化工学报 ›› 2025, Vol. 76 ›› Issue (8): 4228-4238.DOI: 10.11949/0438-1157.20250159
李云昊1,2( ), 徐纯刚1,2(
), 徐纯刚1,2( ), 李小森1,2, 付骏1,2, 王屹1,2, 陈朝阳1,2
), 李小森1,2, 付骏1,2, 王屹1,2, 陈朝阳1,2
收稿日期:2025-02-21
修回日期:2025-05-07
出版日期:2025-08-25
发布日期:2025-09-17
通讯作者:
徐纯刚
作者简介:李云昊(2000—),男,硕士研究生,liyunhao@mail.ustc.edu.cn
基金资助:
Yunhao LI1,2( ), Chungang XU1,2(
), Chungang XU1,2( ), Xiaosen LI1,2, Jun FU1,2, Yi WANG1,2, Zhaoyang CHEN1,2
), Xiaosen LI1,2, Jun FU1,2, Yi WANG1,2, Zhaoyang CHEN1,2
Received:2025-02-21
Revised:2025-05-07
Online:2025-08-25
Published:2025-09-17
Contact:
Chungang XU
摘要:
基于固体促进剂和化学促进剂的作用机理,研究了CP水合物晶种+THF固液复配型促进剂对盐水体系中CO2水合物形成及影响,通过宏观实验结合激光拉曼、扫描电子显微镜(SEM)等表征方式,获得了不同浓度组合固液复配促进剂对CO2水合物生长动力学、气体消耗量以及水合物形貌等影响规律。研究表明,CP水合物晶种+THF的协同作用有效地提升了CO₂水合物的形成效率及形成速率;盐水体系中,最佳摩尔分数组合为2.78% CP+ 2.78%THF时,CO2气体消耗量达到23.90 mmol,水合物的平均生成速率为2.94×10-4 mmol/(mol·s),相较于已报道的结果,分别提高了15.00%和66.67%。此外,由SEM和Raman等微观分析手段可以得出,纯水体系中水合物形成受扩散控制,气体消耗量普遍较高,而盐水体系则受控于界面反应,盐离子的静电效应使得水合物界面更致密,从而显著降低了气体消耗量。模拟海洋环境中的CO2水合物沉降过程实验表明,盐水中合成的CO2水合物密度更大且易于沉降,更有利于海洋碳封存。这些微观结构特征与宏观实验数据一致,验证了固液复配型促进剂体系的有效性。该成果为海洋碳封存技术提供了关键参数优化方案与理论支撑,对实现高效稳定的海洋二氧化碳封存具有重要应用价值。
中图分类号:
李云昊, 徐纯刚, 李小森, 付骏, 王屹, 陈朝阳. 固液复配型促进剂对盐水体系CO2水合物形成影响研究[J]. 化工学报, 2025, 76(8): 4228-4238.
Yunhao LI, Chungang XU, Xiaosen LI, Jun FU, Yi WANG, Zhaoyang CHEN. Study on the effect of solid-liquid blended promoters on the formation of CO2 hydrates in saline water system[J]. CIESC Journal, 2025, 76(8): 4228-4238.
