CIESC Journal ›› 2025, Vol. 76 ›› Issue (12): 6633-6643.DOI: 10.11949/0438-1157.20250410
• Energy and environmental engineering • Previous Articles Next Articles
Feifei HU1,2( ), Cheng WANG3, Baohua WANG4, Hao DU2,5, Jian QI4, Haixu WANG6, Shaona WANG2(
), Cheng WANG3, Baohua WANG4, Hao DU2,5, Jian QI4, Haixu WANG6, Shaona WANG2( )
)
Received:2025-04-17
Revised:2025-06-12
Online:2026-01-23
Published:2025-12-31
Contact:
Shaona WANG
胡飞飞1,2( ), 王程3, 王宝华4, 杜浩2,5, 祁健4, 王海旭6, 王少娜2(
), 王程3, 王宝华4, 杜浩2,5, 祁健4, 王海旭6, 王少娜2( )
)
通讯作者:
王少娜
作者简介:胡飞飞(2000—),女,硕士研究生,18247488929@163.com
基金资助:CLC Number:
Feifei HU, Cheng WANG, Baohua WANG, Hao DU, Jian QI, Haixu WANG, Shaona WANG. Preparation of 3.5-valent vanadium electrolyte via ammonia gas-phase reduction[J]. CIESC Journal, 2025, 76(12): 6633-6643.
胡飞飞, 王程, 王宝华, 杜浩, 祁健, 王海旭, 王少娜. 氨气气相还原法短程制备3.5价钒电解液[J]. 化工学报, 2025, 76(12): 6633-6643.
Add to citation manager EndNote|Ris|BibTeX
| 项目 | 传统工艺 | 本研究新工艺 |
|---|---|---|
| 原料 | 五氧化二钒(71~72 CNY/kg) | 偏钒酸铵(69 CNY/kg) 多钒酸铵(67 CNY/kg) |
| 工序 | 化学还原-电解-复配(V3+∶V4+=1∶1) | 气相还原-溶解 |
能耗结构 | 电解能耗高,单位投资高1.89~3.15 kWh/kg V(电解过程) | 焙烧热能为主,时间集中0.21~0.29 kWh/kg V(焙烧过程) |
| 优点 | 技术成熟 | 工序简化、能耗低 |
| 局限性 | 还原剂/副产物残留、能耗高、流程长 | 氨气高温处理需严格安全措施 |
Table 1 Comparison of 3.5-valent vanadium electrolyte preparation processes
| 项目 | 传统工艺 | 本研究新工艺 |
|---|---|---|
| 原料 | 五氧化二钒(71~72 CNY/kg) | 偏钒酸铵(69 CNY/kg) 多钒酸铵(67 CNY/kg) |
| 工序 | 化学还原-电解-复配(V3+∶V4+=1∶1) | 气相还原-溶解 |
能耗结构 | 电解能耗高,单位投资高1.89~3.15 kWh/kg V(电解过程) | 焙烧热能为主,时间集中0.21~0.29 kWh/kg V(焙烧过程) |
| 优点 | 技术成熟 | 工序简化、能耗低 |
| 局限性 | 还原剂/副产物残留、能耗高、流程长 | 氨气高温处理需严格安全措施 |
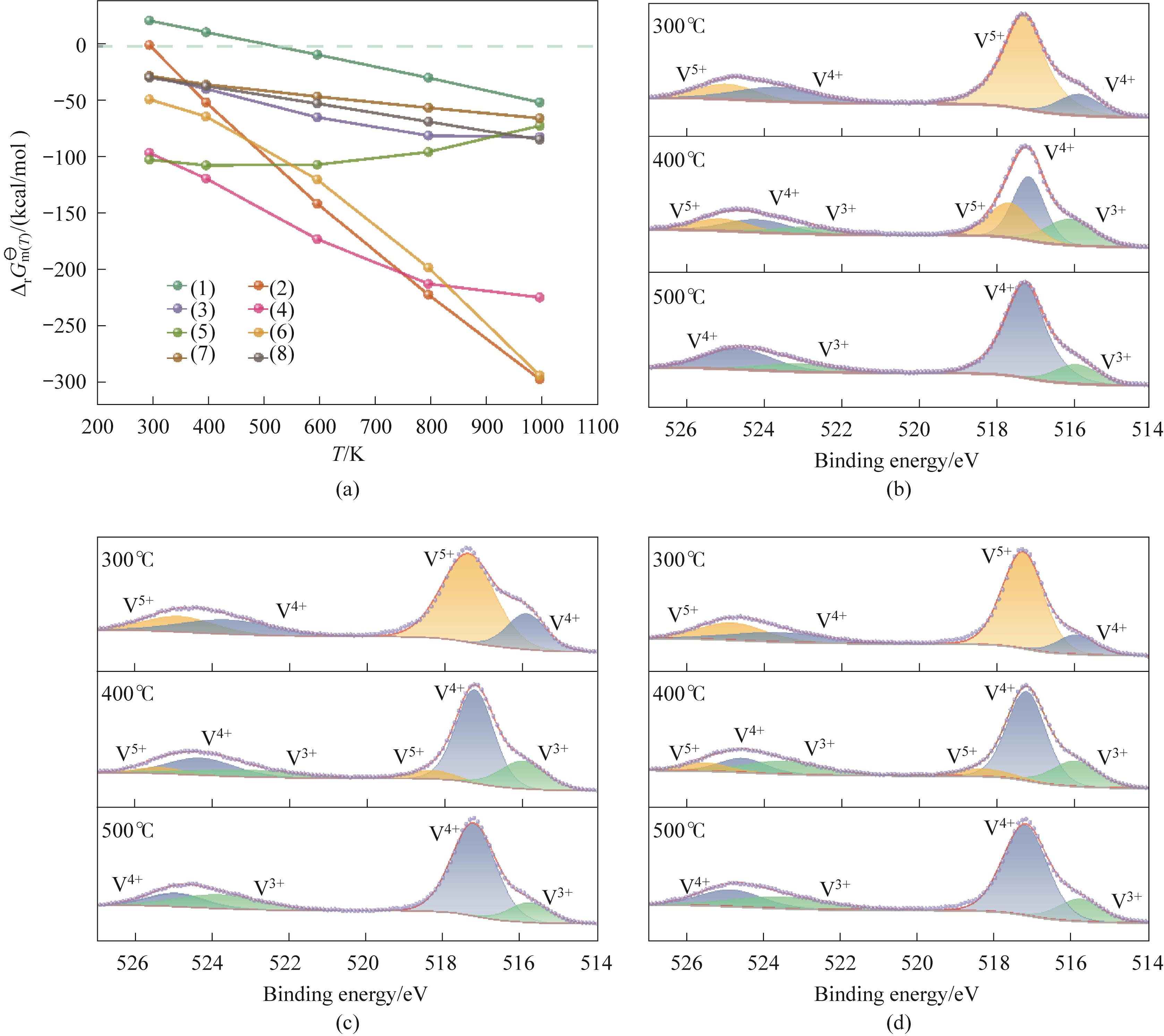
Fig.3 The Gibbs free energy of the reduction of vanadium oxide by ammonia gas(a); XPS spectra of products obtained at 300—500℃: NH3 reduction of NH4VO3 (b); NH3 reduction of (NH4)2V6O16 (c); NH₃ reduction of V2O5 (d)
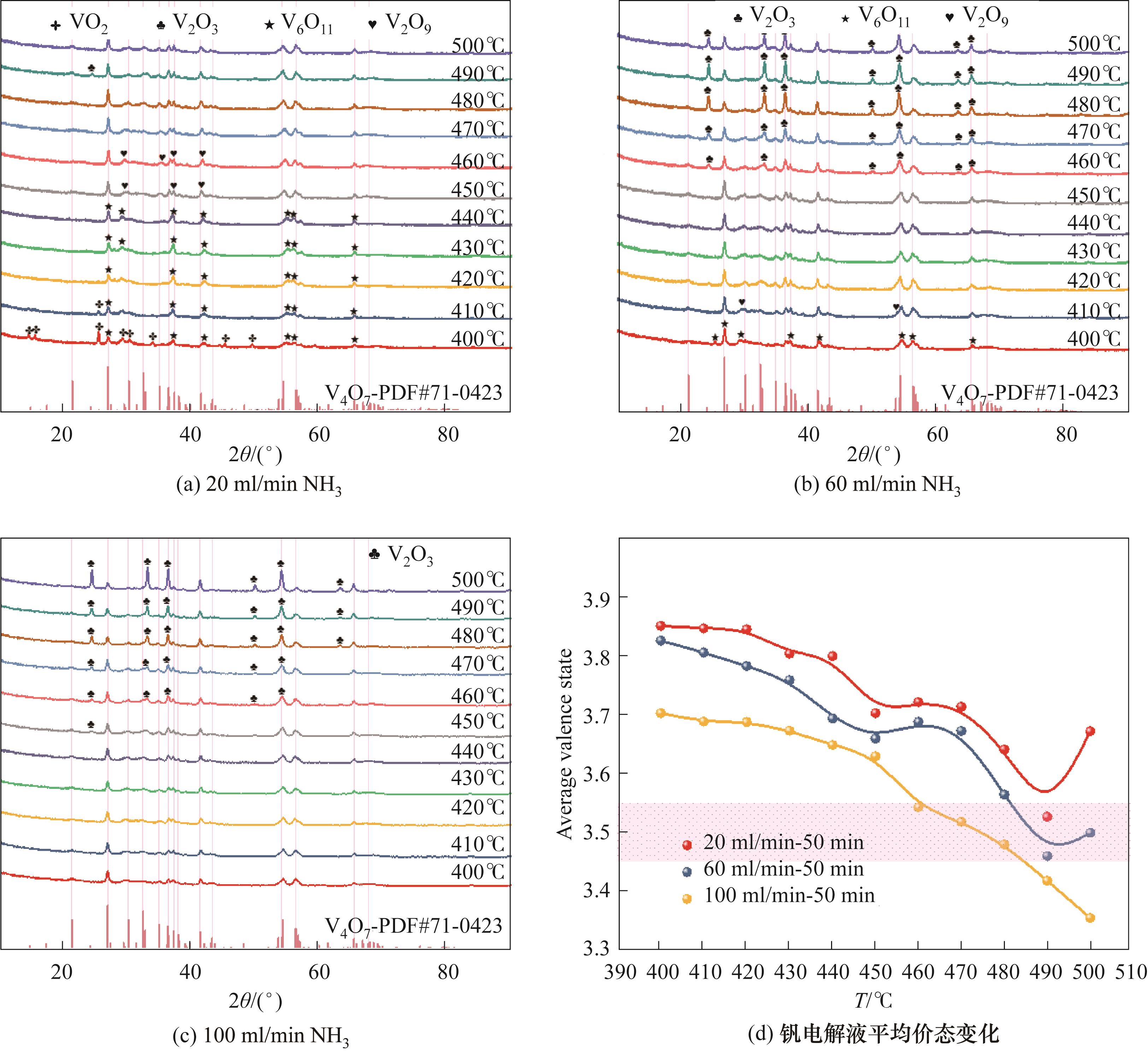
Fig.4 The effects of reaction temperatures and NH3 flow rates on the phase structure of NH4VO3 reduction products and vanadium valence states in the electrolyte
