化工学报 ›› 2025, Vol. 76 ›› Issue (9): 4786-4799.DOI: 10.11949/0438-1157.20250180
邹家庆1( ), 张肇钰1, 张建国2, 张博宇2, 刘定胜1(
), 张肇钰1, 张建国2, 张博宇2, 刘定胜1( ), 毛庆1(
), 毛庆1( ), 王挺3, 李建军3
), 王挺3, 李建军3
收稿日期:2025-02-25
修回日期:2025-03-22
出版日期:2025-09-25
发布日期:2025-10-23
通讯作者:
刘定胜,毛庆
作者简介:邹家庆(1999—),男,硕士研究生,1443608605@qq.com
Jiaqing ZOU1( ), Zhaoyu ZHANG1, Jianguo ZHANG2, Boyu ZHANG2, Dingsheng LIU1(
), Zhaoyu ZHANG1, Jianguo ZHANG2, Boyu ZHANG2, Dingsheng LIU1( ), Qing MAO1(
), Qing MAO1( ), Ting WANG3, Jianjun LI3
), Ting WANG3, Jianjun LI3
Received:2025-02-25
Revised:2025-03-22
Online:2025-09-25
Published:2025-10-23
Contact:
Dingsheng LIU, Qing MAO
摘要:
碱水制氢电解槽极板上气泡的生成和合并会改变电解液的流动状态,影响电解液的速度场、温度场分布,导致局部过电流和热点出现,进而降低电解槽的电解效率。采用水平集耦合流体体积(CLS-VOF)数值技术对极板通道中的气液两相流动状态进行了模拟研究,考察了气泡在极板通道表面上的生成、成长和脱落过程,以及气泡之间的合并规律,研究了电流密度、电解液流量和通道截面形状对极板通道中含气率、表面气体覆盖率和压力降的影响。结果表明,气泡合并尺寸、含气率、表面气体覆盖率随着电流密度增大而增大,随着电解液流量增大而减小。
中图分类号:
邹家庆, 张肇钰, 张建国, 张博宇, 刘定胜, 毛庆, 王挺, 李建军. 碱水制氢电解槽极板通道中气泡的生成及演化性质[J]. 化工学报, 2025, 76(9): 4786-4799.
Jiaqing ZOU, Zhaoyu ZHANG, Jianguo ZHANG, Boyu ZHANG, Dingsheng LIU, Qing MAO, Ting WANG, Jianjun LI. Generation and evolution of bubbles in channels of bipolar plates of alkaline water electrolyzers for producing hydrogen[J]. CIESC Journal, 2025, 76(9): 4786-4799.
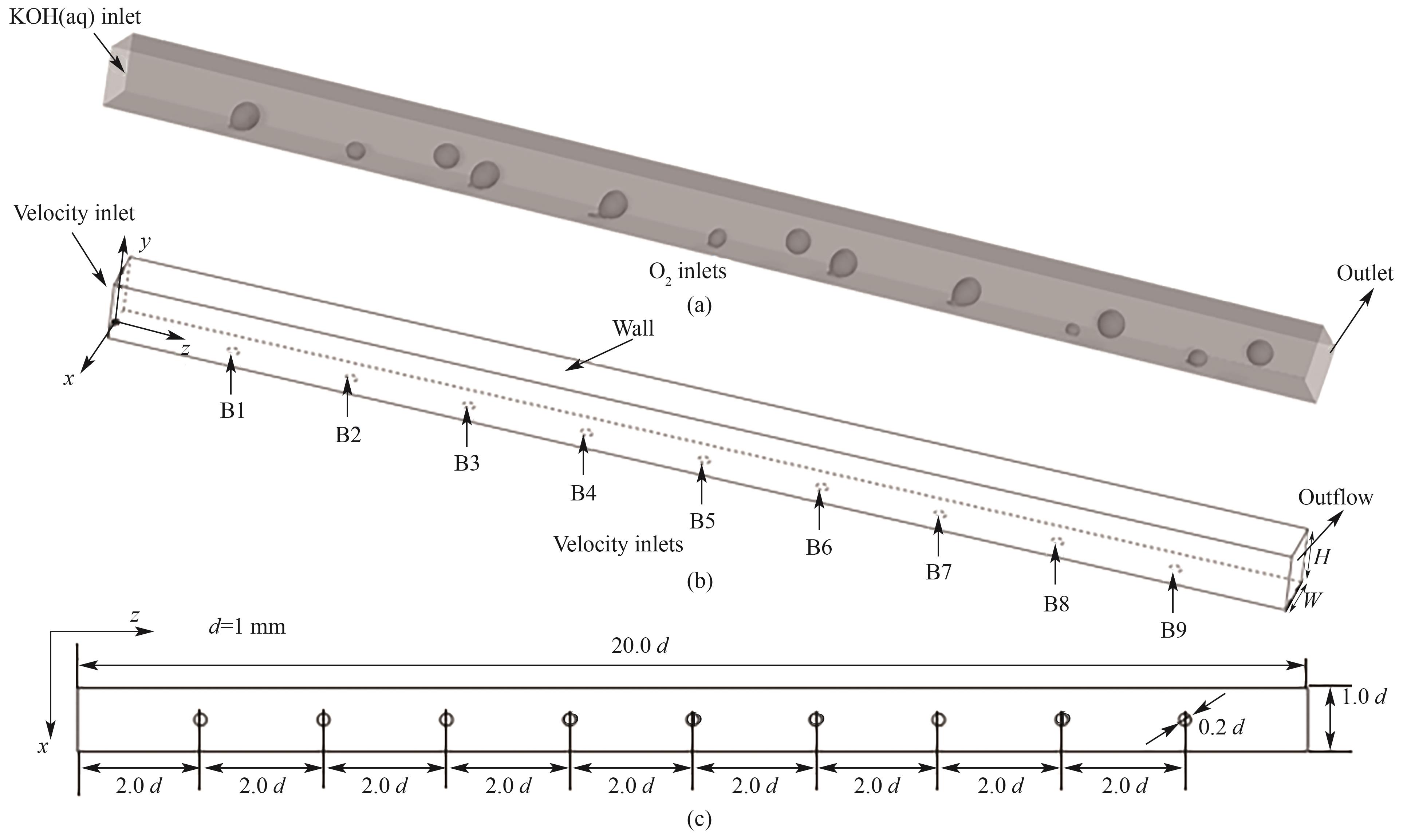
图1 碱性水电解槽的结构示意图:(a)极板模型;(b)边界条件;(c)结构尺寸
Fig.1 Schematic diagrams of the simulations: (a) polar plate model; (b) boundary conditions; (c) geometry dimension
| 参数 | 数值 |
|---|---|
| 工作温度/K | 298 |
| 工作压力/Pa | 101325 |
| KOH溶液密度[ | 1216.11 |
| O2密度/(kg/m3) | 1.29 |
| 5 mol/L KOH动力黏度[ | 1.6549×10-3 |
| O2动力黏度/(kg/(m·s)) | 1.919×10-5 |
| 表面张力[ | 0.0871 |
| KOH入口流量/(ml/min) | 15 |
| 出口表压/Pa | 0 |
| 接触角[ | 150 |