| Materials | Purity/% | Suppliers |
|---|---|---|
| CP | 98.0 | Aladdin Reagent Co., Ltd. (Shanghai, China) |
| THF | 99.0 | Aladdin Reagent Co., Ltd. (Shanghai, China) |
| NaCl | 99.5 | Aladdin Reagent Co., Ltd. (Shanghai, China) |
表1 实验中使用的化学试剂
Table 1 Chemical reagents used in the experiments
| Materials | Purity/% | Suppliers |
|---|---|---|
| CP | 98.0 | Aladdin Reagent Co., Ltd. (Shanghai, China) |
| THF | 99.0 | Aladdin Reagent Co., Ltd. (Shanghai, China) |
| NaCl | 99.5 | Aladdin Reagent Co., Ltd. (Shanghai, China) |
| Item | Liquid | P1/MPa | P2/MPa | t/s | nw/mol | ng/mmol | (ng/nw)/(mmol/mol) | v/(10-5 mmol/(mol·s)) |
|---|---|---|---|---|---|---|---|---|
| Sys1 (2.00%CP + 3.56%THF) | PW | 4.74 | 4.28 | 73000 | 1.11 | 9.01 | 8.11 | 12.3 |
| 4.72 | 4.25 | 71500 | 1.11 | 9.25 | 8.33 | 11.9 | ||
| 4.76 | 4.30 | 74200 | 1.11 | 8.85 | 7.97 | 10.8 | ||
| Sys2 (2.56%CP + 3.00%THF) | PW | 4.84 | 4.05 | 60000 | 1.11 | 26.90 | 24.21 | 44.8 |
| 4.82 | 4.02 | 61500 | 1.11 | 27.50 | 24.77 | 42.1 | ||
| 4.88 | 4.10 | 59900 | 1.11 | 26.20 | 23.60 | 40.3 | ||
| Sys3 (2.78%CP + 2.78%THF) | PW | 4.78 | 3.61 | 60000 | 1.11 | 34.73 | 31.30 | 57.9 |
| 4.80 | 3.58 | 62000 | 1.11 | 35.20 | 31.71 | 55.7 | ||
| 4.75 | 3.64 | 58500 | 1.11 | 34.10 | 30.72 | 53.6 | ||
| Sys4 (3.00%CP + 2.56%THF) | PW | 4.72 | 3.76 | 53000 | 1.11 | 31.64 | 28.48 | 57.7 |
| 4.74 | 3.73 | 54500 | 1.11 | 32.10 | 28.90 | 57.2 | ||
| 4.70 | 3.79 | 51800 | 1.11 | 31.20 | 28.11 | 54.3 | ||
| Sys5 (3.56%CP + 2.00%THF) | PW | 4.71 | 3.87 | 57000 | 1.11 | 28.85 | 25.97 | 50.6 |
| 4.73 | 3.84 | 58200 | 1.11 | 29.30 | 26.40 | 48.1 | ||
| 4.69 | 3.90 | 55800 | 1.11 | 28.40 | 25.59 | 47.3 | ||
| Sys6 (2.00%CP + 3.56%THF) | SW | 4.73 | 4.35 | 52800 | 1.11 | 4.61 | 4.15 | 8.73 |
| 4.71 | 4.32 | 54000 | 1.11 | 4.80 | 4.32 | 8.89 | ||
| 4.75 | 4.38 | 51200 | 1.11 | 4.45 | 4.01 | 7.82 | ||
| Sys7 (2.56%CP + 3.00%THF) | SW | 4.78 | 4.40 | 60565 | 1.11 | 5.41 | 4.87 | 8.44 |
| 4.79 | 4.38 | 62000 | 1.11 | 5.30 | 4.77 | 7.69 | ||
| 4.77 | 4.42 | 59000 | 1.11 | 5.55 | 5.00 | 8.47 | ||
| Sys8 (2.78%CP + 2.78%THF) | SW | 4.70 | 3.87 | 78640 | 1.11 | 23.14 | 20.83 | 29.40 |
| 4.72 | 3.85 | 80000 | 1.11 | 23.90 | 21.53 | 27.50 | ||
