K+, CO
王偲凡, 栗一帆, 陈江波, 周桓. 碳酸盐型卤水Li+, Na+, K+, CO
Add to citation manager EndNote|Ris|BibTeX
| 体系 | T/K | 文献 |
|---|---|---|
| Li2CO3-H2O | 271.04~575.15 | [ |
| Na2CO3-H2O | 273.15~368.15 | [ |
| K2CO3-H2O | 262.15~499.15 | [ |
| Li2CO3-Na2CO3-H2O | 278.15~373.15 | [ |
| Na2CO3-K2CO3-H2O | 273.15~373.15 | [ |
| Li2CO3-K2CO3-H2O | 288.15,298.15 | [ |
| Li2CO3-Na2CO3-K2CO3-H2O | 288.15,298.15 | [ |
Table 1 Solubility data in the Li+, Na+, K+, CO32--H2O system
| 体系 | T/K | 文献 |
|---|---|---|
| Li2CO3-H2O | 271.04~575.15 | [ |
| Na2CO3-H2O | 273.15~368.15 | [ |
| K2CO3-H2O | 262.15~499.15 | [ |
| Li2CO3-Na2CO3-H2O | 278.15~373.15 | [ |
| Na2CO3-K2CO3-H2O | 273.15~373.15 | [ |
| Li2CO3-K2CO3-H2O | 288.15,298.15 | [ |
| Li2CO3-Na2CO3-K2CO3-H2O | 288.15,298.15 | [ |
| 序号 | 液相组成w(B)/% | 密度 ρ/(kg·L-1) | pH | 湿固相组成w(B)/% | 固相 | |||
|---|---|---|---|---|---|---|---|---|
| K2CO3 | Li2CO3 | K2CO3 | Li2CO3 | |||||
| T=273.15 K | ||||||||
| 1, A | 0 | 1.51 | 1.0263 | 11.09 | 0 | 65.25 | S1 | |
| 2 | 8.27 | 1.40 | 1.1450 | 11.58 | 4.00 | 62.58 | S1 | |
| 3 | 16.63 | 1.28 | 1.2153 | 11.72 | 6.32 | 63.43 | S1 | |
| 4 | 24.37 | 1.07 | 1.3037 | 11.94 | 8.97 | 64.58 | S1 | |
| 5 | 31.69 | 0.88 | 1.3561 | 12.36 | 12.21 | 62.33 | S1 | |
| 6 | 37.91 | 0.71 | 1.4014 | 12.56 | 16.61 | 56.56 | S1 | |
| 7 | 44.85 | 0.53 | 1.4255 | 12.67 | 20.53 | 54.86 | S1 | |
| 8, E | 51.32 | 0.43 | 1.4547 | 12.89 | 36.30 | 47.03 | S1+S2 | |
| 9 | 51.22 | 0.28 | 1.4482 | 12.86 | 60.32 | 22.15 | S2 | |
| 10 | 51.13 | 0.14 | 1.4506 | 12.85 | 67.96 | 6.54 | S2 | |
| 11, B | 51.05 | 0 | 1.4512 | 12.88 | 73.00 | 0 | S2 | |
| T=323.15 K | ||||||||
| 1, C | 0 | 1.07 | 1.0212 | 11.01 | 0 | 67.50 | S1 | |
| 2 | 9.31 | 0.92 | 1.1437 | 11.67 | 4.52 | 58.20 | S1 | |
| 3 | 15.83 | 0.89 | 1.1983 | 11.83 | 7.04 | 60.15 | S1 | |
| 4 | 22.94 | 0.79 | 1.2731 | 12.16 | 9.21 | 63.32 | S1 | |
| 5 | 29.06 | 0.71 | 1.3253 | 12.43 | 10.46 | 62.72 | S1 | |
| 6 | 34.18 | 0.63 | 1.3776 | 12.58 | 12.11 | 65.54 | S1 | |
| 7 | 39.62 | 0.47 | 1.4015 | 12.66 | 15.54 | 60.45 | S1 | |
| 8 | 44.53 | 0.39 | 1.4359 | 13.20 | 17.62 | 61.13 | S1 | |
| 9 | 49.83 | 0.32 | 1.4720 | 13.37 | 16.78 | 65.68 | S1 | |
| 10, F | 55.53 | 0.24 | 1.5393 | 13.51 | 37.55 | 45.15 | S1+S2 | |
| 11 | 55.32 | 0.13 | 1.5322 | 13.49 | 60.48 | 21.29 | S2 | |
| 12 | 55.14 | 0.07 | 1.5363 | 13.43 | 66.70 | 12.13 | S2 | |
| 13, D | 54.98 | 0 | 1.5331 | 13.45 | 73.35 | 0 | S2 | |
| T=348.15 K | ||||||||
| 1, M | 0 | 0.88 | 1.0188 | 10.96 | 0 | 63.58 | S1 | |
| 2 | 9.16 | 0.79 | 1.1386 | 11.39 | 3.09 | 65.73 | S1 | |
| 3 | 18.87 | 0.69 | 1.2285 | 11.63 | 7.33 | 63.31 | S1 | |
| 4 | 28.28 | 0.51 | 1.3109 | 11.79 | 10.11 | 64.12 | S1 | |
| 5 | 35.49 | 0.41 | 1.3860 | 12.15 | 14.76 | 58.58 | S1 | |
| 6 | 40.96 | 0.33 | 1.4091 | 12.41 | 16.11 | 61.26 | S1 | |
| 7 | 47.36 | 0.26 | 1.4533 | 12.55 | 20.02 | 58.33 | S1 | |
| 8 | 53.12 | 0.19 | 1.5117 | 12.98 | 23.18 | 57.19 | S1 | |
| 9, G | 57.75 | 0.16 | 1.5677 | 13.68 | 42.36 | 39.35 | S1+S2 | |
| 10 | 57.73 | 0.10 | 1.5619 | 13.67 | 68.59 | 10.12 | S2 | |
| 11, N | 57.71 | 0 | 1.5601 | 13.63 | 72.63 | 0 | S2 | |
Table 2 Solid-liquid phase equilibrium data of Li2CO3-K2CO3-H2O ternary system
| 序号 | 液相组成w(B)/% | 密度 ρ/(kg·L-1) | pH | 湿固相组成w(B)/% | 固相 | |||
|---|---|---|---|---|---|---|---|---|
| K2CO3 | Li2CO3 | K2CO3 | Li2CO3 | |||||
| T=273.15 K | ||||||||
| 1, A | 0 | 1.51 | 1.0263 | 11.09 | 0 | 65.25 | S1 | |
| 2 | 8.27 | 1.40 | 1.1450 | 11.58 | 4.00 | 62.58 | S1 | |
| 3 | 16.63 | 1.28 | 1.2153 | 11.72 | 6.32 | 63.43 | S1 | |
| 4 | 24.37 | 1.07 | 1.3037 | 11.94 | 8.97 | 64.58 | S1 | |
| 5 | 31.69 | 0.88 | 1.3561 | 12.36 | 12.21 | 62.33 | S1 | |
| 6 | 37.91 | 0.71 | 1.4014 | 12.56 | 16.61 | 56.56 | S1 | |
| 7 | 44.85 | 0.53 | 1.4255 | 12.67 | 20.53 | 54.86 | S1 | |