表1 物性参数及操作条件(电流密度为0.5 A/cm2)
Table 1 Physical parameters and operation conditions (current density of 0.5 A/cm2)
| 参数 | 数值 |
|---|---|
| 工作温度/K | 298 |
| 工作压力/Pa | 101325 |
| KOH溶液密度[ | 1216.11 |
| O2密度/(kg/m3) | 1.29 |
| 5 mol/L KOH动力黏度[ | 1.6549×10-3 |
| O2动力黏度/(kg/(m·s)) | 1.919×10-5 |
| 表面张力[ | 0.0871 |
| KOH入口流量/(ml/min) | 15 |
| 出口表压/Pa | 0 |
| 接触角[ | 150 |
| 网格尺寸/mm | 网格总数/个 | B4气泡y方向尺寸/mm | B5气泡y方向尺寸/mm |
|---|---|---|---|
| 0.030 | 528160 | 0.475 | 0.474 |
| 0.025 | 741280 | 0.480 | 0.488 |
| 0.020 | 2000000 | 0.483 | 0.482 |
表2 0.7 ms时B4及B5处生成的气泡大小随网格尺寸的变化
Table 2 Variation of bubble size with mesh size at B4 and B5 at 0.7 ms
| 网格尺寸/mm | 网格总数/个 | B4气泡y方向尺寸/mm | B5气泡y方向尺寸/mm |
|---|---|---|---|
| 0.030 | 528160 | 0.475 | 0.474 |
| 0.025 | 741280 | 0.480 | 0.488 |
| 0.020 | 2000000 | 0.483 | 0.482 |
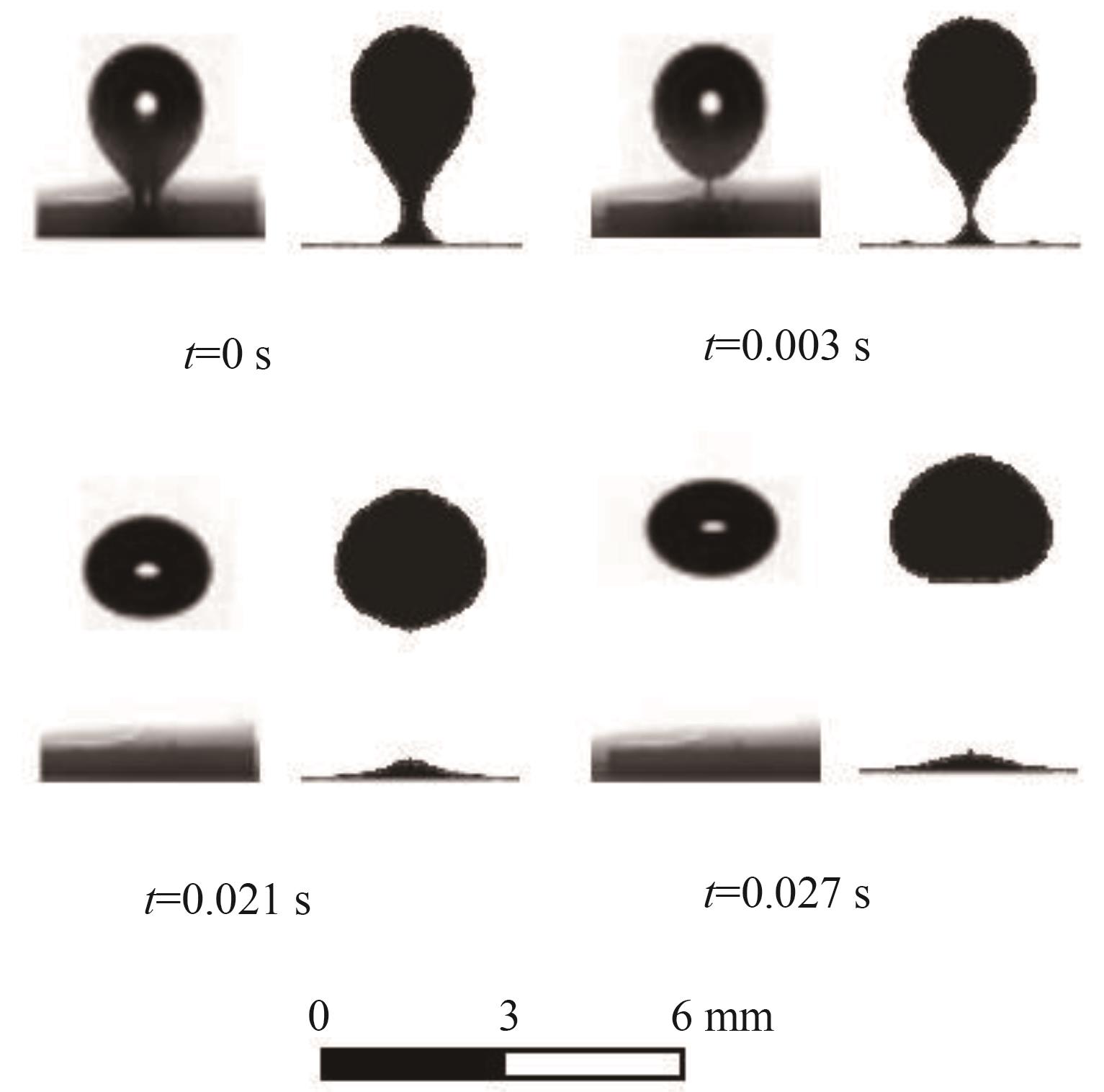
图3 气泡形状的实验[30](左侧)与仿真(右侧)比较
Fig.3 Comparisons of bubble shapes between the experiment[30] (left) and the present simulation (right) for model validation
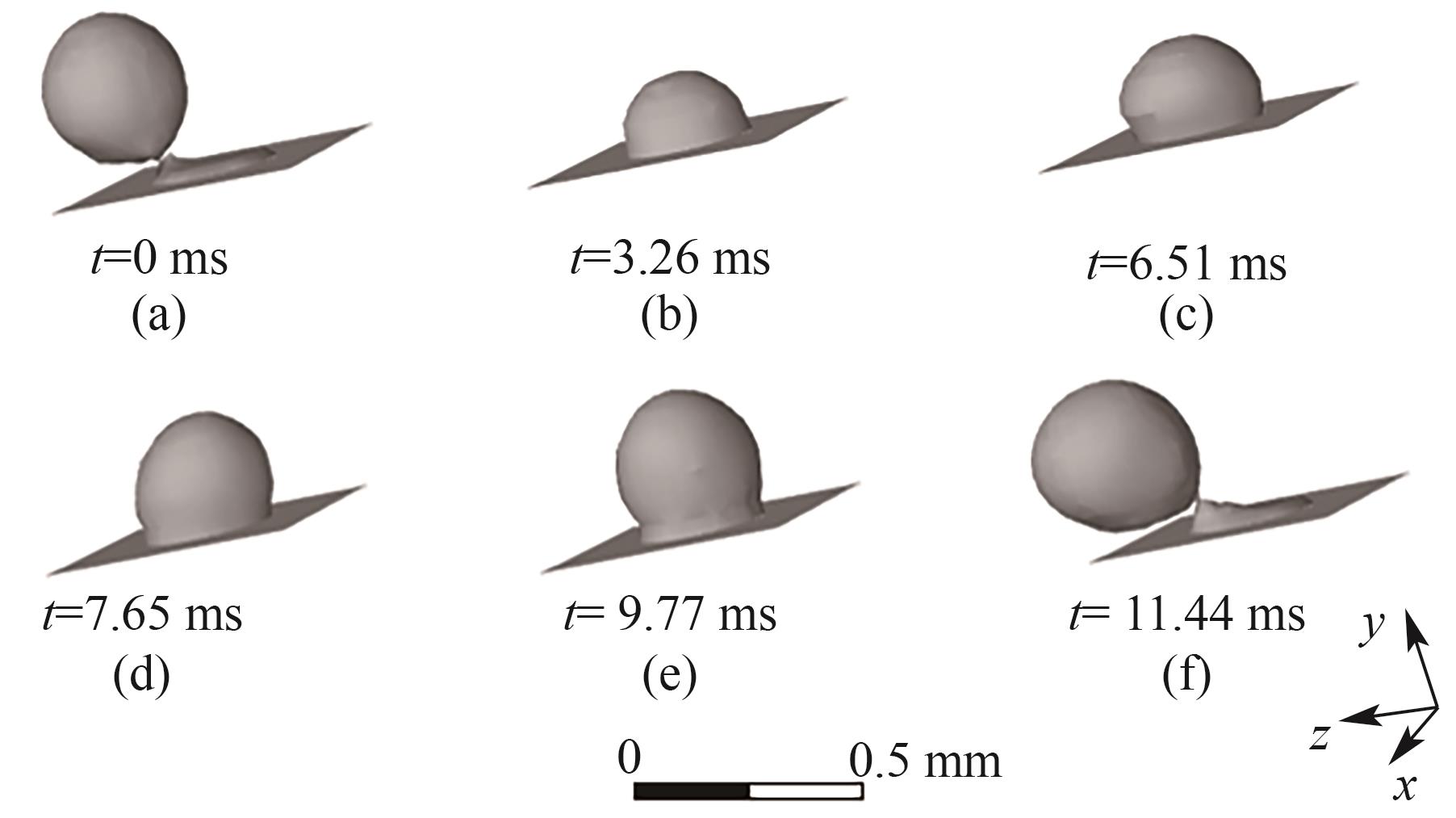
图4 B1处气泡的生成过程(J = 0.5 A/cm2,QL = 15 ml/min,θ = 150°,W/H = 1.0)
Fig.4 Bubble generation process at orifice B1 (J = 0.5 A/cm2, QL = 15 ml/min, θ = 150°, W/H = 1.0)
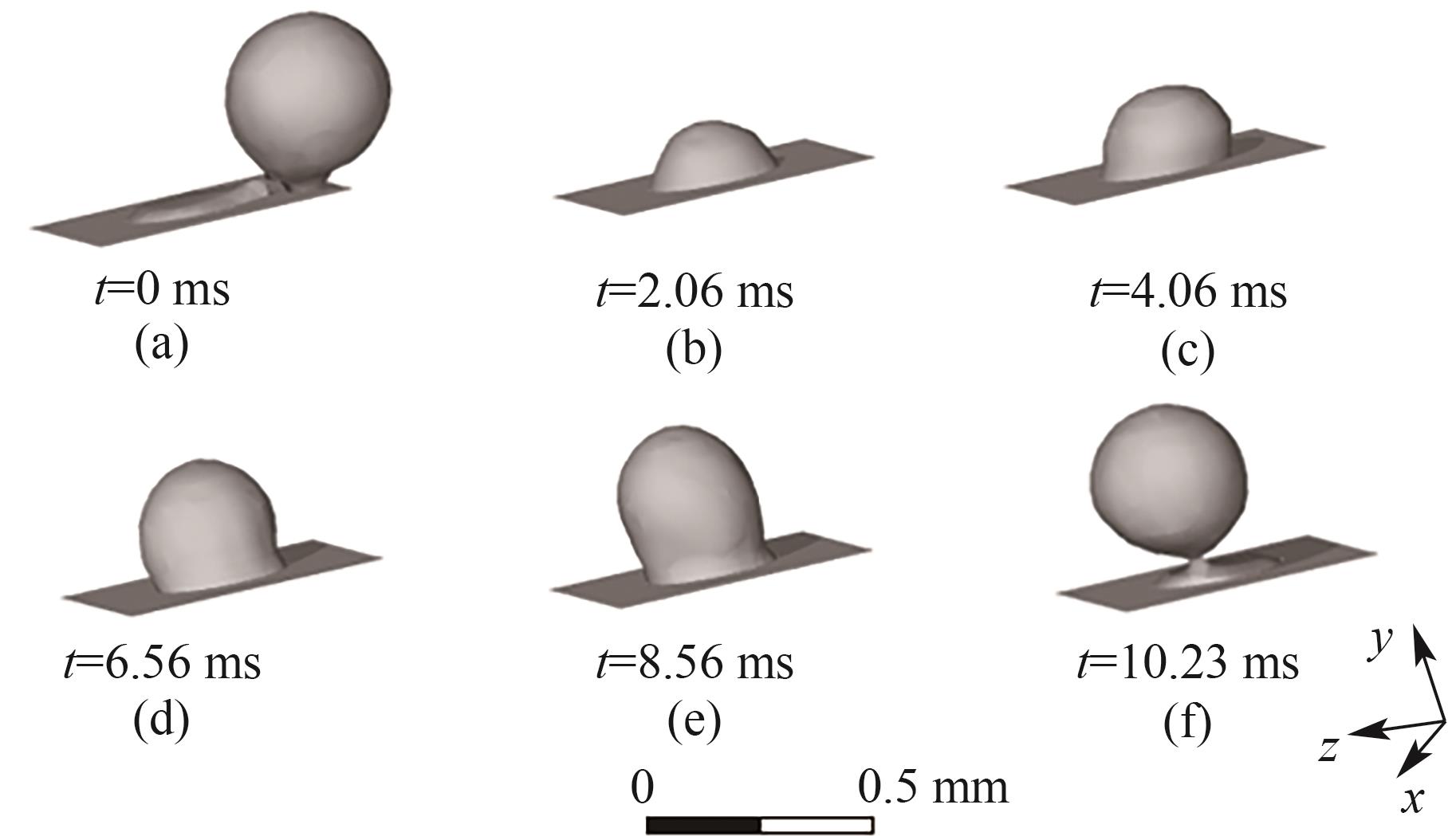
图5 B1处气泡的生成过程(J = 0.5 A/cm2,QL = 5 ml/min,θ = 150°,W/H = 1.0)
Fig.5 Bubble generation process at orifice B1 (J = 0.5 A/cm2, QL = 5 ml/min, θ = 150°, W/H = 1.0)
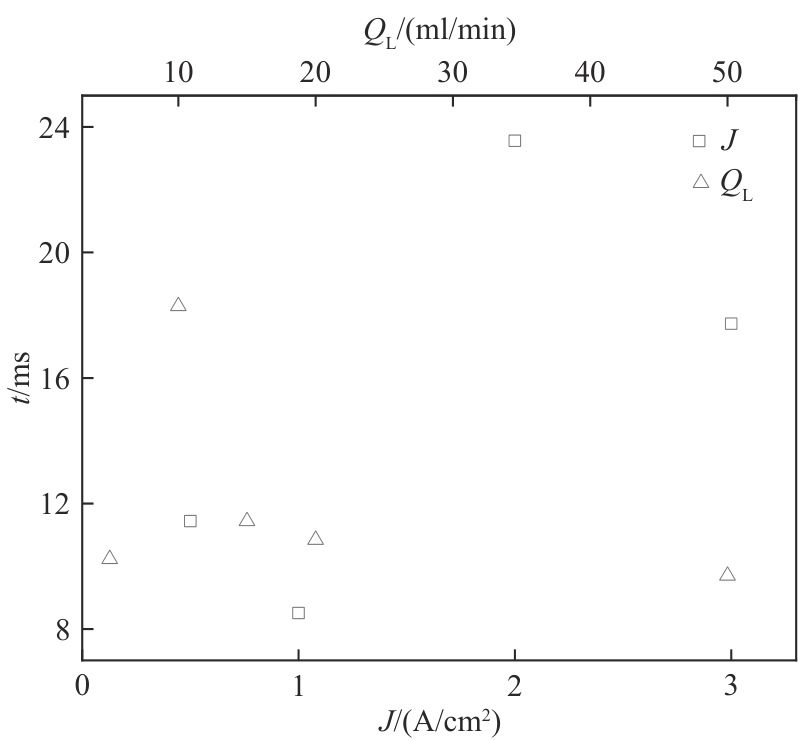
图6 电流密度及电解液流量变化对B1处气泡脱离时间的影响(W/H = 1.0)
Fig.6 Effects of current density and electrolyte flow rate on the detachment time of bubbles at B1 hole (W/H = 1.0)
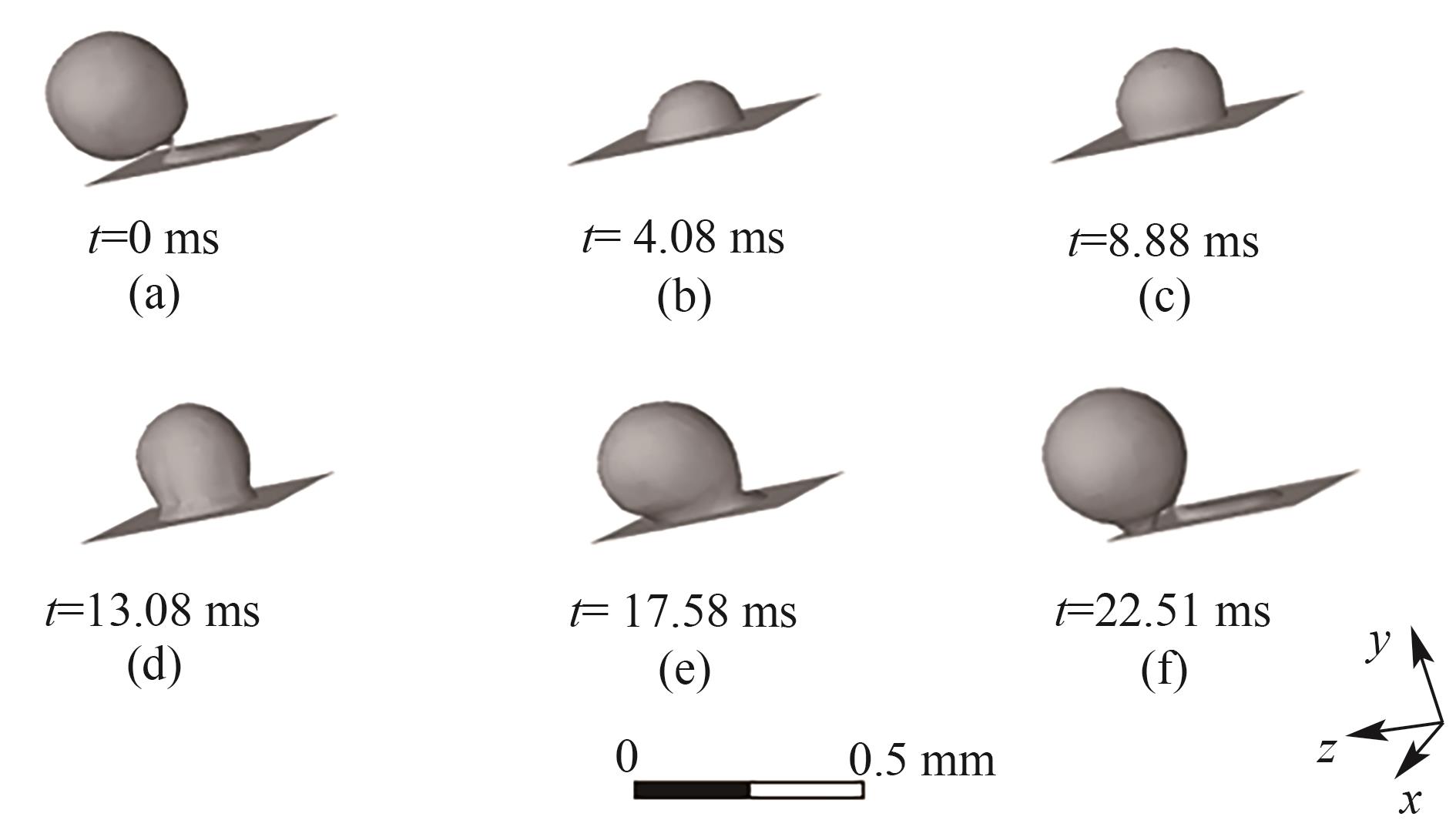
图7 B1处气泡的生成过程(J = 0.5 A/cm2,QL = 15 ml/min,θ = 150°,W/H = 0.5)
