化工学报 ›› 2025, Vol. 76 ›› Issue (11): 5923-5932.DOI: 10.11949/0438-1157.20250307
• 催化、动力学与反应器 • 上一篇
收稿日期:2025-03-25
修回日期:2025-05-15
出版日期:2025-11-25
发布日期:2025-12-19
通讯作者:
张淑芬
作者简介:唐志成(1995—),男,博士研究生,zhichengtang@mail.dlut.edu.cn
基金资助:
Zhicheng TANG( ), Tianwei WANG, Rongwen LYU, Shufen ZHANG(
), Tianwei WANG, Rongwen LYU, Shufen ZHANG( )
)
Received:2025-03-25
Revised:2025-05-15
Online:2025-11-25
Published:2025-12-19
Contact:
Shufen ZHANG
摘要:
为解决1-氨基-4-溴蒽醌-2-磺酸(溴氨酸)芳胺化反应催化剂Cu+易失活、影响溴氨酸芳胺化反应效率的问题,设计并制备了一种促Cu2+释放的含Mn碱式碳酸铜电催化剂,并在两电极体系中采用电化学还原方法进行溴氨酸芳胺化反应。利用循环伏安法(CV)、扫描电镜(SEM)、电化学阻抗谱(EIS)等研究了还原电压、电催化剂组成对芳胺化反应的影响,并进行了底物拓展和放大实验。结果表明,所制备的含Mn碱式碳酸铜电催化剂能有效促进Cu2+释放,并在-0.4 V的还原电压下被还原为Cu+原位催化溴氨酸与2,4,6-三甲基-1,3-二氨基苯磺酸(M酸)的芳胺化,反应收率达93%,脱溴副产仅3%。对于苯胺、间乙酰氨基苯胺及对乙酰氨基苯胺这三种底物,本策略均能使其芳胺化反应收率达90%以上。此外,在20 g(溴氨酸)级的放大反应中,使溴氨酸与M酸的芳胺化反应取得90%的收率,具有优异的放大制备潜力。
