化工学报 ›› 2025, Vol. 76 ›› Issue (7): 3305-3315.DOI: 10.11949/0438-1157.20241406
赵世颖( ), 左志帅, 贺梦颖, 安华良, 赵新强(
), 左志帅, 贺梦颖, 安华良, 赵新强( ), 王延吉
), 王延吉
收稿日期:2024-12-05
修回日期:2025-01-21
出版日期:2025-07-25
发布日期:2025-08-13
通讯作者:
赵新强
作者简介:赵世颖(2001—),女,硕士研究生,zhaoshiying319@163.com
基金资助:
Shiying ZHAO( ), Zhishuai ZUO, Mengying HE, Hualiang AN, Xinqiang ZHAO(
), Zhishuai ZUO, Mengying HE, Hualiang AN, Xinqiang ZHAO( ), Yanji WANG
), Yanji WANG
Received:2024-12-05
Revised:2025-01-21
Online:2025-07-25
Published:2025-08-13
Contact:
Xinqiang ZHAO
摘要:
为克服工业上氨与二卤丙烷反应合成1,2-丙二胺工艺的缺点,制备一系列负载型Co基催化剂,对其催化1,2-丙二醇氨化合成1,2-丙二胺反应性能进行研究。采用H2-TPR、TEM、ICP-MS等方法对催化剂进行了表征,重点考察了载体、第二金属组分、金属负载量等的影响。结果表明,以兼具酸-碱活性位点的羟基磷灰石(HAP)为载体制备的15Co-1.05Pt/HAP催化效果最佳,1,2-丙二醇转化率为68.5%,1,2-丙二胺选择性为27.0%,伯胺总选择性达到95.2%。催化剂中Pt与Co之间存在明显相互作用,Co-Pt纳米颗粒以合金的形式存在;Pt的引入不仅可以促进氧化钴的还原,而且使金属平均粒径更小,从而有效提高了钴基催化剂的性能。利用气相色谱-质谱联用技术对1,2-丙二醇氨化反应体系进行了定性分析,结合设计实验确定了Co-Pt/HAP催化1,2-丙二醇氨化的主要反应路径。
中图分类号:
赵世颖, 左志帅, 贺梦颖, 安华良, 赵新强, 王延吉. Co-Pt/HAP的制备及其催化1,2-丙二醇氨化反应[J]. 化工学报, 2025, 76(7): 3305-3315.
Shiying ZHAO, Zhishuai ZUO, Mengying HE, Hualiang AN, Xinqiang ZHAO, Yanji WANG. Preparation of Co-Pt/HAP catalyst and its catalytic performance for 1,2-propanediol amination[J]. CIESC Journal, 2025, 76(7): 3305-3315.
| Catalyst | X1,2-PDO/% | Selectivity/% | ||||
|---|---|---|---|---|---|---|
| 1A2A | 2A1H | 1A2H | PIP | Primary amine | ||
| Co/SiO2 | 2.7 | 0 | 0 | 0 | 0 | 0 |
| Co/MgO | 32.6 | 3.1 | 87.6 | 3.6 | 3.1 | 94.3 |
| Co/HAP | 37.8 | 9.8 | 81.6 | 3.5 | 4.9 | 94.9 |
| Co/MgAlO x | 34.4 | 0.3 | 82.3 | 10.7 | 3.4 | 93.3 |
| Co/Nb2O5 | 3.2 | 0 | 0 | 0 | 0 | 0 |
表1 载体对Co基催化剂的催化性能的影响
Table 1 Effect of supports on the catalytic performance of Co-based catalysts
| Catalyst | X1,2-PDO/% | Selectivity/% | ||||
|---|---|---|---|---|---|---|
| 1A2A | 2A1H | 1A2H | PIP | Primary amine | ||
| Co/SiO2 | 2.7 | 0 | 0 | 0 | 0 | 0 |
| Co/MgO | 32.6 | 3.1 | 87.6 | 3.6 | 3.1 | 94.3 |
| Co/HAP | 37.8 | 9.8 | 81.6 | 3.5 | 4.9 | 94.9 |
| Co/MgAlO x | 34.4 | 0.3 | 82.3 | 10.7 | 3.4 | 93.3 |
| Co/Nb2O5 | 3.2 | 0 | 0 | 0 | 0 | 0 |
