化工学报 ›› 2025, Vol. 76 ›› Issue (9): 4770-4785.DOI: 10.11949/0438-1157.20250218
收稿日期:2025-03-05
修回日期:2025-05-06
出版日期:2025-09-25
发布日期:2025-10-23
通讯作者:
周桓
作者简介:王偲凡(1999—),女,硕士研究生,952874405@qq.com
基金资助:
Sifan WANG( ), Yifan LI, Jiangbo CHEN, Huan ZHOU(
), Yifan LI, Jiangbo CHEN, Huan ZHOU( )
)
Received:2025-03-05
Revised:2025-05-06
Online:2025-09-25
Published:2025-10-23
Contact:
Huan ZHOU
摘要:
碳酸盐型卤水广泛存在于自然界和各种工业过程,新型资源和过程的开发同样需要相图和热力学模型的支持。碱金属(Li,Na,K)的碳酸盐卤水体系是典型和普遍的,补充必要的相图数据完善卤水体系热力学模型是亟需的。为此,本研究首先对多温相图数据缺乏的Li2CO3-K2CO3-H2O体系实验补充了273.15、323.15、348.15 K的相图数据;而后在完善Li2CO3、Na2CO3、K2CO3三个二元体系热力学模型的基础上,重新获得CO
中图分类号:
王偲凡, 栗一帆, 陈江波, 周桓. 碳酸盐型卤水Li+, Na+, K+, CO
Sifan WANG, Yifan LI, Jiangbo CHEN, Huan ZHOU. Thermodynamics and phase diagram modeling of carbonate-type brines Li+, Na+, K+, CO
| 体系 | T/K | 文献 |
|---|---|---|
| Li2CO3-H2O | 271.04~575.15 | [ |
| Na2CO3-H2O | 273.15~368.15 | [ |
| K2CO3-H2O | 262.15~499.15 | [ |
| Li2CO3-Na2CO3-H2O | 278.15~373.15 | [ |
| Na2CO3-K2CO3-H2O | 273.15~373.15 | [ |
| Li2CO3-K2CO3-H2O | 288.15,298.15 | [ |
| Li2CO3-Na2CO3-K2CO3-H2O | 288.15,298.15 | [ |
表1 Li+, Na+, K+, CO32--H2O相关体系相图的相关文献汇总
Table 1 Solubility data in the Li+, Na+, K+, CO32--H2O system
| 体系 | T/K | 文献 |
|---|---|---|
| Li2CO3-H2O | 271.04~575.15 | [ |
| Na2CO3-H2O | 273.15~368.15 | [ |
| K2CO3-H2O | 262.15~499.15 | [ |
| Li2CO3-Na2CO3-H2O | 278.15~373.15 | [ |
| Na2CO3-K2CO3-H2O | 273.15~373.15 | [ |
| Li2CO3-K2CO3-H2O | 288.15,298.15 | [ |
| Li2CO3-Na2CO3-K2CO3-H2O | 288.15,298.15 | [ |
| 序号 | 液相组成w(B)/% | 密度 ρ/(kg·L-1) | pH | 湿固相组成w(B)/% | 固相 | |||
|---|---|---|---|---|---|---|---|---|
| K2CO3 | Li2CO3 | K2CO3 | Li2CO3 | |||||
| T=273.15 K | ||||||||
| 1, A | 0 | 1.51 | 1.0263 | 11.09 | 0 | 65.25 | S1 | |
| 2 | 8.27 | 1.40 | 1.1450 | 11.58 | 4.00 | 62.58 | S1 | |
| 3 | 16.63 | 1.28 | 1.2153 | 11.72 | 6.32 | 63.43 | S1 | |
| 4 | 24.37 | 1.07 | 1.3037 | 11.94 | 8.97 | 64.58 | S1 | |
| 5 | 31.69 | 0.88 | 1.3561 | 12.36 | 12.21 | 62.33 | S1 | |
| 6 | 37.91 | 0.71 | 1.4014 | 12.56 | 16.61 | 56.56 | S1 | |
| 7 | 44.85 | 0.53 | 1.4255 | 12.67 | 20.53 | 54.86 | S1 | |
| 8, E | 51.32 | 0.43 | 1.4547 | 12.89 | 36.30 | 47.03 | S1+S2 | |
| 9 | 51.22 | 0.28 | 1.4482 | 12.86 | 60.32 | 22.15 | S2 | |
| 10 | 51.13 | 0.14 | 1.4506 | 12.85 | 67.96 | 6.54 | S2 | |
| 11, B | 51.05 | 0 | 1.4512 | 12.88 | 73.00 | 0 | S2 | |
| T=323.15 K | ||||||||
| 1, C | 0 | 1.07 | 1.0212 | 11.01 | 0 | 67.50 | S1 | |
| 2 | 9.31 | 0.92 | 1.1437 | 11.67 | 4.52 | 58.20 | S1 | |
| 3 | 15.83 | 0.89 | 1.1983 | 11.83 | 7.04 | 60.15 | S1 | |
| 4 | 22.94 | 0.79 | 1.2731 | 12.16 | 9.21 | 63.32 | S1 | |
| 5 | 29.06 | 0.71 | 1.3253 | 12.43 | 10.46 | 62.72 | S1 | |
| 6 | 34.18 | 0.63 | 1.3776 | 12.58 | 12.11 | 65.54 | S1 | |
| 7 | 39.62 | 0.47 | 1.4015 | 12.66 | 15.54 | 60.45 | S1 | |
| 8 | 44.53 | 0.39 | 1.4359 | 13.20 | 17.62 | 61.13 | S1 | |
| 9 | 49.83 | 0.32 | 1.4720 | 13.37 | 16.78 | 65.68 | S1 | |
| 10, F | 55.53 | 0.24 | 1.5393 | 13.51 | 37.55 | 45.15 | S1+S2 | |
| 11 | 55.32 | 0.13 | 1.5322 | 13.49 | 60.48 | 21.29 | S2 | |
| 12 | 55.14 | 0.07 | 1.5363 | 13.43 | 66.70 | 12.13 | S2 | |
| 13, D | 54.98 | 0 | 1.5331 | 13.45 | 73.35 | 0 | S2 | |
| T=348.15 K | ||||||||
| 1, M | 0 | 0.88 | 1.0188 | 10.96 | 0 | 63.58 | S1 | |
| 2 | 9.16 | 0.79 | 1.1386 | 11.39 | 3.09 | 65.73 | S1 | |
| 3 | 18.87 | 0.69 | 1.2285 | 11.63 | 7.33 | 63.31 | S1 | |
| 4 | 28.28 | 0.51 | 1.3109 | 11.79 | 10.11 | 64.12 | S1 | |
| 5 | 35.49 | 0.41 | 1.3860 | 12.15 | 14.76 | 58.58 | S1 | |
| 6 | 40.96 | 0.33 | 1.4091 | 12.41 | 16.11 | 61.26 | S1 | |
| 7 | 47.36 | 0.26 | 1.4533 | 12.55 | 20.02 | 58.33 | S1 | |
| 8 | 53.12 | 0.19 | 1.5117 | 12.98 | 23.18 | 57.19 | S1 | |
| 9, G | 57.75 | 0.16 | 1.5677 | 13.68 | 42.36 | 39.35 | S1+S2 | |
| 10 | 57.73 | 0.10 | 1.5619 | 13.67 | 68.59 | 10.12 | S2 | |