| 4.68 | 3.90 | 77000 | 1.11 | 22.80 | 20.54 | 26.70 | ||
| Sys9 (3.00%CP + 2.56%THF) | SW | 4.73 | 4.05 | 59500 | 1.11 | 22.54 | 20.30 | 37.9 |
| 4.75 | 4.02 | 60000 | 1.11 | 23.00 | 21.00 | 34.5 | ||
| 4.71 | 4.08 | 60200 | 1.11 | 22.10 | 20.70 | 34.3 | ||
| Sys10 (3.56%CP + 2.00%THF) | SW | 4.80 | 4.12 | 50080 | 1.11 | 22.69 | 20.43 | 45.3 |
| 4.82 | 4.10 | 51000 | 1.11 | 23.10 | 20.81 | 41.7 | ||
| 4.78 | 4.14 | 49500 | 1.11 | 22.30 | 20.09 | 40.6 | ||
| Yan, et al.[ | PW | 3.80 | 2.20 | 21600 | 9.45 | 260.63 | 27.58 | 142.0 |
| Li, et al.[ | PW | 3.60 | 2.51 | 108000 | 13.11 | 250.00 | 19.06 | 17.64 |
| Khandelwal, et al.[ | SW | 3.40 | 2.37 | 14400 | 2.00 | 37.44 | 18.72 | 139.93 |
表2 不同体系中CO2水合物形成数据
Table 2 Data on hydrate formation indifferent systems
| Item | Liquid | P1/MPa | P2/MPa | t/s | nw/mol | ng/mmol | (ng/nw)/(mmol/mol) | v/(10-5 mmol/(mol·s)) |
|---|---|---|---|---|---|---|---|---|
| Sys1 (2.00%CP + 3.56%THF) | PW | 4.74 | 4.28 | 73000 | 1.11 | 9.01 | 8.11 | 12.3 |
| 4.72 | 4.25 | 71500 | 1.11 | 9.25 | 8.33 | 11.9 | ||
| 4.76 | 4.30 | 74200 | 1.11 | 8.85 | 7.97 | 10.8 | ||
| Sys2 (2.56%CP + 3.00%THF) | PW | 4.84 | 4.05 | 60000 | 1.11 | 26.90 | 24.21 | 44.8 |
| 4.82 | 4.02 | 61500 | 1.11 | 27.50 | 24.77 | 42.1 | ||
| 4.88 | 4.10 | 59900 | 1.11 | 26.20 | 23.60 | 40.3 | ||
| Sys3 (2.78%CP + 2.78%THF) | PW | 4.78 | 3.61 | 60000 | 1.11 | 34.73 | 31.30 | 57.9 |
| 4.80 | 3.58 | 62000 | 1.11 | 35.20 | 31.71 | 55.7 | ||
| 4.75 | 3.64 | 58500 | 1.11 | 34.10 | 30.72 | 53.6 | ||
| Sys4 (3.00%CP + 2.56%THF) | PW | 4.72 | 3.76 | 53000 | 1.11 | 31.64 | 28.48 | 57.7 |
| 4.74 | 3.73 | 54500 | 1.11 | 32.10 | 28.90 | 57.2 | ||
| 4.70 | 3.79 | 51800 | 1.11 | 31.20 | 28.11 | 54.3 | ||
| Sys5 (3.56%CP + 2.00%THF) | PW | 4.71 | 3.87 | 57000 | 1.11 | 28.85 | 25.97 | 50.6 |
| 4.73 | 3.84 | 58200 | 1.11 | 29.30 | 26.40 | 48.1 | ||
| 4.69 | 3.90 | 55800 | 1.11 | 28.40 | 25.59 | 47.3 | ||
| Sys6 (2.00%CP + 3.56%THF) | SW | 4.73 | 4.35 | 52800 | 1.11 | 4.61 | 4.15 | 8.73 |
| 4.71 | 4.32 | 54000 | 1.11 | 4.80 | 4.32 | 8.89 | ||
| 4.75 | 4.38 | 51200 | 1.11 | 4.45 | 4.01 | 7.82 | ||
| Sys7 (2.56%CP + 3.00%THF) | SW | 4.78 | 4.40 | 60565 | 1.11 | 5.41 | 4.87 | 8.44 |
| 4.79 | 4.38 | 62000 | 1.11 | 5.30 | 4.77 | 7.69 | ||