中图分类号:
唐志成, 王添巍, 吕荣文, 张淑芬. 含Mn碱式碳酸铜电催化剂催化溴氨酸芳胺化反应研究[J]. 化工学报, 2025, 76(11): 5923-5932.
Zhicheng TANG, Tianwei WANG, Rongwen LYU, Shufen ZHANG. Amination of bromaminic acid catalyzed by Mn-containing basic copper carbonate electrocatalyst based on electrochemical reduction[J]. CIESC Journal, 2025, 76(11): 5923-5932.
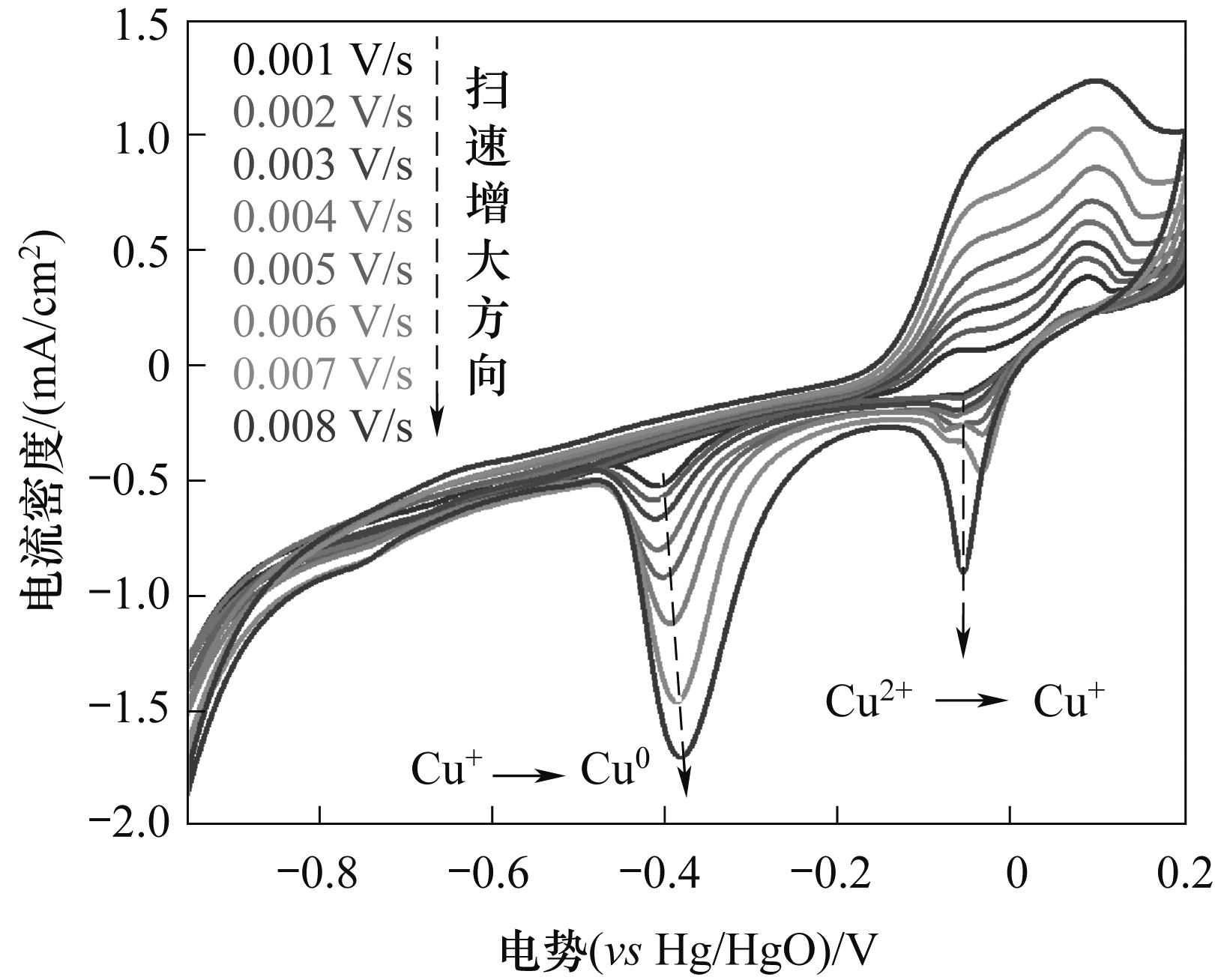
图1 不同扫速下的石墨电极在含0.1 g碱式碳酸铜的0.1 mol/L碳酸氢钠溶液中的循环伏安曲线
Fig.1 Cyclic voltammetry curves of graphite electrodes at different scanning speeds in a 0.1 mol/L sodium bicarbonate solution containing 0.1 g of basic copper carbonate
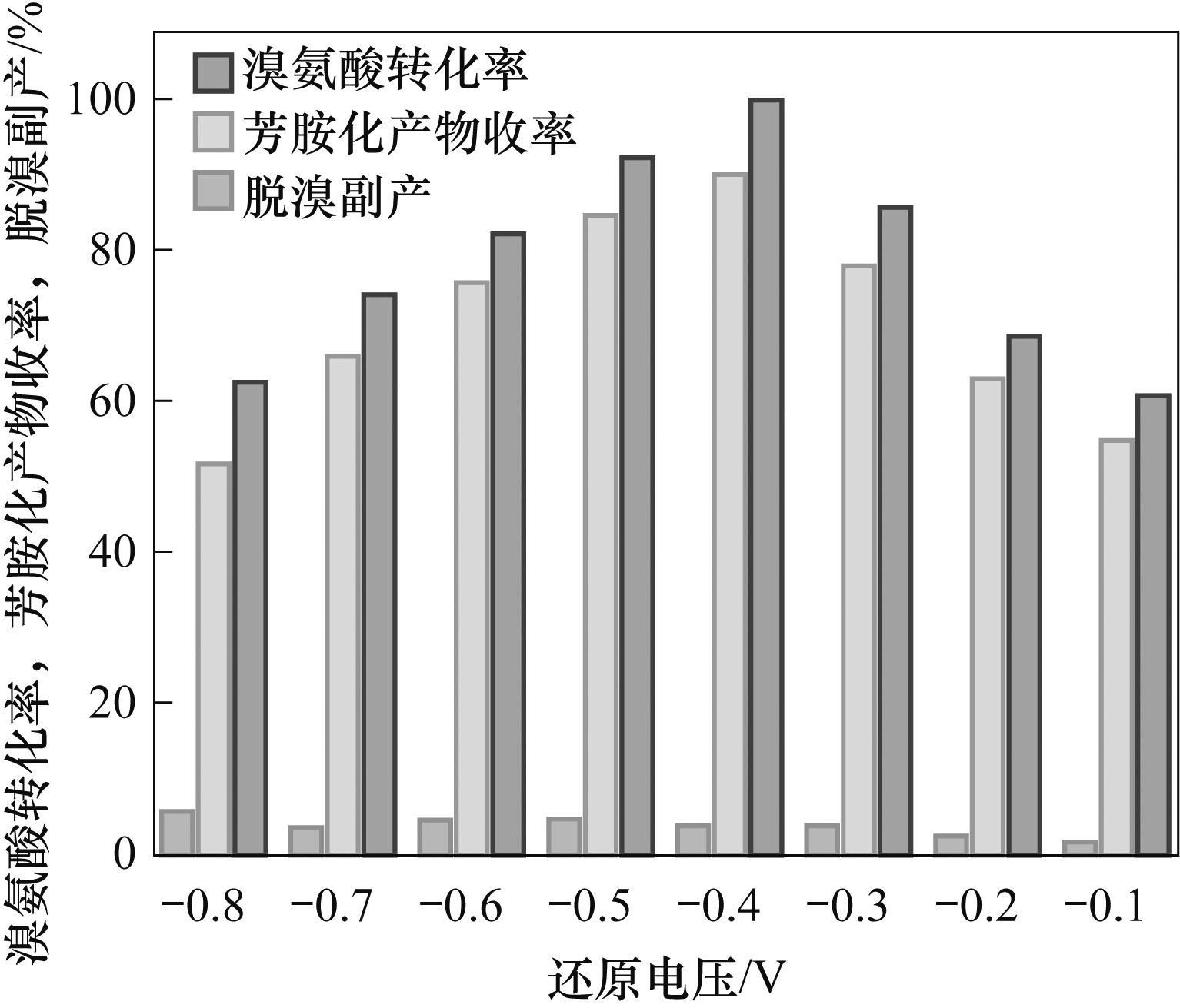
图2 溴氨酸与M酸芳胺化反应中溴氨酸转化率、芳胺化产物收率及脱溴副产与还原电压的关系