| 11, N | 57.71 | 0 | 1.5601 | 13.63 | 72.63 | 0 | S2 | |
表 2 Li2CO3-K2CO3-H2O 三元体系固-液相平衡数据
Table 2 Solid-liquid phase equilibrium data of Li2CO3-K2CO3-H2O ternary system
| 序号 | 液相组成w(B)/% | 密度 ρ/(kg·L-1) | pH | 湿固相组成w(B)/% | 固相 | |||
|---|---|---|---|---|---|---|---|---|
| K2CO3 | Li2CO3 | K2CO3 | Li2CO3 | |||||
| T=273.15 K | ||||||||
| 1, A | 0 | 1.51 | 1.0263 | 11.09 | 0 | 65.25 | S1 | |
| 2 | 8.27 | 1.40 | 1.1450 | 11.58 | 4.00 | 62.58 | S1 | |
| 3 | 16.63 | 1.28 | 1.2153 | 11.72 | 6.32 | 63.43 | S1 | |
| 4 | 24.37 | 1.07 | 1.3037 | 11.94 | 8.97 | 64.58 | S1 | |
| 5 | 31.69 | 0.88 | 1.3561 | 12.36 | 12.21 | 62.33 | S1 | |
| 6 | 37.91 | 0.71 | 1.4014 | 12.56 | 16.61 | 56.56 | S1 | |
| 7 | 44.85 | 0.53 | 1.4255 | 12.67 | 20.53 | 54.86 | S1 | |
| 8, E | 51.32 | 0.43 | 1.4547 | 12.89 | 36.30 | 47.03 | S1+S2 | |
| 9 | 51.22 | 0.28 | 1.4482 | 12.86 | 60.32 | 22.15 | S2 | |
| 10 | 51.13 | 0.14 | 1.4506 | 12.85 | 67.96 | 6.54 | S2 | |
| 11, B | 51.05 | 0 | 1.4512 | 12.88 | 73.00 | 0 | S2 | |
| T=323.15 K | ||||||||
| 1, C | 0 | 1.07 | 1.0212 | 11.01 | 0 | 67.50 | S1 | |
| 2 | 9.31 | 0.92 | 1.1437 | 11.67 | 4.52 | 58.20 | S1 | |
| 3 | 15.83 | 0.89 | 1.1983 | 11.83 | 7.04 | 60.15 | S1 | |
| 4 | 22.94 | 0.79 | 1.2731 | 12.16 | 9.21 | 63.32 | S1 | |
| 5 | 29.06 | 0.71 | 1.3253 | 12.43 | 10.46 | 62.72 | S1 | |
| 6 | 34.18 | 0.63 | 1.3776 | 12.58 | 12.11 | 65.54 | S1 | |
| 7 | 39.62 | 0.47 | 1.4015 | 12.66 | 15.54 | 60.45 | S1 | |
| 8 | 44.53 | 0.39 | 1.4359 | 13.20 | 17.62 | 61.13 | S1 | |
| 9 | 49.83 | 0.32 | 1.4720 | 13.37 | 16.78 | 65.68 | S1 | |
| 10, F | 55.53 | 0.24 | 1.5393 | 13.51 | 37.55 | 45.15 | S1+S2 | |
| 11 | 55.32 | 0.13 | 1.5322 | 13.49 | 60.48 | 21.29 | S2 | |
| 12 | 55.14 | 0.07 | 1.5363 | 13.43 | 66.70 | 12.13 | S2 | |
| 13, D | 54.98 | 0 | 1.5331 | 13.45 | 73.35 | 0 | S2 | |
| T=348.15 K | ||||||||
| 1, M | 0 | 0.88 | 1.0188 | 10.96 | 0 | 63.58 | S1 | |
| 2 | 9.16 | 0.79 | 1.1386 | 11.39 | 3.09 | 65.73 | S1 | |
| 3 | 18.87 | 0.69 | 1.2285 | 11.63 | 7.33 | 63.31 | S1 | |
| 4 | 28.28 | 0.51 | 1.3109 | 11.79 | 10.11 | 64.12 | S1 | |
| 5 | 35.49 | 0.41 | 1.3860 | 12.15 | 14.76 | 58.58 | S1 | |
| 6 | 40.96 | 0.33 | 1.4091 | 12.41 | 16.11 | 61.26 | S1 | |
| 7 | 47.36 | 0.26 | 1.4533 | 12.55 | 20.02 | 58.33 | S1 | |
| 8 | 53.12 | 0.19 | 1.5117 | 12.98 | 23.18 | 57.19 | S1 | |
| 9, G | 57.75 | 0.16 | 1.5677 | 13.68 | 42.36 | 39.35 | S1+S2 | |
| 10 | 57.73 | 0.10 | 1.5619 | 13.67 | 68.59 | 10.12 | S2 | |
| 11, N | 57.71 | 0 | 1.5601 | 13.63 | 72.63 | 0 | S2 | |
| System | Property | T/K | m/(mol·kg-1) | Ref. | MRD/% |
|---|---|---|---|---|---|
| Li2CO3-H2O | Solubility(Li2CO3) | 273.14—575.15 | 0.2128—0.0206 | [ | 0.69 |
| Na2CO3-H2O | Pvap | 283.14—573.15 | 0.3921—2.6611 | [ | 2.46 |
| Cp | 283.13—403.15 | 0.1924—4.0436 | [ | 0.94 | |
| Solubility(Na2CO3) | 271.04—305.15 | 0.5703—4.3186 | [ | 0.51 | |
| Solubility(Na2CO3·H2O) | 305.14—308.52 | 4.2834—4.6703 | [ | 0.60 | |
| Solubility(Na2CO3·7H2O) | 305.52—377.95 | 4.6681—4.1994 | [ | 0.55 | |
| Solubility(Na2CO3·10H2O) | 377.94—453.15 | 4.1994—3.1450 | [ | 0.67 | |
| K2CO3-H2O | Pvap | 313.15—333.15 | 0.1477—3.4050 | [ | 2.15 |
| Cp | 283.15—353.15 | 0.1477—7.2356 | [ | 0.95 | |
| Solubility(K2CO3) | 446.15—499.15 | 17.1628—19.0007 | [ | 0.56 | |
| Solubility(K2CO3·1.5H2O) | 268.15—426.15 | 7.7015—17.1913 | [ | 0.64 | |
| Solubility(K2CO3·6H2O) | 243.15—266.95 | 5.3035—7.5179 | [ | 1.94 |
表3 二元体系的溶液物性与溶解度数据
Table 3 Physical property and solubility data of solution in binary systems
| System | Property | T/K | m/(mol·kg-1) | Ref. | MRD/% |
|---|---|---|---|---|---|
| Li2CO3-H2O | Solubility(Li2CO3) | 273.14—575.15 | 0.2128—0.0206 | [ | 0.69 |
| Na2CO3-H2O | Pvap | 283.14—573.15 | 0.3921—2.6611 | [ | 2.46 |
| Cp | 283.13—403.15 | 0.1924—4.0436 | [ | 0.94 | |