| 4.77 | 4.42 | 59000 | 1.11 | 5.55 | 5.00 | 8.47 | ||
| Sys8 (2.78%CP + 2.78%THF) | SW | 4.70 | 3.87 | 78640 | 1.11 | 23.14 | 20.83 | 29.40 |
| 4.72 | 3.85 | 80000 | 1.11 | 23.90 | 21.53 | 27.50 | ||
| 4.68 | 3.90 | 77000 | 1.11 | 22.80 | 20.54 | 26.70 | ||
| Sys9 (3.00%CP + 2.56%THF) | SW | 4.73 | 4.05 | 59500 | 1.11 | 22.54 | 20.30 | 37.9 |
| 4.75 | 4.02 | 60000 | 1.11 | 23.00 | 21.00 | 34.5 | ||
| 4.71 | 4.08 | 60200 | 1.11 | 22.10 | 20.70 | 34.3 | ||
| Sys10 (3.56%CP + 2.00%THF) | SW | 4.80 | 4.12 | 50080 | 1.11 | 22.69 | 20.43 | 45.3 |
| 4.82 | 4.10 | 51000 | 1.11 | 23.10 | 20.81 | 41.7 | ||
| 4.78 | 4.14 | 49500 | 1.11 | 22.30 | 20.09 | 40.6 | ||
| Yan, et al.[ | PW | 3.80 | 2.20 | 21600 | 9.45 | 260.63 | 27.58 | 142.0 |
| Li, et al.[ | PW | 3.60 | 2.51 | 108000 | 13.11 | 250.00 | 19.06 | 17.64 |
| Khandelwal, et al.[ | SW | 3.40 | 2.37 | 14400 | 2.00 | 37.44 | 18.72 | 139.93 |

图4 在纯水和盐水体系中Sys3和Sys8条件下合成的CO2水合物沉降过程中分解和形态变化
Fig.4 Decomposition times and morphological features of CO2 hydrates formed in Sys3 and Sys8 in pure and saltwater systems
| Hydrates | Liquid | Time (when the hydrate particles start to disperse) | Time (when the hydrate particles disperse completely) |
|---|---|---|---|
| Sys3 | pure water | T1 (15.6 h) | T2 (24.0 h) |
| Sys8 | pure water | T3 (12.4 h) | T4 (20.0 h) |
| Sys3 | saltwater | T5 (4.5 h) | T6 (6.5 h) |
| Sys8 | saltwater | T7 (3.5 h) | T8 (6.0 h) |
表3 纯水和盐水中CO2水合物的稳定性测试结果
Table 3 Stability test outcomes of CO2 hydrates in pure water and saltwater
| Hydrates | Liquid | Time (when the hydrate particles start to disperse) | Time (when the hydrate particles disperse completely) |
|---|---|---|---|
| Sys3 | pure water | T1 (15.6 h) | T2 (24.0 h) |
| Sys8 | pure water | T3 (12.4 h) | T4 (20.0 h) |
| Sys3 | saltwater | T5 (4.5 h) | T6 (6.5 h) |
| Sys8 | saltwater | T7 (3.5 h) | T8 (6.0 h) |
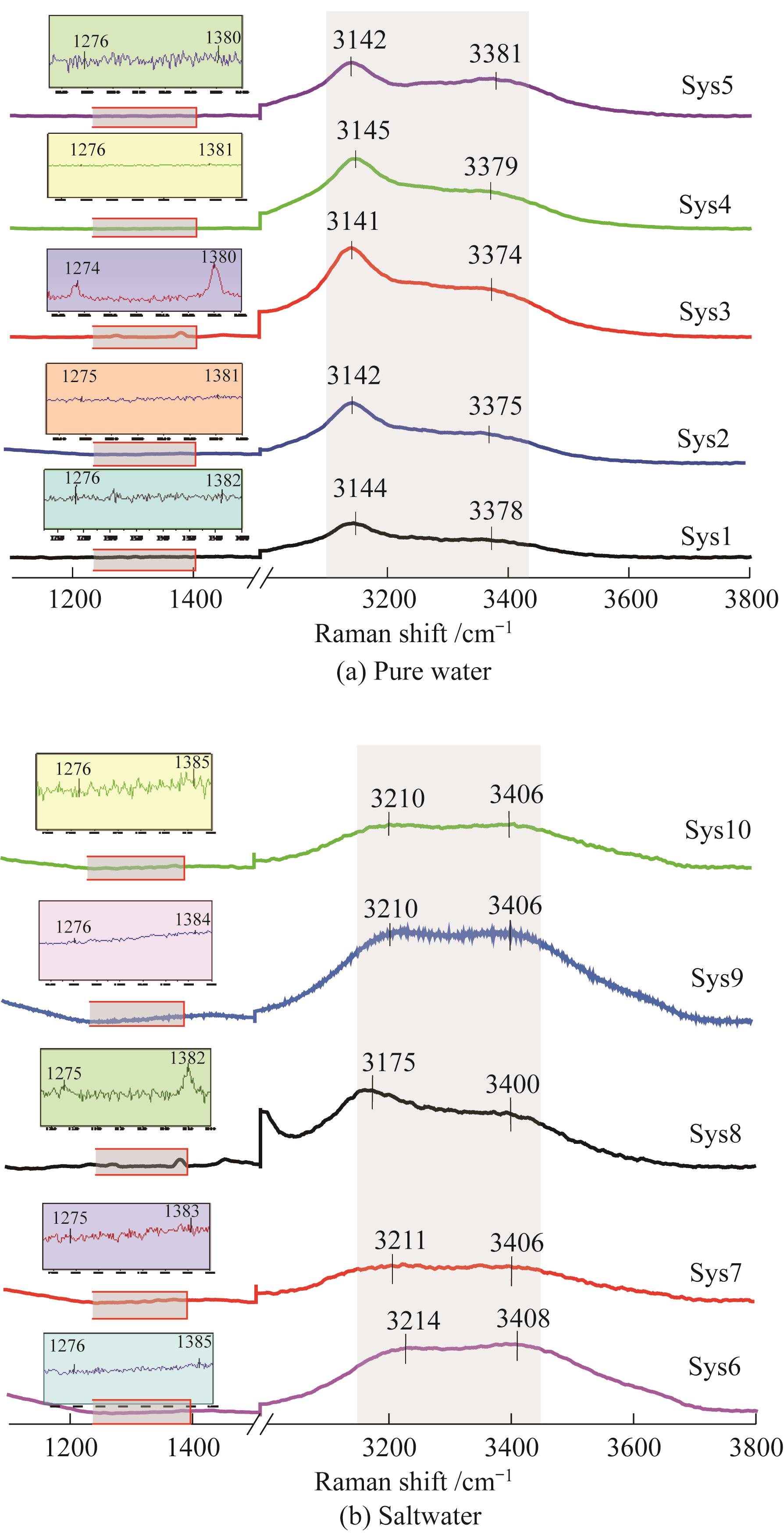
图5 在初始压力为4.70 MPa、温度为277.0 K条件下不同体系中形成的水合物中二氧化碳和水的拉曼光谱
Fig.5 Raman spectra of CO2 and H2O in the hydrates formed from various systems at an initial pressure of 4.70 MPa and temperature of 277.0 K
| [1] | Amstrup S C, Deweaver E T, Douglas D C, et al. Greenhouse gas mitigation can reduce sea-ice loss and increase polar bear persistence[J]. Nature, 2010, 468(7326): 955-958. |