Fig.7 Generation process of a bubble at orifice B1 (J = 0.5 A/cm2, QL = 15 ml/min, θ = 150°, W/H = 0.5)
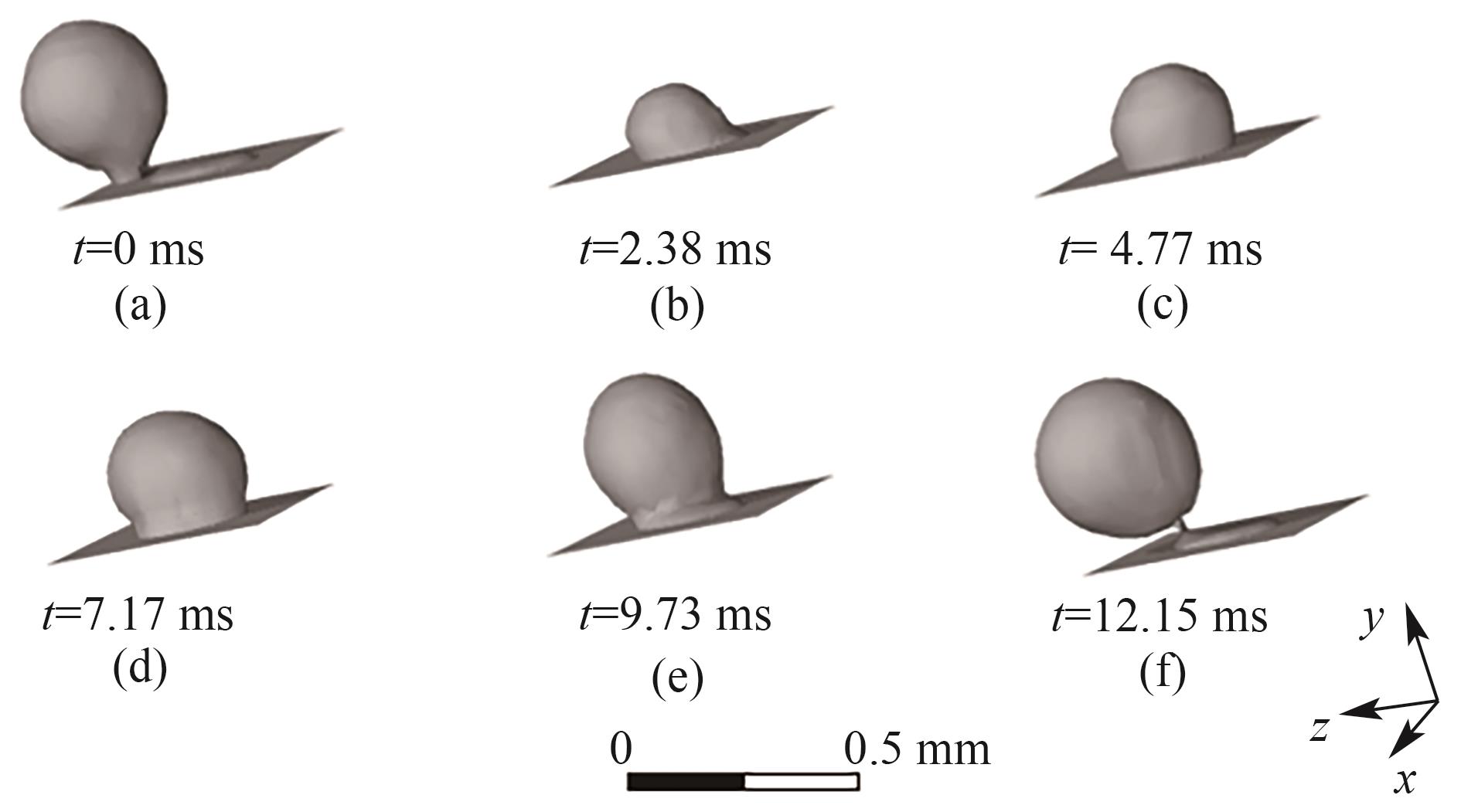
图8 B1处气泡的生成过程(J = 0.5 A/cm2,QL = 15 ml/min,θ = 150°,W/H = 2.0)
Fig.8 Generation process of a bubble at orifice B1 (J = 0.5 A/cm2, QL = 15 ml/min, θ = 150°, W/H = 2.0)
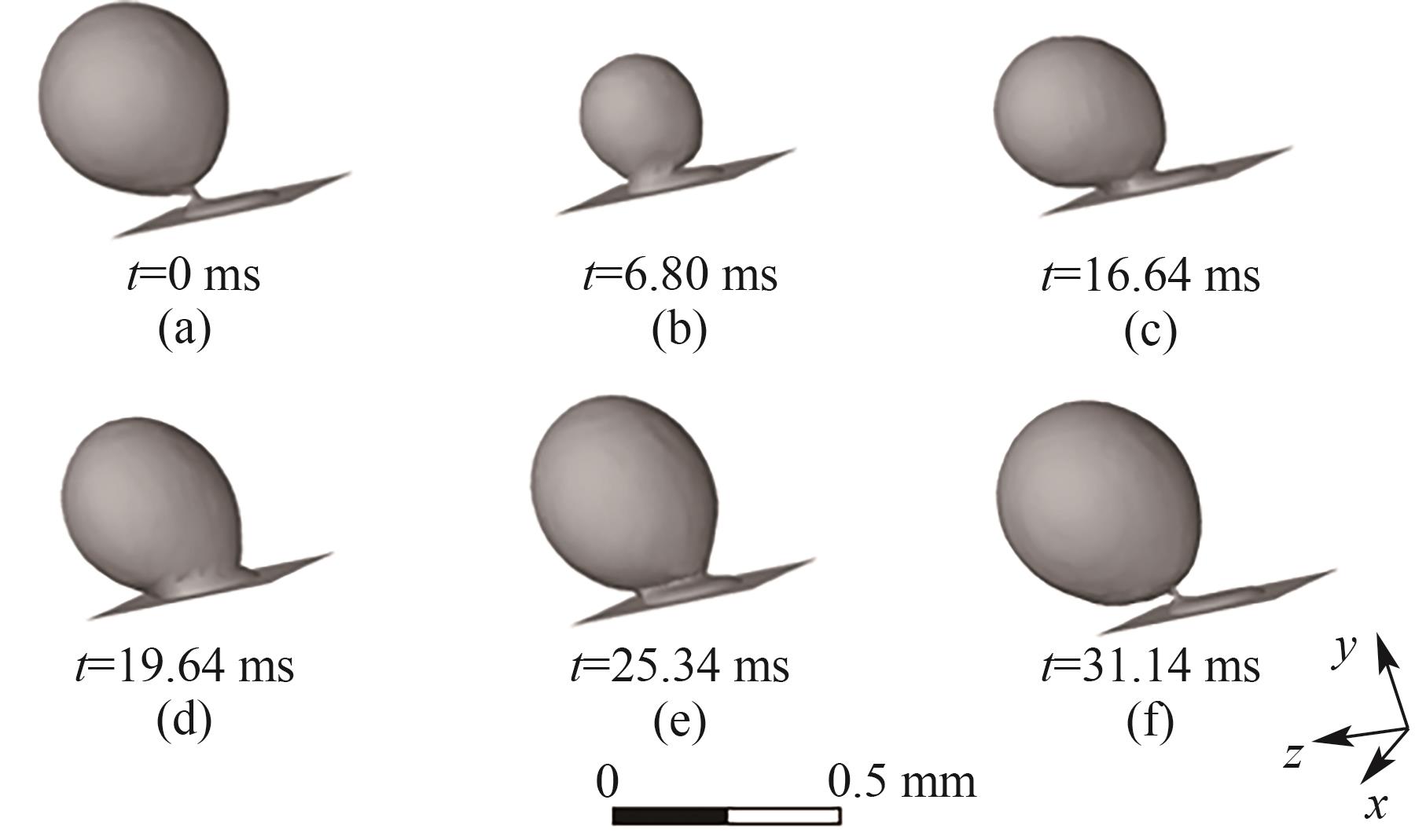
图9 B1处气泡的生成过程(J = 0.5 A/cm2,QL = 15 ml/min,θ = 150°,梯形截面通道)
Fig.9 Generation process of a bubble at orifice B1 (J = 0.5 A/cm2, QL = 15 ml/min, θ = 150°, trapezoidal cross-section channel)
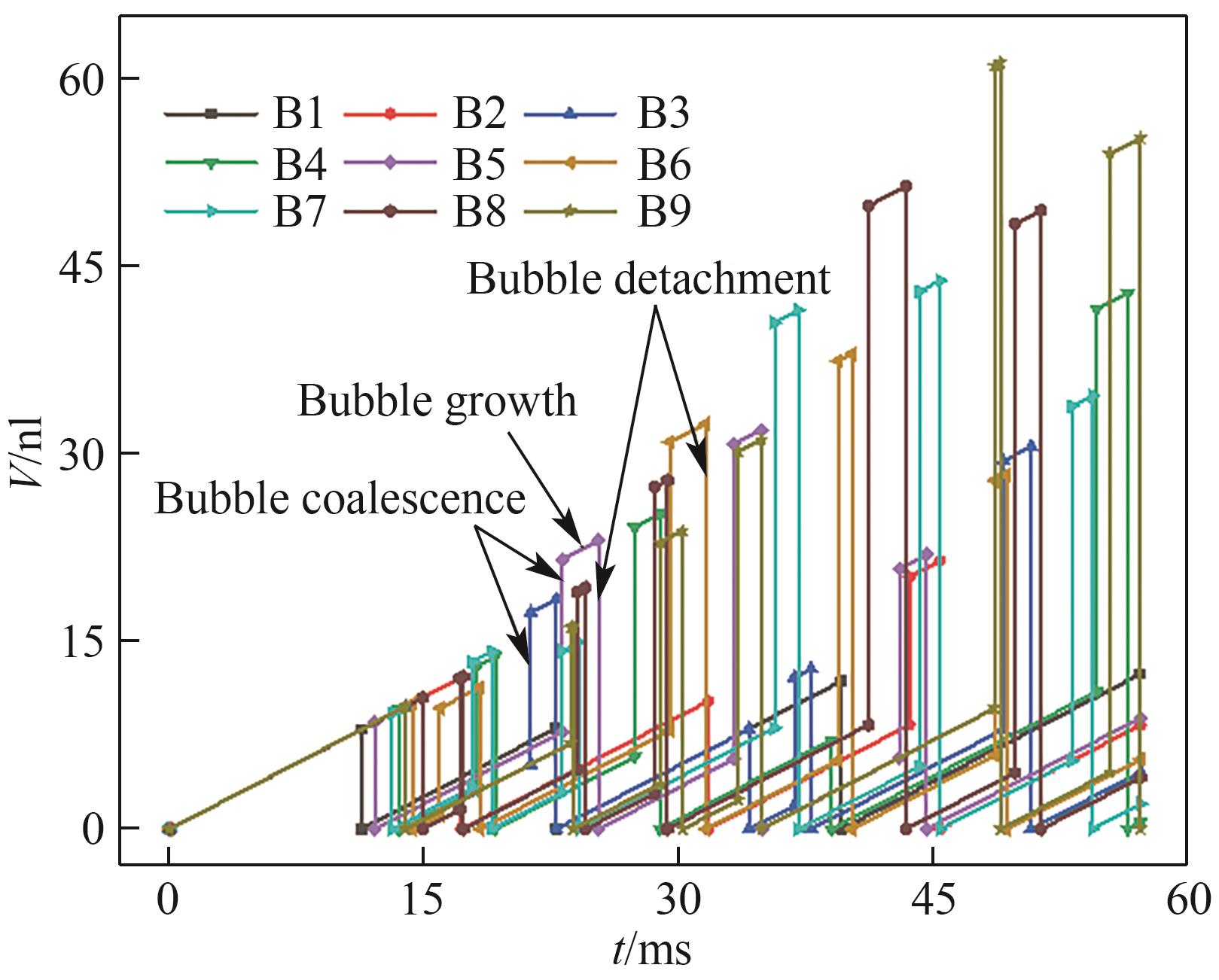
图10 气泡体积随时间的变化(J = 0.5 A/cm2,QL = 15 ml/min,θ = 150°,W/H = 1.0)
Fig.10 Variation of bubble volume with time (J = 0.5 A/cm2, QL = 15 ml/min, θ = 150°, W/H = 1.0)
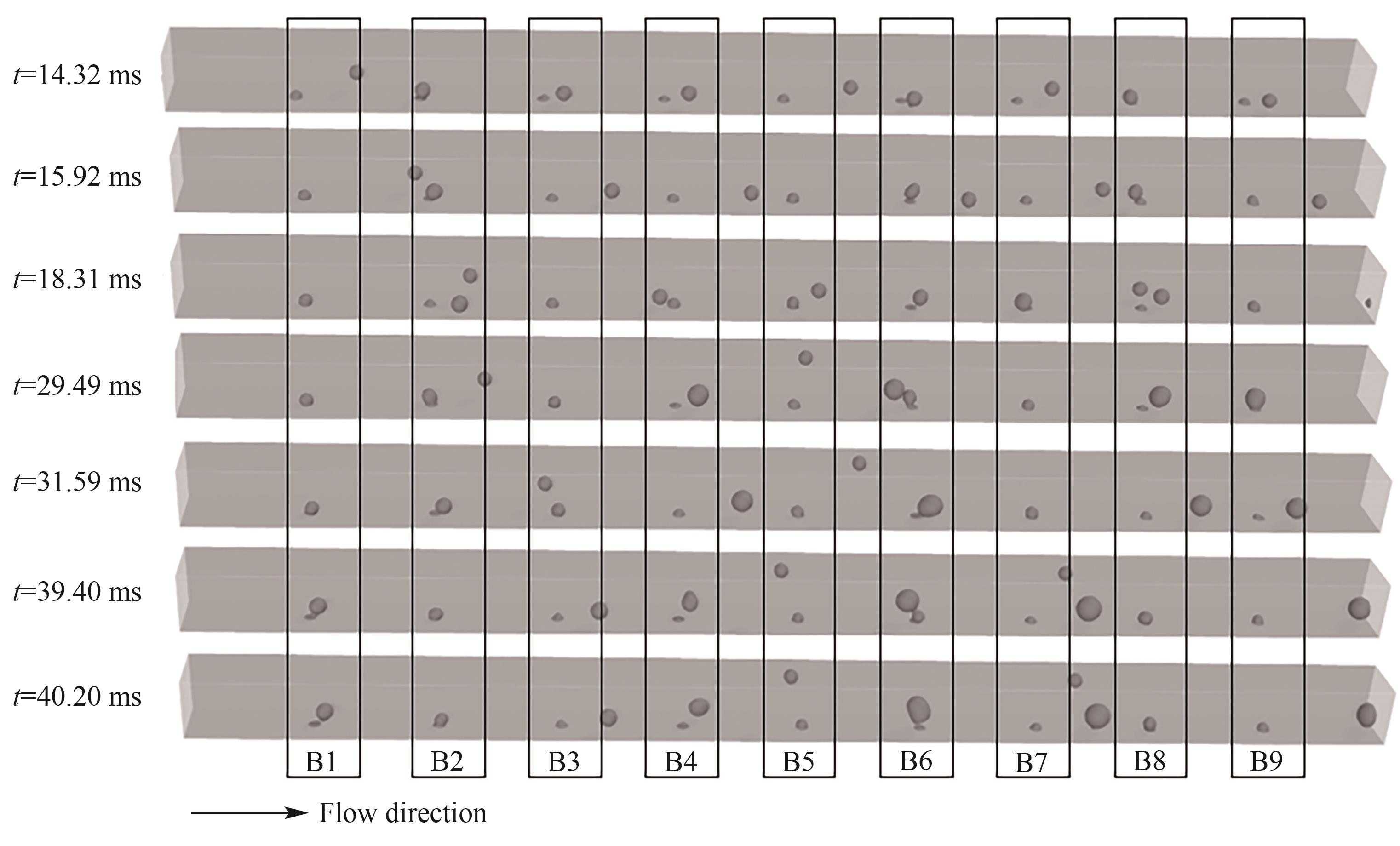
图11 B1~B9处气泡脱离和合并过程(J = 0.5 A/cm2,QL = 15 ml/min,θ = 150°,W/H = 1.0)
Fig.11 Detachment and coalescence of bubbles at orifices B1—B9 (J = 0.5 A/cm2, QL = 15 ml/min, θ = 150°, W/H = 1.0)
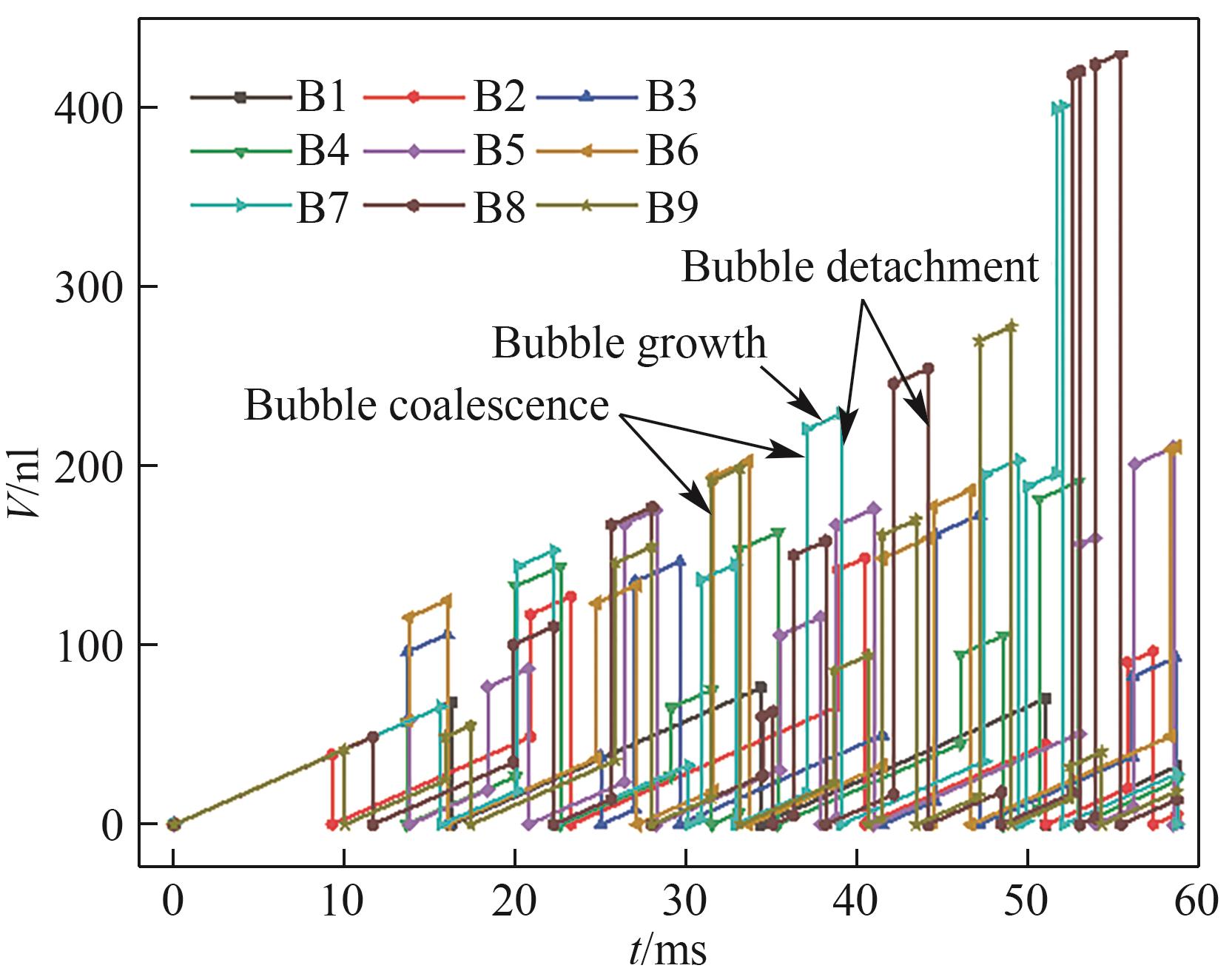
图12 气泡体积随时间的变化(J = 3.0 A/cm2,QL = 15 ml/min,θ = 150°,W/H = 1.0)
Fig.12 Variation of bubble volume with time (J = 3.0 A/cm2, QL = 15 ml/min, θ = 150°, W/H = 1.0)
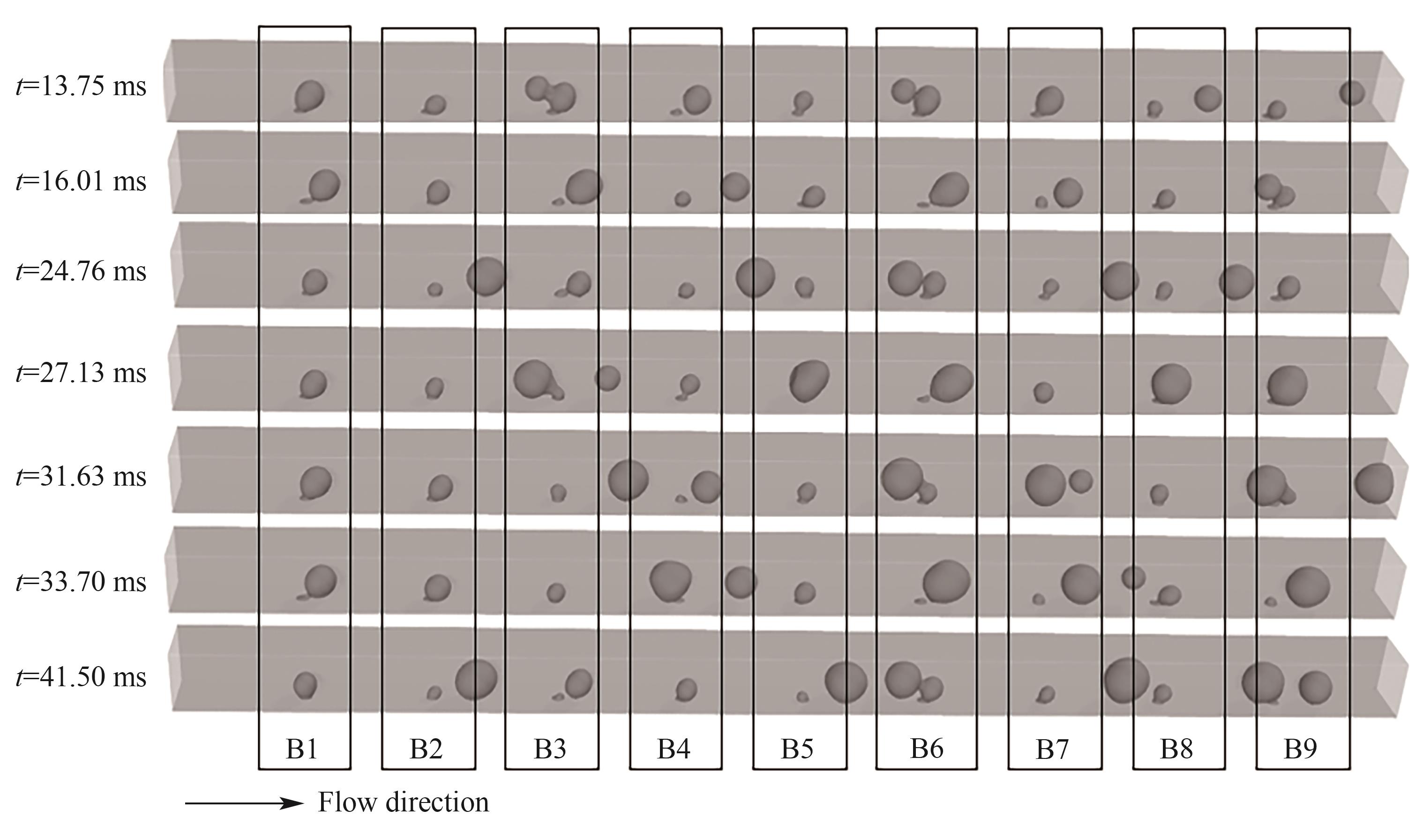
图13 B1~B9处气泡脱离和合并过程(J = 3.0 A/cm2,QL = 15 ml/min,θ = 150°,W/H = 1.0)
Fig.13 Detachment and coalescence of bubbles at orifices B1—B9 (J = 3.0 A/cm2, QL = 15 ml/min, θ = 150°, W/H = 1.0)
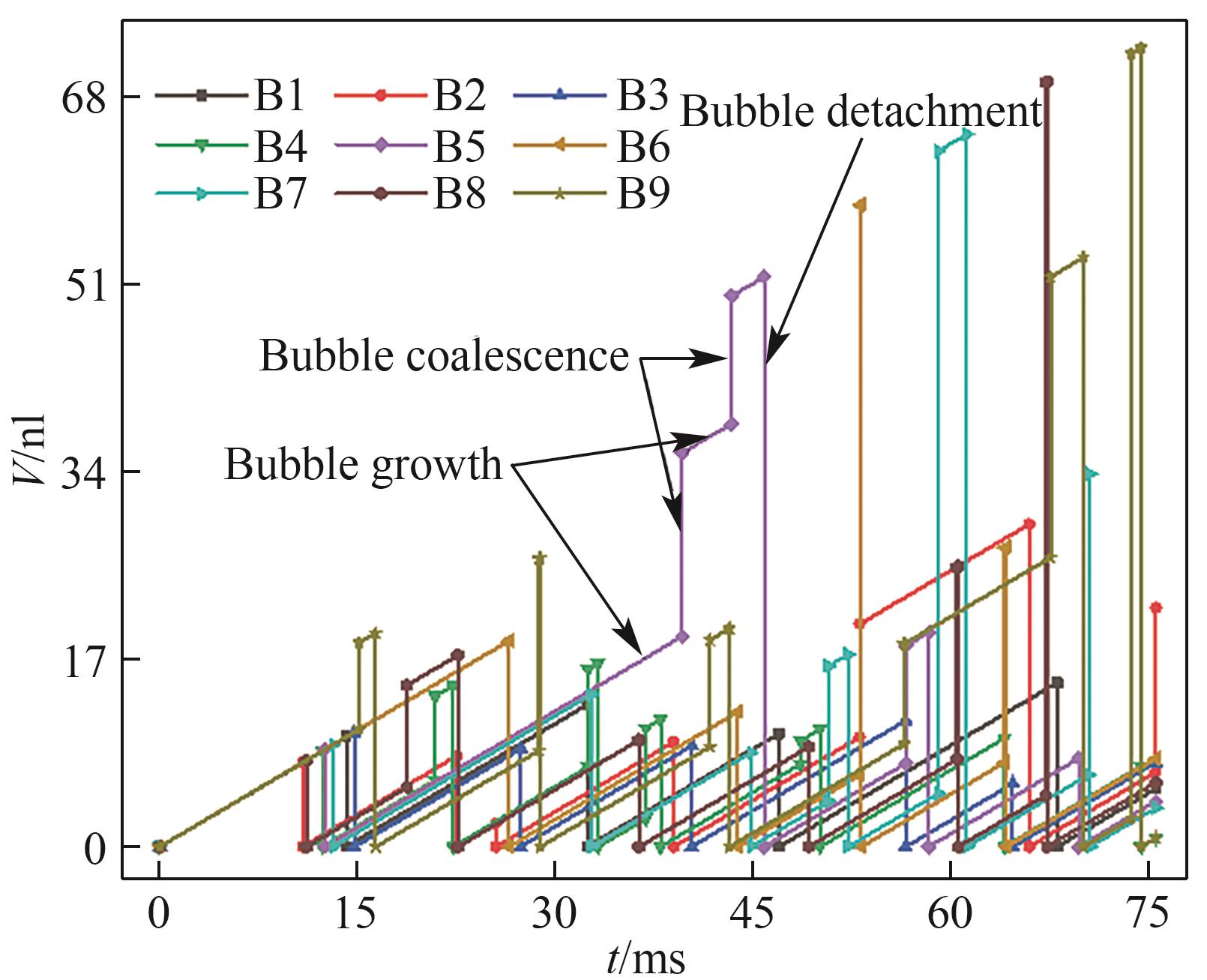
图14 气泡体积随时间的变化(J = 0.5 A/cm2,QL = 10 ml/min,θ = 150°,W/H = 1.0)
Fig.14 Detachment and coalescence of bubbles at B6 (J = 0.5 A/cm2, QL = 10 ml/min, θ = 150°, W/H = 1.0)
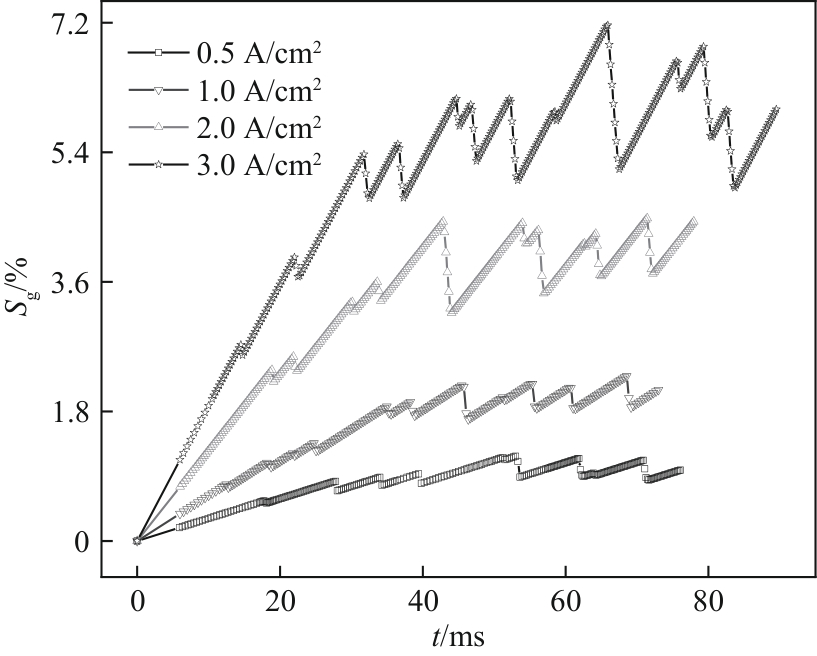
图16 不同电流密度下含气率随时间的变化(W/H = 1.0,QL = 15 ml/min,θ = 150°)