| Catalyst | Acid amount/(μmol·g-1) | Base amount/(μmol·g-1) | ||||||
|---|---|---|---|---|---|---|---|---|
Weak (<250℃) | Medium (250—400℃) | Strong (>400℃) | Total | Weak (<250℃) | Medium (250—400℃) | Strong (>400℃) | Total | |
| Co/SiO2 | 42.6 | 57.4 | 34.4 | 134.4 | 29.5 | 25.9 | 10.9 | 66.4 |
| Co/MgO | 87.4 | 223.3 | 72.1 | 382.8 | 400.3 | 360.2 | 96.4 | 856.9 |
| Co/HAP | 160.0 | 222.1 | 74.9 | 456.9 | 398.1 | 295.3 | 73.8 | 767.1 |
| Co/MgAlO x | 399.6 | 383.5 | 139.6 | 922.7 | 252.8 | 224.1 | 83.1 | 560.1 |
| Co/Nb2O5 | 18.7 | 21.8 | 6.2 | 46.7 | 13.9 | 14.1 | 6.6 | 34.5 |
表2 NH3/CO2-TPD测定的负载型Co基催化剂酸碱性质
Table 2 Acid and base properties of various supported Co-based catalysts measured by NH3/CO2-TPD
| Catalyst | Acid amount/(μmol·g-1) | Base amount/(μmol·g-1) | ||||||
|---|---|---|---|---|---|---|---|---|
Weak (<250℃) | Medium (250—400℃) | Strong (>400℃) | Total | Weak (<250℃) | Medium (250—400℃) | Strong (>400℃) | Total | |
| Co/SiO2 | 42.6 | 57.4 | 34.4 | 134.4 | 29.5 | 25.9 | 10.9 | 66.4 |
| Co/MgO | 87.4 | 223.3 | 72.1 | 382.8 | 400.3 | 360.2 | 96.4 | 856.9 |
| Co/HAP | 160.0 | 222.1 | 74.9 | 456.9 | 398.1 | 295.3 | 73.8 | 767.1 |
| Co/MgAlO x | 399.6 | 383.5 | 139.6 | 922.7 | 252.8 | 224.1 | 83.1 | 560.1 |
| Co/Nb2O5 | 18.7 | 21.8 | 6.2 | 46.7 | 13.9 | 14.1 | 6.6 | 34.5 |
| Catalyst | X1,2-PDO/% | Selectivity/% | ||||
|---|---|---|---|---|---|---|
| 1A2A | 2A1H | 1A2H | PIP | Primary amine | ||
| Co/HAP | 41.6 | 12.6 | 78.4 | 2.8 | 5.6 | 93.8 |
| Co-Fe/HAP | 33.8 | 7.3 | 77.1 | 2.4 | 6.7 | 86.8 |
| Co-Ni/HAP | 39.8 | 18.0 | 63.2 | 4.2 | 11.3 | 85.4 |
| Co-Cu/HAP | 37.7 | 8.8 | 65.3 | 2.6 | 17.1 | 76.7 |
| Co-Ru/HAP | 44.6 | 16.0 | 64.0 | 2.7 | 9.5 | 82.7 |
| Co-Pt/HAP | 63.9 | 18.9 | 62.4 | 2.3 | 14.5 | 83.6 |
| Co-Pd/HAP | 19.9 | 0.2 | 72.2 | 4.1 | 9.7 | 76.5 |
表3 负载型双金属催化剂的催化性能
Table 3 Catalytic performance of various supported bimetallic catalysts
| Catalyst | X1,2-PDO/% | Selectivity/% | ||||
|---|---|---|---|---|---|---|
| 1A2A | 2A1H | 1A2H | PIP | Primary amine | ||
| Co/HAP | 41.6 | 12.6 | 78.4 | 2.8 | 5.6 | 93.8 |