| 原料 | 氧化峰电流(IPO)/mA | 还原峰电流(IPR)/mA | 峰值电流比(IPO/IPR) | 峰值电位差(ΔEP)/V | 电导率/(S/m) |
|---|---|---|---|---|---|
| 偏钒酸铵 | 9.51 | 6.76 | 1.40 | 0.26 | 19.17 |
| 五氧化二钒 | 9.87 | 6.69 | 1.47 | 0.27 | 12.83 |
| 多钒酸铵 | 9.88 | 6.37 | 1.55 | 0.35 | 11.02 |
Table 2 Electrochemical and conductivity analysis of 3.5-valent vanadium electrolytes derived from various vanadium materials
| 原料 | 氧化峰电流(IPO)/mA | 还原峰电流(IPR)/mA | 峰值电流比(IPO/IPR) | 峰值电位差(ΔEP)/V | 电导率/(S/m) |
|---|---|---|---|---|---|
| 偏钒酸铵 | 9.51 | 6.76 | 1.40 | 0.26 | 19.17 |
| 五氧化二钒 | 9.87 | 6.69 | 1.47 | 0.27 | 12.83 |
| 多钒酸铵 | 9.88 | 6.37 | 1.55 | 0.35 | 11.02 |
| [1] | Zhu D, Zhu B Q, et al. Global carbon emissions and decarbonization in 2024[J]. Nature Reviews Earth & Environment, 2025, 6(4): 231-233. |
| [2] | Liu X, Zhang Y, Smith J . et al. Globally interconnected solar-wind system addresses future energy variability[J]. Nature, 2025, 630: 123-130. |
| [3] | Meng Y C, Ye Z, Chen L, et al. Energy storage deployment and benefits in the Chinese electricity market considering renewable energy uncertainty and energy storage life cycle costs[J]. Processes, 2024, 12(1): 130. |
| [4] | Sharmoukh W. Redox flow batteries as energy storage systems: materials, viability, and industrial applications[J]. RSC Advances, 2025, 15(13): 10106-10143. |
| [5] | Huang Z B, Mu A L, Wu L X, et al. Comprehensive analysis of critical issues in all-vanadium redox flow battery[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(24): 7786-7810. |
| [6] | Ashok A, Kumar A. A comprehensive review of metal-based redox flow batteries: progress and perspectives[J]. Green Chemistry Letters and Reviews, 2024, 17(1): 2302834. |
| [7] | Shi Y, Eze C K, Xiong B Y, et al. Recent development of membrane for vanadium redox flow battery applications: a review[J]. Applied Energy, 2019, 238: 202-224. |
| [8] | Jung B Y, Ryu C H, Hwang G J. Characteristics of the all-vanadium redox flow battery using ammonium metavanadate electrolyte[J]. Korean Journal of Chemical Engineering, 2022, 39(9): 2361-2367. |
