化工学报 ›› 2023, Vol. 74 ›› Issue (5): 2179-2185.DOI: 10.11949/0438-1157.20230166
收稿日期:2023-02-27
修回日期:2023-04-10
出版日期:2023-05-05
发布日期:2023-06-29
通讯作者:
孙志高
作者简介:徐文超(1998—),男,硕士研究生,2279067493@qq.com
基金资助:
Wenchao XU( ), Zhigao SUN(
), Zhigao SUN( ), Cuimin LI, Juan LI, Haifeng HUANG
), Cuimin LI, Juan LI, Haifeng HUANG
Received:2023-02-27
Revised:2023-04-10
Online:2023-05-05
Published:2023-06-29
Contact:
Zhigao SUN
摘要:
制冷剂水合物是一种理想的蓄冷工质,一氟二氯乙烷(HCFC-141b)水合物的蓄冷密度可达344 kJ/kg。静态系统中水合物难以成核,晶体生长速度慢,存在随机性等问题。为促进水合物形成,实验研究了环保型非离子表面活性剂异构十三醇聚氧乙烯醚(E-1310)的添加对HCFC-141b水合物生成的影响。研究结果表明,E-1310的添加可有效促进水合物形成,其促进效果与添加量有关。1.0%(质量)的E-1310是最佳添加量,此时的水合物形成诱导时间最短,水合物成核最稳定,水合物生长速率和蓄冷量均达到最大值,降低或提高E-1310的添加量都不能进一步促进水合物生成。E-1310非离子表面活性剂对水合物生成的促进机理主要是胶束效应。
中图分类号:
徐文超, 孙志高, 李翠敏, 李娟, 黄海峰. 静态条件下表面活性剂E-1310对HCFC-141b水合物生成的影响[J]. 化工学报, 2023, 74(5): 2179-2185.
Wenchao XU, Zhigao SUN, Cuimin LI, Juan LI, Haifeng HUANG. Effect of surfactant E-1310 on the formation of HCFC-141b hydrate under static conditions[J]. CIESC Journal, 2023, 74(5): 2179-2185.
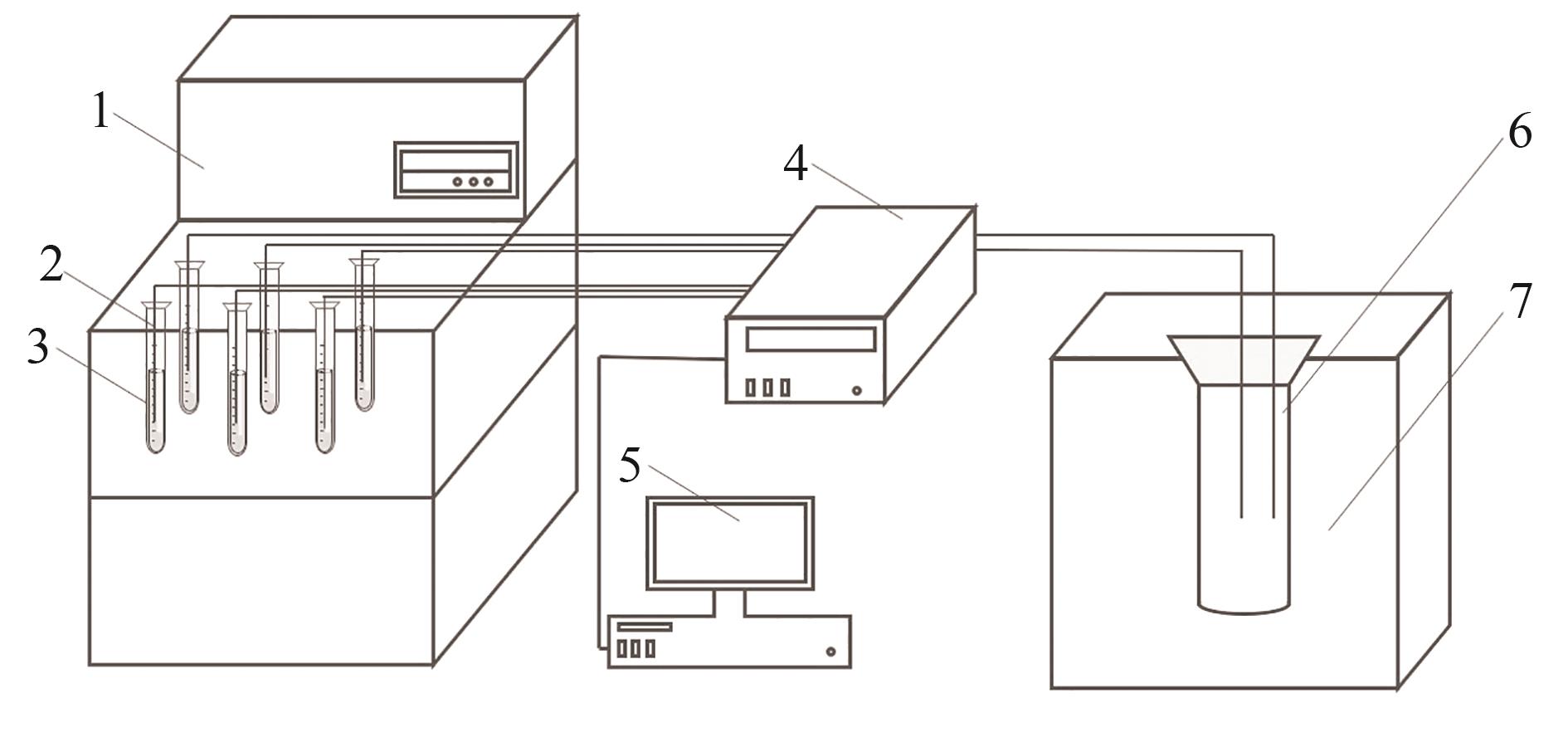
图1 实验装置1—控温装置;2—Pt100;3—试管;4—数据采集器;5—计算机;6—保温杯;7—保温装置
Fig.1 Experimental rigs1—temperature control device; 2—Pt100; 3—test tube; 4—data collector; 5—computer; 6—thermos cup; 7—insulation device
| E-1310添加量/% | 诱导时间/min | 平均诱导时间/min | 诱导时间标准差 | 形成持续时间/min | 平均持续时间/min |
|---|---|---|---|---|---|
| 0 | >1440 | — | — | — | — |
| 0.5 | 762,866,353,846,546 | 675 | 197 | 79,110,63,180,70 | 100 |
| 1.0 | 221,83,191,192,169 | 171 | 47 | 41,45,44,49,40 | 44 |
| 2.0 | 670,519,200,356,373 | 424 | 159 | 31,37,42,33,38 | 36 |
| 3.0 | 490,802,342,533,512 | 536 | 149 | 43,42,42,38,44 | 42 |
| 4.0 | 881,662,439,473,493 | 590 | 165 | 41,45,44,45,46 | 44 |
表1 水合物生成时间
Table 1 Time of hydrate formation
| E-1310添加量/% | 诱导时间/min | 平均诱导时间/min | 诱导时间标准差 | 形成持续时间/min | 平均持续时间/min |
|---|---|---|---|---|---|
| 0 | >1440 | — | — | — | — |
| 0.5 | 762,866,353,846,546 | 675 | 197 | 79,110,63,180,70 | 100 |
| 1.0 | 221,83,191,192,169 | 171 | 47 | 41,45,44,49,40 | 44 |
| 2.0 | 670,519,200,356,373 | 424 | 159 | 31,37,42,33,38 | 36 |
| 3.0 | 490,802,342,533,512 | 536 | 149 | 43,42,42,38,44 | 42 |