| Solubility(Na2CO3) | 271.04—305.15 | 0.5703—4.3186 | [ | 0.51 | |
| Solubility(Na2CO3·H2O) | 305.14—308.52 | 4.2834—4.6703 | [ | 0.60 | |
| Solubility(Na2CO3·7H2O) | 305.52—377.95 | 4.6681—4.1994 | [ | 0.55 | |
| Solubility(Na2CO3·10H2O) | 377.94—453.15 | 4.1994—3.1450 | [ | 0.67 | |
| K2CO3-H2O | Pvap | 313.15—333.15 | 0.1477—3.4050 | [ | 2.15 |
| Cp | 283.15—353.15 | 0.1477—7.2356 | [ | 0.95 | |
| Solubility(K2CO3) | 446.15—499.15 | 17.1628—19.0007 | [ | 0.56 | |
| Solubility(K2CO3·1.5H2O) | 268.15—426.15 | 7.7015—17.1913 | [ | 0.64 | |
| Solubility(K2CO3·6H2O) | 243.15—266.95 | 5.3035—7.5179 | [ | 1.94 |
| 离子对(水) | ||||
|---|---|---|---|---|
| I | J | |||
| (Li+,CO | H2O | 0 | 0 | 0 |
| H2O | (Li+,CO | 0 | 0 | 0 |
| (Na+,CO | H2O | -3.79 | 154.56 | 0.90 |
| H2O | (Na+,CO | 7.41 | -621.23 | 0.31 |
| (K+,CO | H2O | -4.69 | 67.11 | 0.91 |
| H2O | (K+,CO | 9.54 | -667.88 | -1.94 |
| (Li+,CO | (Na+,CO | 0.60 | -620.77 | 0 |
| (Na+,CO | (Li+,CO | -5.32 | 1451.19 | 0 |
| (Li+,CO | (K+,CO | 0.10 | -139.11 | 0 |
| (K+,CO | (Li+,CO | -8.23 | 160.24 | 0 |
| (Na+,CO | (K+,CO | -0.45 | -980.33 | 0 |
| (K+,CO | (Na+,CO | 2.88 | 1611.80 | 0 |
表4 Li+, Na+, K+, CO32--H2O体系液相特征参数
Table 4 Liquid parameters of Li+, Na+, K+, CO32--H2O system
| 离子对(水) | ||||
|---|---|---|---|---|
| I | J | |||
| (Li+,CO | H2O | 0 | 0 | 0 |
| H2O | (Li+,CO | 0 | 0 | 0 |
| (Na+,CO | H2O | -3.79 | 154.56 | 0.90 |
| H2O | (Na+,CO | 7.41 | -621.23 | 0.31 |
| (K+,CO | H2O | -4.69 | 67.11 | 0.91 |
| H2O | (K+,CO | 9.54 | -667.88 | -1.94 |
| (Li+,CO | (Na+,CO | 0.60 | -620.77 | 0 |
| (Na+,CO | (Li+,CO | -5.32 | 1451.19 | 0 |
| (Li+,CO | (K+,CO | 0.10 | -139.11 | 0 |
| (K+,CO | (Li+,CO | -8.23 | 160.24 | 0 |
| (Na+,CO | (K+,CO | -0.45 | -980.33 | 0 |
| (K+,CO | (Na+,CO | 2.88 | 1611.80 | 0 |
| 物种 | c1 | c2 | c3 | 文献 | |||
|---|---|---|---|---|---|---|---|
| CO | -527.46 | -677.48 | -130.58 | -344.85 | 1759.55 | -3491.17 | 本文 |
| Li2CO3 | -1130.56 | -1219.27 | 99.12 | 104.76 | 177.32 | 0.00 | 本文 |
| -1132.06 | -1215.90 | 99.12 | — | — | — | NBS[ | |
| Na2CO3 | -1043.25 | -1130.03 | 112.30 | 12.01 | 244.01 | 24.48 | 本文 |
| -1044.44 | -1130.00 | 112.30 | — | — | — | NBS[ | |
| Na2CO3·H2O | -1285.37 | -1435.20 | 145.60 | 45.31 | 244.01 | 24.48 | 本文 |
| -1285.31 | -1431.26 | 145.60 | — | — | — | NBS[ | |
| Na2CO3·7H2O | -2712.19 | -3178.93 | 374.56 | 274.27 | 244.01 | 24.48 | 本文 |
| -2714.20 | -3199.96 | — | — | — | — | NBS[ | |
| Na2CO3·10H2O | -3425.64 | -4088.86 | 550.29 | 450.00 | 244.01 | 24.48 | 本文 |
| -3427.66 | -4081.32 | 550.32 | — | — | — | NBS[ | |
| K2CO3 | -1064.04 | -1169.21 | 114.25 | 97.91 | 92.05 | -9.87 | 本文 |
| -1063.5 | -1151.02 | 114.43 | — | — | — | NBS[ | |
| K2CO3·1.5H2O | -1434.55 | -1630.27 | 198.76 | 198.76 | 0 | 0 | 本文 |
| -1432.5 | -1609.20 | — | — | — | — | NBS[ | |
| K2CO3·6H2O | -1434.64 | -1630.63 | 107.74 | 28.57 | 302.80 | -9.87 | 本文 |
| Li2CO3·Na2CO3 | -2179.86 | -2371.48 | 269.93 | 116.77 | 421.33 | 24.48 | 本文 |
| Na2CO3·K2CO3 | -2125.32 | 2335.81 | 226.55 | 109.92 | 336.06 | 14.61 | 本文 |
| Na2CO3·K2CO3·H2O | -2363.36 | -2629.56 | 379.77 | 379.77 | 0 | 0 | 本文 |
| Na2CO3·K2CO3·6H2O | -3560.81 | -4160.11 | 476.44 | 476.44 | 0 | 0 | 本文 |
| Na2CO3·K2CO3·12H2O | -5013.40 | -5559.91 | -7404.63 | -7404.63 | 0 | 0 | 本文 |
| NaKCO3·6H2O | -2494.87 | -2972.26 | 207.81 | 207.81 | 0 | 0 | 本文 |
表5 Li+, Na+, K+, CO32--H2O体系的物种热力学参数
Table 5 Thermodynamic parameters of species in Li+, Na+, K+, CO32--H2O system
| 物种 | c1 | c2 | c3 | 文献 | |||
|---|---|---|---|---|---|---|---|
| CO | -527.46 | -677.48 | -130.58 | -344.85 | 1759.55 | -3491.17 | 本文 |
| Li2CO3 | -1130.56 | -1219.27 | 99.12 | 104.76 | 177.32 | 0.00 | 本文 |
| -1132.06 | -1215.90 | 99.12 | — | — | — | NBS[ | |
| Na2CO3 | -1043.25 | -1130.03 | 112.30 | 12.01 | 244.01 | 24.48 | 本文 |