Fig.16 Variation of gas fraction with time under different current densities (W/H = 1.0, QL = 15 ml/min, θ = 150°)
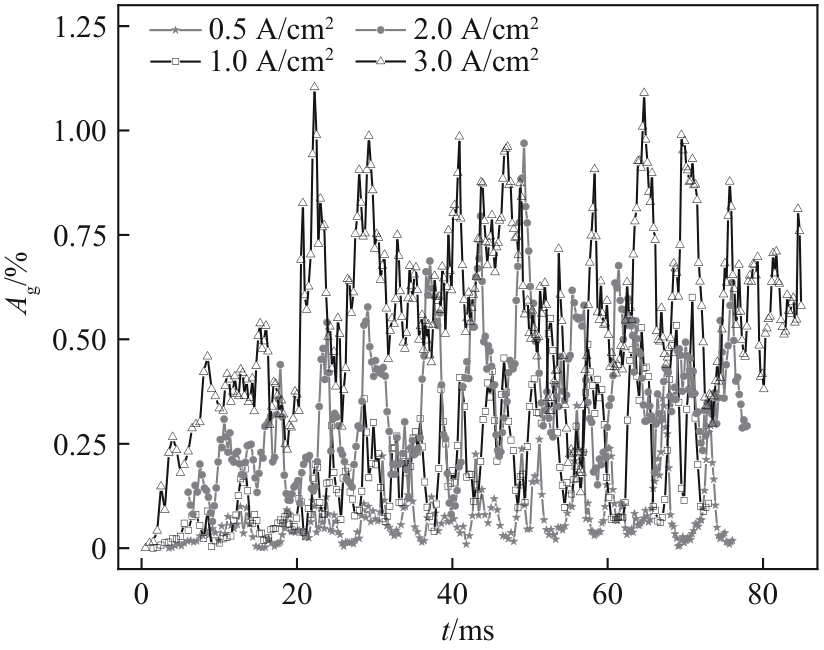
图17 不同电流密度下GDL表面气体覆盖率随时间的变化(W/H = 1.0,QL = 15 ml/min,θ = 150°)
Fig.17 Variation of surface gas fraction on GDLwith time under different current densities (W/H = 1.0, QL = 15 ml/min, θ = 150°)
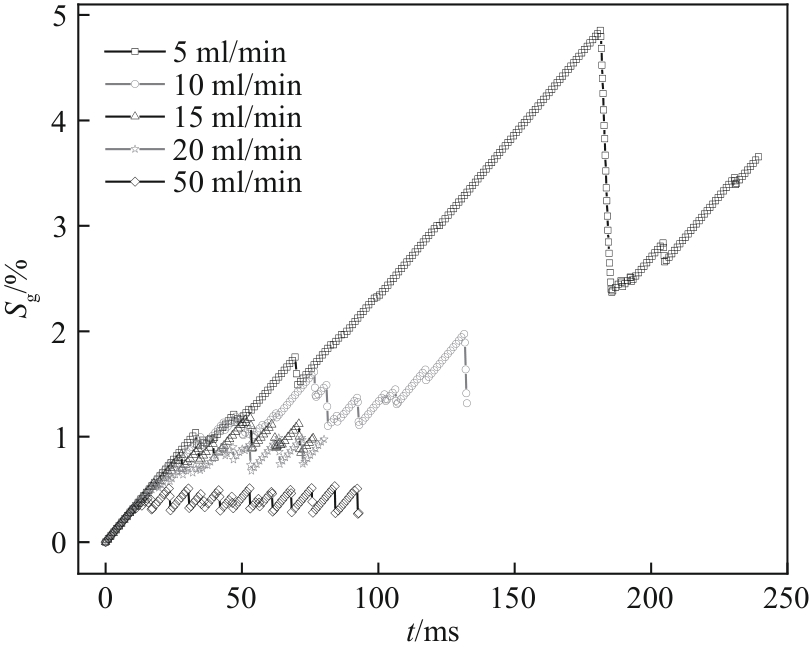
图19 不同电解液流量下含气率随时间的变化(W/H = 1.0,J = 0.5 A/cm2,θ = 150°)
Fig.19 Variation of gas fraction with time under different electrolyte flow rates (W/H = 1.0, J = 0.5 A/cm2, θ = 150°)
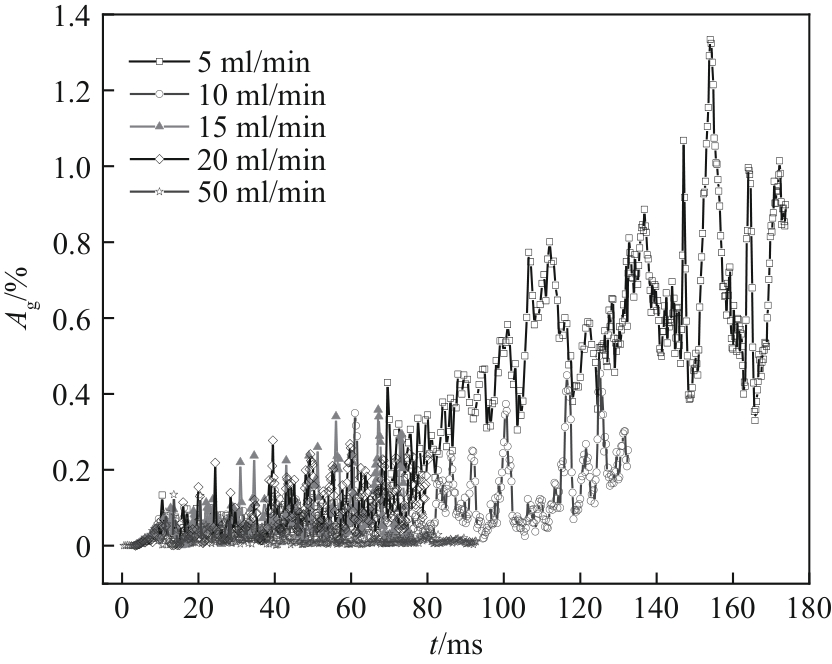
图20 不同电解液流量下表面气体覆盖率随时间的变化(W/H = 1.0,J = 0.5 A/cm2,θ = 150°)
Fig.20 Variation of surface gas fraction on GDL with time under different electrolyte flow rates (W/H = 1.0, J = 0.5 A/cm2, θ = 150°)
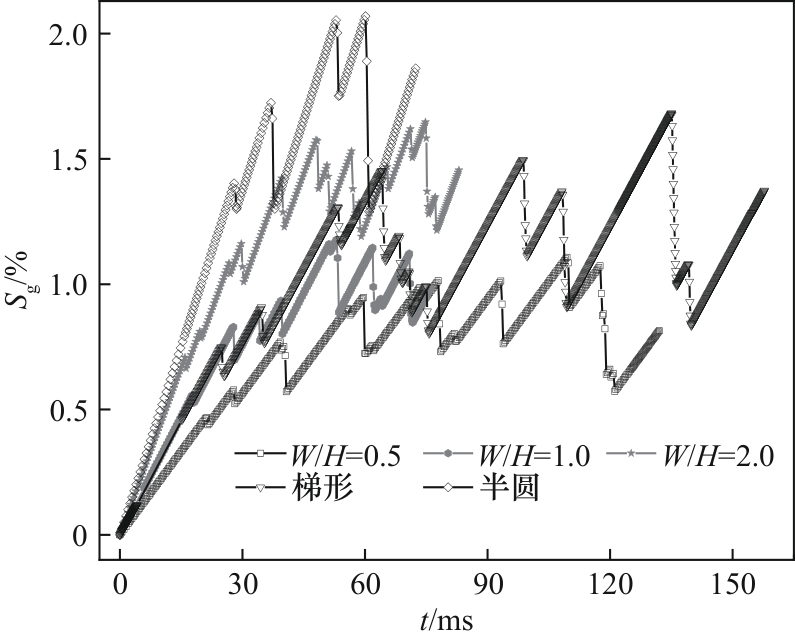
图22 不同通道截面形状下含气率随时间的变化(J = 0.5 A/cm2,QL = 15 ml/min,θ = 150°)
Fig.22 Variation of gas fraction with time in channels with different cross-sections (J = 0.5 A/cm2, QL = 15 ml/min, θ = 150°)
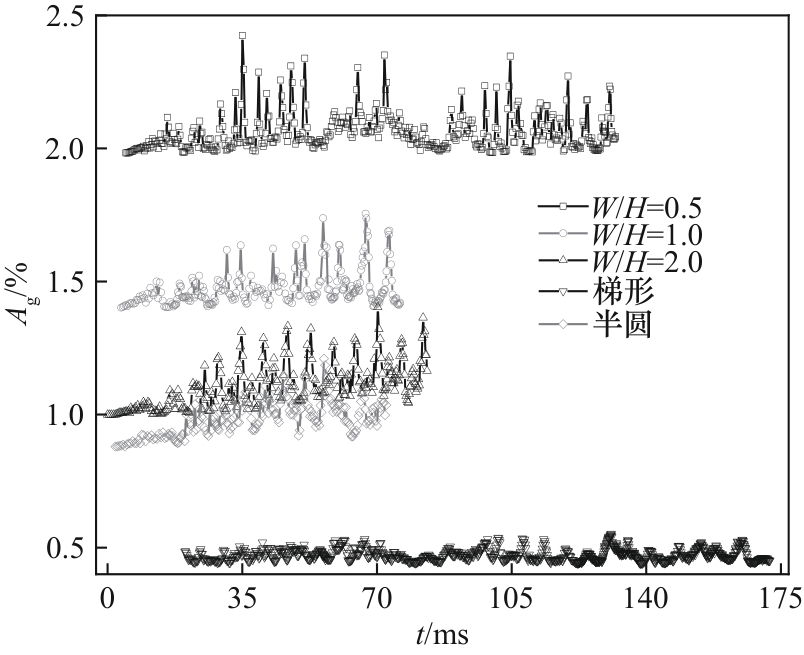
图23 不同通道截面形状下表面气体覆盖率随时间的变化(J = 0.5 A/cm2,QL = 15 ml/min,θ = 150°)
Fig.23 Variation of surface gas coverage with time in channels with different cross-sections (J = 0.5 A/cm2, QL = 15 ml/min, θ = 150°)
| [1] | Shimada H, Yamaguchi T, Kishimoto H, et al. Nanocomposite electrodes for high current density over 3 A·cm-2 in solid oxide electrolysis cells[J]. Nature Communications, 2019, 10(1): 5432. |
| [2] | Hauch A, Küngas R, Blennow P, et al. Recent advances in solid oxide cell technology for electrolysis[J]. Science, 2020, 370(6513): eaba6118. |
| [3] | Flamm B, Peter C, Büchi F N, et al. Electrolyzer modeling and real-time control for optimized production of hydrogen gas[J]. Applied Energy, 2021, 281: 116031. |