| Co-Fe/HAP | 33.8 | 7.3 | 77.1 | 2.4 | 6.7 | 86.8 |
| Co-Ni/HAP | 39.8 | 18.0 | 63.2 | 4.2 | 11.3 | 85.4 |
| Co-Cu/HAP | 37.7 | 8.8 | 65.3 | 2.6 | 17.1 | 76.7 |
| Co-Ru/HAP | 44.6 | 16.0 | 64.0 | 2.7 | 9.5 | 82.7 |
| Co-Pt/HAP | 63.9 | 18.9 | 62.4 | 2.3 | 14.5 | 83.6 |
| Co-Pd/HAP | 19.9 | 0.2 | 72.2 | 4.1 | 9.7 | 76.5 |
| Catalyst | X1,2-PDO/% | Selectivity/% | ||||
|---|---|---|---|---|---|---|
| 1A2A | 2A1H | 1A2H | PIP | Primary amine | ||
| Co/HAP | 41.6 | 12.6 | 78.4 | 2.8 | 5.6 | 93.8 |
| 10Co-0.1Pt/HAP | 59.5 | 15.7 | 64.7 | 2.8 | 15.3 | 83.2 |
| 10Co-0.3Pt/HAP | 62.1 | 16.9 | 62.5 | 2.6 | 14.7 | 82.0 |
| 10Co-0.5Pt/HAP | 63.9 | 18.9 | 62.4 | 2.3 | 14.5 | 83.6 |
| 10Co-0.7Pt/HAP | 66.3 | 24.1 | 62.1 | 2.7 | 9.5 | 88.9 |
| 10Co-1Pt/HAP | 67.6 | 19.3 | 57.1 | 2.3 | 14.3 | 78.7 |
表4 Co与Pt质量比对Co-Pt/HAP催化性能的影响
Table 4 Effect of mass ratio of Co to Pt on catalytic performance of Co-Pt/HAP
| Catalyst | X1,2-PDO/% | Selectivity/% | ||||
|---|---|---|---|---|---|---|
| 1A2A | 2A1H | 1A2H | PIP | Primary amine | ||
| Co/HAP | 41.6 | 12.6 | 78.4 | 2.8 | 5.6 | 93.8 |
| 10Co-0.1Pt/HAP | 59.5 | 15.7 | 64.7 | 2.8 | 15.3 | 83.2 |
| 10Co-0.3Pt/HAP | 62.1 | 16.9 | 62.5 | 2.6 | 14.7 | 82.0 |
| 10Co-0.5Pt/HAP | 63.9 | 18.9 | 62.4 | 2.3 | 14.5 | 83.6 |
| 10Co-0.7Pt/HAP | 66.3 | 24.1 | 62.1 | 2.7 | 9.5 | 88.9 |
| 10Co-1Pt/HAP | 67.6 | 19.3 | 57.1 | 2.3 | 14.3 | 78.7 |
| Catalyst | X1,2-PDO/% | Selectivity/% | ||||
|---|---|---|---|---|---|---|
| 1A2A | 2A1H | 1A2H | PIP | Primary amine | ||
| 5Co-0.35Pt/HAP | 63.7 | 19.5 | 55.4 | 2.5 | 10.3 | 77.4 |
| 10Co-0.7Pt/HAP | 66.3 | 24.1 | 62.1 | 2.7 | 9.5 | 88.9 |
| 15Co-1.05Pt/HAP | 68.5 | 27.0 | 65.0 | 3.2 | 3.9 | 95.2 |
| 20Co-1.4Pt/HAP | 70.8 | 25.0 | 62.9 | 3.0 | 9.0 | 90.9 |
表5 金属总负载量对Co-Pt/HAP催化性能的影响
Table 5 Effect of metal loading amount on the catalytic performance of Co-Pt/HAP
| Catalyst | X1,2-PDO/% | Selectivity/% | ||||
|---|---|---|---|---|---|---|
| 1A2A | 2A1H | 1A2H | PIP | Primary amine | ||
| 5Co-0.35Pt/HAP | 63.7 | 19.5 | 55.4 | 2.5 | 10.3 | 77.4 |