| -1044.44 | -1130.00 | 112.30 | — | — | — | NBS[ | |
| Na2CO3·H2O | -1285.37 | -1435.20 | 145.60 | 45.31 | 244.01 | 24.48 | 本文 |
| -1285.31 | -1431.26 | 145.60 | — | — | — | NBS[ | |
| Na2CO3·7H2O | -2712.19 | -3178.93 | 374.56 | 274.27 | 244.01 | 24.48 | 本文 |
| -2714.20 | -3199.96 | — | — | — | — | NBS[ | |
| Na2CO3·10H2O | -3425.64 | -4088.86 | 550.29 | 450.00 | 244.01 | 24.48 | 本文 |
| -3427.66 | -4081.32 | 550.32 | — | — | — | NBS[ | |
| K2CO3 | -1064.04 | -1169.21 | 114.25 | 97.91 | 92.05 | -9.87 | 本文 |
| -1063.5 | -1151.02 | 114.43 | — | — | — | NBS[ | |
| K2CO3·1.5H2O | -1434.55 | -1630.27 | 198.76 | 198.76 | 0 | 0 | 本文 |
| -1432.5 | -1609.20 | — | — | — | — | NBS[ | |
| K2CO3·6H2O | -1434.64 | -1630.63 | 107.74 | 28.57 | 302.80 | -9.87 | 本文 |
| Li2CO3·Na2CO3 | -2179.86 | -2371.48 | 269.93 | 116.77 | 421.33 | 24.48 | 本文 |
| Na2CO3·K2CO3 | -2125.32 | 2335.81 | 226.55 | 109.92 | 336.06 | 14.61 | 本文 |
| Na2CO3·K2CO3·H2O | -2363.36 | -2629.56 | 379.77 | 379.77 | 0 | 0 | 本文 |
| Na2CO3·K2CO3·6H2O | -3560.81 | -4160.11 | 476.44 | 476.44 | 0 | 0 | 本文 |
| Na2CO3·K2CO3·12H2O | -5013.40 | -5559.91 | -7404.63 | -7404.63 | 0 | 0 | 本文 |
| NaKCO3·6H2O | -2494.87 | -2972.26 | 207.81 | 207.81 | 0 | 0 | 本文 |
| 体系 | 固相 | T/K | 液相组成 / % | |||
|---|---|---|---|---|---|---|
| LC | NC | KC | ||||
| Na2CO3–H2O | A | 冰+ NC10 | 270.78 | — | 5.81 | — |
| B | NC10 + NC7 | 300.00 | — | 29.83 | — | |
| C | NC7 + NC1 | 308.91 | — | 33.44 | — | |
| D | NC1 + NC | 385.09 | — | 30.99 | — | |
| K2CO3-H2O | E | 冰+ KC6 | 242.95 | — | — | 42.30 |
| F | KC6+ KC1.5 | 266.01 | — | — | 50.96 | |
| G | KC1.5 + KC | 429.75 | — | — | 70.38 | |
| Li2CO3–K2CO3–H2O | H | 冰+ LC + KC1.5 | 223.15 | 0.14 | — | 45.08 |
| Li2CO3–Na2CO3–H2O | I | 冰+ LC + NC10 | 269.83 | 1.28 | 6.46 | — |
| J | LC + NC10 + LNC | 300.72 | 0.97 | 32.77 | — | |
| K | NC10+NC7+LNC | 301.27 | 0.90 | 34.62 | — | |
| L | NC7+NC1+LNC | 308.02 | 0.72 | 39.67 | — | |
| Na2CO3–K2CO3–H2O | M | 冰+ KC6 + NKC6 | 236.15 | — | 0.04 | 40.28 |
| N | 冰+ NC10 + NKC6 | 251.51 | — | 1.70 | 27.27 | |
| O | KC6+NKC6+KC1.5 | 262.15 | — | 0.25 | 49.75 | |
| P | NC10 + NKC6 + NC1 | 295.32 | — | 21.05 | 17.24 | |
| Q | NC10 + NC7 + NC1 | 296.03 | — | 21.74 | 16.11 | |
| R | NKC6 + KC1.5 + NKC | 304.24 | — | 4.77 | 49.18 | |
| S | NKC6 + NC1 + NKC | 310.67 | — | 11.71 | 37.48 | |
表6 二元体系和三元体系零变量点的预测结果
Table 6 The predicted invariant points in binary and ternary systems
| 体系 | 固相 | T/K | 液相组成 / % | |||
|---|---|---|---|---|---|---|
| LC | NC | KC | ||||
| Na2CO3–H2O | A | 冰+ NC10 | 270.78 | — | 5.81 | — |
| B | NC10 + NC7 | 300.00 | — | 29.83 | — | |
| C | NC7 + NC1 | 308.91 | — | 33.44 | — | |
| D | NC1 + NC | 385.09 | — | 30.99 | — | |
| K2CO3-H2O | E | 冰+ KC6 | 242.95 | — | — | 42.30 |
| F | KC6+ KC1.5 | 266.01 | — | — | 50.96 | |
| G | KC1.5 + KC | 429.75 | — | — | 70.38 | |
| Li2CO3–K2CO3–H2O | H | 冰+ LC + KC1.5 | 223.15 | 0.14 | — | 45.08 |
| Li2CO3–Na2CO3–H2O | I | 冰+ LC + NC10 | 269.83 | 1.28 | 6.46 | — |
| J | LC + NC10 + LNC | 300.72 | 0.97 | 32.77 | — | |
| K | NC10+NC7+LNC | 301.27 | 0.90 | 34.62 | — | |
| L | NC7+NC1+LNC | 308.02 | 0.72 | 39.67 | — | |
| Na2CO3–K2CO3–H2O | M | 冰+ KC6 + NKC6 | 236.15 | — | 0.04 | 40.28 |
| N | 冰+ NC10 + NKC6 | 251.51 | — | 1.70 | 27.27 | |
| O | KC6+NKC6+KC1.5 | 262.15 | — | 0.25 | 49.75 | |
| P | NC10 + NKC6 + NC1 | 295.32 | — | 21.05 | 17.24 | |
| Q | NC10 + NC7 + NC1 | 296.03 | — | 21.74 | 16.11 | |
| R | NKC6 + KC1.5 + NKC | 304.24 | — | 4.77 | 49.18 | |
| S | NKC6 + NC1 + NKC | 310.67 | — | 11.71 | 37.48 | |
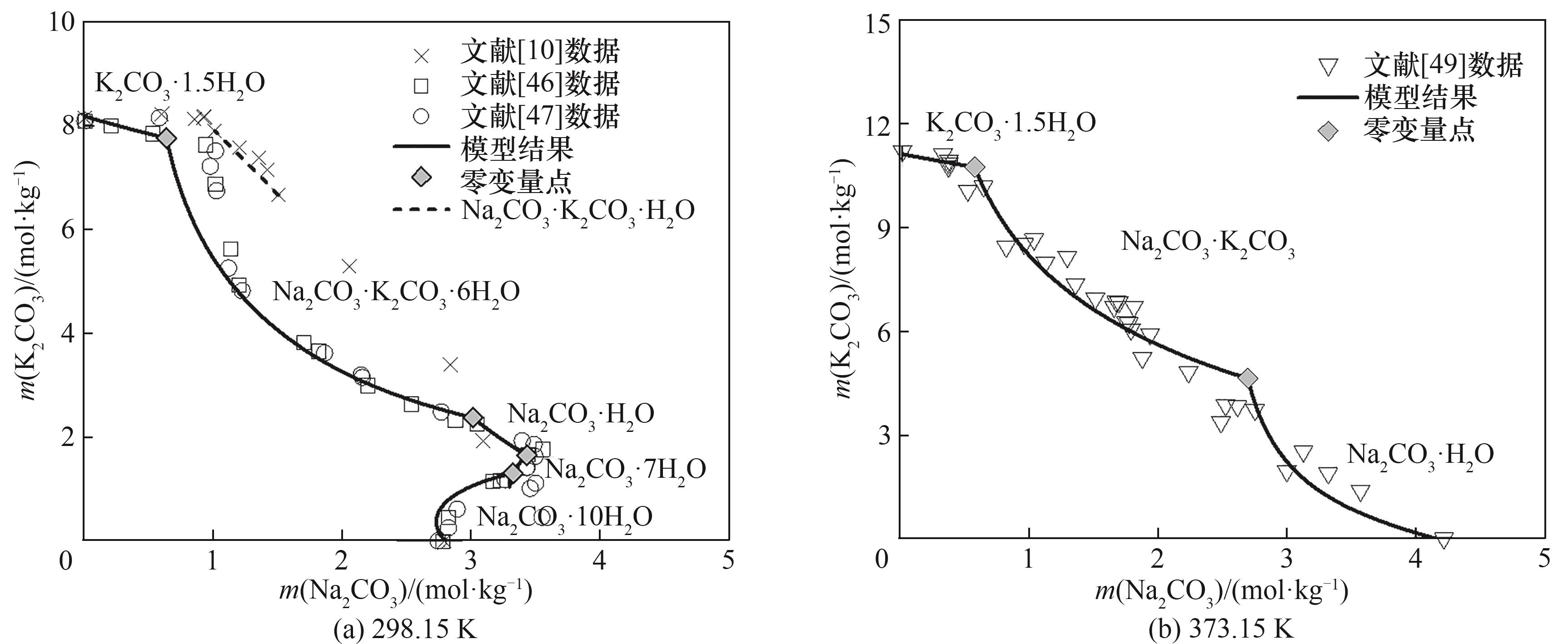
图10 Na2CO3-K2CO3-H2O三元体系在298.15 K和373.15 K下的等温溶解度
Fig. 10 Calculated results of isothermal solubility for Na2CO3-K2CO3-H2O ternary system at 298.15 K and 373.15 K
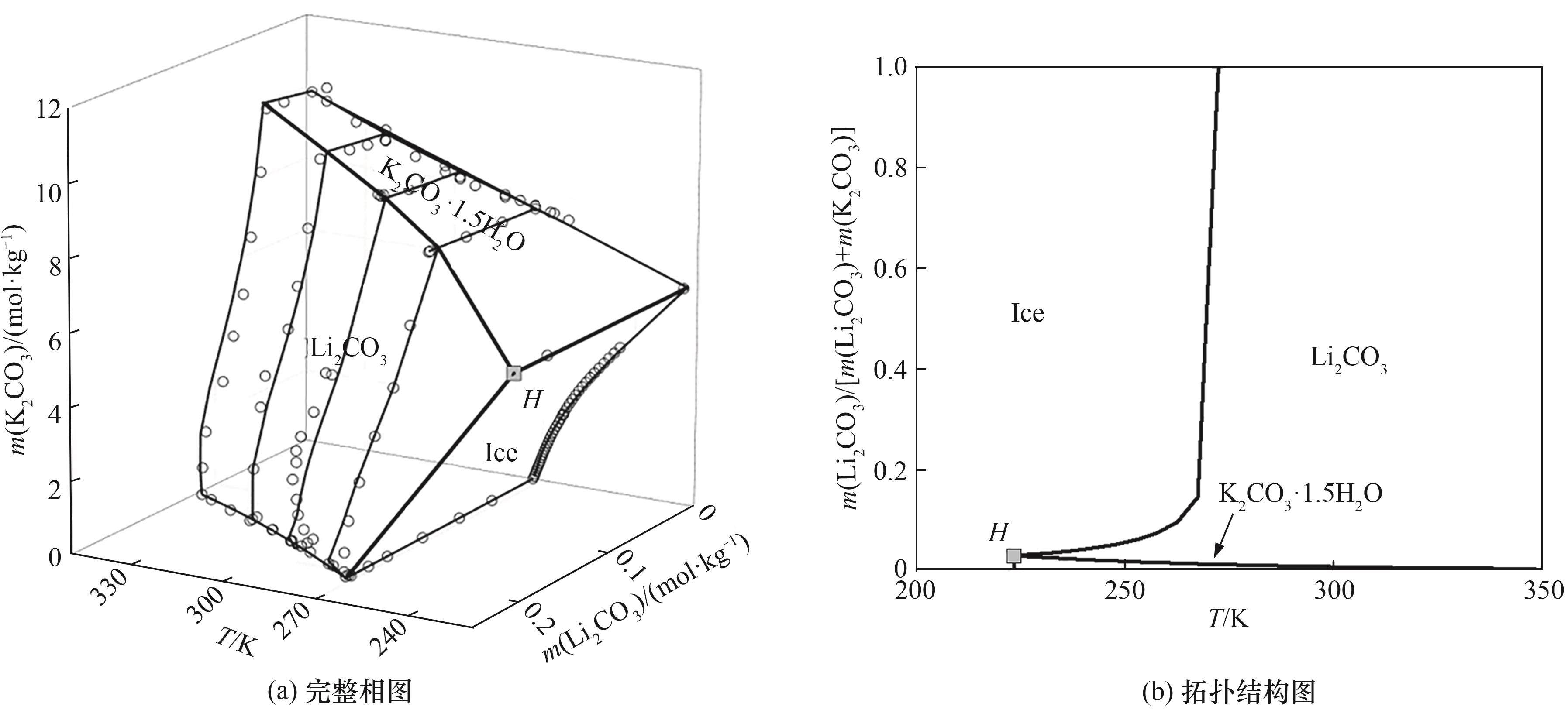
