化工学报 ›› 2025, Vol. 76 ›› Issue (8): 4095-4107.DOI: 10.11949/0438-1157.20250093
张荟钦1( ), 赵泓竣2, 付正军3, 庄力4, 董凯5, 贾添智1(
), 赵泓竣2, 付正军3, 庄力4, 董凯5, 贾添智1( ), 曹雪丽2(
), 曹雪丽2( ), 孙世鹏1,2
), 孙世鹏1,2
收稿日期:2025-01-22
修回日期:2025-03-10
出版日期:2025-08-25
发布日期:2025-09-17
通讯作者:
贾添智,曹雪丽
作者简介:张荟钦(1984—),男,博士,zhanghuiqin@jsfmtic.com
基金资助:
Huiqin ZHANG1( ), Hongjun ZHAO2, Zhengjun FU3, Li ZHUANG4, Kai DONG5, Tianzhi JIA1(
), Hongjun ZHAO2, Zhengjun FU3, Li ZHUANG4, Kai DONG5, Tianzhi JIA1( ), Xueli CAO2(
), Xueli CAO2( ), Shipeng SUN1,2
), Shipeng SUN1,2
Received:2025-01-22
Revised:2025-03-10
Online:2025-08-25
Published:2025-09-17
Contact:
Tianzhi JIA, Xueli CAO
摘要:
本研究旨在评估纳滤膜技术在稀土浸出液膜法提浓过程中的应用潜力。从稀土离子截留与抗污染性能方面,对商业膜进行了筛选,发现提高硫酸钙饱和溶解度是提高浓缩倍率的关键。进一步深入研究了硫酸钙溶解度与纳滤膜过程的关系,并对纳滤膜的分离工艺进行了优化。通过调节溶液的pH,有效控制了硫酸钙在膜表面的沉积现象,进而提高了膜处理的效率以及稀土离子的浓缩倍率。此外,应用Hermia模型分析了不同运行条件下膜污染的影响,为膜清洗和延长使用寿命提供了理论依据。本研究为稀土浸出液的提浓提供了一种高效且环保的解决方案,具有显著的应用前景。
中图分类号:
张荟钦, 赵泓竣, 付正军, 庄力, 董凯, 贾添智, 曹雪丽, 孙世鹏. 纳滤膜在离子型稀土浸出液提浓中的应用研究[J]. 化工学报, 2025, 76(8): 4095-4107.
Huiqin ZHANG, Hongjun ZHAO, Zhengjun FU, Li ZHUANG, Kai DONG, Tianzhi JIA, Xueli CAO, Shipeng SUN. Application of nanofiltration membrane in concentration of ionic rare earth leach solution[J]. CIESC Journal, 2025, 76(8): 4095-4107.
| 离子 | 离子浓度/ (mg/L) |
|---|---|
| Ca2+ | 158.6 |
| Mg2+ | 1106 |
| Ce3+ | 3.415 |
| La3+ | 56.92 |
表1 真实稀土浸出液主要离子成分
Table 1 The main ionic components of the actual rare earth leaching solution
| 离子 | 离子浓度/ (mg/L) |
|---|---|
| Ca2+ | 158.6 |
| Mg2+ | 1106 |
| Ce3+ | 3.415 |
| La3+ | 56.92 |
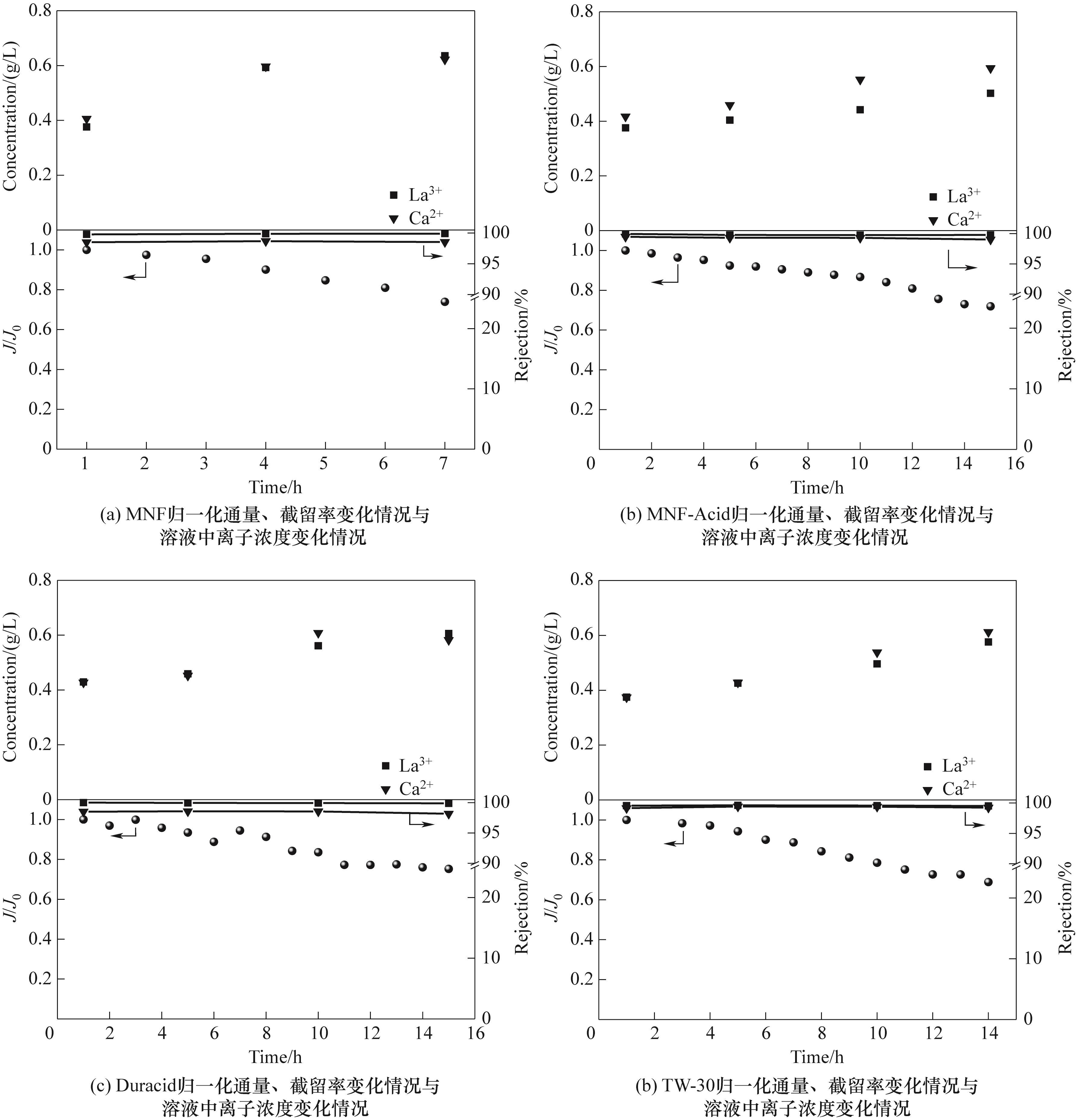
图2 稀土浸出液浓缩过程商业膜归一化通量变化、镧离子截留率变化
Fig.2 Change of normalized flux of commercial membrane and the rejection of La3+ in the concentration process of rare earth leaching solution
| 离子 | MNF | MNF-Acid | Duracid | TW-30 | |
|---|---|---|---|---|---|
| Ca2+ | 实验组/(10-2 mg/L) | 4.35 | 5.10 | 3.01 | 6.00 |
| 浸泡组/(10-2 mg/L) | 低于检测限 | ||||
| La3+ | 实验组/(10-2 mg/L) | 0.14 | 0.15 | 0.11 | 0.23 |
| 浸泡组/(10-2 mg/L) | 低于检测限 | ||||
表2 膜表面残留离子浓度
Table 2 Residual ion concentration on the membrane surface
| 离子 | MNF | MNF-Acid | Duracid | TW-30 | |