Fig.2 The relationship between the conversion rate of bromamic acid, the yields and the by-product of debromination with the reduction voltage in amination reaction of bromamic acid and M-acid
| 编号 | 还原电压/V | 溴氨酸转化率/% | 收率/% | 脱溴副产/% |
|---|---|---|---|---|
| 1 | -0.1 | 61 | 55 | 2 |
| 2 | -0.2 | 69 | 63 | 3 |
| 3 | -0.3 | 86 | 78 | 4 |
| 4 | -0.4 | 100 | 90 | 4 |
| 5 | -0.5 | 93 | 85 | 5 |
| 6 | -0.6 | 82 | 76 | 5 |
| 7 | -0.7 | 74 | 66 | 4 |
| 8 | -0.8 | 63 | 52 | 6 |
表1 还原电压对溴氨酸与M酸芳胺化反应的影响
Table 1 Effect of reduction voltage on amination of bromaminic acid with M acid
| 编号 | 还原电压/V | 溴氨酸转化率/% | 收率/% | 脱溴副产/% |
|---|---|---|---|---|
| 1 | -0.1 | 61 | 55 | 2 |
| 2 | -0.2 | 69 | 63 | 3 |
| 3 | -0.3 | 86 | 78 | 4 |
| 4 | -0.4 | 100 | 90 | 4 |
| 5 | -0.5 | 93 | 85 | 5 |
| 6 | -0.6 | 82 | 76 | 5 |
| 7 | -0.7 | 74 | 66 | 4 |
| 8 | -0.8 | 63 | 52 | 6 |
| 编号 | 催化方式 | 溴氨酸转化率/% | 收率/% | 脱溴副产/% |
|---|---|---|---|---|
| 1 | 含Mn碱式碳酸铜,电化学还原,-0.4 V | 100 | 93 | 3 |
| 2 | 硫酸铜,电化学还原,-0.4 V | 97 | 88 | 3 |
| 3 | 无外加铜源,电化学还原,-0.4 V | 0 | 0 | 0 |
| 4 | 硫酸铜 | 69 | 63 | 3 |
| 5 | 硫酸铜+抗坏血酸 | 98 | 87 | 8 |
| 6 | 硫酸铜+葡萄糖 | 99 | 90 | 6 |
表2 电还原与化学还原催化溴氨酸芳胺化结果比较
Table 2 Comparison of results of amination of bromaminic acid catalyzed by electroreduction and chemical reduction
| 编号 | 催化方式 | 溴氨酸转化率/% | 收率/% | 脱溴副产/% |
|---|---|---|---|---|
| 1 | 含Mn碱式碳酸铜,电化学还原,-0.4 V | 100 | 93 | 3 |
| 2 | 硫酸铜,电化学还原,-0.4 V | 97 | 88 | 3 |
| 3 | 无外加铜源,电化学还原,-0.4 V | 0 | 0 | 0 |