图11 Li2CO3-K2CO3-H2O体系348.15 K 以下温度的完整相图和拓扑结构
Fig.11 Complete phase diagrams and topology diagrams of Li2CO3-K2CO3-H2O system at temperature under 348.15 K
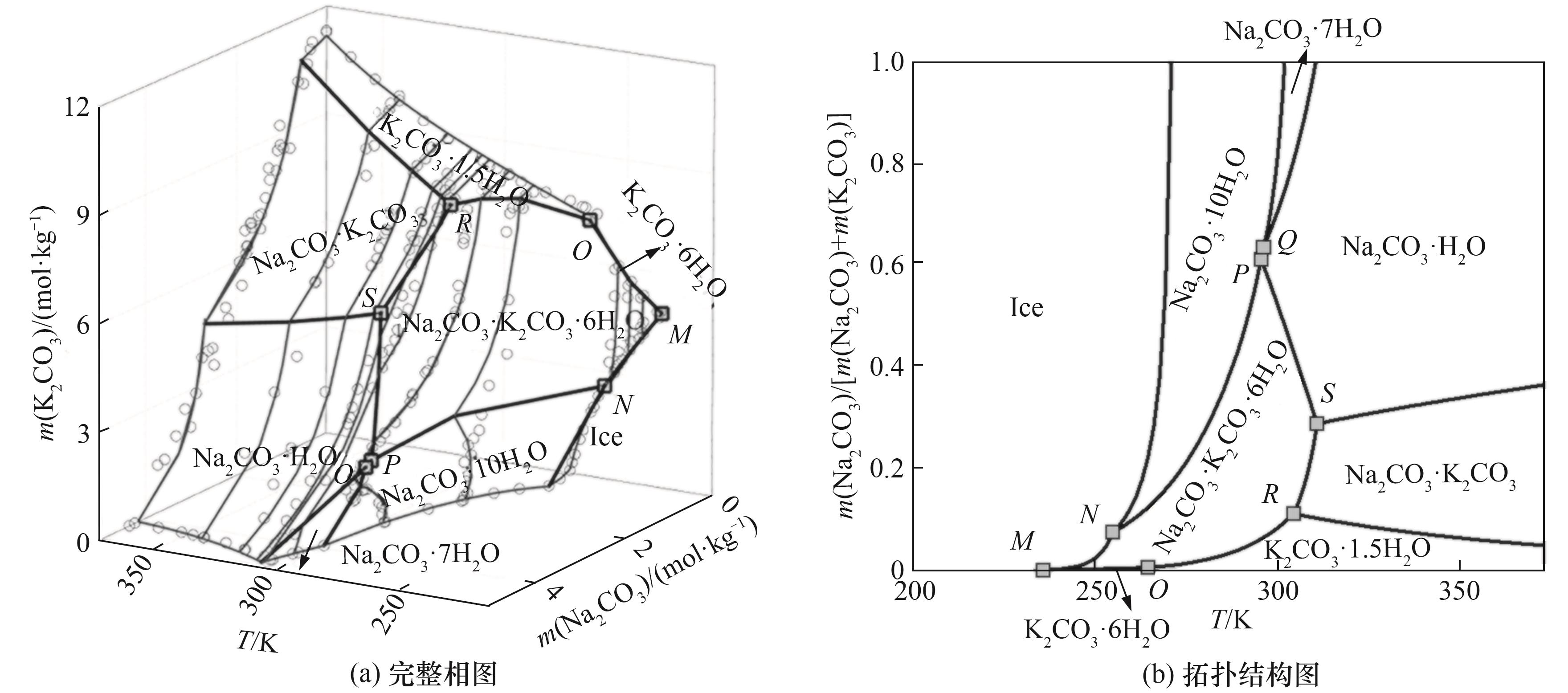
图13 Na2CO3-K2CO3-H2O体系在共晶点至373.15 K温度的完整相图和拓扑结构图
Fig. 13 Calculated isothermal solubility and predicted complete topology phase diagrams of Na 2CO3-K2CO3-H2O system at temperature range from eutectic point to 373.15 K
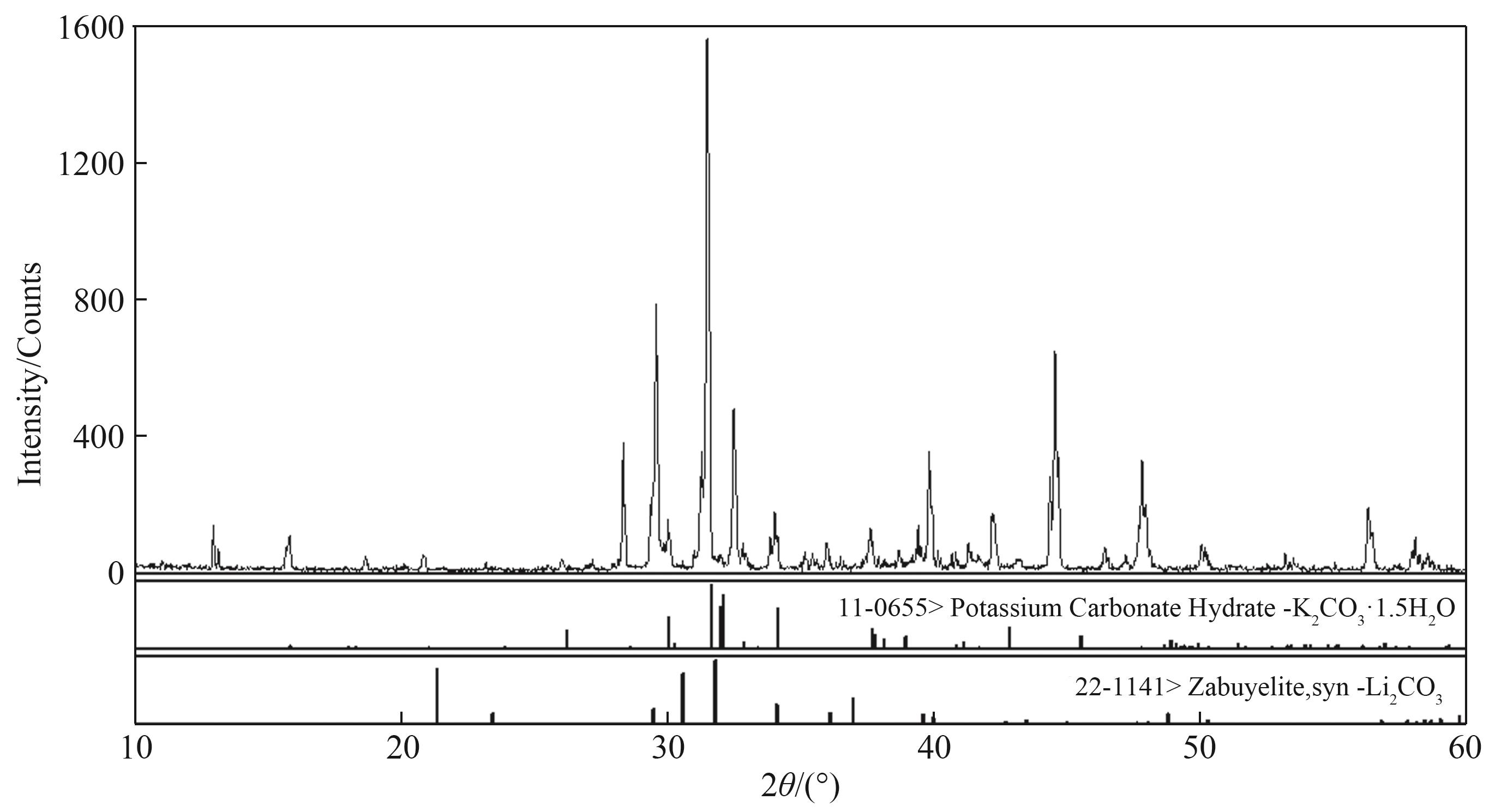
图s1 三元体系Li2CO3-K2CO3-H2O 273.15 K 零变量点XRD 谱图(S1+S2)
Fig. s1 X-ray diffraction patterns of invariant points in Li2CO3-K2CO3-H2O ternary system at 273.15 K S1—Li2CO3; S2—K2CO3·1.5 H2O
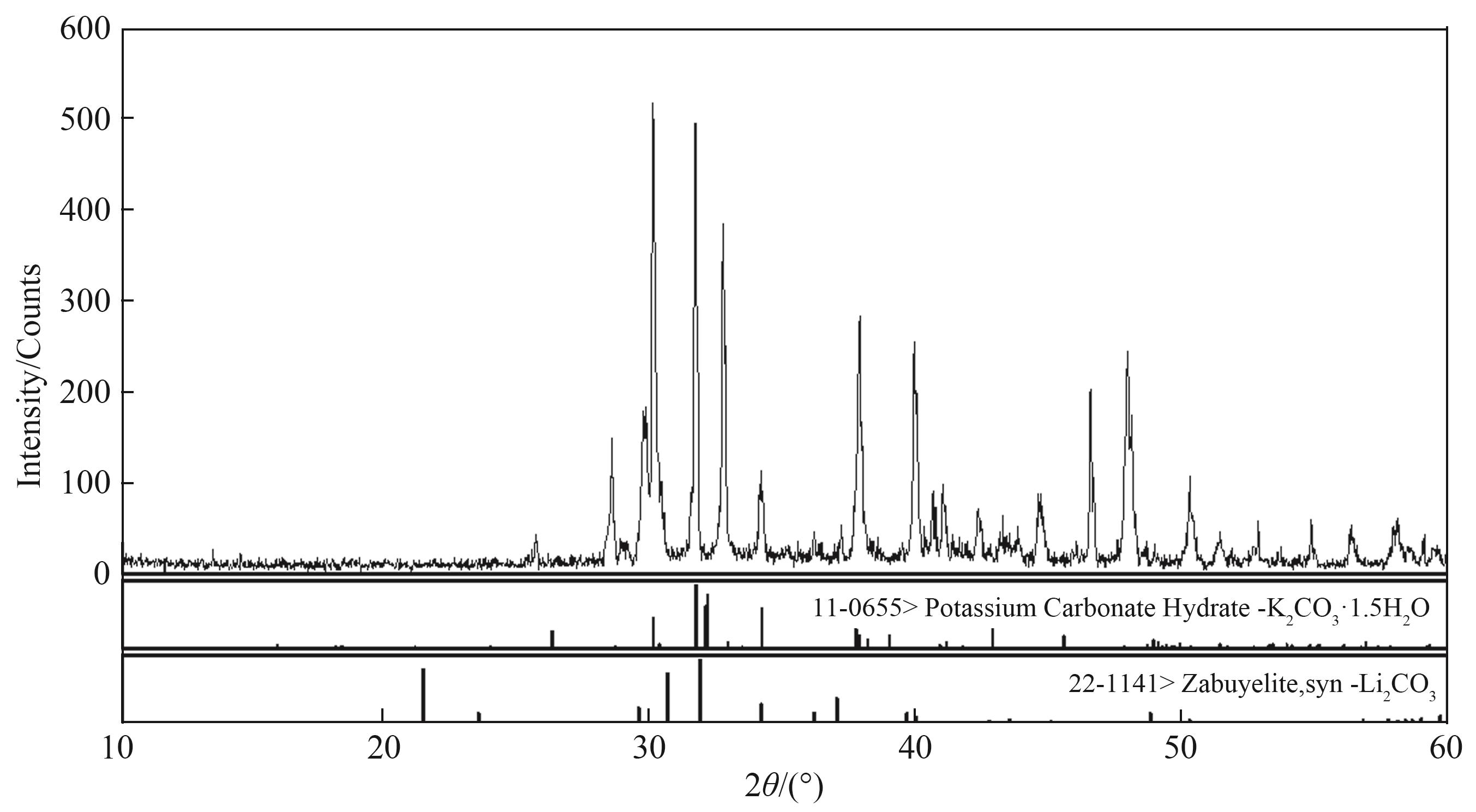
图s2 三元体系Li2CO3-K2CO3-H2O 323.15 K 零变量点XRD 谱图(S1+S2)
Fig. s2 X-ray diffraction patterns of invariant points in Li2CO3-K2CO3-H2O ternary system at 323.15 K S1—Li2CO3; S2—K2CO3·1.5 H2O
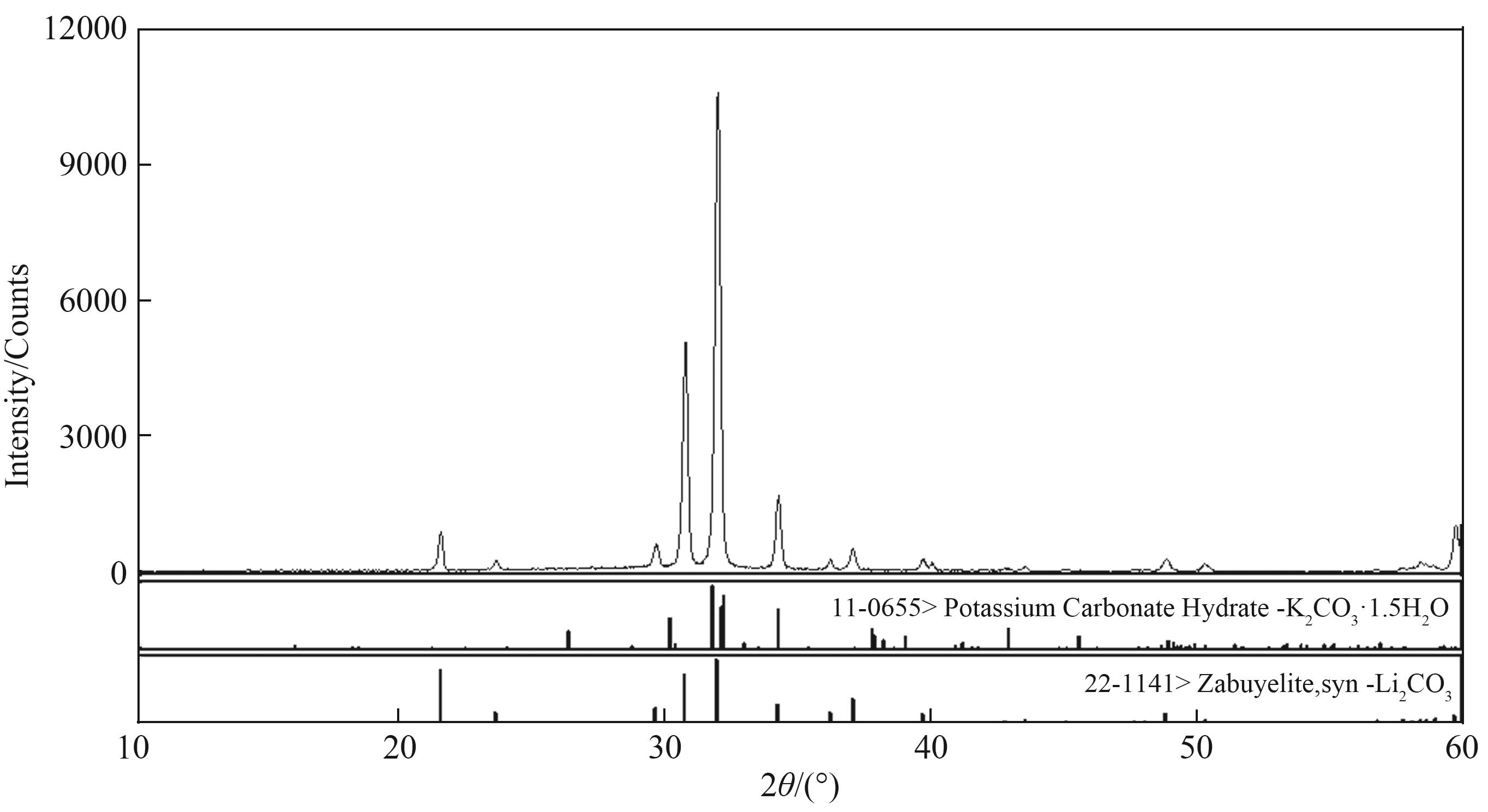
图s3 三元体系Li2CO3-K2CO3-H2O 348.15 K 零变量点XRD 谱图(S1+S2)
Fig. s3 X-ray diffraction patterns of invariant points in Li2CO3-K2CO3-H2O ternary system at 348.15 K S1—Li2CO3; S2—K2CO3·1.5 H2O
| [1] | 郑绵平. 论中国盐湖[J]. 矿床地质, 2001, 20(2): 181-189, 128. |
| Zheng M P. On saline lakes of China[J]. Mineral Deposits, 2001, 20(2): 181-189, 128. | |
| [2] | Zhou Y C, Chen Z Z, Gong H J, et al. Study on the zinc porphyrins as potential carbonic anhydrase mimics for promoting CO2 absorption in K2CO3 solution[J]. Chemical Engineering Journal, 2024, 481: 148690. |
| [3] | 高世扬, 宋彭生, 夏树屏, 等. 盐湖化学 : 新类型硼锂盐湖[M]. 北京: 科学出版社, 2007. |
| Gao S Y, Song P S, Xia S P, et al. Salt Llake Chemistry: a New Type of Boron-lithium Salt Lake[M]. Beijing: Science Press, 2007. | |
| [4] | Kindyakov P S. In Solubilities of Inorganic and Organic Compounds[M]. Silcock H. London: Pergamon Press, 1979, 3(1): 674-675. |
| [5] | Itkina L S. In Solubilities of Inorganic and Organic Compounds[M]. Silcock H. London: Pergamon Press, 1979, 3(1): 676. |
| [6] | Urazov G G. In Solubilities of Inorganic and Organic Compounds[M]. Silcock H. London: Pergamon Press, 1979, 3(1): 674. |
| [7] | 殷辉安, 郝丽芳, 曾英, 等. Li⁺, Na⁺//CO 3 2 -, B₄O 7 2 -, Cl⁻-H₂O五元体系298 K相平衡及平衡液相物化性质的研究[J]. 高校化学工程学报, 2003, 16(1): 1-5. |
| Yin H A, Hao L F, Zeng Y, et al. Phase equilibrium and physicochemical properties of aqueous solutions for the quinary system Li⁺, Na⁺//CO 3 2 -, B₄O 7 2 -, Cl⁻-H₂O at 298 K[J]. Journal of Chemical Engineering of China Universities, 2003, 16(1): 1-5. | |
| [8] | 殷辉安, 桑世华, 唐明林, 等. K2CO3-Li2CO3-H2O三元体系288 K相平衡研究[C]//中国物理学会相图专业委员会. 第十二届全国相图学术会议论文集. 成都, 2004: 4. |
| Yin H A, Sang S H, Tang M L, et al. Study on phase equilibrium of K2CO3-Li2CO3-H2O ternary system at 288 K[C]//Chinese Physical Society, Phase Diagram Professional Committee. Proceedings of the 12th National Conference on Phase Diagrams. Chengdu, 2004: 4. | |
| [9] | 殷辉安, 桑世华, 唐明林, 等. 288K下Li+, K+//CO 3 2 -, B4O 7 2 --H₂O四元体系的相平衡[J]. 化工学报, 2004, 55(3): 464-467. |
| Yin H A, Sang S H, Tang M L, et al. Equilibrium of the quaternary system Li+, K+//CO 3 2 -, B4O 7 2 --H₂O at 288 K[J]. Journal of Chemical Industry and Engineering (China), 2004, 55(3): 464-467. | |
| [10] | 曾英, 殷辉安, 唐明林, 等. 三元体系Na2CO3-K2CO3-H2O 298 K 相关系和溶液物化性质的研究[J]. 四川联合大学学报(工程科学版), 1999, 31(6): 135-139. |
| Zeng Y, Yin H A, Tang M L, et al. An experimental study on the equilibrium phase diagram and solution properties of the Na2CO3-K2CO3-H2O ternary system at 298 K[J]. Advanced Engineering Sciences, 1999, 31(6): 135-139. | |
| [11] | 曾英, 殷辉安, 唐明林, 等. 298K时Li+, Na+, K+/CO 3 2 --H2O四元体系相图和液相物化性质测定[J]. 化学工程, 1999, 27(5): 45-47. |
| Zeng Y, Yin H A, Tang M L, et al. Phase diagram of Li+, Na+, K+/CO 3 2 --H2O quaternary system at 298 K and determination of physicochemical properties in liquid phase[J]. Chemical Engineering, 1999, 27(5): 45-47. | |