|---|---|---|---|---|---|
| Ca2+ | 实验组/(10-2 mg/L) | 4.35 | 5.10 | 3.01 | 6.00 |
| 浸泡组/(10-2 mg/L) | 低于检测限 | ||||
| La3+ | 实验组/(10-2 mg/L) | 0.14 | 0.15 | 0.11 | 0.23 |
| 浸泡组/(10-2 mg/L) | 低于检测限 | ||||
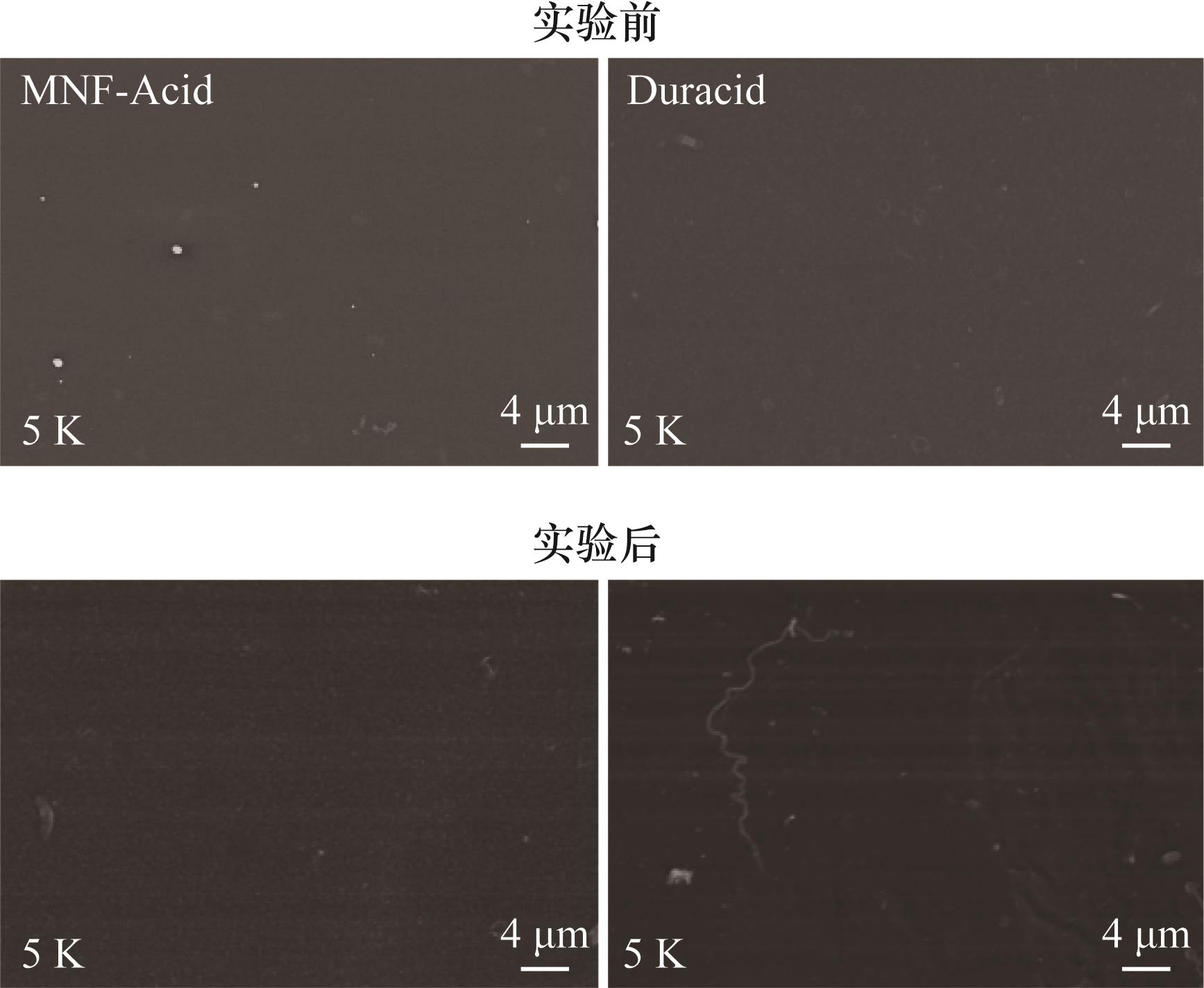
图6 在不同pH条件模拟浓缩实验前后MNF-Acid、Duracid膜表面SEM图像
Fig.6 SEM images of the surface of MNF-Acid, and Duracid membranes before and after experiments under different pH conditions
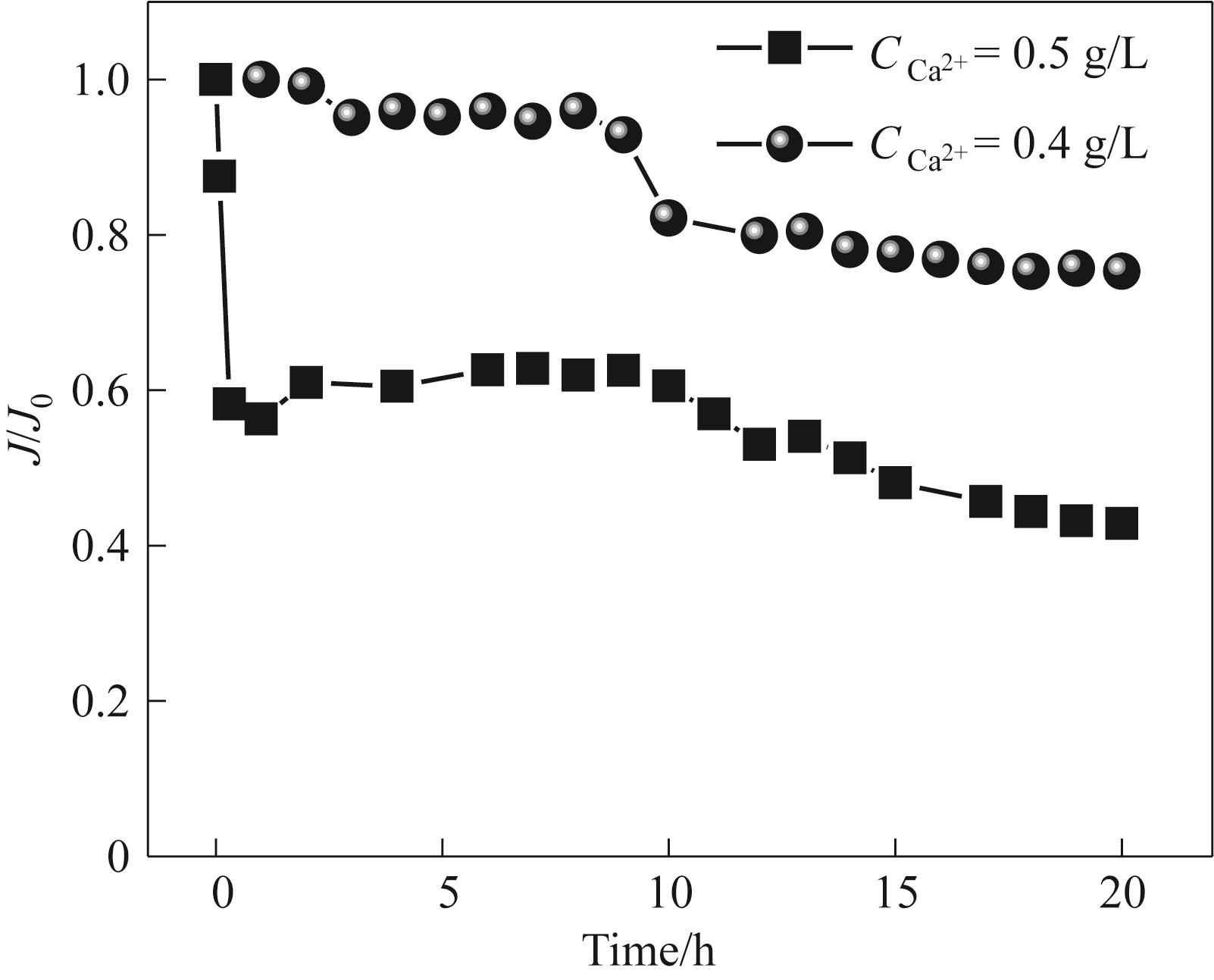
图7 不同浓度的模拟稀土浸出液浓缩过程中Duracid归一化通量变化
Fig.7 The normalized flux changes of Duracid during the concentration process of simulated rare earth leaching solutions with different concentrations
| 项目 | 浓度/(g/L) | 饱和溶解度/(g/L) | |||
|---|---|---|---|---|---|
| Mg2+ | MgSO4 | La3+ | Ca2+ | CaSO4 | |
| pH=5.8 | 1.4 | 7 | 0.4 | 0.51 | 1.73 |
| pH=3 | 0.54 | 1.84 | |||
| pH=1 | 0.82 | 2.79 | |||
| 1%(质量分数)H2SO4 | 0.66 | 2.24 | |||
| pH=5.8 | 1.4 | 7 | 0.4 | 0.51 | 1.73 |
| 2 | 10 | 0.48 | 1.63 | ||
| 2.8 | 14 | 0.46 | 1.56 | ||
| pH=1 | 1.4 | 7 | 0.05 | 0.58 | 1.97 |
| 0.2 | 0.73 | 2.48 | |||
| 0.4 | 0.82 | 2.79 | |||
表3 不同条件下硫酸钙溶解度
Table 3 Solubility of CaSO4 under different conditions
| 项目 | 浓度/(g/L) | 饱和溶解度/(g/L) | |||
|---|---|---|---|---|---|
| Mg2+ | MgSO4 | La3+ | Ca2+ | CaSO4 | |
| pH=5.8 | 1.4 | 7 | 0.4 | 0.51 | 1.73 |
| pH=3 | 0.54 | 1.84 | |||
| pH=1 | 0.82 | 2.79 | |||
| 1%(质量分数)H2SO4 | 0.66 | 2.24 | |||
| pH=5.8 | 1.4 | 7 | 0.4 | 0.51 | 1.73 |
| 2 | 10 | 0.48 | 1.63 | ||
| 2.8 | 14 | 0.46 | 1.56 | ||
| pH=1 | 1.4 | 7 | 0.05 | 0.58 | 1.97 |
| 0.2 | 0.73 | 2.48 | |||
| 0.4 | 0.82 | 2.79 | |||
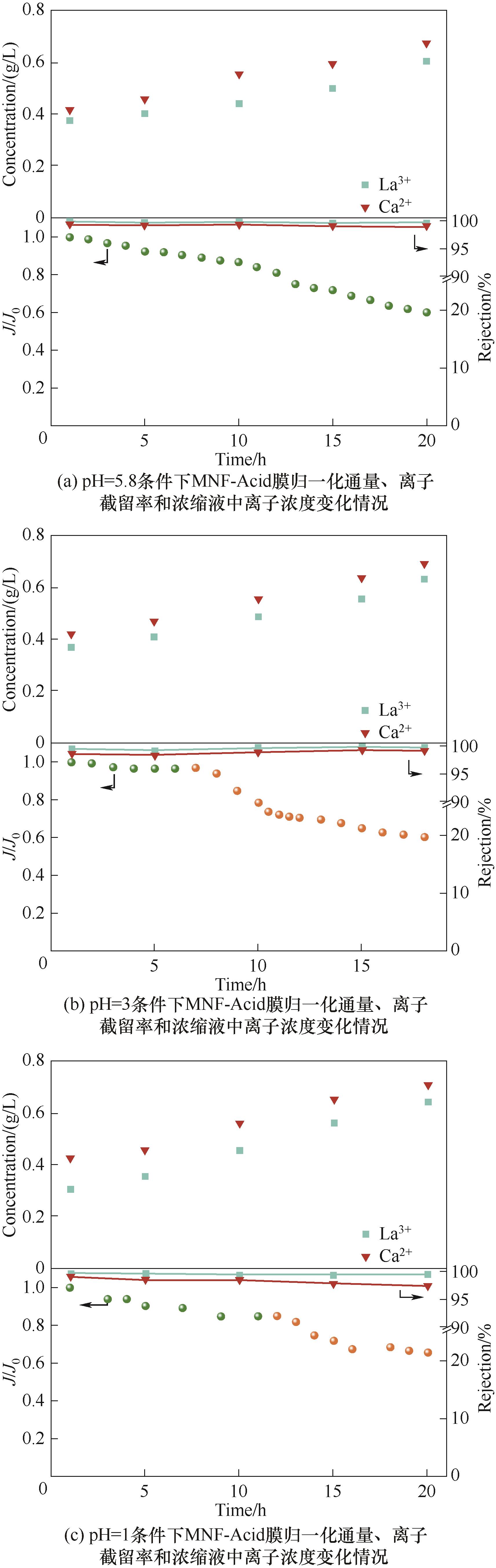
图8 不同pH条件下稀土浸出液浓缩过程中MNF-Acid膜的性能变化情况(进料液:1.4 g/L Mg2+、0.4 g/L La3+、0.4 g/L Ca2+,横流速度为90 L/h,黄色散点表示Hermia模型拟合段)
Fig.8 The performance changes of MNF-Acid membrane during the concentration process of rare earth leaching solution under different pH conditions
| pH | Hermia模型拟合精度 | ||
|---|---|---|---|
| 完全孔堵塞 | 中间孔堵塞 | 标准孔堵塞 | |
| 5.8 | 0.9169 | 0.8806 | 0.9472 |
| 3 | 0.9779 | 0.9702 | 0.9360 |
| 1 | 0.9648 | 0.9640 | 0.9284 |
表4 不同pH条件下膜污染过程的Hermia模型拟合结果
Table 4 The fitting results of Hermia model for membrane fouling process under different pH conditions