| 4.0 | 881,662,439,473,493 | 590 | 165 | 41,45,44,45,46 | 44 |
| No. | 冰质量/g | 温水 质量/g | 冰融化 前后温度/℃ | 温水放热 前后温度/℃ | 冰融化热/(kJ/kg) | 融化热 相对误差ε/% |
|---|---|---|---|---|---|---|
| 1 | 20.2 | 255.1 | -5.1/24.5 | 33.8/24.7 | 329.1 | -1.36 |
| 2 | 17.9 | 246.1 | -4.8/25.9 | 34.4/25.9 | 325.3 | -2.53 |
| 3 | 18.8 | 252.8 | -5.1/25.1 | 33.9/25.2 | 331.5 | -0.67 |
表2 冰的融化热测量计算参数与结果
Table 2 Calculation parameters and results of ice melting heat measurement
| No. | 冰质量/g | 温水 质量/g | 冰融化 前后温度/℃ | 温水放热 前后温度/℃ | 冰融化热/(kJ/kg) | 融化热 相对误差ε/% |
|---|---|---|---|---|---|---|
| 1 | 20.2 | 255.1 | -5.1/24.5 | 33.8/24.7 | 329.1 | -1.36 |
| 2 | 17.9 | 246.1 | -4.8/25.9 | 34.4/25.9 | 325.3 | -2.53 |
| 3 | 18.8 | 252.8 | -5.1/25.1 | 33.9/25.2 | 331.5 | -0.67 |
| E-1310 浓度/% | 蓄冷量/(kJ/kg) | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 平均值 | |
| 0.5 | 73.2 | 65.3 | 68.7 | 72.0 | 56.3 | 67.1 |
| 1.0 | 194.0 | 192.9 | 189.4 | 187.3 | 187.4 | 190.2 |
| 2.0 | 150.0 | 151.4 | 151.4 | 153.7 | 151.5 | 151.6 |
| 3.0 | 161.0 | 165.8 | 162.9 | 163.8 | 162.8 | 163.3 |
| 4.0 | 149.9 | 151.0 | 144.9 | 152.5 | 147.0 | 149.1 |
表3 水合物蓄冷量测量结果
Table 3 Results of hydrate cold storage capacity
| E-1310 浓度/% | 蓄冷量/(kJ/kg) | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 平均值 | |
| 0.5 | 73.2 | 65.3 | 68.7 | 72.0 | 56.3 | 67.1 |
| 1.0 | 194.0 | 192.9 | 189.4 | 187.3 | 187.4 | 190.2 |
| 2.0 | 150.0 | 151.4 | 151.4 | 153.7 | 151.5 | 151.6 |
| 3.0 | 161.0 | 165.8 | 162.9 | 163.8 | 162.8 | 163.3 |
| 4.0 | 149.9 | 151.0 | 144.9 | 152.5 | 147.0 | 149.1 |
| 1 | Koh C A, Sum A K, Sloan E D. State of the art: natural gas hydrates as a natural resource[J]. Journal of Natural Gas Science and Engineering, 2012, 8: 132-138. |
| 2 | Li G, Liu D P, Xie Y M, et al. Study on effect factors for CO2 hydrate rapid formation in a water-spraying apparatus[J]. Energy & Fuels, 2010, 24(8): 4590-4597. |
| 3 | Cheng C X, Wang F, Tian Y J, et al. Review and prospects of hydrate cold storage technology[J]. Renewable and Sustainable Energy Reviews, 2020, 117: 109492. |
| 4 | Linga P, Kumar R, Lee J D, et al. A new apparatus to enhance the rate of gas hydrate formation: application to capture of carbon dioxide[J]. International Journal of Greenhouse Gas Control, 2010, 4(4): 630-637. |