| [12] | 曾英, 殷辉安, 唐明林. 四元体系Na⁺, K⁺//CO 3 2 -,B₄O 7 2 --H₂O 298 K相平衡研究[J]. 无机化学学报, 2001, 27(5): 665-668. |
| Zeng Y, Yin H A, Tang M L. Phase equilibrium study on the quaternary system Na⁺,K⁺//CO 3 2 -,B₄O 7 2 --H₂O at 298 K[J]. Journal of Inorganic Chemistry, 2001, 27(5): 665-668. | |
| [13] | 曾英, 肖霞, 殷辉安, 等. 交互四元体系Li+, K+//CO 3 2 -, B4O 7 2 --H2O 298K相关系及平衡液相物化性质的研究[J]. 高校化学工程学报, 2002, 16(6): 591-596. |
| Zeng Y, Xiao X, Yin H A, et al. A study on the phase equilibrium and solution properties of the quaternary system Li+, K+// CO 3 2 -, B4O 7 2 --H2O 298K[J]. Journal of Chemical Engineering of Chinese Universities, 2002, 16(6): 591-596. | |
| [14] | 曾英, 殷辉安, 唐明林, 等. 五元交互体系Li+, Na+, K+//CO 3 2 -, Cl--: H2O在298.15K的相平衡研究[J]. 高等学校化学学报, 2003, 24(6): 968-972. |
| Zeng Y, Yin H A, Tang M L, et al. A study of the phase equilibrium for quinary system Li+, Na+, K+// CO 3 2 -, Cl--: H2O at 298.15 K[J]. Chemical Journal of Chinese Universities, 2003, 24(6): 968-972. | |
| [15] | 桑世华, 殷辉安, 唐明林, 等. Li+, Na+∥CO 3 2 -, B4O 7 2 --H2O四元交互体系288K的相平衡[J]. 物理化学学报, 2002, 18(9): 835-837. |
| Sang S H, Yin H A, Tang M L, et al. Equilibrium phase diagram of Li+, Na+∥CO 3 2 -, B4O 7 2 --H2O quaternary system at 288 K[J]. Acta Physico-Chimica Sinica, 2002, 18(9): 835-837. | |
| [16] | 桑世华, 殷辉安, 唐明林. K2CO3-Na2CO3-H2O三元体系288K相平衡研究[J]. 无机盐工业, 2003, 35(1): 23-24, 55. |
| Sang S H, Yin H A, Tang M L. Experimental study on the phase equilibrium of the ternary system K2CO3-Na2CO3-H2O at 288 K[J]. Inorganic Chemicals Industry, 2003, 35(1): 23-24, 55. | |
| [17] | 桑世华, 唐明林, 殷辉安, 等. K2CO3-Na2CO3-Li2CO3-H2O四元体系288K的相平衡[J]. 应用化学, 2004, 21(5): 509-511. |
| Sang S H, Tang M L, Yin H A, et al. Phase equilibrium of the quaternary system K2CO3-Na2CO3-Li2CO3-H2O at 288 K[J]. Chinese Journal of Applied Chemistry, 2004, 21(5): 509-511. | |
| [18] | 李以圭, 陆九芳. 电解质溶液理论[M]. 北京: 清华大学出版社, 2005. |
| Li Y G, Lu J F. Electrolyte Solution Theory[M]. Beijing: Tsinghua University Press, 2005. | |
| [19] | Pitzer K S. Thermodynamics of electrolytes I: Theoretical basis and general equations[J]. The Journal of Physical Chemistry, 1973, 77(2): 268-277. |
| [20] | Clegg S L, Pitzer K S. Thermodynamics of multicomponent, miscible, ionic solutions: generalized equations for symmetrical electrolytes[J]. The Journal of Physical Chemistry, 1992, 96(8): 3513-3520. |
| [21] | Li D D, Zeng D W, Yin X, et al. Phase diagrams and thermochemical modeling of salt lake brine systems( Ⅳ ): Thermodynamic framework and program implementation for multicomponent systems[J]. Calphad, 2020, 71: 101806. |
| [22] | Thomsen K. Modeling electrolyte solutions with the extended universal quasichemical (UNIQUAC) model[J]. Pure and Applied Chemistry, 2005, 77(3): 531-542. |
| [23] | Chen C C, Britt H I, Boston J F, et al. Local composition model for excess Gibbs energy of electrolyte systems(Part Ⅰ ): Single solvent, single completely dissociated electrolyte systems[J]. AIChE Journal, 1982, 28(4): 588-596. |
| [24] | Song Y H, Chen C C. Symmetric electrolyte nonrandom two-liquid activity coefficient model[J]. Industrial & Engineering Chemistry Research, 2009, 48(16): 7788-7797. |
| [25] | Wang P, Springer R D, Anderko A, et al. Modeling phase equilibria and speciation in mixed-solvent electrolyte systems[J]. Fluid Phase Equilibria, 2004, 222: 11-17. |
| [26] | Wang P, Anderko A, Springer R D, et al. Modeling phase equilibria and speciation in mixed-solvent electrolyte systems ( Ⅱ ) : Liquid-liquid equilibria and properties of associating electrolyte solutions[J]. Journal of Molecular Liquids, 2006, 125(1): 37-44. |
| [27] | Tanveer S, Chen C C. Molecular thermodynamic modeling of aqueous Na+-K+-Mg2+-Ca2+-SO 4 2 - quinary system[J]. Fluid Phase Equilibria, 2019, 491: 77-87. |