| [2] | Ajayi T, Gomes J S, Bera A. A review of CO2 storage in geological formations emphasizing modeling, monitoring and capacity estimation approaches[J]. Petroleum Science, 2019, 16(5): 1028-1063. |
| [3] | Stainforth D A, Aina T, Christensen C, et al. Uncertainty in predictions of the climate response to rising levels of greenhouse gases[J]. Nature, 2005, 433: 403-406. |
| [4] | Siegel D A, DeVries T, Doney S C, et al. Assessing the sequestration time scales of some ocean-based carbon dioxide reduction strategies[J]. Environmental Research Letters, 2021, 16(10): 104003. |
| [5] | Orr J C, Fabry V J, Aumont O, et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms[J]. Nature, 2005, 437(7059): 681-686. |
| [6] | Ricke K L, Orr J C, Schneider K, et al. Risks to coral reefs from ocean carbonate chemistry changes in recent earth system model projections[J]. Environmental Research Letters, 2013, 8(3): 034003. |
| [7] | Teng Y, Zhang D. Long-term viability of carbon sequestration in deep-sea sediments[J]. Sci. Adv., 2018, 4(7): eaao6588. |
| [8] | Cao X W, Wang H C, Yang K R, et al. Hydrate-based CO2 sequestration technology: feasibilities, mechanisms, influencing factors, and applications[J]. Journal of Petroleum Science and Engineering, 2022, 219: 111121. |
| [9] | Kumar Y, Sangwai J S. A perspective on the effect of physicochemical parameters, macroscopic environment, additives, and economics to harness the large-scale hydrate-based CO2 sequestration potential in oceans[J]. ACS Sustainable Chemistry & Engineering, 2023, 11(30): 10950-10979. |
| [10] | Zulqarnain, Mohd Yusoff M H, Keong L K, et al. Recent development of integrating CO2 hydrogenation into methanol with ocean thermal energy conversion (OTEC) as potential source of green energy[J]. Green Chemistry Letters and Reviews, 2023, 16(1): 2152740. |
| [11] | Yang M J, Shan X Y, Sun H R, et al. Review of thermodynamic and kinetic properties of CO2 hydrate phase transition process[J]. Chemical Engineering Science, 2025, 308: 121383. |
| [12] | Gholinezhad J, Chapoy A, Tohidi B. Separation and capture of carbon dioxide from CO2/H2 syngas mixture using semi-clathrate hydrates[J]. Chemical Engineering Research and Design, 2011, 89(9): 1747-1751. |