| 8, E | 51.32 | 0.43 | 1.4547 | 12.89 | 36.30 | 47.03 | S1+S2 | |
| 9 | 51.22 | 0.28 | 1.4482 | 12.86 | 60.32 | 22.15 | S2 | |
| 10 | 51.13 | 0.14 | 1.4506 | 12.85 | 67.96 | 6.54 | S2 | |
| 11, B | 51.05 | 0 | 1.4512 | 12.88 | 73.00 | 0 | S2 | |
| T=323.15 K | ||||||||
| 1, C | 0 | 1.07 | 1.0212 | 11.01 | 0 | 67.50 | S1 | |
| 2 | 9.31 | 0.92 | 1.1437 | 11.67 | 4.52 | 58.20 | S1 | |
| 3 | 15.83 | 0.89 | 1.1983 | 11.83 | 7.04 | 60.15 | S1 | |
| 4 | 22.94 | 0.79 | 1.2731 | 12.16 | 9.21 | 63.32 | S1 | |
| 5 | 29.06 | 0.71 | 1.3253 | 12.43 | 10.46 | 62.72 | S1 | |
| 6 | 34.18 | 0.63 | 1.3776 | 12.58 | 12.11 | 65.54 | S1 | |
| 7 | 39.62 | 0.47 | 1.4015 | 12.66 | 15.54 | 60.45 | S1 | |
| 8 | 44.53 | 0.39 | 1.4359 | 13.20 | 17.62 | 61.13 | S1 | |
| 9 | 49.83 | 0.32 | 1.4720 | 13.37 | 16.78 | 65.68 | S1 | |
| 10, F | 55.53 | 0.24 | 1.5393 | 13.51 | 37.55 | 45.15 | S1+S2 | |
| 11 | 55.32 | 0.13 | 1.5322 | 13.49 | 60.48 | 21.29 | S2 | |
| 12 | 55.14 | 0.07 | 1.5363 | 13.43 | 66.70 | 12.13 | S2 | |
| 13, D | 54.98 | 0 | 1.5331 | 13.45 | 73.35 | 0 | S2 | |
| T=348.15 K | ||||||||
| 1, M | 0 | 0.88 | 1.0188 | 10.96 | 0 | 63.58 | S1 | |
| 2 | 9.16 | 0.79 | 1.1386 | 11.39 | 3.09 | 65.73 | S1 | |
| 3 | 18.87 | 0.69 | 1.2285 | 11.63 | 7.33 | 63.31 | S1 | |
| 4 | 28.28 | 0.51 | 1.3109 | 11.79 | 10.11 | 64.12 | S1 | |
| 5 | 35.49 | 0.41 | 1.3860 | 12.15 | 14.76 | 58.58 | S1 | |
| 6 | 40.96 | 0.33 | 1.4091 | 12.41 | 16.11 | 61.26 | S1 | |
| 7 | 47.36 | 0.26 | 1.4533 | 12.55 | 20.02 | 58.33 | S1 | |
| 8 | 53.12 | 0.19 | 1.5117 | 12.98 | 23.18 | 57.19 | S1 | |
| 9, G | 57.75 | 0.16 | 1.5677 | 13.68 | 42.36 | 39.35 | S1+S2 | |
| 10 | 57.73 | 0.10 | 1.5619 | 13.67 | 68.59 | 10.12 | S2 | |
| 11, N | 57.71 | 0 | 1.5601 | 13.63 | 72.63 | 0 | S2 | |
| System | Property | T/K | m/(mol·kg-1) | Ref. | MRD/% |
|---|---|---|---|---|---|
| Li2CO3-H2O | Solubility(Li2CO3) | 273.14—575.15 | 0.2128—0.0206 | [ | 0.69 |
| Na2CO3-H2O | Pvap | 283.14—573.15 | 0.3921—2.6611 | [ | 2.46 |
| Cp | 283.13—403.15 | 0.1924—4.0436 | [ | 0.94 | |
| Solubility(Na2CO3) | 271.04—305.15 | 0.5703—4.3186 | [ | 0.51 | |
| Solubility(Na2CO3·H2O) | 305.14—308.52 | 4.2834—4.6703 | [ | 0.60 | |
| Solubility(Na2CO3·7H2O) | 305.52—377.95 | 4.6681—4.1994 | [ | 0.55 | |
| Solubility(Na2CO3·10H2O) | 377.94—453.15 | 4.1994—3.1450 | [ | 0.67 | |
| K2CO3-H2O | Pvap | 313.15—333.15 | 0.1477—3.4050 | [ | 2.15 |
| Cp | 283.15—353.15 | 0.1477—7.2356 | [ | 0.95 | |
| Solubility(K2CO3) | 446.15—499.15 | 17.1628—19.0007 | [ | 0.56 | |
| Solubility(K2CO3·1.5H2O) | 268.15—426.15 | 7.7015—17.1913 | [ | 0.64 | |
| Solubility(K2CO3·6H2O) | 243.15—266.95 | 5.3035—7.5179 | [ | 1.94 |
Table 3 Physical property and solubility data of solution in binary systems
| System | Property | T/K | m/(mol·kg-1) | Ref. | MRD/% |
|---|---|---|---|---|---|
| Li2CO3-H2O | Solubility(Li2CO3) | 273.14—575.15 | 0.2128—0.0206 | [ | 0.69 |
| Na2CO3-H2O | Pvap | 283.14—573.15 | 0.3921—2.6611 | [ | 2.46 |
| Cp | 283.13—403.15 | 0.1924—4.0436 | [ | 0.94 | |
| Solubility(Na2CO3) | 271.04—305.15 | 0.5703—4.3186 | [ | 0.51 | |
| Solubility(Na2CO3·H2O) | 305.14—308.52 | 4.2834—4.6703 | [ | 0.60 | |
| Solubility(Na2CO3·7H2O) | 305.52—377.95 | 4.6681—4.1994 | [ | 0.55 | |
| Solubility(Na2CO3·10H2O) | 377.94—453.15 | 4.1994—3.1450 | [ | 0.67 | |
| K2CO3-H2O | Pvap | 313.15—333.15 | 0.1477—3.4050 | [ | 2.15 |
| Cp | 283.15—353.15 | 0.1477—7.2356 | [ | 0.95 | |
| Solubility(K2CO3) | 446.15—499.15 | 17.1628—19.0007 | [ | 0.56 | |
| Solubility(K2CO3·1.5H2O) | 268.15—426.15 | 7.7015—17.1913 | [ | 0.64 | |
| Solubility(K2CO3·6H2O) | 243.15—266.95 | 5.3035—7.5179 | [ | 1.94 |
| 离子对(水) | ||||
|---|---|---|---|---|
| I | J | |||
| (Li+,CO | H2O | 0 | 0 | 0 |
| H2O | (Li+,CO | 0 | 0 | 0 |