| [28] | Zhou H, Gu X L, Dai Y, et al. Thermodynamic modeling and phase diagram prediction of salt lake brine systems ( Ⅰ ) : Aqueous Mg2+-Ca2+-Cl- binary and ternary systems[J]. Chinese Journal of Chemical Engineering, 2020, 28(9): 2391-2408. |
| [29] | Zhou H, Wu P, Li W X, et al. Thermodynamic modeling and phase diagram prediction of salt lake brine systems ( Ⅱ ) : Aqueous Li+-Na+-K+-SO 4 2 - and its subsystems[J]. Chinese Journal of Chemical Engineering, 2021, 34: 134-149. |
| [30] | 王星帆, 周桓, 周阔, 等. 硝酸盐型卤水体系的综合热力学模型与多温相图预测 [J]. 高等学校化学学报, 2021, 42(10):12. |
| Wang X F, Zhou H, Zhou K, et al. Comprehensive thermodynamic model and polythermal phase diagram prediction of nitrate-type brine systems [J]. Chemical Journal of Chinese Universities, 2021, 42(10):12. | |
| [31] | 常静宇, 周桓, 杨洁, 等. NaF-Na3PO4-H2O体系相图与热力学模型 [J]. 天津科技大学学报, 2024, 39(1):30-41. |
| Chang J Y, Zhou H, Yang J, et al. Phase diagram and thermodynamic model of the NaF-Na3PO4-H2O system [J]. Journal of Tianjin University of Science & Technology, 2024, 39(1):30-41. | |
| [32] | Kaur H, Chen C C. Thermodynamic modeling of CO2 absorption in aqueous potassium carbonate solution with electrolyte NRTL model[J]. Fluid Phase Equilibria, 2020, 505: 112339. |
| [33] | Chen T Y, Honarparvar S, Reible D, et al. Thermodynamic modeling of calcium carbonate scale precipitation: aqueous Na+-Ca2+-Cl–-HCO 3 - -CO 3 2 --CO2 system[J]. Fluid Phase Equilibria, 2022, 552: 113263. |
| [34] | Miller R R, Smith S H, Williams D D. Solubility of lithium carbonate at elevated temperatures[J]. Journal of Chemical & Engineering Data, 1971, 16(1): 74-75. |
| [35] | Cheng W T, Li Z B, Cheng F Q. Solubility of Li2CO3 in Na-K-Li-Cl brines from 20 to 90℃[J]. The Journal of Chemical Thermodynamics, 2013, 67: 74-82. |
| [36] | Wang J F, Wu X W, Zhang S J. Development of a thermodynamic model for the Li2CO3-NaCl-Na2SO4-H2O system and its application[J]. The Journal of Chemical Thermodynamics, 2018, 123: 62-73. |
| [37] | 邓天龙, 周桓, 陈侠. 水盐体系相图及应用[M]. 2版. 北京: 化学工业出版社, 2020. |
| Deng T L, Zhou H, Chen X. Salt-water System Phase Diagrams and Applications[M]. 2nd ed. Beijing: Chemical Industry Press, 2020. | |
| [38] | Waldeck W F, Lynn G, Hill A E. Aqueous solubility of salts at high temperatures ( Ⅰ ): Solubility of sodium carbonate from 50 to 348℃[J]. Journal of the American Chemical Society, 1932, 54(3): 928-936. |
| [39] | Kobe K A, Sheehy T M. Thermochemistry of sodium carbonate and its solutions[J]. Industrial & Engineering Chemistry, 1948, 40(1): 99-102. |
| [40] | Seidell A. Solubilities of Inorganic and Metal Organic Compounds[M]. New York: Van Nostrand Company, Inc., 1953. |
| [41] | Takahashi G. In Solubilities of Inorganic and Organic Compounds[M]. Silcock H. London: Pergamon Press, 1979, 1(1): 158. |
| [42] | Moore R C, Mesmer R E, Simonson J M. Solubility of potassium carbonate in water between 384 and 529 K measured using the synthetic method[J]. Journal of Chemical & Engineering Data, 1997, 42(6): 1078-1081. |
| [43] | 伍倩, 乜贞, 卜令忠, 等. 盐湖卤水Li+, Na+/CO 3 2 --H2O三元体系298.15K稳定相平衡研究[J]. 化工矿物与加工, 2018, 47(2): 15-18, 45. |
| Wu Q, Nie Z, Bu L Z, et al. Research on stable phase equilibrium in ternary system Li+, Na+/CO 3 2 --H2O at 298.15K[J]. Industrial Minerals & Processing, 2018, 47(2): 15-18, 45. | |
| [44] | Ge H W, Wang M, Zhao Y J, et al. Solid-liquid phase equilibria of the aqueous ternary system (Li2CO3 + Na2CO3 + H2O) at (278.15 to 308.15) K[J]. Journal of Chemical & Engineering Data, 2020, 65(11): 5553-5558. |
| [45] | 郑志远, 曾英, 林晓峰. K2CO3-Na2CO3-H2O三元体系273K相平衡实验研究[J]. 盐业与化工, 2007, 36(1): 7-9. |
| Zheng Z Y, Zeng Y, Lin X F. Experimental study on the phase equilibrium of the ternary system K2CO3-Na2CO3-H2O at 273K[J]. Sea-Lake Salt and Chemical Industry, 2007, 36(1): 7-9. | |
| [46] | Osaka J. In Solubilities of Inorganic and Organic Compounds[M]. Silcock H. London: Pergamon Press, 1979, 3(2): 693-695. |