| [13] | Hayama H, Mitarai M, Mori H, et al. Surfactant effects on crystal growth dynamics and crystal morphology of methane hydrate formed at gas/liquid interface[J]. Crystal Growth & Design, 2016, 16(10): 6084-6088. |
| [14] | Hazas M, Hopper A. Broadband ultrasonic location systems for improved indoor positioning[J]. IEEE Transactions on Mobile Computing, 2006, 5(5): 536-547. |
| [15] | Liu N, Huang J L, Meng F, et al. Experimental study on the mechanism of enhanced CO2 hydrate generation by thermodynamic promoters[J]. ACS Sustainable Chemistry & Engineering, 2023, 11(14): 5367-5375. |
| [16] | Nesterov A N, Reshetnikov A M. New combination of thermodynamic and kinetic promoters to enhance carbon dioxide hydrate formation under static conditions[J]. Chemical Engineering Journal, 2019, 378: 122165. |
| [17] | Przybyla R J, Shelton S E, Guedes A, et al. In-air rangefinding with an AlN piezoelectric micromachined ultrasound transducer[J]. IEEE Sensors Journal, 2011, 11(11): 2690-2697. |
| [18] | Saad M M, Bleakley C J, Ballal T, et al. High-accuracy reference-free ultrasonic location estimation[J]. IEEE Transactions on Instrumentation and Measurement, 2012, 61(6): 1561-1570. |
| [19] | Adisasmito S, R J Ⅲ Frank, Sloan E D. Hydrates of carbon dioxide and methane mixtures[J]. Journal of Chemical & Engineering Data, 1991, 36(1): 68-71. |
| [20] | Sagidullin A K, Skiba S S, Adamova T P, et al. Investigation of the formation processes of CO2 hydrate films on the interface of liquid carbon dioxide with humic acids solutions[J]. Chinese Journal of Chemical Engineering, 2025, 79: 53-61. |
| [21] | Sandru F D, Ungureanu V I, Silea I. Ultrasonic and VCSEL sensor fusion for distance measurement in parking assistance[C]//2023 24th International Conference on Control Systems and Computer Science (CSCS). Bucharest, Romania: IEEE, 2023: 35-40. |
| [22] | Di Profio P, Arca S, Germani R, et al. Surfactant promoting effects on clathrate hydrate formation: Are micelles really involved?[J]. Chemical Engineering Science, 2005, 60(15): 4141-4145. |
| [23] | Watanabe K, Imai S, Mori Y H. Surfactant effects on hydrate formation in an unstirred gas/liquid system: an experimental study using HFC-32 and sodium dodecyl sulfate[J]. Chemical Engineering Science, 2005, 60(17): 4846-4857. |