| 10Co-0.7Pt/HAP | 66.3 | 24.1 | 62.1 | 2.7 | 9.5 | 88.9 |
| 15Co-1.05Pt/HAP | 68.5 | 27.0 | 65.0 | 3.2 | 3.9 | 95.2 |
| 20Co-1.4Pt/HAP | 70.8 | 25.0 | 62.9 | 3.0 | 9.0 | 90.9 |
| Catalyst | Specific surface area/(m2·g-1) | Pore volume/(cm3·g-1) | Average pore size/nm |
|---|---|---|---|
| 5Co-0.35Pt/HAP | 138.1 | 0.47 | 11.8 |
| 10Co-0.7Pt/HAP | 125.7 | 0.41 | 11.6 |
| 15Co-1.05Pt/HAP | 113.1 | 0.34 | 11.2 |
| 20Co-1.4Pt/HAP | 100.0 | 0.23 | 8.6 |
表6 不同金属负载量Co-Pt/HAP催化剂的织构性质
Table 6 Textural properties of Co-Pt/HAP catalysts with different metal loading amount
| Catalyst | Specific surface area/(m2·g-1) | Pore volume/(cm3·g-1) | Average pore size/nm |
|---|---|---|---|
| 5Co-0.35Pt/HAP | 138.1 | 0.47 | 11.8 |
| 10Co-0.7Pt/HAP | 125.7 | 0.41 | 11.6 |
| 15Co-1.05Pt/HAP | 113.1 | 0.34 | 11.2 |
| 20Co-1.4Pt/HAP | 100.0 | 0.23 | 8.6 |
| Catalyst | X1,2-PDO/% | Selectivity/% | ||||
|---|---|---|---|---|---|---|
| 1A2A | 2A1H | 1A2H | PIP | Primary amine | ||
| HAP | 0.6 | 0 | 0 | 0 | 0 | 0 |
| 15Co/HAP | 48.4 | 21.2 | 69.4 | 2.8 | 6.0 | 93.4 |
| 1.05Pt/HAP | 5.0 | 0 | 24.9 | 0 | 0 | 24.9 |
| 15Co-1.05Pt/HAP | 68.5 | 27.0 | 65.0 | 3.2 | 3.9 | 95.2 |
15Co/HAP与 1.05Pt/HAP物理混合 | 55.8 | 19.9 | 71.8 | 2.6 | 5.3 | 94.3 |
表7 HAP负载金属催化剂上的1,2-丙二醇氨化反应性能
Table 7 Performance of 1,2-propanediol amination reaction over various HAP supported metal catalysts
| Catalyst | X1,2-PDO/% | Selectivity/% | ||||
|---|---|---|---|---|---|---|
| 1A2A | 2A1H | 1A2H | PIP | Primary amine | ||
| HAP | 0.6 | 0 | 0 | 0 | 0 | 0 |
| 15Co/HAP | 48.4 | 21.2 | 69.4 | 2.8 | 6.0 | 93.4 |
| 1.05Pt/HAP | 5.0 | 0 | 24.9 | 0 | 0 | 24.9 |
| 15Co-1.05Pt/HAP | 68.5 | 27.0 | 65.0 | 3.2 | 3.9 | 95.2 |
15Co/HAP与 1.05Pt/HAP物理混合 | 55.8 | 19.9 | 71.8 | 2.6 | 5.3 | 94.3 |
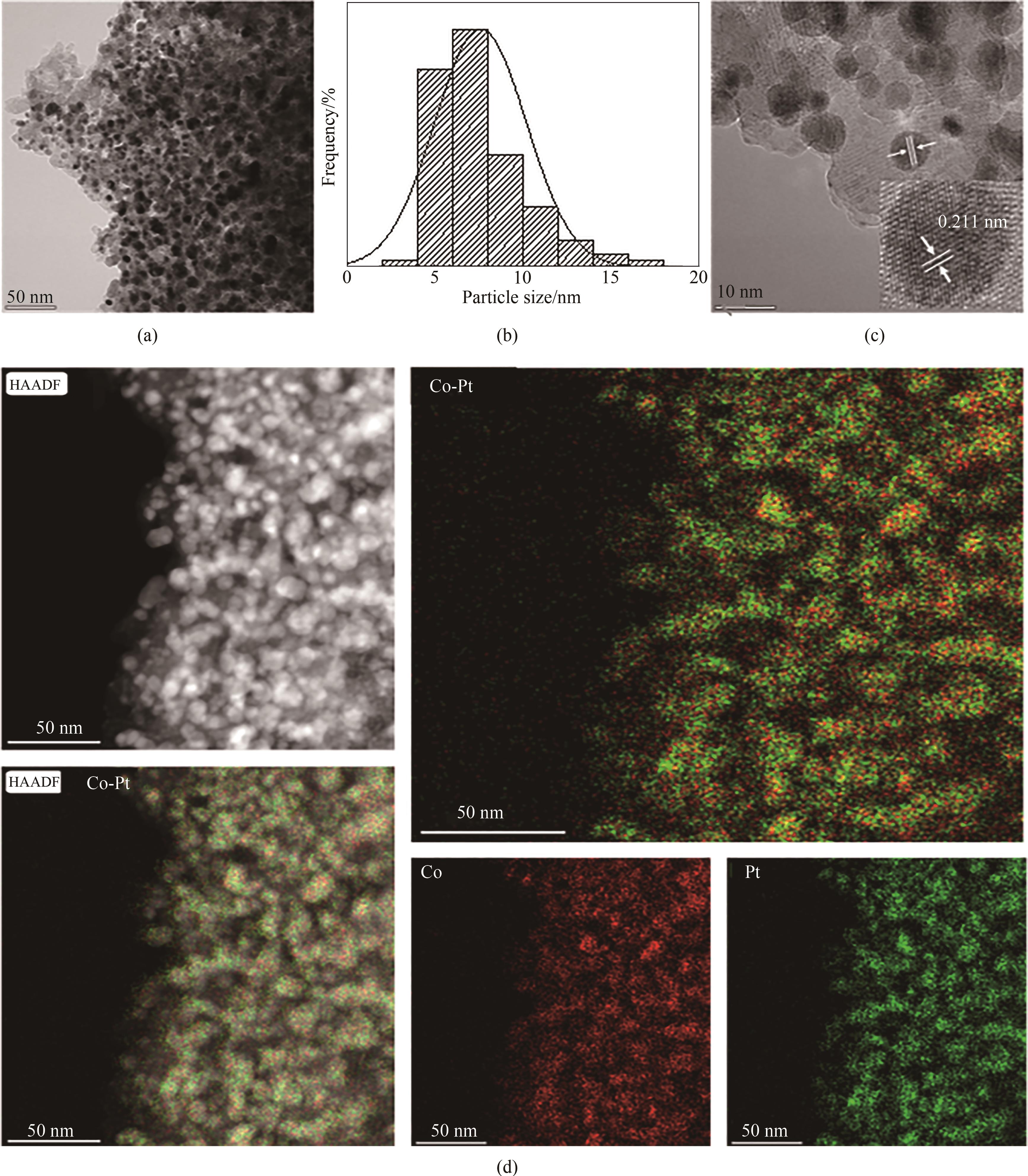
图6 15Co-1.05Pt/HAP催化剂的TEM[(a),(b)]、HRTEM(c)、HAADF-STEM 和EDS(d)照片
Fig. 6 TEM [(a), (b)], HRTEM (c), HAADF-STEM and EDS-mapping (d) of 15Co-1.05Pt/HAP catalysts
| Raw material | X/% | Yield/% | Selectivity/% | ||
|---|---|---|---|---|---|
| 1A2A | 2,5-DMP | 1A2A | 2,5-DMP | ||
| 2A1H | 34.5 | 21.9 | 12.4 | 63.6 | 34.1 |