| 4 | 硫酸铜 | 69 | 63 | 3 |
| 5 | 硫酸铜+抗坏血酸 | 98 | 87 | 8 |
| 6 | 硫酸铜+葡萄糖 | 99 | 90 | 6 |
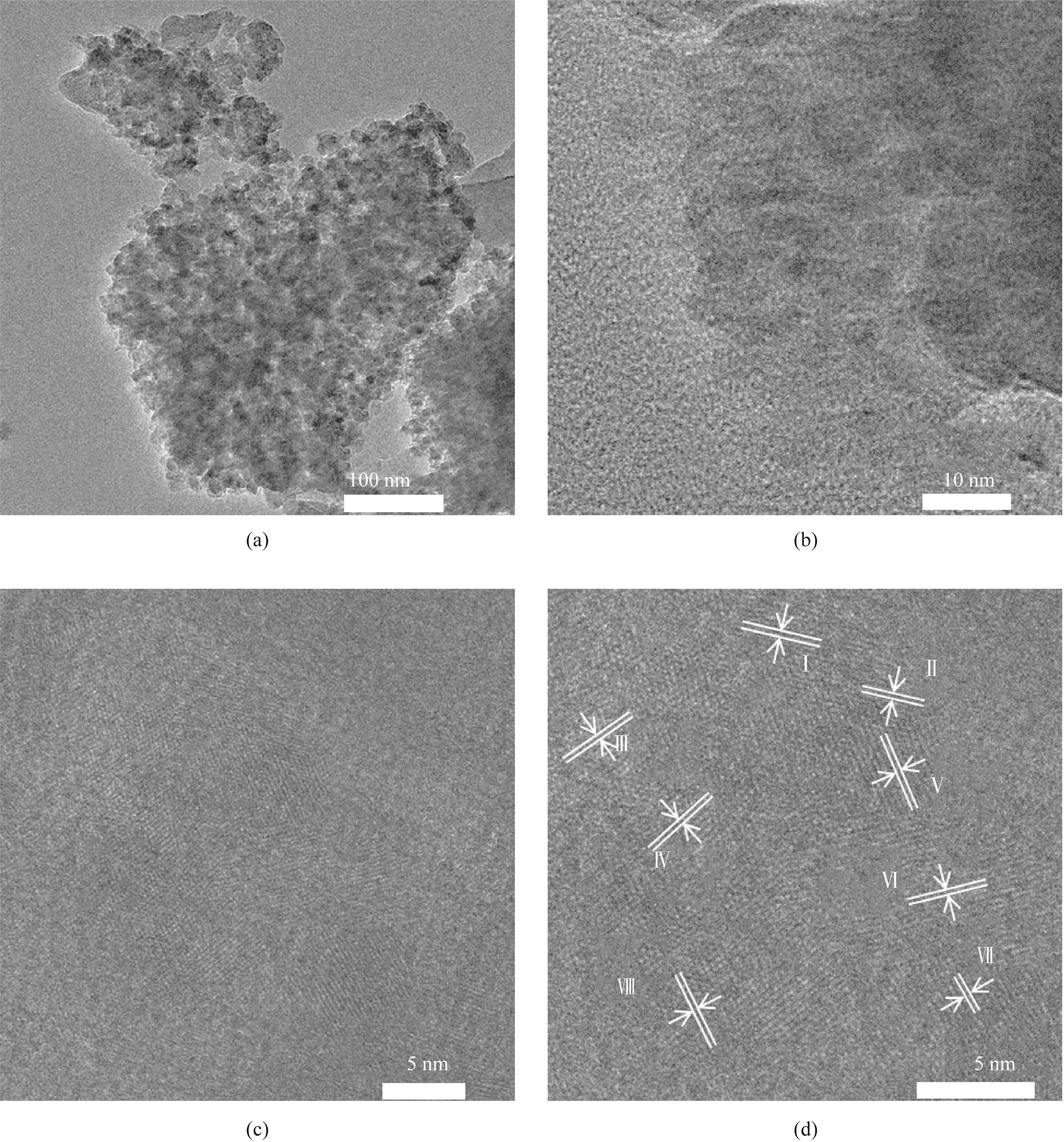
图4 含Mn电催化剂在100 nm(a)、10 nm(b)、5 nm[(c), (d)]尺度下的SEM图
Fig.4 SEM images of manganese containing electrocatalysts at scale of 100 nm (a), 10 nm (b), and 5 nm[(c), (d)]
| 物种 | 条纹编号 | 晶格尺寸/nm | 晶面 |
|---|---|---|---|
| CuCO3 | Ⅲ | 0.286 | (20-1) |
Cu2(OH)2CO3 | Ⅱ | 0.218 | (041) |
| Ⅳ | 0.278 | (21-1) | |
| Ⅷ | 0.267 | (122) | |
| Na2Cu(CO3)2·3H2O | Ⅳ | 0.278 | (311) |
| Ⅶ | 0.284 | (114) | |
| Na3Cu2(CO3)3(OH)4·H2O | Ⅴ, Ⅵ | 0.267 | — |
| MnCO3 | Ⅰ | 0.239 | (110) |
表3 含Mn碱式碳酸铜电催化剂的晶格条纹信息
Table 3 Lattice fringe information of Mn containing basic copper carbonate electrocatalysts
| 物种 | 条纹编号 | 晶格尺寸/nm | 晶面 |
|---|---|---|---|