| pH | Hermia模型拟合精度 | ||
|---|---|---|---|
| 完全孔堵塞 | 中间孔堵塞 | 标准孔堵塞 | |
| 5.8 | 0.9169 | 0.8806 | 0.9472 |
| 3 | 0.9779 | 0.9702 | 0.9360 |
| 1 | 0.9648 | 0.9640 | 0.9284 |

图9 pH=1条件下MNF-Acid与Duracid模拟浓缩实验前后膜表面SEM图像
Fig.9 SEM images of the membrane surface before and after the experiment with MNF-Acid and Duracid under the condition of pH=1
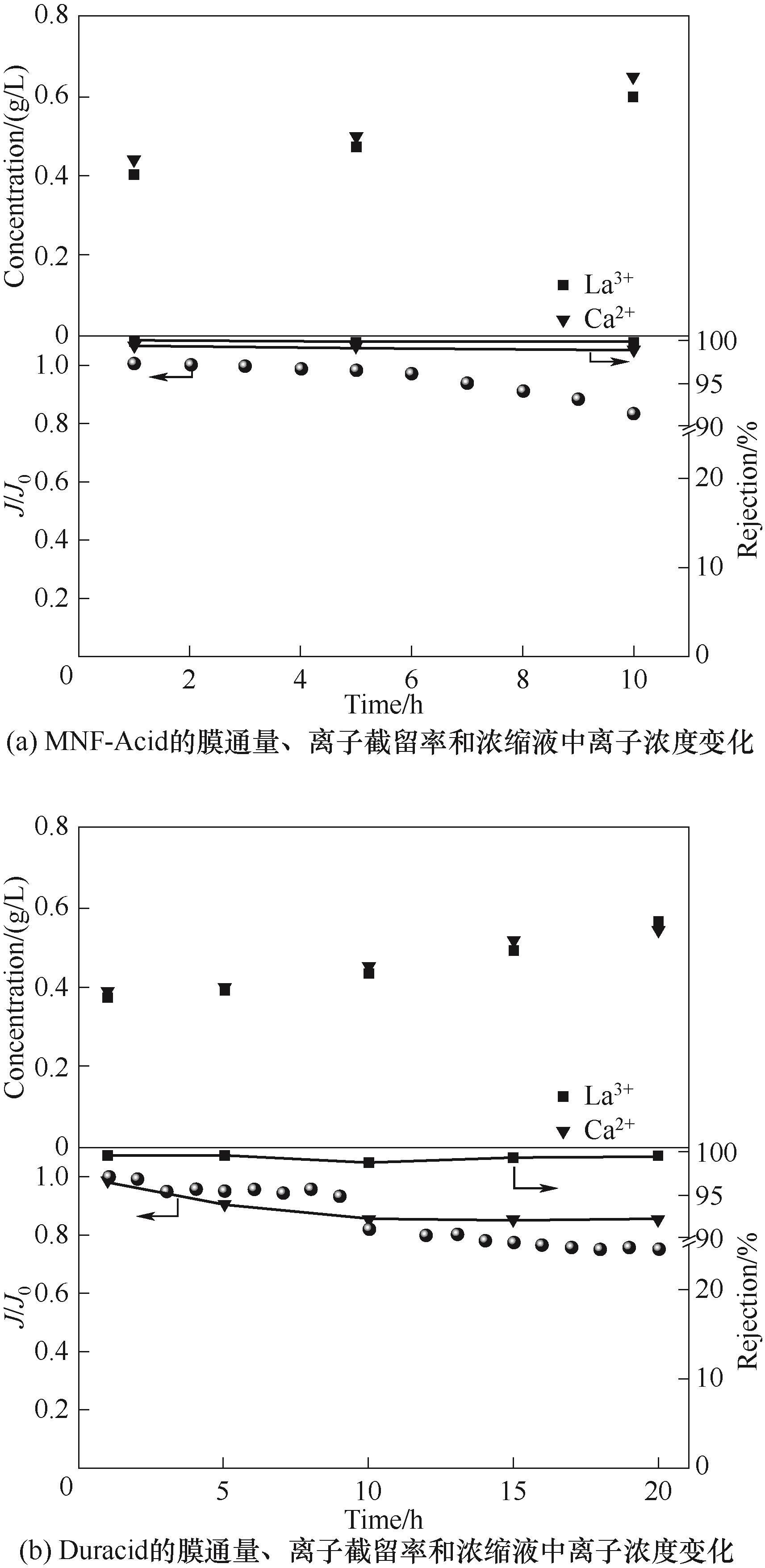
图10 在pH=1条件下的稀土浸出液浓缩过程中MNF-Acid与Duracid膜性能变化情况(进料液:1.4 g/L Mg2+、0.4 g/L La3+、0.4 g/L Ca2+)
Fig.10 The performance changes of MNF-Acid and Duracid membranes during the concentration process of rare earth leaching solution under the condition of pH=1
| pH | 浓度/(g/L) | 饱和溶解度/(g/L) | |||
|---|---|---|---|---|---|
| Mg2+ | MgSO4 | La3+ | Ca2+ | CaSO4 | |
| 5.8 | 1.4 | 7 | 0.4 | 0.51 | 1.73 |
| 5.8 | 1.75 | 8.75 | 0.5 | 0.50 | 1.7 |
| 5.8 | 2.1 | 10.5 | 0.6 | 0.48 | 1.63 |
| 5.8 | 2 | 10 | 0.57 | 0.50 | 1.7 |
| 5.8 | 2.8 | 14 | 0.8 | 0.49 | 1.67 |
| 3 | 1.4 | 7 | 0.4 | 0.54 | 1.84 |
| 3 | 2 | 10 | 0.57 | 0.51 | 1.73 |
| 3 | 2.8 | 14 | 0.8 | 0.5 | 1.7 |
| 1 | 1.4 | 7 | 0.4 | 0.82 | 2.79 |
| 1 | 2 | 10 | 0.57 | 0.75 | 2.55 |
| 1 | 2.8 | 14 | 0.8 | 0.75 | 2.55 |
表5 不同回收率时硫酸钙溶解度
Table 5 Solubility of CaSO4 under different recovery rates
| pH | 浓度/(g/L) | 饱和溶解度/(g/L) | |||
|---|---|---|---|---|---|
| Mg2+ | MgSO4 | La3+ | Ca2+ | CaSO4 | |
| 5.8 | 1.4 | 7 | 0.4 | 0.51 | 1.73 |
| 5.8 | 1.75 | 8.75 | 0.5 | 0.50 | 1.7 |
| 5.8 | 2.1 | 10.5 | 0.6 | 0.48 | 1.63 |
| 5.8 | 2 | 10 | 0.57 | 0.50 | 1.7 |
| 5.8 | 2.8 | 14 | 0.8 | 0.49 | 1.67 |
| 3 | 1.4 | 7 | 0.4 | 0.54 | 1.84 |
| 3 | 2 | 10 | 0.57 | 0.51 | 1.73 |
| 3 | 2.8 | 14 | 0.8 | 0.5 | 1.7 |
| 1 | 1.4 | 7 | 0.4 | 0.82 | 2.79 |
| 1 | 2 | 10 | 0.57 | 0.75 | 2.55 |
| 1 | 2.8 | 14 | 0.8 | 0.75 | 2.55 |
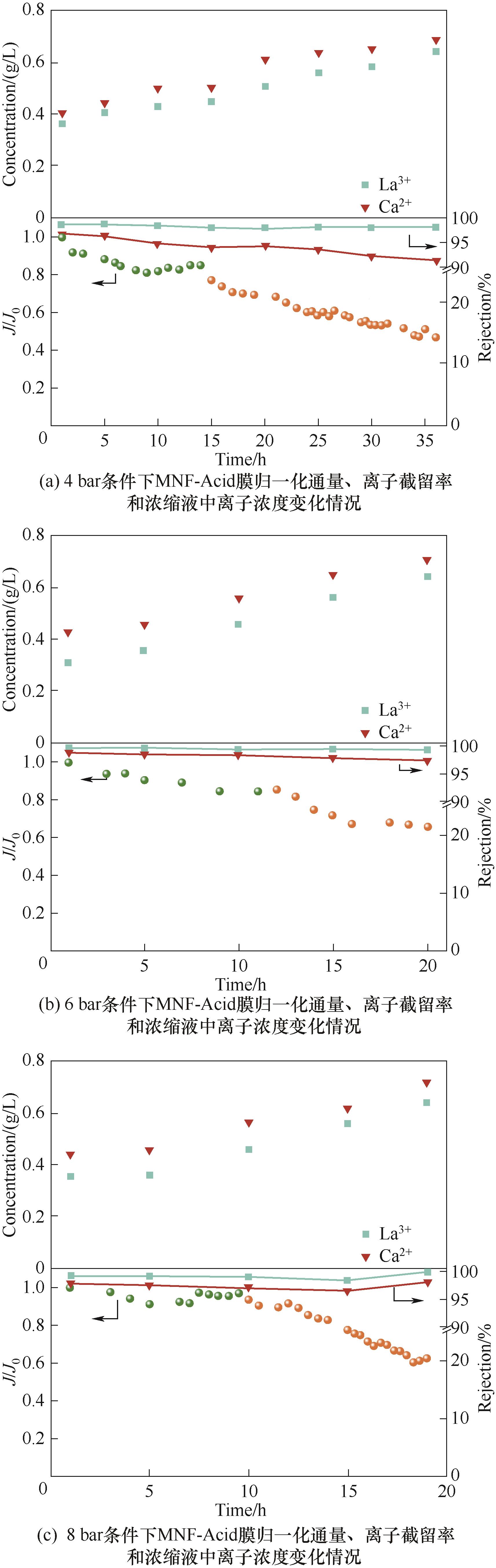
图11 不同压力条件下稀土浸出液浓缩过程中MNF-Acid膜的性能变化情况
Fig.11 The performance changes of MNF-Acid membrane during the concentration process of rare earth leaching solution under different pressure conditions
| 压力/bar | Hermia模型拟合精度 | ||
|---|---|---|---|
| 完全孔堵塞 | 中间孔堵塞 | 标准孔堵塞 | |
| 4 | 0.9423 | 0.9336 | 0.9464 |