| 5 | 徐婷婷. 二氧化硅表面或电场存在下的气体水合物生成分解模拟研究[D]. 广州: 华南理工大学, 2019. |
| Xu T T. The effect of silica surface or electric field on gas hydrate formation and dissociation: MD simulation[D]. Guangzhou: South China University of Technology, 2019. | |
| 6 | 刘卫国, 陈兵兵, 杨明军, 等. 弱电场下THF水合物生成特性[J]. 工程热物理学报, 2019, 40(12): 2763-2768. |
| Liu W G, Chen B B, Yang M J, et al. The influence of weak electric field on the THF hydrate formation characteristics[J]. Journal of Engineering Thermophysics, 2019, 40(12): 2763-2768. | |
| 7 | Firoozabadi S R, Bonyadi M, Lashanizadegan A. Experimental investigation of Fe3O4 nanoparticles effect on the carbon dioxide hydrate formation in the presence of magnetic field[J]. Journal of Natural Gas Science and Engineering, 2018, 59: 374-386. |
| 8 | 谢育博, 杨亮, 刘道平, 等. 表面活性剂促进气体水合物生成的研究[J]. 制冷学报, 2016, 37(3): 35-41. |
| Xie Y B, Yang L, Liu D P, et al. Research in surfactant effect on promoting gas hydrates formation[J]. Journal of Refrigeration, 2016, 37(3): 35-41. | |
| 9 | Chaturvedi E, Prasad N, Mandal A. Enhanced formation of methane hydrate using a novel synthesized anionic surfactant for application in storage and transportation of natural gas[J]. Journal of Natural Gas Science and Engineering, 2018, 56: 246-257. |
| 10 | Daniel-David D, Guerton F, Dicharry C, et al. Hydrate growth at the interface between water and pure or mixed CO2/CH4 gases: influence of pressure, temperature, gas composition and water-soluble surfactants[J]. Chemical Engineering Science, 2015, 132: 118-127. |
| 11 | Kwon Y A, Park J M, Jeong K E, et al. Synthesis of anionic multichain type surfactant and its effect on methane gas hydrate formation[J]. Journal of Industrial and Engineering Chemistry, 2011, 17(1): 120-124. |
| 12 | Jeenmuang K, Viriyakul C, Inkong K, et al. Enhanced hydrate formation by natural-like hydrophobic side chain amino acids at ambient temperature: a kinetics and morphology investigation[J]. Fuel, 2021, 299: 120828. |
| 13 | 李荣, 孙志高, 宋佳. 氨基酸侧链对HCFC-141b水合物形成的影响[J]. 储能科学与技术, 2022, 11(7): 2126-2132. |