| (Na+,CO | H2O | -3.79 | 154.56 | 0.90 |
| H2O | (Na+,CO | 7.41 | -621.23 | 0.31 |
| (K+,CO | H2O | -4.69 | 67.11 | 0.91 |
| H2O | (K+,CO | 9.54 | -667.88 | -1.94 |
| (Li+,CO | (Na+,CO | 0.60 | -620.77 | 0 |
| (Na+,CO | (Li+,CO | -5.32 | 1451.19 | 0 |
| (Li+,CO | (K+,CO | 0.10 | -139.11 | 0 |
| (K+,CO | (Li+,CO | -8.23 | 160.24 | 0 |
| (Na+,CO | (K+,CO | -0.45 | -980.33 | 0 |
| (K+,CO | (Na+,CO | 2.88 | 1611.80 | 0 |
Table 4 Liquid parameters of Li+, Na+, K+, CO32--H2O system
| 离子对(水) | ||||
|---|---|---|---|---|
| I | J | |||
| (Li+,CO | H2O | 0 | 0 | 0 |
| H2O | (Li+,CO | 0 | 0 | 0 |
| (Na+,CO | H2O | -3.79 | 154.56 | 0.90 |
| H2O | (Na+,CO | 7.41 | -621.23 | 0.31 |
| (K+,CO | H2O | -4.69 | 67.11 | 0.91 |
| H2O | (K+,CO | 9.54 | -667.88 | -1.94 |
| (Li+,CO | (Na+,CO | 0.60 | -620.77 | 0 |
| (Na+,CO | (Li+,CO | -5.32 | 1451.19 | 0 |
| (Li+,CO | (K+,CO | 0.10 | -139.11 | 0 |
| (K+,CO | (Li+,CO | -8.23 | 160.24 | 0 |
| (Na+,CO | (K+,CO | -0.45 | -980.33 | 0 |
| (K+,CO | (Na+,CO | 2.88 | 1611.80 | 0 |
| 物种 | c1 | c2 | c3 | 文献 | |||
|---|---|---|---|---|---|---|---|
| CO | -527.46 | -677.48 | -130.58 | -344.85 | 1759.55 | -3491.17 | 本文 |
| Li2CO3 | -1130.56 | -1219.27 | 99.12 | 104.76 | 177.32 | 0.00 | 本文 |
| -1132.06 | -1215.90 | 99.12 | — | — | — | NBS[ | |
| Na2CO3 | -1043.25 | -1130.03 | 112.30 | 12.01 | 244.01 | 24.48 | 本文 |
| -1044.44 | -1130.00 | 112.30 | — | — | — | NBS[ | |
| Na2CO3·H2O | -1285.37 | -1435.20 | 145.60 | 45.31 | 244.01 | 24.48 | 本文 |
| -1285.31 | -1431.26 | 145.60 | — | — | — | NBS[ | |
| Na2CO3·7H2O | -2712.19 | -3178.93 | 374.56 | 274.27 | 244.01 | 24.48 | 本文 |
| -2714.20 | -3199.96 | — | — | — | — | NBS[ | |
| Na2CO3·10H2O | -3425.64 | -4088.86 | 550.29 | 450.00 | 244.01 | 24.48 | 本文 |
| -3427.66 | -4081.32 | 550.32 | — | — | — | NBS[ | |
| K2CO3 | -1064.04 | -1169.21 | 114.25 | 97.91 | 92.05 | -9.87 | 本文 |
| -1063.5 | -1151.02 | 114.43 | — | — | — | NBS[ | |
| K2CO3·1.5H2O | -1434.55 | -1630.27 | 198.76 | 198.76 | 0 | 0 | 本文 |
| -1432.5 | -1609.20 | — | — | — | — | NBS[ | |
| K2CO3·6H2O | -1434.64 | -1630.63 | 107.74 | 28.57 | 302.80 | -9.87 | 本文 |
| Li2CO3·Na2CO3 | -2179.86 | -2371.48 | 269.93 | 116.77 | 421.33 | 24.48 | 本文 |
| Na2CO3·K2CO3 | -2125.32 | 2335.81 | 226.55 | 109.92 | 336.06 | 14.61 | 本文 |
| Na2CO3·K2CO3·H2O | -2363.36 | -2629.56 | 379.77 | 379.77 | 0 | 0 | 本文 |
| Na2CO3·K2CO3·6H2O | -3560.81 | -4160.11 | 476.44 | 476.44 | 0 | 0 | 本文 |
| Na2CO3·K2CO3·12H2O | -5013.40 | -5559.91 | -7404.63 | -7404.63 | 0 | 0 | 本文 |
| NaKCO3·6H2O | -2494.87 | -2972.26 | 207.81 | 207.81 | 0 | 0 | 本文 |
Table 5 Thermodynamic parameters of species in Li+, Na+, K+, CO32--H2O system
| 物种 | c1 | c2 | c3 | 文献 | |||
|---|---|---|---|---|---|---|---|
| CO | -527.46 | -677.48 | -130.58 | -344.85 | 1759.55 | -3491.17 | 本文 |
| Li2CO3 | -1130.56 | -1219.27 | 99.12 | 104.76 | 177.32 | 0.00 | 本文 |
| -1132.06 | -1215.90 | 99.12 | — | — | — | NBS[ | |
| Na2CO3 | -1043.25 | -1130.03 | 112.30 | 12.01 | 244.01 | 24.48 | 本文 |
| -1044.44 | -1130.00 | 112.30 | — | — | — | NBS[ | |
| Na2CO3·H2O | -1285.37 | -1435.20 | 145.60 | 45.31 | 244.01 | 24.48 | 本文 |
| -1285.31 | -1431.26 | 145.60 | — | — | — | NBS[ | |
| Na2CO3·7H2O | -2712.19 | -3178.93 | 374.56 | 274.27 | 244.01 | 24.48 | 本文 |
| -2714.20 | -3199.96 | — | — | — | — | NBS[ | |
| Na2CO3·10H2O | -3425.64 | -4088.86 | 550.29 | 450.00 | 244.01 | 24.48 | 本文 |
| -3427.66 | -4081.32 | 550.32 | — | — | — | NBS[ | |
| K2CO3 | -1064.04 | -1169.21 | 114.25 | 97.91 | 92.05 | -9.87 | 本文 |
| -1063.5 | -1151.02 | 114.43 | — | — | — | NBS[ | |
| K2CO3·1.5H2O | -1434.55 | -1630.27 | 198.76 | 198.76 | 0 | 0 | 本文 |
| -1432.5 | -1609.20 | — | — | — | — | NBS[ | |
| K2CO3·6H2O | -1434.64 | -1630.63 | 107.74 | 28.57 | 302.80 | -9.87 | 本文 |