| [9] | Zheng N B, Qiu X M, Jin S Q, et al. Preparation of V4+ electrolyte by nanofluid-based electrocatalytic reduction of V2O5 for vanadium redox flow batteries[J]. Electrochimica Acta, 2025, 513: 145532. |
| [10] | Du J Y, Lin H T, Zhang L Y, et al. Advanced materials for vanadium redox flow batteries: major obstacles and optimization strategies[J]. Advanced Functional Materials, 2025: 2501689. |
| [11] | 国家市场监督管理总局, 国家标准化管理委员会. 全钒液流电池用电解液: [S]. 北京: 中国标准出版社, 2018. |
| State Administration for Market Regulation, Standardization Administration of the People's Republic of China. Electrolyte for vanadium flow battery: [S]. Beijing: Standards Press of China, 2018. | |
| [12] | Maurice A A, Quintero A E, Vera M. A comprehensive guide for measuring total vanadium concentration and state of charge of vanadium electrolytes using UV-Visible spectroscopy[J]. Electrochimica Acta, 2024, 482: 144003. |
| [13] | 胡超, 董玉明, 张伟, 等. 浓硫酸活化五氧化二钒制备高浓度全钒液流电池正极电解液[J]. 化工学报, 2023, 74(S1): 338-345. |
| Hu C, Dong Y M, Zhang W, et al. Preparation of high concentration positive electrolyte for all-vanadium flow battery by activating vanadium pentoxide with concentrated sulfuric acid[J]. CIESC Journal, 2023, 74(S1): 338-345. | |
| [14] | Guo Y, Yang Y D, Li W J, et al. Novel process to prepare a vanadium electrolyte from a calcification roasting-acid leaching solution of vanadium slag[J]. Industrial & Engineering Chemistry Research, 2023, 62(40): 16411-16418. |
| [15] | Khaki B, Das P. Definition of multi-objective operation optimization of vanadium redox flow and lithium-ion batteries considering levelized cost of energy, fast charging, and energy efficiency based on current density[J]. Journal of Energy Storage, 2023, 64: 107246. |
| [16] | Hu C, Dong Y M, Zhang W, et al. Clean preparation of mixed trivalent and quadrivalent vanadium electrolyte for vanadium redox flow batteries by catalytic reduction with hydrogen[J]. Journal of Power Sources, 2023, 555: 232330. |
| [17] | Skyllas-Kazacos M, Kazacos G, Poon G, et al. Recent advances with UNSW vanadium-based redox flow batteries[J]. International Journal of Energy Research, 2010, 34(2): 182-189. |