| Li R, Sun Z G, Song J. Effect of amino acid side chains on HCFC-141b hydrate formation[J]. Energy Storage Science and Technology, 2022, 11(7): 2126-2132. | |
| 14 | Sun Z G, Dai M L, Zhu M G, et al. Improving THF hydrate formation in the presence of nonanoic acid[J]. Journal of Molecular Liquids, 2020, 299: 112118. |
| 15 | Song X F, Xin F, Yan H C, et al. Intensification and kinetics of methane hydrate formation under heat removal by phase change of n-tetradecane[J]. AIChE Journal, 2015, 61(10): 3441-3450. |
| 16 | 陈彬, 辛峰, 宋小飞, 等. 相变浆液中甲烷水合物的生成过程强化[J]. 化工学报, 2016, 67(8): 3202-3208. |
| Chen B, Xin F, Song X F, et al. Enhancement of methane hydrate formation process in phase change slurry[J]. CIESC Journal, 2016, 67(8): 3202-3208. | |
| 17 | Rahmati-Abkenar M, Manteghian M, Pahlavanzadeh H. Nucleation of ethane hydrate in water containing silver nanoparticles[J]. Materials & Design, 2017, 126: 190-196. |
| 18 | Park S S, An E J, Lee S B, et al. Characteristics of methane hydrate formation in carbon nanofluids[J]. Journal of Industrial and Engineering Chemistry, 2012, 18(1): 443-448. |
| 19 | Song Y M, Wang F, Liu G Q, et al. Promotion effect of carbon nanotubes-doped SDS on methane hydrate formation[J]. Energy & Fuels, 2017, 31(2): 1850-1857. |
| 20 | 刘妮, 张亚楠, 柳秀婷, 等. 纳米流体中CO2水合物生成特性实验研究[J]. 制冷学报, 2015, 36(2): 41-45, 58. |
| Liu N, Zhang Y N, Liu X T, et al. Experimental study on characteristics of CO2 hydrate formation in nanofluids[J]. Journal of Refrigeration, 2015, 36(2): 41-45, 58. | |
| 21 | 张雪艳, 周诗岽, 姬浩洋, 等. 氧化石墨烯/纳米石墨颗粒与SDS复配对CO2水合物生成特性的影响[J]. 天然气化工(C1化学与化工), 2021, 46(2): 53-58. |
| Zhang X Y, Zhou S D, Ji H Y, et al. Effect of GO/GN and SDS compound system on formation characteristics of CO2 hydrate[J]. Natural Gas Chemical Industry (C1 Chemistry and Chemical Engineering), 2021, 46(2): 53-58. | |
| 22 | Ando N, Kuwabara Y, Mori Y H. Surfactant effects on hydrate formation in an unstirred gas/liquid system: an experimental study using methane and micelle-forming surfactants[J]. Chemical Engineering Science, 2012, 73: 79-85. |
| 23 | 李文昭, 潘振, 马贵阳, 等. 表面活性剂吸附对促进甲烷水合物生成效果的影响[J]. 化工学报, 2017, 68(4): 1542-1549. |