| Li2CO3·Na2CO3 | -2179.86 | -2371.48 | 269.93 | 116.77 | 421.33 | 24.48 | 本文 |
| Na2CO3·K2CO3 | -2125.32 | 2335.81 | 226.55 | 109.92 | 336.06 | 14.61 | 本文 |
| Na2CO3·K2CO3·H2O | -2363.36 | -2629.56 | 379.77 | 379.77 | 0 | 0 | 本文 |
| Na2CO3·K2CO3·6H2O | -3560.81 | -4160.11 | 476.44 | 476.44 | 0 | 0 | 本文 |
| Na2CO3·K2CO3·12H2O | -5013.40 | -5559.91 | -7404.63 | -7404.63 | 0 | 0 | 本文 |
| NaKCO3·6H2O | -2494.87 | -2972.26 | 207.81 | 207.81 | 0 | 0 | 本文 |
| 体系 | 固相 | T/K | 液相组成 / % | |||
|---|---|---|---|---|---|---|
| LC | NC | KC | ||||
| Na2CO3–H2O | A | 冰+ NC10 | 270.78 | — | 5.81 | — |
| B | NC10 + NC7 | 300.00 | — | 29.83 | — | |
| C | NC7 + NC1 | 308.91 | — | 33.44 | — | |
| D | NC1 + NC | 385.09 | — | 30.99 | — | |
| K2CO3-H2O | E | 冰+ KC6 | 242.95 | — | — | 42.30 |
| F | KC6+ KC1.5 | 266.01 | — | — | 50.96 | |
| G | KC1.5 + KC | 429.75 | — | — | 70.38 | |
| Li2CO3–K2CO3–H2O | H | 冰+ LC + KC1.5 | 223.15 | 0.14 | — | 45.08 |
| Li2CO3–Na2CO3–H2O | I | 冰+ LC + NC10 | 269.83 | 1.28 | 6.46 | — |
| J | LC + NC10 + LNC | 300.72 | 0.97 | 32.77 | — | |
| K | NC10+NC7+LNC | 301.27 | 0.90 | 34.62 | — | |
| L | NC7+NC1+LNC | 308.02 | 0.72 | 39.67 | — | |
| Na2CO3–K2CO3–H2O | M | 冰+ KC6 + NKC6 | 236.15 | — | 0.04 | 40.28 |
| N | 冰+ NC10 + NKC6 | 251.51 | — | 1.70 | 27.27 | |
| O | KC6+NKC6+KC1.5 | 262.15 | — | 0.25 | 49.75 | |
| P | NC10 + NKC6 + NC1 | 295.32 | — | 21.05 | 17.24 | |
| Q | NC10 + NC7 + NC1 | 296.03 | — | 21.74 | 16.11 | |
| R | NKC6 + KC1.5 + NKC | 304.24 | — | 4.77 | 49.18 | |
| S | NKC6 + NC1 + NKC | 310.67 | — | 11.71 | 37.48 | |
Table 6 The predicted invariant points in binary and ternary systems
| 体系 | 固相 | T/K | 液相组成 / % | |||
|---|---|---|---|---|---|---|
| LC | NC | KC | ||||
| Na2CO3–H2O | A | 冰+ NC10 | 270.78 | — | 5.81 | — |
| B | NC10 + NC7 | 300.00 | — | 29.83 | — | |
| C | NC7 + NC1 | 308.91 | — | 33.44 | — | |
| D | NC1 + NC | 385.09 | — | 30.99 | — | |
| K2CO3-H2O | E | 冰+ KC6 | 242.95 | — | — | 42.30 |
| F | KC6+ KC1.5 | 266.01 | — | — | 50.96 | |
| G | KC1.5 + KC | 429.75 | — | — | 70.38 | |
| Li2CO3–K2CO3–H2O | H | 冰+ LC + KC1.5 | 223.15 | 0.14 | — | 45.08 |
| Li2CO3–Na2CO3–H2O | I | 冰+ LC + NC10 | 269.83 | 1.28 | 6.46 | — |
| J | LC + NC10 + LNC | 300.72 | 0.97 | 32.77 | — | |
| K | NC10+NC7+LNC | 301.27 | 0.90 | 34.62 | — | |
| L | NC7+NC1+LNC | 308.02 | 0.72 | 39.67 | — | |
| Na2CO3–K2CO3–H2O | M | 冰+ KC6 + NKC6 | 236.15 | — | 0.04 | 40.28 |
| N | 冰+ NC10 + NKC6 | 251.51 | — | 1.70 | 27.27 | |
| O | KC6+NKC6+KC1.5 | 262.15 | — | 0.25 | 49.75 | |
| P | NC10 + NKC6 + NC1 | 295.32 | — | 21.05 | 17.24 | |
| Q | NC10 + NC7 + NC1 | 296.03 | — | 21.74 | 16.11 | |
| R | NKC6 + KC1.5 + NKC | 304.24 | — | 4.77 | 49.18 | |
| S | NKC6 + NC1 + NKC | 310.67 | — | 11.71 | 37.48 | |
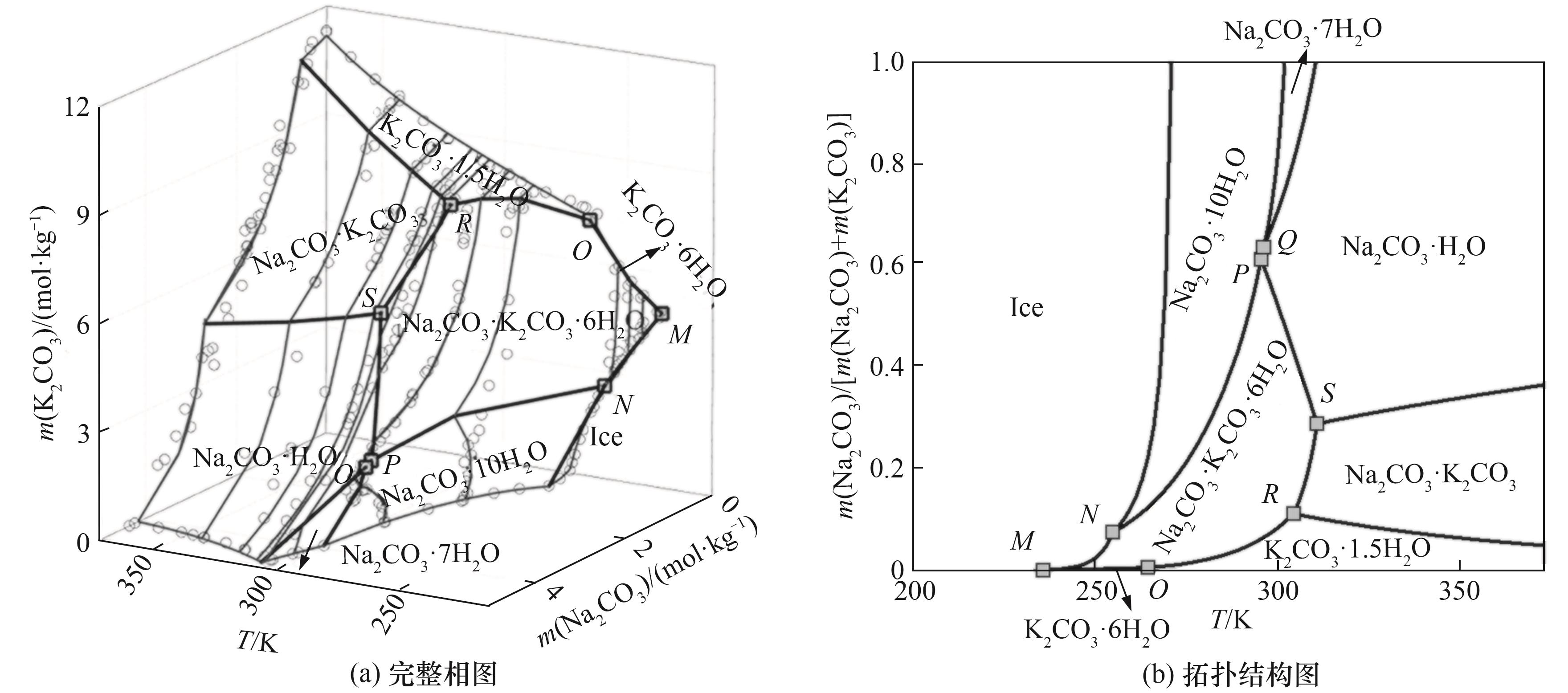
Fig. 13 Calculated isothermal solubility and predicted complete topology phase diagrams of Na 2CO3-K2CO3-H2O system at temperature range from eutectic point to 373.15 K
| [1] | 郑绵平. 论中国盐湖[J]. 矿床地质, 2001, 20(2): 181-189, 128. |
| Zheng M P. On saline lakes of China[J]. Mineral Deposits, 2001, 20(2): 181-189, 128. | |
| [2] | Zhou Y C, Chen Z Z, Gong H J, et al. Study on the zinc porphyrins as potential carbonic anhydrase mimics for promoting CO2 absorption in K2CO3 solution[J]. Chemical Engineering Journal, 2024, 481: 148690. |
| [3] | 高世扬, 宋彭生, 夏树屏, 等. 盐湖化学 : 新类型硼锂盐湖[M]. 北京: 科学出版社, 2007. |
| Gao S Y, Song P S, Xia S P, et al. Salt Llake Chemistry: a New Type of Boron-lithium Salt Lake[M]. Beijing: Science Press, 2007. | |
| [4] | Kindyakov P S. In Solubilities of Inorganic and Organic Compounds[M]. Silcock H. London: Pergamon Press, 1979, 3(1): 674-675. |
| [5] | Itkina L S. In Solubilities of Inorganic and Organic Compounds[M]. Silcock H. London: Pergamon Press, 1979, 3(1): 676. |