| [18] | 郭辉. 全钒液流电池用电解液的制备与性能研究[D]. 北京: 北京化工大学, 2022. |
| Guo H. Preparation and properties of electrolyte for vanadium flow battery[D]. Beijing: Beijing University of Chemical Technology, 2022. | |
| [19] | Guo Y, Huang J, Feng J K. Research progress in preparation of electrolyte for all-vanadium redox flow battery[J]. Journal of Industrial and Engineering Chemistry, 2023, 118: 33-43. |
| [20] | 叶涛, 王怡君, 唐子龙, 等. 全钒液流电池电解液容量衰减及草酸恢复研究[J]. 储能科学与技术, 2025, 14(3): 1177-1186. |
| Ye T, Wang Y J, Tang Z L, et al. Investigation of capacity fading in vanadium flow battery electrolytes and recovery via oxalic acid[J]. Energy Storage Science and Technology, 2025, 14(3): 1177-1186. | |
| [21] | Wang Y H, Chen P, He H. Review: preparation and modification of all-vanadium redox flow battery electrolyte for green development[J]. Ionics, 2025, 31(1): 23-40. |
| [22] | 河钟郁, 黄德炫. 钒电解液的制备方法以及包含钒电解液的电池: 117397075A[P]. 2024-01-12. |
| He Z Y, Huang D X. Preparation method of vanadium electrolyte and battery containing vanadium electrolyte: 117397075A[P]. 2024-01-12. | |
| [23] | 胡超. 全钒液流电池电解液的制备及其性能研究[D]. 秦皇岛: 燕山大学, 2023. |
| Hu C. Preparation and properties of electrolyte for vanadium flow battery[D]. Qinhuangdao: Yanshan University, 2023. | |
| [24] | 王程. 氨气气相还原短程制备钒电解液应用基础研究[D]. 北京: 中国科学院大学, 2024. |
| Wang C. Basic research on the application of ammonia gas phase reduction in the short-range preparation of vanadium electrolytes[D]. Beijing: Uniuersity of Chinese Academy of Sciences, 2024. | |
| [25] | 谢浩, 尹兴荣, 吴雄伟, 等. 用于全钒液流电池的钒电解液的制备方法及钒电解液和应用: 120089772A[P]. 2025-06-03. |
| Xie H, Ying X R, Wu X W, et al. Preparation method of vanadium electrolyte for all-vanadium redox flow batteries and application: 120089772A[P].2025-06-03. | |
| [26] | Wang C, Li L J, Du H. Cleaner production of 3.5 valent vanadium electrolyte from ammonium metavanadate by ammonia reduction-sulfuric acid dissolution method[J]. Tungsten, 2024, 6(3): 555-560. |
| [27] | 魏冬, 林友斌, 杨霖霖. 一种以多钒酸铵为原料制备全钒电解液的制备方法: 119674156A[P]. 2025-03-21. |