| [4] | Qu W J, Zhang J, Jiang R H, et al. An energy storage approach for storing surplus power into hydrogen in a cogeneration system[J]. Energy Conversion and Management, 2022, 268: 116032. |
| [5] | Shiva Kumar S, Lim H. An overview of water electrolysis technologies for green hydrogen production[J]. Energy Reports, 2022, 8: 13793-13813. |
| [6] | Okolie J A, Patra B R, Mukherjee A, et al. Futuristic applications of hydrogen in energy, biorefining, aerospace, pharmaceuticals and metallurgy[J]. International Journal of Hydrogen Energy, 2021, 46(13): 8885-8905. |
| [7] | Naqvi S A H, Taner T, Ozkaymak M, et al. Hydrogen production through alkaline electrolyzers: a techno-economic and enviro-economic analysis[J]. Chemical Engineering & Technology, 2023, 46(3): 474-481. |
| [8] | David M, Ocampo-Martínez C, Sánchez-Peña R. Advances in alkaline water electrolyzers: a review[J]. Journal of Energy Storage, 2019, 23: 392-403. |
| [9] | Koumi Ngoh S, Njomo D. An overview of hydrogen gas production from solar energy[J]. Renewable and Sustainable Energy Reviews, 2012, 16(9): 6782-6792. |
| [10] | Dincer I, Acar C. Review and evaluation of hydrogen production methods for better sustainability[J]. International Journal of Hydrogen Energy, 2015, 40(34): 11094-11111. |
| [11] | Hreiz R, Abdelouahed L, Fünfschilling D, et al. Electrogenerated bubbles induced convection in narrow vertical cells: PIV measurements and Euler-Lagrange CFD simulation[J]. Chemical Engineering Science, 2015, 134: 138-152. |
| [12] | Majasan J O, Cho J I S, Dedigama I, et al. Two-phase flow behaviour and performance of polymer electrolyte membrane electrolysers: electrochemical and optical characterisation[J]. International Journal of Hydrogen Energy, 2018, 43(33): 15659-15672. |