| [6] | Urazov G G. In Solubilities of Inorganic and Organic Compounds[M]. Silcock H. London: Pergamon Press, 1979, 3(1): 674. |
| [7] | 殷辉安, 郝丽芳, 曾英, 等. Li⁺, Na⁺//CO 3 2 -, B₄O 7 2 -, Cl⁻-H₂O五元体系298 K相平衡及平衡液相物化性质的研究[J]. 高校化学工程学报, 2003, 16(1): 1-5. |
| Yin H A, Hao L F, Zeng Y, et al. Phase equilibrium and physicochemical properties of aqueous solutions for the quinary system Li⁺, Na⁺//CO 3 2 -, B₄O 7 2 -, Cl⁻-H₂O at 298 K[J]. Journal of Chemical Engineering of China Universities, 2003, 16(1): 1-5. | |
| [8] | 殷辉安, 桑世华, 唐明林, 等. K2CO3-Li2CO3-H2O三元体系288 K相平衡研究[C]//中国物理学会相图专业委员会. 第十二届全国相图学术会议论文集. 成都, 2004: 4. |
| Yin H A, Sang S H, Tang M L, et al. Study on phase equilibrium of K2CO3-Li2CO3-H2O ternary system at 288 K[C]//Chinese Physical Society, Phase Diagram Professional Committee. Proceedings of the 12th National Conference on Phase Diagrams. Chengdu, 2004: 4. | |
| [9] | 殷辉安, 桑世华, 唐明林, 等. 288K下Li+, K+//CO 3 2 -, B4O 7 2 --H₂O四元体系的相平衡[J]. 化工学报, 2004, 55(3): 464-467. |
| Yin H A, Sang S H, Tang M L, et al. Equilibrium of the quaternary system Li+, K+//CO 3 2 -, B4O 7 2 --H₂O at 288 K[J]. Journal of Chemical Industry and Engineering (China), 2004, 55(3): 464-467. | |
| [10] | 曾英, 殷辉安, 唐明林, 等. 三元体系Na2CO3-K2CO3-H2O 298 K 相关系和溶液物化性质的研究[J]. 四川联合大学学报(工程科学版), 1999, 31(6): 135-139. |
| Zeng Y, Yin H A, Tang M L, et al. An experimental study on the equilibrium phase diagram and solution properties of the Na2CO3-K2CO3-H2O ternary system at 298 K[J]. Advanced Engineering Sciences, 1999, 31(6): 135-139. | |
| [11] | 曾英, 殷辉安, 唐明林, 等. 298K时Li+, Na+, K+/CO 3 2 --H2O四元体系相图和液相物化性质测定[J]. 化学工程, 1999, 27(5): 45-47. |
| Zeng Y, Yin H A, Tang M L, et al. Phase diagram of Li+, Na+, K+/CO 3 2 --H2O quaternary system at 298 K and determination of physicochemical properties in liquid phase[J]. Chemical Engineering, 1999, 27(5): 45-47. | |
| [12] | 曾英, 殷辉安, 唐明林. 四元体系Na⁺, K⁺//CO 3 2 -,B₄O 7 2 --H₂O 298 K相平衡研究[J]. 无机化学学报, 2001, 27(5): 665-668. |
| Zeng Y, Yin H A, Tang M L. Phase equilibrium study on the quaternary system Na⁺,K⁺//CO 3 2 -,B₄O 7 2 --H₂O at 298 K[J]. Journal of Inorganic Chemistry, 2001, 27(5): 665-668. | |
| [13] | 曾英, 肖霞, 殷辉安, 等. 交互四元体系Li+, K+//CO 3 2 -, B4O 7 2 --H2O 298K相关系及平衡液相物化性质的研究[J]. 高校化学工程学报, 2002, 16(6): 591-596. |
| Zeng Y, Xiao X, Yin H A, et al. A study on the phase equilibrium and solution properties of the quaternary system Li+, K+// CO 3 2 -, B4O 7 2 --H2O 298K[J]. Journal of Chemical Engineering of Chinese Universities, 2002, 16(6): 591-596. | |
| [14] | 曾英, 殷辉安, 唐明林, 等. 五元交互体系Li+, Na+, K+//CO 3 2 -, Cl--: H2O在298.15K的相平衡研究[J]. 高等学校化学学报, 2003, 24(6): 968-972. |
| Zeng Y, Yin H A, Tang M L, et al. A study of the phase equilibrium for quinary system Li+, Na+, K+// CO 3 2 -, Cl--: H2O at 298.15 K[J]. Chemical Journal of Chinese Universities, 2003, 24(6): 968-972. | |
| [15] | 桑世华, 殷辉安, 唐明林, 等. Li+, Na+∥CO 3 2 -, B4O 7 2 --H2O四元交互体系288K的相平衡[J]. 物理化学学报, 2002, 18(9): 835-837. |
| Sang S H, Yin H A, Tang M L, et al. Equilibrium phase diagram of Li+, Na+∥CO 3 2 -, B4O 7 2 --H2O quaternary system at 288 K[J]. Acta Physico-Chimica Sinica, 2002, 18(9): 835-837. | |
| [16] | 桑世华, 殷辉安, 唐明林. K2CO3-Na2CO3-H2O三元体系288K相平衡研究[J]. 无机盐工业, 2003, 35(1): 23-24, 55. |
| Sang S H, Yin H A, Tang M L. Experimental study on the phase equilibrium of the ternary system K2CO3-Na2CO3-H2O at 288 K[J]. Inorganic Chemicals Industry, 2003, 35(1): 23-24, 55. | |
| [17] | 桑世华, 唐明林, 殷辉安, 等. K2CO3-Na2CO3-Li2CO3-H2O四元体系288K的相平衡[J]. 应用化学, 2004, 21(5): 509-511. |
| Sang S H, Tang M L, Yin H A, et al. Phase equilibrium of the quaternary system K2CO3-Na2CO3-Li2CO3-H2O at 288 K[J]. Chinese Journal of Applied Chemistry, 2004, 21(5): 509-511. | |
| [18] | 李以圭, 陆九芳. 电解质溶液理论[M]. 北京: 清华大学出版社, 2005. |
| Li Y G, Lu J F. Electrolyte Solution Theory[M]. Beijing: Tsinghua University Press, 2005. | |