| 1A2H | 91.6 | 73.1 | 16.4 | 79.8 | 17.9 |
表8 15Co-1.05Pt/HAP对不同中间产物氨化反应的催化性能
Table 8 The catalytic performance of 15Co-1.05Pt/HAP on the amination of different intermediates
| Raw material | X/% | Yield/% | Selectivity/% | ||
|---|---|---|---|---|---|
| 1A2A | 2,5-DMP | 1A2A | 2,5-DMP | ||
| 2A1H | 34.5 | 21.9 | 12.4 | 63.6 | 34.1 |
| 1A2H | 91.6 | 73.1 | 16.4 | 79.8 | 17.9 |
| [1] | Yu Q W, Li Y N, Zhang Q, et al. Synthesis of 1,2-propanediamine via reductive amination of isopropanolamine over Raney Ni under the promotion of K2CO3 [J]. Chemical Papers, 2019, 73(8): 2019-2026. |
| [2] | Wu Y J, Huang Y J, Dai X C, et al. Alcohol amination catalyzed by copper powder as a self-supported catalyst[J]. ChemSusChem, 2019, 12(13): 3185-3191. |
| [3] | 余秦伟, 李亚妮, 王伟, 等. 催化合成1,2-丙二胺研究进展[J]. 化学世界, 2015, 56(3): 179-183. |
| Yu Q W, Li Y N, Wang W, et al. Progress in catalytic synthesis of 1,2-propanediamine[J]. Chemical World, 2015, 56(3): 179-183. | |
| [4] | Rivera A, Pacheco D J, Ríos-Motta J, et al. Synthesis of a new chiral cyclic aminal derived from rac-1,2-propanediamine[J]. Tetrahedron Letters, 2012, 53(45): 6132-6135. |
| [5] | Pelckmans M, Renders T, van de Vyver S, et al. Bio-based amines through sustainable heterogeneous catalysis[J]. Green Chemistry, 2017, 19(22): 5303-5331. |
| [6] | Yue C J, Di K, Gu L P, et al. Selective amination of 1,2-propanediol over Co/La3O4 catalyst prepared by liquid-phase reduction[J]. Molecular Catalysis, 2019, 477: 110539. |
| [7] | Takanashi T, Nakagawa Y, Tomishige K. Amination of alcohols with ammonia in water over Rh-In catalyst[J]. Chemistry Letters, 2014, 43(6): 822-824. |
| [8] | Xie Z Y, An H L, Zhao X Q, et al. Catalytic activity of nickel and cobalt for amination of ethylene glycol: which is better?[J]. Molecular Catalysis, 2022, 522: 112243. |
| [9] | Xie Z Y, An H L, Zhao X Q, et al. Influence of different microstructures of cobalt on the catalytic activity for amination of ethylene glycol: comparison of HCP cobalt and FCC cobalt[J]. Catalysis Science & Technology, 2022, 12(10): 3148-3157 |