| Wei D, Lin Y B, Yang L L. Method for preparing all-vanadium electrolyte from ammonium polyvanadate: 119674156A[P]. 2025-03-21. | |
| [28] | 杨晓武, 李伟达, 袁爱武. 钒电解液的工业化制备技术分析[J]. 稀有金属与硬质合金, 2025, 53(3): 127-134. |
| Yang X W, Li W D, Yuan A W. Analysis of industrial preparation technology for vanadium electrolyte[J]. Rare Metals and Cemented Carbides, 2025, 53(3): 127-134. | |
| [29] | Cao L Y, Skyllas-Kazacos M, Menictas C, et al. A review of electrolyte additives and impurities in vanadium redox flow batteries[J]. Journal of Energy Chemistry, 2018, 27(5): 1269-1291. |
| [30] | Kang U I. Preparation of vanadium (3.5+) electrolyte by hydrothermal reduction process using citric acid for vanadium redox flow battery[J]. Electrochem, 2024, 5(4): 470-481. |
| [1] | Guoxiang HU, Yikui ZHU, Hua LONG, Xiaowen LIU, Qingang XIONG. Study on the underlying mechanism of choline chloride-lactic acid molar ratio influencing alkali lignin solubility in choline chloride-lactic acid deep eutectic solvents [J]. CIESC Journal, 2025, 76(9): 4449-4461. |
| [2] | Huiqin ZHANG, Hongjun ZHAO, Zhengjun FU, Li ZHUANG, Kai DONG, Tianzhi JIA, Xueli CAO, Shipeng SUN. Application of nanofiltration membrane in concentration of ionic rare earth leach solution [J]. CIESC Journal, 2025, 76(8): 4095-4107. |
| [3] | Xin LIU, Haoren ZHENG, Qiang CHEN, Jingyi DING, Kang HUANG, Zhi XU. Cellulose nanocrystals-doped hybrid matrix membranes for vanadium flow battery [J]. CIESC Journal, 2025, 76(5): 2294-2303. |
| [4] | Junying WANG, Hui JIN. Molecular dynamics investigation on the solubility parameters of supercritical CO2 and petroleum hydrocarbon [J]. CIESC Journal, 2025, 76(11): 5788-5798. |
| [5] | Zhicheng TANG, Tianwei WANG, Rongwen LYU, Shufen ZHANG. Amination of bromaminic acid catalyzed by Mn-containing basic copper carbonate electrocatalyst based on electrochemical reduction [J]. CIESC Journal, 2025, 76(11): 5923-5932. |
| [6] | Yansong HU, Zhao YANG, Lei GAO, Bujian ZHANG. Experimental study of solubility and viscosity of R513A and PVE lubricants [J]. CIESC Journal, 2025, 76(10): 5015-5023. |