| [19] | Pitzer K S. Thermodynamics of electrolytes I: Theoretical basis and general equations[J]. The Journal of Physical Chemistry, 1973, 77(2): 268-277. |
| [20] | Clegg S L, Pitzer K S. Thermodynamics of multicomponent, miscible, ionic solutions: generalized equations for symmetrical electrolytes[J]. The Journal of Physical Chemistry, 1992, 96(8): 3513-3520. |
| [21] | Li D D, Zeng D W, Yin X, et al. Phase diagrams and thermochemical modeling of salt lake brine systems( Ⅳ ): Thermodynamic framework and program implementation for multicomponent systems[J]. Calphad, 2020, 71: 101806. |
| [22] | Thomsen K. Modeling electrolyte solutions with the extended universal quasichemical (UNIQUAC) model[J]. Pure and Applied Chemistry, 2005, 77(3): 531-542. |
| [23] | Chen C C, Britt H I, Boston J F, et al. Local composition model for excess Gibbs energy of electrolyte systems(Part Ⅰ ): Single solvent, single completely dissociated electrolyte systems[J]. AIChE Journal, 1982, 28(4): 588-596. |
| [24] | Song Y H, Chen C C. Symmetric electrolyte nonrandom two-liquid activity coefficient model[J]. Industrial & Engineering Chemistry Research, 2009, 48(16): 7788-7797. |
| [25] | Wang P, Springer R D, Anderko A, et al. Modeling phase equilibria and speciation in mixed-solvent electrolyte systems[J]. Fluid Phase Equilibria, 2004, 222: 11-17. |
| [26] | Wang P, Anderko A, Springer R D, et al. Modeling phase equilibria and speciation in mixed-solvent electrolyte systems ( Ⅱ ) : Liquid-liquid equilibria and properties of associating electrolyte solutions[J]. Journal of Molecular Liquids, 2006, 125(1): 37-44. |
| [27] | Tanveer S, Chen C C. Molecular thermodynamic modeling of aqueous Na+-K+-Mg2+-Ca2+-SO 4 2 - quinary system[J]. Fluid Phase Equilibria, 2019, 491: 77-87. |
| [28] | Zhou H, Gu X L, Dai Y, et al. Thermodynamic modeling and phase diagram prediction of salt lake brine systems ( Ⅰ ) : Aqueous Mg2+-Ca2+-Cl- binary and ternary systems[J]. Chinese Journal of Chemical Engineering, 2020, 28(9): 2391-2408. |
| [29] | Zhou H, Wu P, Li W X, et al. Thermodynamic modeling and phase diagram prediction of salt lake brine systems ( Ⅱ ) : Aqueous Li+-Na+-K+-SO 4 2 - and its subsystems[J]. Chinese Journal of Chemical Engineering, 2021, 34: 134-149. |
| [30] | 王星帆, 周桓, 周阔, 等. 硝酸盐型卤水体系的综合热力学模型与多温相图预测 [J]. 高等学校化学学报, 2021, 42(10):12. |
| Wang X F, Zhou H, Zhou K, et al. Comprehensive thermodynamic model and polythermal phase diagram prediction of nitrate-type brine systems [J]. Chemical Journal of Chinese Universities, 2021, 42(10):12. | |
| [31] | 常静宇, 周桓, 杨洁, 等. NaF-Na3PO4-H2O体系相图与热力学模型 [J]. 天津科技大学学报, 2024, 39(1):30-41. |
| Chang J Y, Zhou H, Yang J, et al. Phase diagram and thermodynamic model of the NaF-Na3PO4-H2O system [J]. Journal of Tianjin University of Science & Technology, 2024, 39(1):30-41. | |
| [32] | Kaur H, Chen C C. Thermodynamic modeling of CO2 absorption in aqueous potassium carbonate solution with electrolyte NRTL model[J]. Fluid Phase Equilibria, 2020, 505: 112339. |
| [33] | Chen T Y, Honarparvar S, Reible D, et al. Thermodynamic modeling of calcium carbonate scale precipitation: aqueous Na+-Ca2+-Cl–-HCO 3 - -CO 3 2 --CO2 system[J]. Fluid Phase Equilibria, 2022, 552: 113263. |
| [34] | Miller R R, Smith S H, Williams D D. Solubility of lithium carbonate at elevated temperatures[J]. Journal of Chemical & Engineering Data, 1971, 16(1): 74-75. |
| [35] | Cheng W T, Li Z B, Cheng F Q. Solubility of Li2CO3 in Na-K-Li-Cl brines from 20 to 90℃[J]. The Journal of Chemical Thermodynamics, 2013, 67: 74-82. |
| [36] | Wang J F, Wu X W, Zhang S J. Development of a thermodynamic model for the Li2CO3-NaCl-Na2SO4-H2O system and its application[J]. The Journal of Chemical Thermodynamics, 2018, 123: 62-73. |