| 6 | 0.9648 | 0.9640 | 0.9284 |
| 8 | 0.9601 | 0.9488 | 0.9554 |
表6 不同压力条件下膜污染过程的Hermia模型拟合结果
Table 6 The performance changes of MNF-Acid membrane during the concentration process of rare earth leaching solution under different pressure conditions
| 压力/bar | Hermia模型拟合精度 | ||
|---|---|---|---|
| 完全孔堵塞 | 中间孔堵塞 | 标准孔堵塞 | |
| 4 | 0.9423 | 0.9336 | 0.9464 |
| 6 | 0.9648 | 0.9640 | 0.9284 |
| 8 | 0.9601 | 0.9488 | 0.9554 |
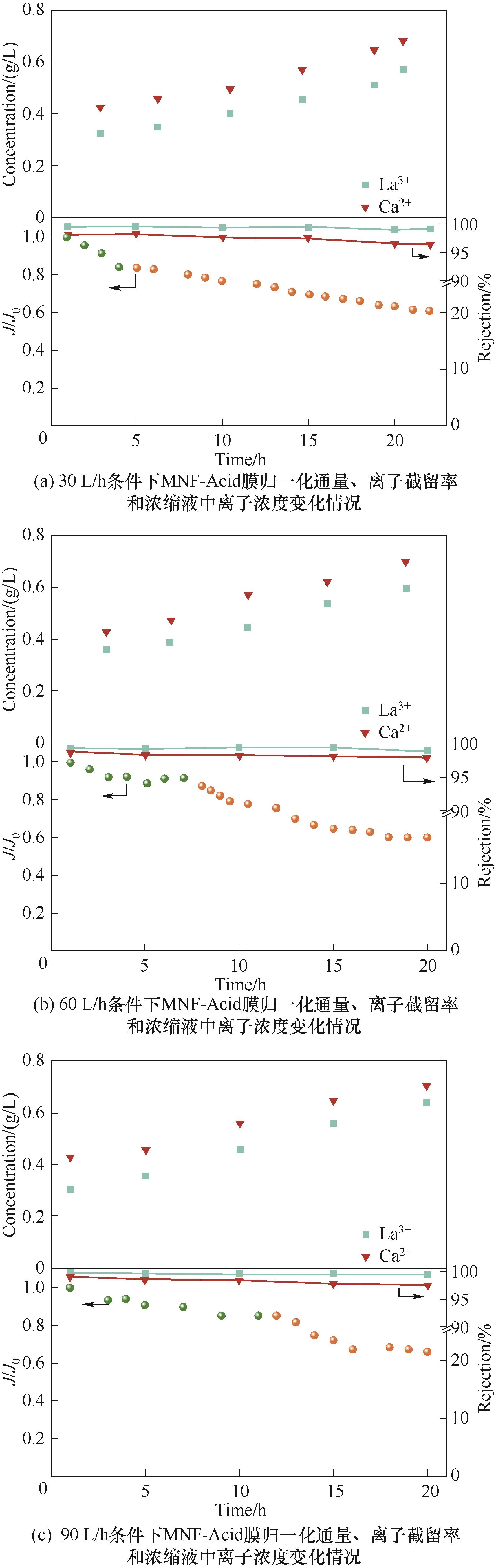
图12 不同进料流量下稀土浸出液浓缩过程中MNF-Acid膜的性能变化情况
Fig.12 The performance changes of MNF-Acid membrane during the concentration process of rare earth leaching solution under different feed flow rate
| 流量/(L/h) | Hermia模型拟合精度 | ||
|---|---|---|---|
| 完全孔堵塞 | 中间孔堵塞 | 标准孔堵塞 | |
| 30 | 不适用 | 0.9154 | 0.9893 |
| 60 | 0.9864 | 0.9681 | 0.9740 |
| 90 | 0.9626 | 0.9562 | 0.7371 |
表7 不同进料流量下膜污染过程的Hermia模型拟合结果
Table 7 The performance changes of MNF-Acid membrane during the concentration process of rare earth leaching solution under different feed flow rate
| 流量/(L/h) | Hermia模型拟合精度 | ||
|---|---|---|---|
| 完全孔堵塞 | 中间孔堵塞 | 标准孔堵塞 | |
| 30 | 不适用 | 0.9154 | 0.9893 |
| 60 | 0.9864 | 0.9681 | 0.9740 |
| 90 | 0.9626 | 0.9562 | 0.7371 |
| pH | 处理效率/(L/h) | 最大通量衰减 程度/% | 不可逆通量 衰减程度/% |
|---|---|---|---|
| 5.8 | 2.22 | 39 | 9.50 |
| 1 | 3.56 | 34 | 1.10 |
表8 膜组件浓缩效率与通量衰减程度
Table 8 Component concentration efficiency and flux attenuation degree
| pH | 处理效率/(L/h) | 最大通量衰减 程度/% | 不可逆通量 衰减程度/% |
|---|---|---|---|
| 5.8 | 2.22 | 39 | 9.50 |
| 1 | 3.56 | 34 | 1.10 |
| pH | 离子 | 时间/(min) | 浓度/(g/L) | |
|---|---|---|---|---|
| 真实 | 理论 | |||
| 5.8 | Ca2+ | 35 | 0.797 | 0.791 |
| 65 | 1.247 | 1.4 | ||
| La3+ | 35 | 0.528 | 0.525 | |
| 65 | 1.062 | 0.96 | ||
| 1 | Ca2+ | 55 | 1.359 | 1.3 |
| La3+ | 0.925 | 0.893 | ||
表9 组件浓缩过程中真实离子浓度与理论浓度
Table 9 Real ion concentration and theoretical concentration during the concentration process of components
| pH | 离子 | 时间/(min) | 浓度/(g/L) | |
|---|---|---|---|---|
| 真实 | 理论 | |||
| 5.8 | Ca2+ | 35 | 0.797 | 0.791 |
| 65 | 1.247 | 1.4 | ||
| La3+ | 35 | 0.528 | 0.525 | |
| 65 | 1.062 | 0.96 | ||
| 1 | Ca2+ | 55 | 1.359 | 1.3 |