| [10] | Xie Z Y, An H L, Zhao X Q, et al. Insight into highly catalytic performance of Co/γ-Al2O3 for ethylene glycol amination: promotion of catalytic activity of Co by acid sites and base sites[J]. Molecular Catalysis, 2022, 528: 112492. |
| [11] | An H L, Li J P, Zheng G Z, et al. Amination of ethylene glycol to ethylenediamine catalyzed by Co-Cu/γ-Al2O3 [J]. ChemistrySelect, 2022, 7(31): e202201303. |
| [12] | 郭浩林, 李健朋, 赵新强, 等. Co-Ru/HAP 的制备及其催化乙二醇氨化反应[J]. 高校化学工程学报, 2024, 38(4): 566-577. |
| Guo H L, Li J P, Zhao X Q, et al. Preparation of a Co-Ru/HAP catalyst and its catalytic performance for ethylene glycol amination [J]. Journal of Chemical Engineering of Chinese Universities, 2024, 38(4): 566-577. | |
| [13] | Shimizu K I, Kon K, Onodera W, et al. Heterogeneous Ni catalyst for direct synthesis of primary amines from alcohols and ammonia[J]. ACS Catalysis, 2013, 3(1): 112-117. |
| [14] | Fischer A, Maciejewski M, Bürgi T, et al. Cobalt-catalyzed amination of 1,3-propanediol: effects of catalyst promotion and use of supercritical ammonia as solvent and reactant[J]. Journal of Catalysis, 1999, 183(2): 373-383. |
| [15] | Campbell C T. Ultrathin metal films and particles on oxide surfaces: structural, electronic and chemisorptive properties[J]. Surface Science Reports, 1997, 27(1/2/3): 1-111. |
| [16] | Deng L D, Miura H, Shishido T, et al. Elucidating strong metal-support interactions in Pt-Sn/SiO2 catalyst and its consequences for dehydrogenation of lower alkanes[J]. Journal of Catalysis, 2018, 365: 277-291. |
| [17] | Liu D Y, Li Y Y, Kottwitz M, et al. Identifying dynamic structural changes of active sites in Pt-Ni bimetallic catalysts using multimodal approaches[J]. ACS Catalysis, 2018, 8(5): 4120-4131. |
| [18] | Wu C H, Liu C, Su D, et al. Bimetallic synergy in cobalt-palladium nanocatalysts for CO oxidation[J]. Nature Catalysis, 2019, 2: 78-85. |
| [19] | Tong T, Guo W J, Liu X H, et al. Dual functions of CoO x decoration in PtCo/CeO2 catalysts for the hydrogen-borrowing amination of alcohols to primary amines[J]. Journal of Catalysis, 2019, 378: 392-401. |