| [37] | 邓天龙, 周桓, 陈侠. 水盐体系相图及应用[M]. 2版. 北京: 化学工业出版社, 2020. |
| Deng T L, Zhou H, Chen X. Salt-water System Phase Diagrams and Applications[M]. 2nd ed. Beijing: Chemical Industry Press, 2020. | |
| [38] | Waldeck W F, Lynn G, Hill A E. Aqueous solubility of salts at high temperatures ( Ⅰ ): Solubility of sodium carbonate from 50 to 348℃[J]. Journal of the American Chemical Society, 1932, 54(3): 928-936. |
| [39] | Kobe K A, Sheehy T M. Thermochemistry of sodium carbonate and its solutions[J]. Industrial & Engineering Chemistry, 1948, 40(1): 99-102. |
| [40] | Seidell A. Solubilities of Inorganic and Metal Organic Compounds[M]. New York: Van Nostrand Company, Inc., 1953. |
| [41] | Takahashi G. In Solubilities of Inorganic and Organic Compounds[M]. Silcock H. London: Pergamon Press, 1979, 1(1): 158. |
| [42] | Moore R C, Mesmer R E, Simonson J M. Solubility of potassium carbonate in water between 384 and 529 K measured using the synthetic method[J]. Journal of Chemical & Engineering Data, 1997, 42(6): 1078-1081. |
| [43] | 伍倩, 乜贞, 卜令忠, 等. 盐湖卤水Li+, Na+/CO 3 2 --H2O三元体系298.15K稳定相平衡研究[J]. 化工矿物与加工, 2018, 47(2): 15-18, 45. |
| Wu Q, Nie Z, Bu L Z, et al. Research on stable phase equilibrium in ternary system Li+, Na+/CO 3 2 --H2O at 298.15K[J]. Industrial Minerals & Processing, 2018, 47(2): 15-18, 45. | |
| [44] | Ge H W, Wang M, Zhao Y J, et al. Solid-liquid phase equilibria of the aqueous ternary system (Li2CO3 + Na2CO3 + H2O) at (278.15 to 308.15) K[J]. Journal of Chemical & Engineering Data, 2020, 65(11): 5553-5558. |
| [45] | 郑志远, 曾英, 林晓峰. K2CO3-Na2CO3-H2O三元体系273K相平衡实验研究[J]. 盐业与化工, 2007, 36(1): 7-9. |
| Zheng Z Y, Zeng Y, Lin X F. Experimental study on the phase equilibrium of the ternary system K2CO3-Na2CO3-H2O at 273K[J]. Sea-Lake Salt and Chemical Industry, 2007, 36(1): 7-9. | |
| [46] | Osaka J. In Solubilities of Inorganic and Organic Compounds[M]. Silcock H. London: Pergamon Press, 1979, 3(2): 693-695. |
| [47] | Bain H W. In Solubilities of Inorganic and Organic Compounds[M]. Silcock H. London: Pergamon Press, 1979, 3(2): 697. |
| [48] | Yin C C, Liu M T, Yang J, et al. (Solid+liquid) phase equilibrium for the ternary system (K2CO3-Na2CO3-H2O) at T=(323.15, 343.15, and 363.15) K[J]. The Journal of Chemical Thermodynamics, 2017, 108: 1-6. |
| [49] | Erving J. In Solubilities of Inorganic and Organic Compounds[M]. Silcock H. London: Pergamon Press, 1979, 3(2): 702-703. |
| [50] | 伍倩, 乜贞, 卜令忠, 等. 三元体系Li+,K+/CO 3 2 --H2O 298.15 K稳定相平衡研究[J]. 无机盐工业, 2018, 50(6): 4. |
| Wu Q, Nie Z, Bu L Z, et al. Study on stable phase equilibrium of Li+,K+/CO 3 2 --H2O ternary system at 298.15 K[J]. Inorganic Chemicals Industry, 2018, 50(6): 4. | |
| [51] | Zaytsev I D, Aseyev G G. Properties of Aqueous Solutions of Electrolytes[M]. Boca Raton: CRC Press, 1992. |
| [52] | Magalhães M C F, Königsberger E, May P M, et al. Heat capacities of concentrated aqueous solutions of sodium sulfate, sodium carbonate, and sodium hydroxide at 25 ℃[J]. Journal of Chemical & Engineering Data, 2002, 47(3): 590-598. |
| [53] | Sukhotin A M. Spravochnik po Elektrokhimii (Handbook on Electrochemistry)[M]. Leningrad: Khimiya Press, 1981. |
| [54] | Timmermans J. Physico-Chemical Constants of Binary System in Concentrated Solutions Vols. 3, 4[M]: New York: Interscience Publishers, 1960. |
| [55] | Wagman D D, William H E, Parker B, et al. The NBS Tables of Chemical Thermodynamic Properties: selected Values for Inorganic and C1 and C2 Organic Substances in SI Units[R]. Washington, DC: American Chemical Society; New York: American Institute of Physics for the National Bureau of Standards, 1982. |
| [56] | Aspen Physical Property System: version 7.3[DB]. Burlington, MA:Aspen Technology, Inc., 2011. |