| CuCO3 | Ⅲ | 0.286 | (20-1) |
Cu2(OH)2CO3 | Ⅱ | 0.218 | (041) |
| Ⅳ | 0.278 | (21-1) | |
| Ⅷ | 0.267 | (122) | |
| Na2Cu(CO3)2·3H2O | Ⅳ | 0.278 | (311) |
| Ⅶ | 0.284 | (114) | |
| Na3Cu2(CO3)3(OH)4·H2O | Ⅴ, Ⅵ | 0.267 | — |
| MnCO3 | Ⅰ | 0.239 | (110) |
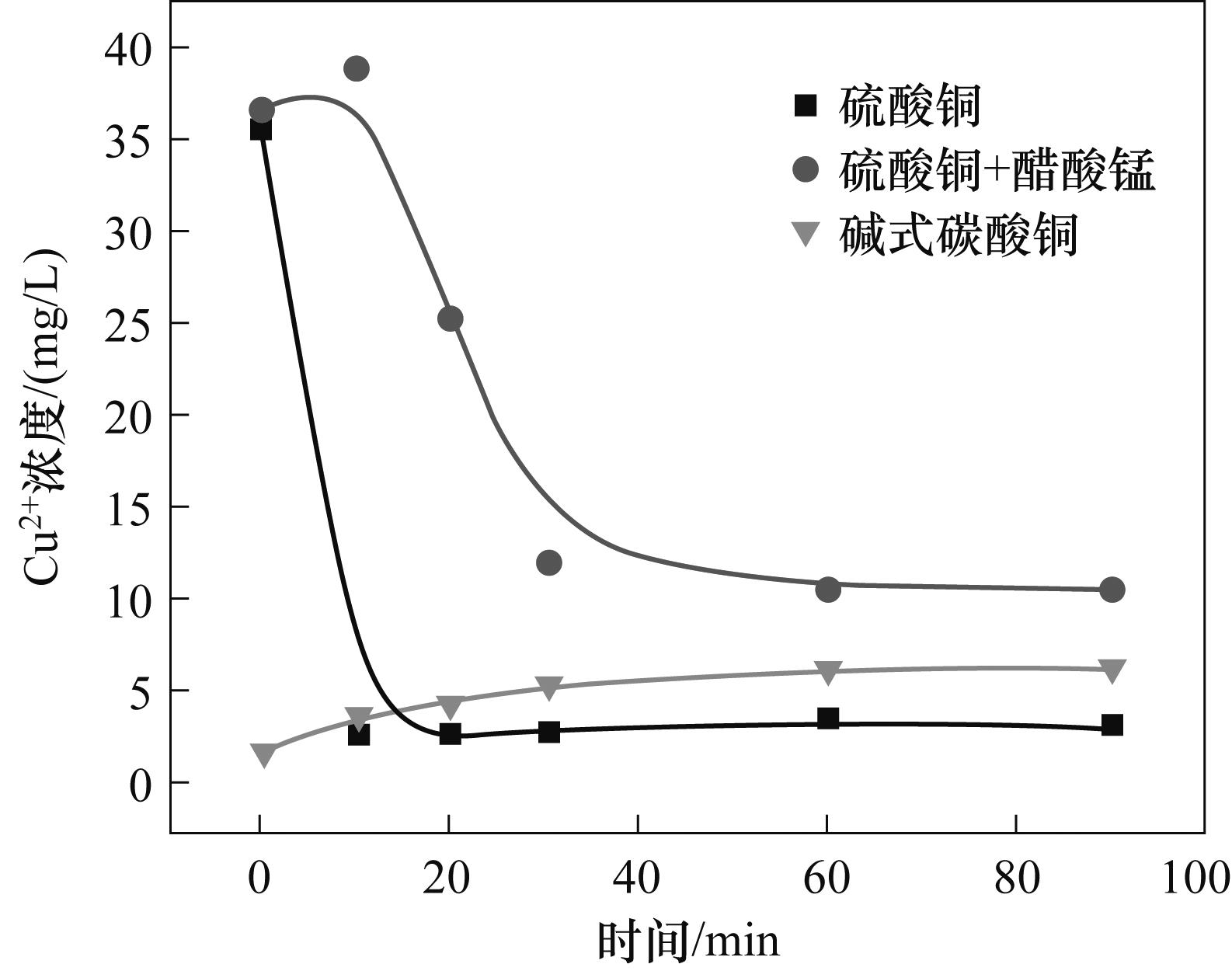
图6 80℃下不同铜催化剂在0.1 mol/L NaHCO3溶液中释放的Cu2+浓度随时间变化
Fig.6 Variation of Cu2+ concentration with time released by different copper catalysts in 0.1 mol/L NaHCO3 solution at 80℃
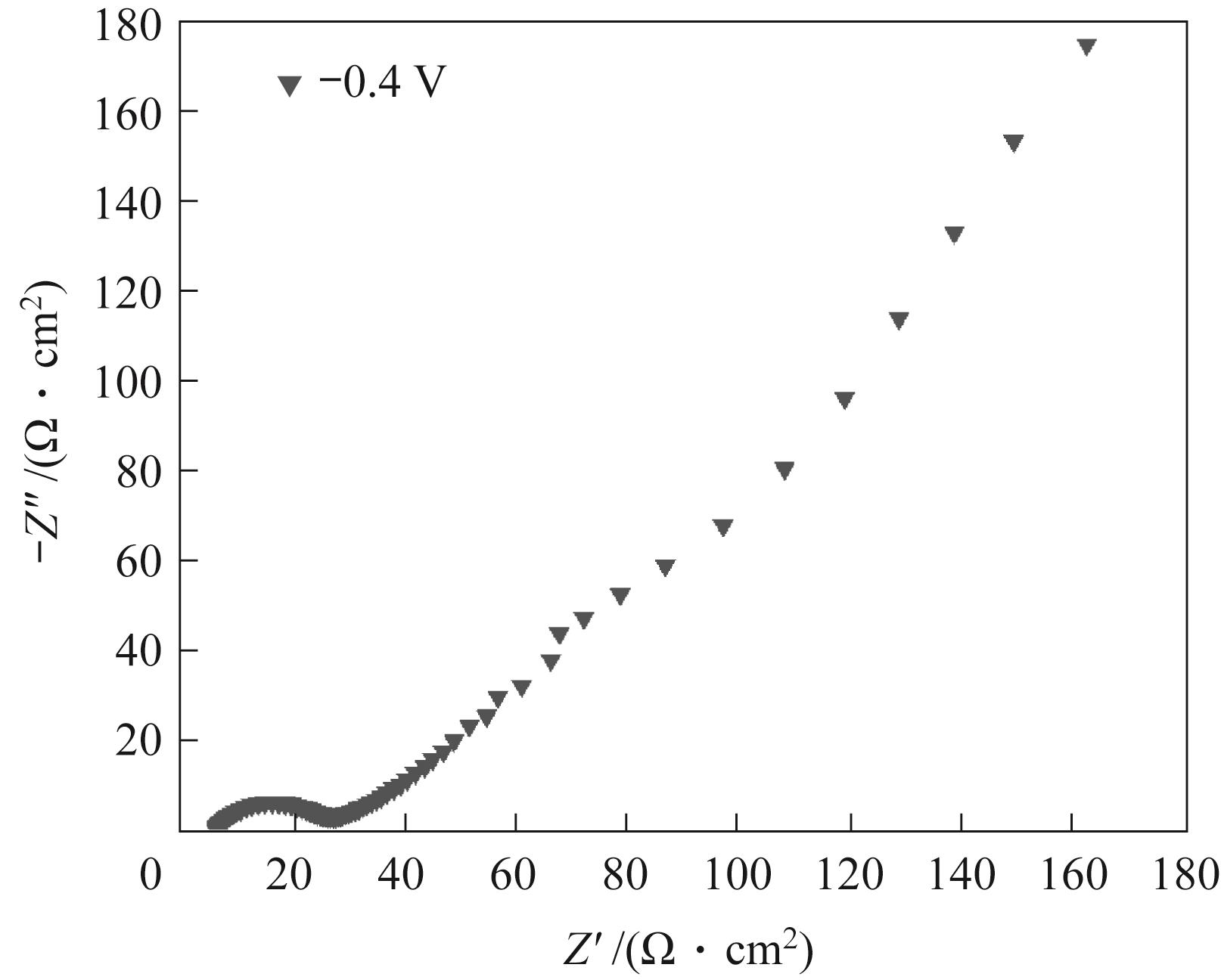
图7 石墨电极在含0.1 g电催化剂的10 ml 0.1 mol/L碳酸氢钠溶液中以-0.4 V电解3 h后的Nyquist图
Fig.7 Nyquist diagram of graphite electrode after electrolysis at -0.4 V for 3 h in 10 ml 0.1 mol/L sodium bicarbonate solution containing 0.1 g electrocatalyst