| [24] | Choi S U S, Zhang Z G, Yu W, et al. Anomalous thermal conductivity enhancement in nanotube suspensions[J]. Applied Physics Letters, 2001, 79(14): 2252-2254. |
| [25] | Reddy M C S, Rao V V. Experimental studies on thermal conductivity of blends of ethylene glycol-water-based TiO2 nanofluids[J]. International Communications in Heat and Mass Transfer, 2013, 46: 31-36. |
| [26] | Xu C G, Yu Y S, Ding Y L, et al. The effect of hydrate promoters on gas uptake[J]. Physical Chemistry Chemical Physics, 2017, 19(32): 21769-21776. |
| [27] | Mok J, Choi W, Kim S, et al. NaCl-induced enhancement of thermodynamic and kinetic CO2 selectivity in CO2 + N2 hydrate formation and its significance for CO2 sequestration[J]. Chemical Engineering Journal, 2023, 451: 138633. |
| [28] | Lee W, Kang D W, Ahn Y H, et al. Blended hydrate seed and liquid promoter for the acceleration of hydrogen hydrate formation[J]. Renewable and Sustainable Energy Reviews, 2023, 177: 113217. |
| [29] | John E, Matschei T, Stephan D. Nucleation seeding with calcium silicate hydrate—a review[J]. Cement and Concrete Research, 2018, 113: 74-85. |
| [30] | Kashchiev D, Firoozabadi A. Induction time in crystallization of gas hydrates[J]. Journal of Crystal Growth, 2003, 250(3/4): 499-515. |
| [31] | Khandelwal H, Qureshi M F, Zheng J J, et al. Effect of L-tryptophan in promoting the kinetics of carbon dioxide hydrate formation[J]. Energy & Fuels, 2021, 35(1): 649-658. |
| [32] | Baek S, Lee W, Min J, et al. Hydrate seeding effect on the metastability of CH4 hydrate[J]. Korean Journal of Chemical Engineering, 2020, 37(2): 341-349. |
| [33] | Huang Z Y, Xu C G, Li X S, et al. Investigation of the influence mechanism of CO2 hydrate formation in seawater systems in the presence of solid promoters by combining in situ Raman analysis with macroscopic experiments[J]. Energy & Fuels, 2024, 38(8): 7137-7147. |
| [34] | Linga P, Kumar R, Lee J D, et al. A new apparatus to enhance the rate of gas hydrate formation: application to capture of carbon dioxide[J]. International Journal of Greenhouse Gas Control, 2010, 4(4): 630-637. |