| [20] | Deng L D, Liu X C, Wang R Q, et al. Unsupported Ni-Co alloy as efficient catalysts for CO2 methanation[J]. Journal of Alloys and Compounds, 2022, 918: 165472. |
| [21] | Chen L, Huang Q Y, Wang Y C, et al. Tailoring performance of Co-Pt/MgO-Al2O3 bimetallic aerogel catalyst for methane oxidative carbon dioxide reforming: effect of Pt/Co ratio[J]. International Journal of Hydrogen Energy, 2019, 44(36): 19878-19889. |
| [22] | Carvalho D C, de Souza H S A, Filho J M, et al. A study on the modification of mesoporous mixed oxides supports for dry reforming of methane by Pt or Ru[J]. Applied Catalysis A: General, 2014, 473: 132-145. |
| [23] | Ayodele B V, Khan M R, Cheng C K. Catalytic performance of ceria-supported cobalt catalyst for CO-rich hydrogen production from dry reforming of methane[J]. International Journal of Hydrogen Energy, 2016, 41(1): 198-207. |
| [24] | Su Y H, Zhu Y H, Jiang H L, et al. Cobalt nanoparticles embedded in N-doped carbon as an efficient bifunctional electrocatalyst for oxygen reduction and evolution reactions[J]. Nanoscale, 2014, 6(24): 15080-15089. |
| [25] | Zhang J K, Chen W Y, Ge H B, et al. Synergistic effects in atomic-layer-deposited PtCo x /CNTs catalysts enhancing hydrolytic dehydrogenation of ammonia borane[J]. Applied Catalysis B: Environmental, 2018, 235: 256-263. |
| [26] | Li Y P, Zhang H, Zhang L H, et al. Bimetallic NiPd/SBA-15 alloy as an effective catalyst for selective hydrogenation of CO2 to methane[J]. International Journal of Hydrogen Energy, 2019, 44(26): 13354-13363. |
| [27] | Zhang X, Cui G Q, Feng H S, et al. Platinum-copper single atom alloy catalysts with high performance towards glycerol hydrogenolysis[J]. Nature Communications, 2019, 10(1): 5812. |
| [28] | Vankudoth K, Gutta N, Velisoju V K, et al. CuCr2O4 derived by the sol-gel method as a highly active and selective catalyst for the conversion of glycerol to 2,6-dimethylpyrazine: a benign and eco-friendly process[J]. Catalysis Science & Technology, 2017, 7(15): 3399-3407. |