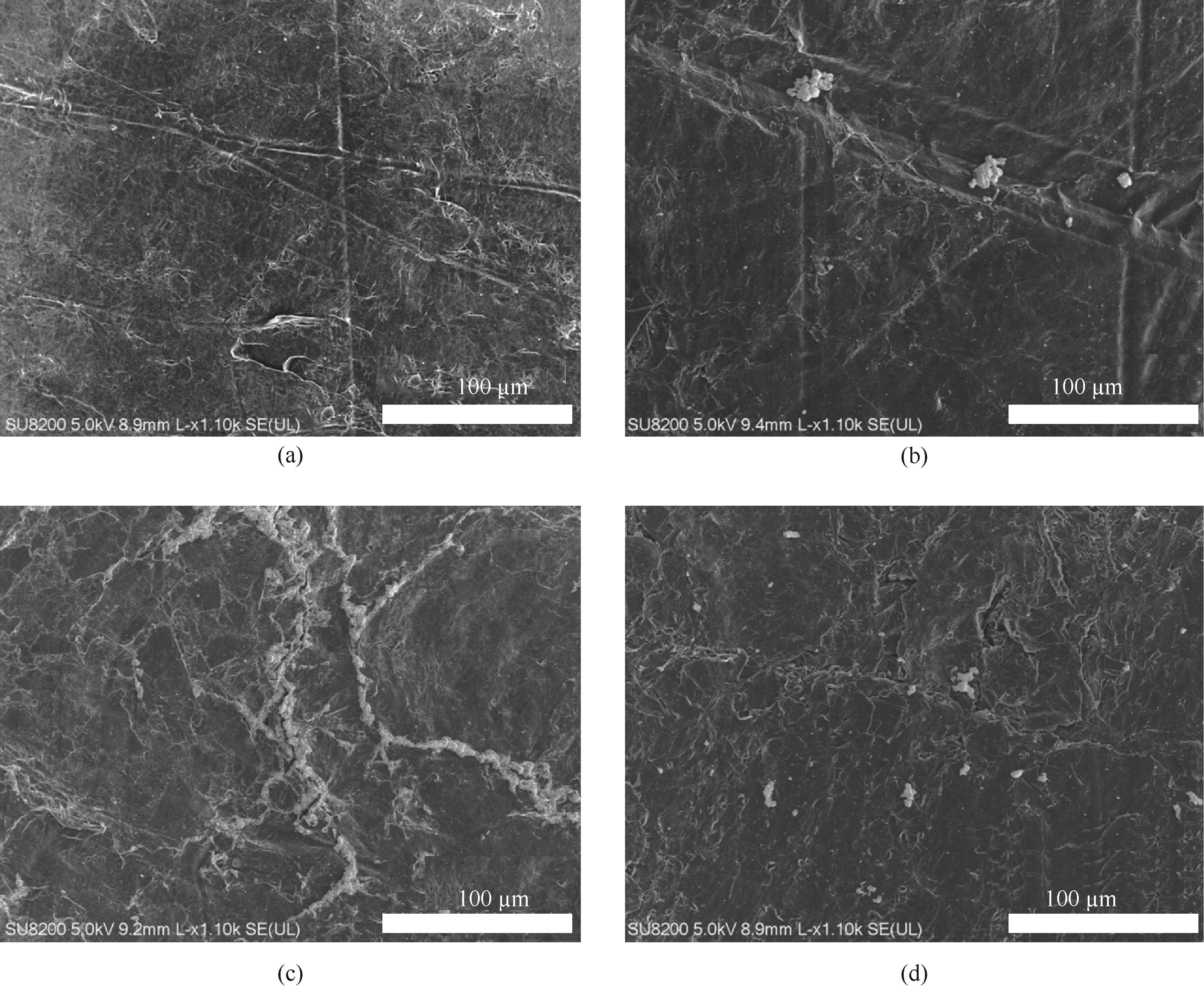
图8 石墨电极在含0.1 g电催化剂的10 ml 0.1 mol/L碳酸氢钠溶液中以0 V(a)、-0.1 V(b)、-0.4 V(c)、-1 V(d)电解3 h后的TEM图
Fig.8 TEM images of graphite electrodes after electrolysis for 3 h in 10 ml 0.1 mol/L sodium bicarbonate solution containing 0.1 g of electrocatalyst at 0 V (a), -0.1 V (b), -0.4 V (c), -1 V (d)
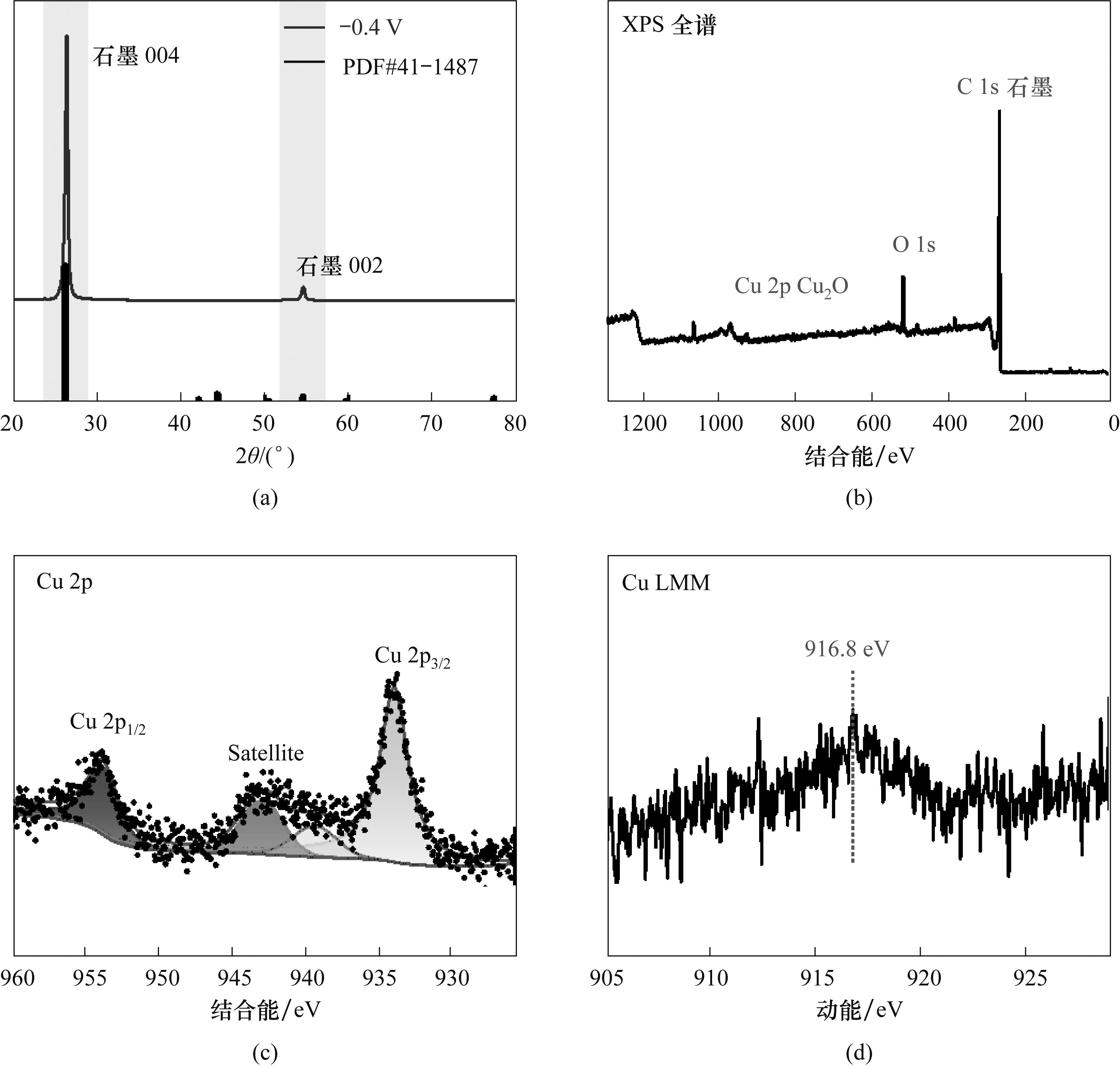
图9 石墨电极在含0.1 g电催化剂的10 ml 0.1 mol/L碳酸氢钠溶液中以-0.4 V电解3 h后的XRD谱图(a)及XPS全谱(b)、Cu 2p(c)、Cu LMM(d)图
Fig.9 XRD pattern (a) and XPS survey (b), Cu 2p (c), Cu LMM(d) patterns of the graphite electrode after electrolysis in 10 ml 0.1 mol/L NaHCO3 solution containing 0.1 g of electrocatalyst at -0.4 V for 3 h
| 底物 | 还原 电压/V | 溴氨酸 转化率/% | 收率/% | 脱溴 副产/% |
|---|---|---|---|---|
 | -0.2 | 95 | 89 | 3 |