| [35] | Linga P, Kumar R, Englezos P. The clathrate hydrate process for post and pre-combustion capture of carbon dioxide[J]. Journal of Hazardous Materials, 2007, 149(3): 625-629. |
| [36] | Yan S, Dai W J, Wang S L, et al. Graphene oxide: an effective promoter for CO2 hydrate formation[J]. Energies, 2018, 11(7): 1756. |
| [37] | Li Y, Gambelli A M, Rossi F. Experimental study on the effect of SDS and micron copper particles mixture on carbon dioxide hydrates formation[J]. Energies, 2022, 15(18): 6540. |
| [38] | Lo C, Zhang J S, Somasundaran P, et al. Raman spectroscopic studies of surfactant effect on the water structure around hydrate guest molecules[J]. The Journal of Physical Chemistry Letters, 2010, 1(18): 2676-2679. |
| [1] | 吴馨, 龚建英, 李祥宇, 王宇涛, 杨小龙, 蒋震. 超声波激励疏水表面液滴运动的实验研究[J]. 化工学报, 2025, 76(S1): 133-139. |
| [2] | 曹庆泰, 郭松源, 李建强, 蒋赞, 汪彬, 耑锐, 吴静怡, 杨光. 负过载下多孔隔板对液氧贮箱蓄液性能的影响研究[J]. 化工学报, 2025, 76(S1): 217-229. |
| [3] | 佘海龙, 胡光忠, 崔晓钰, 柳忠彬, 彭帝, 李航. 不同节流工质下叠层微通道分布式节流制冷器性能研究[J]. 化工学报, 2025, 76(8): 4017-4029. |
| [4] | 田宇红, 杜壮壮, 徐慧芳, 祝自强, 王宇聪. ZIF-8基多孔液体制备及其SO2吸附性能[J]. 化工学报, 2025, 76(8): 4284-4296. |
| [5] | 刘璐, 杨莹, 杨浩文, 王太, 王腾, 董新宇, 闫润. 星形亲水区组合表面冷凝液滴脱落特性实验研究[J]. 化工学报, 2025, 76(8): 3905-3914. |
| [6] | 何晨, 陆明飞, 王令金, 许晓颖, 董鹏博, 赵文涛, 隆武强. 氨-甲醇高压混合气稀燃层流实验与模拟研究[J]. 化工学报, 2025, 76(8): 4248-4258. |
| [7] | 常心泉, 张克学, 王军, 夏国栋. 自由分子区内不规则颗粒的热泳力计算[J]. 化工学报, 2025, 76(8): 3944-3953. |
| [8] | 卢煦旸, 徐强, 康浩鹏, 史健, 曹泽水, 郭烈锦. 化学链制氢系统中磁铁矿氧载体的CO还原特性研究[J]. 化工学报, 2025, 76(7): 3286-3294. |
| [9] | 乔亮, 李尚, 刘新亮, 王明, 张沛, 侯影飞. 三元共聚物稠油降黏剂的合成及分子模拟研究[J]. 化工学报, 2025, 76(7): 3686-3695. |
| [10] | 周臣儒, 刘陈伟, 王志远, 綦民辉, 董三宝, 王翔宇, 李明忠. 甲醇和乙二醇对甲烷水合物黏附强度的影响[J]. 化工学报, 2025, 76(7): 3596-3604. |
| [11] | 徐鹏国, 孟子衡, 朱干宇, 李会泉, 王晨晔, 孙振华, 田国才. 粗碳酸锂CO2微气泡深度碳化工艺与动力学研究[J]. 化工学报, 2025, 76(7): 3325-3338. |
| [12] | 刘沁雯, 叶恒冰, 张逸伟, 朱法华, 钟文琪. 煤与禽类粪便混合燃料的加压富氧燃烧特性研究[J]. 化工学报, 2025, 76(7): 3487-3497. |
| [13] | 刘峰, 韩春硕, 张益, 刘彦成, 郁林军, 申家伟, 高晓泉, 杨凯. 高温高盐环境下单烃链和双烃链表面活性剂对油水界面性质影响的微观机理研究[J]. 化工学报, 2025, 76(6): 2939-2957. |
| [14] | 颜成辉, 谢应明, 庞治海, 翁盛乔. 泡沫多孔材料对R134a水合物蓄冷的强化研究[J]. 化工学报, 2025, 76(6): 3084-3092. |
| [15] | 赵清萍, 张敏, 赵辉, 王刚, 邱永福. 乙烯氢甲酯化合成丙酸甲酯的氢键作用机制及反应动力学研究[J]. 化工学报, 2025, 76(6): 2701-2713. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号