| [29] | Krishna V, Kumar S N, Reema S, et al. Bio-glycerol utilization: synthesis of 2,6-dimethylpyrazine over M x O y -MCr2O4 (M=Mg, Fe, Co, Ni, Cu and Zn) catalysts[J]. Applied Catalysis A: General, 2014, 488: 275-284. |
| [30] | Li X, Xu C H, Liu C Q, et al. Reaction pathway in vapor-phase synthesis of pyrazinyl compounds from glycerol and 1,2-propanediamine over ZnO-based catalysts[J]. Journal of Molecular Catalysis A: Chemical, 2013, 371: 104-110. |
| [31] | Klyuev M V, Khidekel M L. Catalytic amination of alcohols, aldehydes, and ketones[J]. Russian Chemical Reviews, 1980, 49(1): 14-27. |
| [1] | 刘豪, 王林, 丁昊, 耿嘉怡. R1150+R1234ze(E)二元体系223.15~253.15 K汽液相平衡研究[J]. 化工学报, 2025, 76(S1): 1-8. |
| [2] | 吴迪, 胡斌, 姜佳彤. R1233zd(E)高温热泵实验研究与应用分析[J]. 化工学报, 2025, 76(S1): 377-383. |
| [3] | 刘沁雯, 叶恒冰, 张逸伟, 朱法华, 钟文琪. 煤与禽类粪便混合燃料的加压富氧燃烧特性研究[J]. 化工学报, 2025, 76(7): 3487-3497. |
| [4] | 陆学瑞, 周帼彦, 方琦, 俞孟正, 张秀成, 涂善东. 固体氧化物燃料电池外重整器积炭效应数值模拟研究[J]. 化工学报, 2025, 76(7): 3295-3304. |
| [5] | 何军, 李勇, 赵楠, 何孝军. 碳负载硒掺杂硫化钴在锂硫电池中的性能研究[J]. 化工学报, 2025, 76(6): 2995-3008. |
| [6] | 宋粉红, 王文光, 郭亮, 范晶. C元素修饰g-C3N4对TiO2的调控及复合材料光催化产氢性能研究[J]. 化工学报, 2025, 76(6): 2983-2994. |
| [7] | 李长宇, 曾强, 肖杰, 张阳杰, 张政, 林元华. PVDF对LATP基固态电解质膜界面修饰研究[J]. 化工学报, 2025, 76(6): 2974-2982. |
| [8] | 李愽龙, 蒋雨希, 任傲天, 秦雯琪, 傅杰, 吕秀阳. TS-1/In-TS-1催化果糖一步法醇解制备乳酸甲酯连续化试验[J]. 化工学报, 2025, 76(6): 2678-2686. |
| [9] | 茅雨洁, 路晓飞, 锁显, 杨立峰, 崔希利, 邢华斌. 工业气体中微量氧深度脱除催化剂研究进展[J]. 化工学报, 2025, 76(5): 1997-2010. |
| [10] | 刘璐, 万开, 王文玥, 王太, 汤建成, 王少恒. 基于氦膨胀制冷的正仲氢转化耦合流动换热研究[J]. 化工学报, 2025, 76(4): 1513-1522. |
| [11] | 田浩辰, 马志先, 王之浩. R1234ze(E)水平三维肋管外膜状凝结特性实验研究[J]. 化工学报, 2025, 76(3): 975-984. |
| [12] | 赵丽文, 刘桂莲. 基于系统集成的复杂催化反应系统性能强化及参数优化[J]. 化工学报, 2025, 76(3): 1111-1119. |
| [13] | 万俊, 宋佳芮, 范春煌, 魏乐乐, 聂依娜, 刘琳. 高效空穴转移助力光催化碱性甲醇-水溶液制氢[J]. 化工学报, 2025, 76(3): 1064-1075. |
| [14] | 何传超, 周静红, 曹约强, 施尧, 周兴贵. Ag/SiO2催化草酸酯加氢制乙醇酸甲酯的床层-颗粒双尺度耦合模拟研究[J]. 化工学报, 2025, 76(2): 654-666. |
| [15] | 钟晓航, 许卫, 张文, 许莉, 王宇新. 碱性水电解制氢中铁杂质的影响研究进展[J]. 化工学报, 2025, 76(2): 519-531. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号