| [57] | Criss C M, Cobble J W. The thermodynamic properties of high temperature aqueous solutions (V): The calculation of ionic heat capacities up to 200°. Entropies and heat capacities above 200°[J]. Journal of the American Chemical Society, 1964, 86(24): 5390-5393. |
| [58] | Barin I, Knacke O, Kubaschewski O. Thermochemical Properties of Inorganic Substances[M]. Berlin, Heidelberg: Springer Berlin Heidelberg, 1977. |
| [1] | Han LIU, Jiaxin CUI, Mengfan YIN, Tao ZHENG, Rui ZHANG, Xianghai MENG, Zhichang LIU, Haiyan LIU, Chunming XU. Crystal structure of xylene·CuAlCl4 and measurement of solid-liquid equilibrium of binary system [J]. CIESC Journal, 2025, 76(5): 2241-2250. |
| [2] | Yewei DING, Wenbo KANG, Yutong SONG, Qinxi FAN, Yuanhui JI. Mechanism and screening of indomethacin self-assembled nanomedical drugs [J]. CIESC Journal, 2024, 75(11): 4141-4151. |
| [3] | Han TANG, Jin CAI, Haihang QIN, Guangjin CHEN, Changyu SUN. Predictive model on gas solubility in water-rich phase coexisted with gas hydrates [J]. CIESC Journal, 2024, 75(11): 4348-4358. |
| [4] | Wenxin MEN, Qingshou PENG, Xia GUI. Phase equilibrium of CO2 hydrate in the presence of four different quaternary ammonium salts [J]. CIESC Journal, 2022, 73(4): 1472-1482. |
| [5] | Changwei PENG, Shihua SANG, Ruizhi CUI, Hongbao REN. Studies on three-dimensional phase diagram of the quinary system NaBr-KBr-MgBr2-CaBr2-H2O at 298.15 K [J]. CIESC Journal, 2022, 73(11): 4850-4858. |
| [6] | WEI Xiaolan, XIE Pei, WANG Weilong, LU Jianfeng, DING Jing. Calculation of phase diagram and thermal stability of molten salt for ternary chloride systems containing calcium [J]. CIESC Journal, 2021, 72(6): 3074-3083. |
| [7] | LI Dongchan, WANG Jiayu, WANG Shiqiang. Phase equilibria and phase diagram of the quaternary system (Li+, Mg2+//Cl-, borate-H2O) at 308.15 K [J]. CIESC Journal, 2021, 72(6): 3170-3178. |
| [8] | LI Dan, SUN Shuaiqi, ZHANG Tao, ZHAO Yihui, MENG Lingzong, GUO Yafei, DENG Tianlong. Pitzer thermodynamic model of the system HCl-NaCl-CaCl2-H3BO3-H2O at 298.15 K and its application [J]. CIESC Journal, 2021, 72(6): 3160-3169. |
| [9] | CHEN Boya, LI Mingyan, ZHU Yuhang, PENG Changjun, LIU Honglai. Calculating adsorption isotherm of gas mixture at solid interface using molecular thermodynamic model of two-dimensional fluid [J]. CIESC Journal, 2021, 72(2): 913-920. |
| [10] | Pan LI, Hui KONG, Zhuodong SONG, Zuoyi ZHANG, Yunfang WANG. Vapor-liquid equilibrium for methanol-formaldehyde-polyoxymethylene dimethyl ethers ternary system [J]. CIESC Journal, 2020, 71(S1): 7-14. |
| [11] | Xiaoxue CAO, Shaochang JI, Wenjie KUANG, Anping LIAO, Ping LAN, Jinyan ZHANG. Crystallization thermodynamics of L-phenylalanine in methanol-water solvent [J]. CIESC Journal, 2019, 70(4): 1255-1262. |
| [12] | Xiaoxue CAO, Shaochang JI, Wenjie KUANG, Anping LIAO, Ping LAN, Jinyan ZHANG. Solubility and ternary phase diagram of azithromycin dihydrate in water-organic solvent [J]. CIESC Journal, 2019, 70(3): 817-829. |
| [13] | FAN Kai, CHEN Changxu, ZHOU Feng, ZHENG Hui, XU Chunjian. Measurement and correlation of vapor-liquid equilibrium for ternary system of n-butanol-chlorobenzene-acetophenone [J]. CIESC Journal, 2018, 69(2): 578-585. |
| [14] | ZONG Jie, MA Qinglan, CHEN Guangjin, SUN Changyu. Simulation of solubility for separating carbon dioxide from gas mixture using ZIF-8/glycol slurry [J]. CIESC Journal, 2018, 69(10): 4276-4283. |
| [15] | LU Xiaohua, JIANG Guancong, ZHU Yudan, FENG Xin, LÜ Linghong. Preliminary study on controlling nanoconfined fluid behavior and modelling molecular thermodynamics: progress in development of high-specific surface area TiO2 [J]. CIESC Journal, 2018, 69(1): 1-8. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||