| [7] | Lingyu LI, Xin HU, Huaigang CHENG, Yun ZHAO, Dong AN, Yujun MA, Jiahao JIN, Xudong YU, Weidong ZHANG. Isothermal evaporation salt-forming regions of the ternary water-salt systems K+(Mg2+), Ca2+//Cl--H2O [J]. CIESC Journal, 2025, 76(1): 120-130. |
| [8] | Xinyue WANG, Xiaohu XU, Haiyang ZHANG, Chunhua YIN. Study on encapsulation and properties vitamin A acetate/cyclodextrin [J]. CIESC Journal, 2024, 75(S1): 321-328. |
| [9] | Zhixing ZHAO, Zhihao YAO, Xuefeng YU, Yousheng YANG, Ying ZENG, Xudong YU. Multi-temperature phase diagram of lithium-sodium-magnesium coexistence sulfate system and its application [J]. CIESC Journal, 2024, 75(6): 2123-2133. |
| [10] | Youming SI, Lingfeng ZHENG, Pengzhong CHEN, Jiangli FAN, Xiaojun PENG. Performance and mechanism of novel antimony oxo cluster photoresist [J]. CIESC Journal, 2024, 75(4): 1705-1717. |
| [11] | Yuxiang CHEN, Chuanlei LIU, Zijun GONG, Qiyue ZHAO, Guanchu GUO, Hao JIANG, Hui SUN, Benxian SHEN. Machine learning-assisted solvent molecule design for efficient absorption of ethanethiol [J]. CIESC Journal, 2024, 75(3): 914-923. |
| [12] | Han TANG, Jin CAI, Haihang QIN, Guangjin CHEN, Changyu SUN. Predictive model on gas solubility in water-rich phase coexisted with gas hydrates [J]. CIESC Journal, 2024, 75(11): 4348-4358. |
| [13] | Chao HU, Yuming DONG, Wei ZHANG, Hongling ZHANG, Peng ZHOU, Hongbin XU. Preparation of high-concentration positive electrolyte of vanadium redox flow battery by activating vanadium pentoxide with highly concentrated sulfuric acid [J]. CIESC Journal, 2023, 74(S1): 338-345. |
| [14] | Xudong YU, Qi LI, Niancu CHEN, Li DU, Siying REN, Ying ZENG. Phase equilibria and calculation of aqueous ternary system KCl + CaCl2 + H2O at 298.2, 323.2, and 348.2 K [J]. CIESC Journal, 2023, 74(8): 3256-3265. |
| [15] | Ke CHEN, Li DU, Ying ZENG, Siying REN, Xudong YU. Phase equilibria and calculation of quaternary system LiCl+MgCl2+CaCl2+H2O at 323.2 K [J]. CIESC Journal, 2023, 74(5): 1896-1903. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||