| La3+ | 0.925 | 0.893 | ||
| [1] | Xu Z G, Li G, Yang H F, et al. Development review on leaching technology and leaching agents of weathered crust elution-deposited rare earth ores[J]. Minerals, 2023, 13(9): 1223. |
| [2] | Xiao Y F, Feng Z Y, Huang X W, et al. Recovery of rare earth from the ion-adsorption type rare earths ore(Ⅱ): Compound leaching[J]. Hydrometallurgy, 2016, 163: 83-90. |
| [3] | He Q, Qiu J, Chen J F, et al. Progress in green and efficient enrichment of rare earth from leaching liquor of ion adsorption type rare earth ores[J]. Journal of Rare Earths, 2022, 40(3): 353-364. |
| [4] | 王道广, 王均凤, 张香平, 等. 离子液体在稀土萃取分离中的应用[J]. 化工学报, 2020, 71(10): 4379-4394. |
| Wang D G, Wang J F, Zhang X P, et al. Application of ionic liquid in extraction and separation of rare earth[J]. CIESC Journal, 2020, 71(10): 4379-4394. | |
| [5] | Liu L L, Liu Y X, Luo J Q, et al. Membrane pre-concentration as an efficient strategy to enhance the enrichment of rare earth by MgO precipitation[J]. Chemical Engineering Journal, 2024, 491: 152083. |
| [6] | Zhu D M, Qiu T S, Zhong J F, et al. Molecular dynamics simulation of aluminum inhibited leaching during ion-adsorbed type rare earth ore leaching process[J]. Journal of Rare Earths, 2019, 37(12): 1334-1340. |
| [7] | 刘宁, 褚昌辉, 王乾, 等. 用于混合一价盐分离的纳滤膜的制备及性能研究[J]. 化工学报, 2021, 72(1): 578-588. |
| Liu N, Chu C H, Wang Q, et al. Preparation of nanofiltration membrane for separation of mixed monovalent salts[J]. CIESC Journal, 2021, 72(1): 578-588. | |
| [8] | Shirazi S, Lin C J, Chen D. Inorganic fouling of pressure-driven membrane processes: a critical review[J]. Desalination, 2010, 250(1): 236-248. |
| [9] | Zhu X B, Jassby D. Electroactive membranes for water treatment: enhanced treatment functionalities, energy considerations, and future challenges[J]. Accounts of Chemical Research, 2019, 52(5): 1177-1186. |
| [10] | Ashfaq M Y, Al-Ghouti M A, Da'na D A, et al. Investigating the effect of temperature on calcium sulfate scaling of reverse osmosis membranes using FTIR, SEM-EDX and multivariate analysis[J]. Science of the Total Environment, 2020, 703: 134726. |