| [13] | Boissonneau P, Byrne P. An experimental investigation of bubble-induced free convection in a small electrochemical cell[J]. Journal of Applied Electrochemistry, 2000, 30(7): 767-775. |
| [14] | Abdelouahed L, Valentin G, Poncin S, et al. Current density distribution and gas volume fraction in the gap of lantern blade electrodes[J]. Chemical Engineering Research and Design, 2014, 92(3): 559-570. |
| [15] | El-Askary W A, Sakr I M, Ibrahim K A, et al. Hydrodynamics characteristics of hydrogen evolution process through electrolysis: numerical and experimental studies[J]. Energy, 2015, 90: 722-737. |
| [16] | Aldas K. Application of a two-phase flow model for hydrogen evolution in an electrochemical cell[J]. Applied Mathematics and Computation, 2004, 154(2): 507-519. |
| [17] | Abdelouahed L, Hreiz R, Poncin S, et al. Hydrodynamics of gas bubbles in the gap of lantern blade electrodes without forced flow of electrolyte: experiments and CFD modelling[J]. Chemical Engineering Science, 2014, 111: 255-265. |
| [18] | Arbabi F, Montazeri H, Abouatallah R, et al. Three-dimensional computational fluid dynamics modelling of oxygen bubble transport in polymer electrolyte membrane electrolyzer porous transport layers[J]. Journal of the Electrochemical Society, 2016, 163(11): F3062-F3069. |
| [19] | Vachaparambil K J, Einarsrud K E. Numerical simulation of continuum scale electrochemical hydrogen bubble evolution[J]. Applied Mathematical Modelling, 2021, 98: 343-377. |
| [20] | Zarghami A, Deen N G, Vreman A W. CFD modeling of multiphase flow in an alkaline water electrolyzer[J]. Chemical Engineering Science, 2020, 227: 115926. |