| Li W Z, Pan Z, Ma G Y, et al. Promotion effects of surfactant adsorption on formation of methane hydrates[J]. CIESC Journal, 2017, 68(4): 1542-1549. | |
| 24 | 周锡堂, 樊栓狮, 梁德青, 等. HCFC-141b乳化液生成气体水合物[J]. 化工学报, 2007, 58(3): 728-732. |
| Zhou X T, Fan S S, Liang D Q, et al. HCFC-141b hydrate formation from its emulsion[J]. Journal of Chemical Industry and Engineering (China), 2007, 58(3): 728-732. | |
| 25 | 周麟晨, 孙志高, 陆玲, 等. 静态条件下表面活性剂促进HCFC-141b水合物生成[J]. 高校化学工程学报, 2020, 34(2): 402-410. |
| Zhou L C, Sun Z G, Lu L, et al. Enhancement of HCFC-141b hydrate formation with surfactants in a static system[J]. Journal of Chemical Engineering of Chinese Universities, 2020, 34(2): 402-410. | |
| 26 | 赵郁梅, 秦勇, 张高勇. 表面活性剂生物降解度的测定[J]. 日用化学工业, 2002, 32(6): 60-62. |
| Zhao Y M, Qin Y, Zhang G Y. Determination of surfactant biodegradation[J]. China Surfactant Detergent & Cosmetics, 2002, 32(6): 60-62. | |
| 27 | 李映雪, 孙永强, 周婧洁, 等. 异构与直链醇聚氧乙烯醚的合成与性能研究[J]. 应用化工, 2022, 51(8): 2271-2274. |
| Li Y X, Sun Y Q, Zhou J J, et al. Synthesis and properties of isomeric and straight-chain alcohols polyoxyethylene ethers[J]. Applied Chemical Industry, 2022, 51(8): 2271-2274. | |
| 28 | 苏连建, 王慧. 异构醇型特种表面活性剂的合成及应用[J]. 日用化学品科学, 2011, 34(8): 23-26. |
| Su L J, Wang H. Synthesis and application of special surfactants of isomerized alcohol-type[J]. Detrgent & Cosmetics, 2011, 34(8): 23-26. | |
| 29 | 张龙明, 李璞, 李娜, 等. 混合量热法测定水合物浆体蓄冷密度[J]. 制冷学报, 2014, 35(6): 47-52. |
| Zhang L M, Li P, Li N, et al. Determination of hydrate slurry’s cool-storage density with mixing calorimetry method[J]. Journal of Refrigeration, 2014, 35(6): 47-52. | |
| 30 | 陈光进, 孙长宇, 马庆兰. 气体水合物科学与技术[M]. 北京: 化学工业出版社, 2008: 121. |
| Chen G J, Sun C Y, Ma Q L. Gas Hydrate Science and Technology[M]. Beijing: Chemical Industry Press, 2008: 121. | |
| 31 | Kashchiev D. Nucleation: Basic Theory with Applications[M]. Oxford: Butterworth Heinemann, 2000. |
| 32 | 闫乐乐, 梁生康, 宋丹丹, 等. 鼠李糖脂生物表面活性剂胶束性质研究[J]. 中国海洋大学学报(自然科学版), 2016, 46(12): 68-72. |
| Yan L L, Liang S K, Song D D, et al. Studies on some micelle properties of rhamnolipid biosurfactant[J]. Periodical of Ocean University of China (Natural Science Edition), 2016, 46(12): 68-72. | |