| [11] | Wang Y B, Mao X Y, Chen C, et al. Effect of sulfuric acid concentration on morphology of calcium sulfate hemihydrate crystals[J]. Materials Research Express, 2020, 7(10): 105501. |
| [12] | Clampett J B, Fowler R T. Equilibrium solubility of calcium sulphate hemihydrate in sodium chloride: magnesium sulphate solutions at elevated temperatures[J]. Journal of Applied Chemistry, 1964, 14(2): 81-86. |
| [13] | Zhang T, Wang Q Y, Yang Y, et al. Revealing the contradiction between DLVO/XDLVO theory and membrane fouling propensity for oil-in-water emulsion separation[J]. Journal of Hazardous Materials, 2024, 466: 133594. |
| [14] | 唐和礼, 张冰, 黄冬梅, 等. XDLVO理论在膜污染解析中的应用研究[J]. 化工学报, 2021, 72(3): 1230-1241. |
| Tang H L, Zhang B, Huang D M, et al. Advances in membrane fouling analysis based on XDLVO theory[J]. CIESC Journal, 2021, 72(3): 1230-1241. | |
| [15] | Wang L, Li Z H, Fan J H, et al. The intelligent prediction of membrane fouling during membrane filtration by mathematical models and artificial intelligence models[J]. Chemosphere, 2024, 349: 141031. |
| [16] | Zheng W J, Chen Y, Xu X H, et al. Research on the factors influencing nanofiltration membrane fouling and the prediction of membrane fouling[J]. Journal of Water Process Engineering, 2024, 59: 104876. |
| [17] | Gao M F, He Q L, Dong H Z, et al. Identification of the coupled fouling mechanism involved in microfiltration of tobacco extracts liquid by multistage Hermia model[J]. Journal of Food Process Engineering, 2022, 45(2): e13961. |
| [18] | Ahmad A, Raish M, Alkharfy K M, et al. Solubility, solubility parameters and solution thermodynamics of thymoquinone in different mono solvents[J]. Journal of Molecular Liquids, 2018, 272: 912-918. |
| [19] | Fan C Y, Yan J M, Liu H D, et al. Performance and membrane fouling characteristics of a drinking water multistage NF system based on membrane autopsy from a full-scale system[J]. Journal of Water Process Engineering, 2024, 58: 104909. |
| [20] | Field R W, Wu D, Howell J A, et al. Critical flux concept for microfiltration fouling[J]. Journal of Membrane Science, 1995, 100(3): 259-272. |