| [21] | Mat M D, Aldas K. Application of a two-phase flow model for natural convection in an electrochemical cell[J]. International Journal of Hydrogen Energy, 2005, 30(4): 411-420. |
| [22] | Mat M D, Aldas K, Ilegbusi O J. A two-phase flow model for hydrogen evolution in an electrochemical cell[J]. International Journal of Hydrogen Energy, 2004, 29(10): 1015-1023. |
| [23] | Mandin P, Hamburger J, Bessou S, et al. Modelling and calculation of the current density distribution evolution at vertical gas-evolving electrodes[J]. Electrochimica Acta, 2005, 51(6): 1140-1156. |
| [24] | Eigeldinger J, Vogt H. The bubble coverage of gas-evolving electrodes in a flowing electrolyte[J]. Electrochimica Acta, 2000, 45(27): 4449-4456. |
| [25] | Li Y F, Kang Z Y, Mo J K, et al. In-situ investigation of bubble dynamics and two-phase flow in proton exchange membrane electrolyzer cells[J]. International Journal of Hydrogen Energy, 2018, 43(24): 11223-11233. |
| [26] | Gilliam R J, Graydon J W, Kirk D W, et al. A review of specific conductivities of potassium hydroxide solutions for various concentrations and temperatures[J]. International Journal of Hydrogen Energy, 2007, 32(3): 359-364. |
| [27] | Sipos P M, Hefter G, May P M. Viscosities and densities of highly concentrated aqueous MOH solutions (M+ = Na+, K+, Li+, Cs+, (CH3)4N+) at 25.0 ℃[J]. Journal of Chemical & Engineering Data, 2000, 45(4): 613-617. |
| [28] | Dunlap P M, Faris S R. Surface tension of aqueous solutions of potassium hydroxide[J]. Nature, 1962, 196: 1312-1313. |
| [29] | German S R, Edwards M A, Ren H, et al. Critical nuclei size, rate, and activation energy of H2 gas nucleation[J]. Journal of the American Chemical Society, 2018, 140(11): 4047-4053. |