| 33 | 陈丰, 李雄耀, 唐红, 等. 水(冰): 宇宙矿物[J]. 矿物学报, 2015, 35(2): 255-266. |
| Chen F, Li X Y, Tang H, et al. Water ice: cosmic mineral[J]. Acta Mineralogica Sinica, 2015, 35(2): 255-266. | |
| 34 | Zhong Y, Rogers R E. Surfactant effects on gas hydrate formation[J]. Chemical Engineering Science, 2000, 55(19): 4175-4187. |
| 35 | Nguyen N N, Nguyen A V. Hydrophobic effect on gas hydrate formation in the presence of additives[J]. Energy & Fuels, 2017, 31(10): 10311-10323. |
| 36 | He Y, Sun M T, Chen C, et al. Surfactant-based promotion to gas hydrate formation for energy storage[J]. Journal of Materials Chemistry A, 2019, 7(38): 21634-21661. |
| [1] | 邵苛苛, 宋孟杰, 江正勇, 张旋, 张龙, 高润淼, 甄泽康. 水平方向上冰中受陷气泡形成和分布实验研究[J]. 化工学报, 2023, 74(S1): 161-164. |
| [2] | 江河, 袁俊飞, 王林, 邢谷雨. 均流腔结构对微细通道内相变流动特性影响的实验研究[J]. 化工学报, 2023, 74(S1): 235-244. |
| [3] | 孟令玎, 崇汝青, 孙菲雪, 孟子晖, 刘文芳. 改性聚乙烯膜和氧化硅固定化碳酸酐酶[J]. 化工学报, 2023, 74(8): 3472-3484. |
| [4] | 于旭东, 李琪, 陈念粗, 杜理, 任思颖, 曾英. 三元体系KCl + CaCl2 + H2O 298.2、323.2及348.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(8): 3256-3265. |
| [5] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [6] | 郑玉圆, 葛志伟, 韩翔宇, 王亮, 陈海生. 中高温钙基材料热化学储热的研究进展与展望[J]. 化工学报, 2023, 74(8): 3171-3192. |
| [7] | 张澳, 罗英武. 低模量、高弹性、高剥离强度丙烯酸酯压敏胶[J]. 化工学报, 2023, 74(7): 3079-3092. |
| [8] | 汤晓玲, 王嘉瑞, 朱玄烨, 郑仁朝. 基于Pickering乳液的卤醇脱卤酶催化合成手性环氧氯丙烷[J]. 化工学报, 2023, 74(7): 2926-2934. |
| [9] | 毛磊, 刘冠章, 袁航, 张光亚. 可捕集CO2的纳米碳酸酐酶粒子的高效制备及性能研究[J]. 化工学报, 2023, 74(6): 2589-2598. |
| [10] | 龙臻, 王谨航, 任俊杰, 何勇, 周雪冰, 梁德青. 离子液体协同PVCap抑制天然气水合物生成实验研究[J]. 化工学报, 2023, 74(6): 2639-2646. |
| [11] | 蔡斌, 张效林, 罗倩, 党江涛, 左栗源, 刘欣梅. 导电薄膜材料的研究进展[J]. 化工学报, 2023, 74(6): 2308-2321. |
| [12] | 李艳辉, 丁邵明, 白周央, 张一楠, 于智红, 邢利梅, 高鹏飞, 王永贞. 非常规服役超临界锅炉的微纳尺度腐蚀动力学模型建立及应用[J]. 化工学报, 2023, 74(6): 2436-2446. |
| [13] | 葛运通, 王玮, 李楷, 肖帆, 于志鹏, 宫敬. 多相分散体系中微油滴与改性二氧化硅表面间作用力的AFM研究[J]. 化工学报, 2023, 74(4): 1651-1659. |
| [14] | 龙臻, 王谨航, 何勇, 梁德青. 离子液体与动力学抑制剂作用下混合气体水合物生成特性研究[J]. 化工学报, 2023, 74(4): 1703-1711. |
| [15] | 王子健, 柯明, 李佳涵, 李舒婷, 孙巾茹, 童燕兵, 赵治平, 刘加英, 任璐. 短b轴ZSM-5分子筛制备方法及应用研究进展[J]. 化工学报, 2023, 74(4): 1457-1473. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号