| [21] | Xie D Y, Li J, Zhang H, et al. A novel electrochemical method for the removal of aluminum from ionic rare earth leachate[J]. Separation and Purification Technology, 2024, 345: 127296. |
| [22] | 鹿钦礼, 刘德俊, 马贵阳, 等. 注汽锅炉高含盐回用水引发爆管分析[J]. 辽宁石油化工大学学报, 2010, 30(2): 19-22. |
| Lu Q L, Liu D J, Ma G Y, et al. Analysis of explosion of tube with high salt recycling water in steam injection boiler[J]. Journal of Liaoning Shihua University, 2010, 30(2): 19-22. | |
| [23] | Zhang Z X, Bright V M, Greenberg A R. Use of capacitive microsensors and ultrasonic time-domain reflectometry for in-situ quantification of concentration polarization and membrane fouling in pressure-driven membrane filtration[J]. Sensors and Actuators B: Chemical, 2006, 117(2): 323-331. |
| [24] | Cui C, Luo C, Tian T, et al. Comparative evaluation of acid-resistant nanofiltration membranes for heavy metal removal in acidic wastewater[J]. Desalination, 2024, 576: 117349. |
| [25] | Lee J, Shin Y, Boo C, et al. Performance, limitation, and opportunities of acid-resistant nanofiltration membranes for industrial wastewater treatment[J]. Journal of Membrane Science, 2023, 666: 121142. |
| [26] | Bai Y, Gao P, Fang R, et al. Constructing positively charged acid-resistant nanofiltration membranes via surface postgrafting for efficient removal of metal ions from electroplating rinse wastewater[J]. Separation and Purification Technology, 2022, 297: 121500. |
| [27] | Hoang T A, Ang H M, Rohl A L. Effects of temperature on the scaling of calcium sulphate in pipes[J]. Powder Technology, 2007, 179(1/2): 31-37. |
| [28] | Li J F, Liu P P, Wu C L, et al. Common ion effect in the hydrolysis reaction of MgCa alloy hydride-salt composites[J]. International Journal of Hydrogen Energy, 2017, 42(2): 1429-1435. |
| [29] | Dutrizac J E. The behaviour of the rare earth elements during gypsum (CaSO4·2H2O) precipitation[J]. Hydrometallurgy, 2017, 174: 38-46. |
| [30] | Barati N, Husein M M, Azaiez J. Iron oxide doped ceramic membranes for combined organic-inorganic colloidal fouling mitigation[J]. Separation and Purification Technology, 2023, 323: 124498. |