| [30] | Kong G, Mirsandi H, Buist K A, et al. Oscillation dynamics of a bubble rising in viscous liquid[J]. Experiments in Fluids, 2019, 60(8): 130. |
| [1] | 燕子腾, 詹飞龙, 丁国良. 空调用套管式分流器结构设计及分流效果验证[J]. 化工学报, 2025, 76(S1): 152-159. |
| [2] | 赵子祥, 段钟弟, 孙浩然, 薛鸿祥. 大温差两相流动诱导水锤冲击的数值模型[J]. 化工学报, 2025, 76(S1): 170-180. |
| [3] | 曹庆泰, 郭松源, 李建强, 蒋赞, 汪彬, 耑锐, 吴静怡, 杨光. 负过载下多孔隔板对液氧贮箱蓄液性能的影响研究[J]. 化工学报, 2025, 76(S1): 217-229. |
| [4] | 孔俊龙, 毕扬, 赵耀, 代彦军. 储能电池直冷热管理系统的模拟实验[J]. 化工学报, 2025, 76(S1): 289-296. |
| [5] | 段浩磊, 陈浩远, 梁坤峰, 王林, 陈彬, 曹勇, 张晨光, 李硕鹏, 朱登宇, 何亚茹, 杨大鹏. 纯电动车热管理系统低GWP工质替代方案性能分析与综合评价[J]. 化工学报, 2025, 76(S1): 54-61. |
| [6] | 王俊鹏, 冯佳琪, 张恩搏, 白博峰. 曲折式与阵列式迷宫阀芯结构内流动与空化特性研究[J]. 化工学报, 2025, 76(S1): 93-105. |
| [7] | 贾志勇, 沈宪琨, 蓝晓程, 王铁峰. 气体密度对高压流态化影响的CFD-DEM模拟[J]. 化工学报, 2025, 76(9): 4383-4397. |
| [8] | 段炼, 周星睿, 袁文君, 陈飞. 连续相速度脉动对微通道内聚合物液滴生成和形貌的影响规律[J]. 化工学报, 2025, 76(9): 4578-4585. |
| [9] | 刘奕扬, 邢志祥, 刘烨铖, 彭明, 李玉洋, 李云浩, 沈宁舟. 加氢站液氢泄漏扩散特性与安全监测数值模拟研究[J]. 化工学报, 2025, 76(9): 4694-4708. |
| [10] | 梁晓江, 陈薇薇, 罗佳南, 费浩天, 叶雪蕾, 李文豪, 聂勇. 电分散管式填充床中荷电气泡的分散特性研究[J]. 化工学报, 2025, 76(8): 3915-3931. |
| [11] | 张淇栋, 艾立强, 马原, 吴胜宝, 王磊, 厉彦忠. 基于一维漂移流模型的低温管路预冷过程两相流动与换热特性研究[J]. 化工学报, 2025, 76(8): 3842-3852. |
| [12] | 朱紫橙, 焦云鹏, 刘梦溪, 陈建华. 三相流化床内分布器与挡板效应的模拟分析[J]. 化工学报, 2025, 76(8): 3873-3884. |
| [13] | 周奕彤, 周明熙, 刘若晨, 叶爽, 黄伟光. 光伏与电网协同驱动氢基直接还原铁炼钢的技术经济分析[J]. 化工学报, 2025, 76(8): 4318-4330. |
| [14] | 范夏雨, 孙建辰, 李可莹, 姚馨雅, 商辉. 机器学习驱动液态有机储氢技术的系统优化[J]. 化工学报, 2025, 76(8): 3805-3821. |
| [15] | 杨宁, 李皓男, LIN Xiao, GEORGIADOU Stella, LIN Wen-Feng. 从塑料废弃物到能源催化剂:塑料衍生碳@CoMoO4复合材料在电解水析氢反应中的应用[J]. 化工学报, 2025, 76(8): 4081-4094. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号