| [47] | Bain H W. In Solubilities of Inorganic and Organic Compounds[M]. Silcock H. London: Pergamon Press, 1979, 3(2): 697. |
| [48] | Yin C C, Liu M T, Yang J, et al. (Solid+liquid) phase equilibrium for the ternary system (K2CO3-Na2CO3-H2O) at T=(323.15, 343.15, and 363.15) K[J]. The Journal of Chemical Thermodynamics, 2017, 108: 1-6. |
| [49] | Erving J. In Solubilities of Inorganic and Organic Compounds[M]. Silcock H. London: Pergamon Press, 1979, 3(2): 702-703. |
| [50] | 伍倩, 乜贞, 卜令忠, 等. 三元体系Li+,K+/CO 3 2 --H2O 298.15 K稳定相平衡研究[J]. 无机盐工业, 2018, 50(6): 4. |
| Wu Q, Nie Z, Bu L Z, et al. Study on stable phase equilibrium of Li+,K+/CO 3 2 --H2O ternary system at 298.15 K[J]. Inorganic Chemicals Industry, 2018, 50(6): 4. | |
| [51] | Zaytsev I D, Aseyev G G. Properties of Aqueous Solutions of Electrolytes[M]. Boca Raton: CRC Press, 1992. |
| [52] | Magalhães M C F, Königsberger E, May P M, et al. Heat capacities of concentrated aqueous solutions of sodium sulfate, sodium carbonate, and sodium hydroxide at 25 ℃[J]. Journal of Chemical & Engineering Data, 2002, 47(3): 590-598. |
| [53] | Sukhotin A M. Spravochnik po Elektrokhimii (Handbook on Electrochemistry)[M]. Leningrad: Khimiya Press, 1981. |
| [54] | Timmermans J. Physico-Chemical Constants of Binary System in Concentrated Solutions Vols. 3, 4[M]: New York: Interscience Publishers, 1960. |
| [55] | Wagman D D, William H E, Parker B, et al. The NBS Tables of Chemical Thermodynamic Properties: selected Values for Inorganic and C1 and C2 Organic Substances in SI Units[R]. Washington, DC: American Chemical Society; New York: American Institute of Physics for the National Bureau of Standards, 1982. |
| [56] | Aspen Physical Property System: version 7.3[DB]. Burlington, MA:Aspen Technology, Inc., 2011. |
| [57] | Criss C M, Cobble J W. The thermodynamic properties of high temperature aqueous solutions (V): The calculation of ionic heat capacities up to 200°. Entropies and heat capacities above 200°[J]. Journal of the American Chemical Society, 1964, 86(24): 5390-5393. |
| [58] | Barin I, Knacke O, Kubaschewski O. Thermochemical Properties of Inorganic Substances[M]. Berlin, Heidelberg: Springer Berlin Heidelberg, 1977. |
| [1] | 刘晗, 崔家馨, 殷梦凡, 郑涛, 张睿, 孟祥海, 刘植昌, 刘海燕, 徐春明. CuAlCl4-二甲苯络合物晶体结构及二元固液相平衡测定[J]. 化工学报, 2025, 76(5): 2241-2250. |
| [2] | 丁叶薇, 康文博, 宋昱潼, 樊钦习, 吉远辉. 吲哚美辛纳米药物筛选及自组装机制的理论研究[J]. 化工学报, 2024, 75(11): 4141-4151. |
| [3] | 汤涵, 蔡进, 覃海航, 陈光进, 孙长宇. 水合物共存体系中气体溶解度预测模型[J]. 化工学报, 2024, 75(11): 4348-4358. |
| [4] | 门文欣, 彭庆收, 桂霞. 不同季铵盐作用下的CO2水合物相平衡[J]. 化工学报, 2022, 73(4): 1472-1482. |
| [5] | 彭昌炜, 桑世华, 崔瑞芝, 任红保. 五元体系NaBr-KBr-MgBr2-CaBr2-H2O在298.15 K下的空间立体相图研究[J]. 化工学报, 2022, 73(11): 4850-4858. |
| [6] | 魏小兰, 谢佩, 王维龙, 陆建峰, 丁静. 含钙三元氯化物体系相图计算与熔盐热稳定性[J]. 化工学报, 2021, 72(6): 3074-3083. |
| [7] | 李栋婵, 王嘉宇, 王士强. 四元体系(Li+, Mg2+//Cl-, borate-H2O)308.15 K相平衡与相图研究[J]. 化工学报, 2021, 72(6): 3170-3178. |
| [8] | 李丹, 孙帅琦, 张涛, 赵一慧, 孟令宗, 郭亚飞, 邓天龙. 五元体系HCl-NaCl-CaCl2-H3BO3-H2O在298.15 K的Pitzer模型及其应用研究[J]. 化工学报, 2021, 72(6): 3160-3169. |
| [9] | 陈博亚, 李明宴, 朱雨航, 彭昌军, 刘洪来. 利用二维流体分子热力学模型计算气体混合物在固体界面的吸附等温线[J]. 化工学报, 2021, 72(2): 913-920. |
| [10] | 李攀, 孔慧, 宋卓栋, 张作毅, 王云芳. 甲醇-甲醛-聚甲氧基二甲醚三元体系汽液平衡[J]. 化工学报, 2020, 71(S1): 7-14. |
| [11] | 曹小雪, 吉绍长, 匡雯婕, 廖安平, 蓝平, 张金彦. 甲醇-水溶剂中L-苯丙氨酸结晶热力学[J]. 化工学报, 2019, 70(4): 1255-1262. |
| [12] | 曹小雪, 吉绍长, 匡雯婕, 廖安平, 蓝平, 张金彦. 阿奇霉素二水合物在水-有机溶剂中溶解度及三元相图测定[J]. 化工学报, 2019, 70(3): 817-829. |
| [13] | 范凯, 陈长旭, 周峰, 郑晖, 许春建. 正丁醇-氯苯-苯乙酮三元体系等压汽液平衡的测定与关联[J]. 化工学报, 2018, 69(2): 578-585. |
| [14] | 宗杰, 马庆兰, 陈光进, 孙长宇. ZIF-8/乙二醇体系分离捕集CO2溶解度的模拟计算[J]. 化工学报, 2018, 69(10): 4276-4283. |
| [15] | 陆小华, 蒋管聪, 朱育丹, 冯新, 吕玲红. 受限界面处流体分子行为的调控及相关分子热力学模型初探:基于高比表面氧化钛的研究进展[J]. 化工学报, 2018, 69(1): 1-8. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号