| [31] | Hoek E M V, Kim A S, Elimelech M. Influence of crossflow membrane filter geometry and shear rate on colloidal fouling in reverse osmosis and nanofiltration separations[J]. Environmental Engineering Science, 2002, 19(6): 357-372. |
| [32] | Chong Y K, Fletcher D F, Liang Y Y. CFD simulation of hydrodynamics and concentration polarization in osmotically assisted reverse osmosis membrane systems[J]. Journal of Water Process Engineering, 2024, 57: 104535. |
| [1] | 向昕辰, 鲁丹, 赵影, 姚之侃, 寇瑞强, 郑丹军, 周志军, 张林. 聚酰胺纳滤膜表面季铵化提高荷正电性及其锂镁分离性能[J]. 化工学报, 2025, 76(5): 2377-2386. |
| [2] | 陆艳秋, 狄扬, 石文博, 殷聪聪, 汪勇. 基于新型有机多孔聚合物的智能响应膜研究进展[J]. 化工学报, 2025, 76(5): 2101-2118. |
| [3] | 李玲玉, 胡鑫, 成怀刚, 赵云, 安东, 马玉军, 金家豪, 于旭东, 张卫东. K+(Mg2+), Ca2+//Cl--H2O三元水盐体系的等温蒸发成盐相区[J]. 化工学报, 2025, 76(1): 120-130. |
| [4] | 王新月, 徐小虎, 张海洋, 尹春华. 维生素A醋酸酯/环糊精包合及性质研究[J]. 化工学报, 2024, 75(S1): 321-328. |
| [5] | 赵志星, 姚智豪, 于雪峰, 杨游胜, 曾英, 于旭东. 锂钠镁共存硫酸盐体系多温相图及其应用[J]. 化工学报, 2024, 75(6): 2123-2133. |
| [6] | 司友明, 郑凌峰, 陈鹏忠, 樊江莉, 彭孝军. 新型锑氧簇光刻胶的性能与机理研究[J]. 化工学报, 2024, 75(4): 1705-1717. |
| [7] | 王灵洁, 高海龙, 靳继鹏, 王志浩, 李见波. 海水中的污染物对逆电渗析电堆性能的影响[J]. 化工学报, 2024, 75(2): 695-705. |
| [8] | 吴雨茜, 唐元晖, 郭强, 林亚凯, 余立新, 王晓琳. 纳滤膜对硫酸型解吸液锂镁分离的实验研究与模拟[J]. 化工学报, 2024, 75(12): 4563-4575. |
| [9] | 杨百玉, 寇悦, 姜峻韬, 詹亚力, 王庆宏, 陈春茂. 炼化碱渣湿式氧化预处理过程DOM的化学转化特征[J]. 化工学报, 2023, 74(9): 3912-3920. |
| [10] | 于旭东, 李琪, 陈念粗, 杜理, 任思颖, 曾英. 三元体系KCl + CaCl2 + H2O 298.2、323.2及348.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(8): 3256-3265. |
| [11] | 陈科, 杜理, 曾英, 任思颖, 于旭东. 四元体系LiCl+MgCl2+CaCl2+H2O 323.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(5): 1896-1903. |
| [12] | 吕阳光, 左培培, 杨正金, 徐铜文. 三嗪框架聚合物膜用于有机纳滤甲醇/正己烷分离[J]. 化工学报, 2023, 74(4): 1598-1606. |
| [13] | 王思琪, 顾天宇, 陈献富, 王通, 李佳, 柯威, 李小锋, 范益群. 陶瓷膜用于杜仲叶提取液澄清的分离特性与膜污染机制研究[J]. 化工学报, 2023, 74(3): 1113-1125. |
| [14] | 程文婷, 李杰, 徐丽, 程芳琴, 刘国际. AlCl3·6H2O在FeCl3、CaCl2、KCl及KCl–FeCl3溶液中溶解度的实验及预测[J]. 化工学报, 2023, 74(2): 642-652. |
| [15] | 齐欣欣, 贾辉, 高菲, 王琦, 尹延梅, 王捷. 基于双侧阵列电极的电阻抗成像原位膜污染监测[J]. 化工学报, 2023, 74(11): 4720-4729. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号