| -0.3 | 100 | 90 | 3 | |
| -0.4 | 94 | 89 | 4 | |
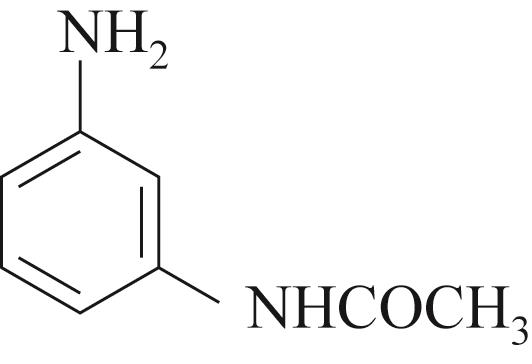 | -0.3 | 91 | 80 | 3 |
| -0.4 | 100 | 93 | 3 | |
| -0.5 | 95 | 89 | 4 | |
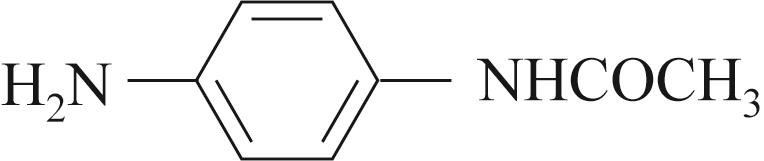 | -0.4 | 90 | 82 | 5 |
| -0.5 | 100 | 91 | 5 | |
| -0.6 | 94 | 85 | 6 |
表4 含Mn碱式碳酸铜及电化学还原催化溴氨酸芳胺化反应底物拓展
Table 4 Substrate scope for Mn containing basic copper carbonate and electrochemical reduction catalyzed amination of bromaminic acid
| 底物 | 还原 电压/V | 溴氨酸 转化率/% | 收率/% | 脱溴 副产/% |
|---|---|---|---|---|
 | -0.2 | 95 | 89 | 3 |
| -0.3 | 100 | 90 | 3 | |
| -0.4 | 94 | 89 | 4 | |
 | -0.3 | 91 | 80 | 3 |
| -0.4 | 100 | 93 | 3 | |
| -0.5 | 95 | 89 | 4 | |
 | -0.4 | 90 | 82 | 5 |
| -0.5 | 100 | 91 | 5 | |
| -0.6 | 94 | 85 | 6 |
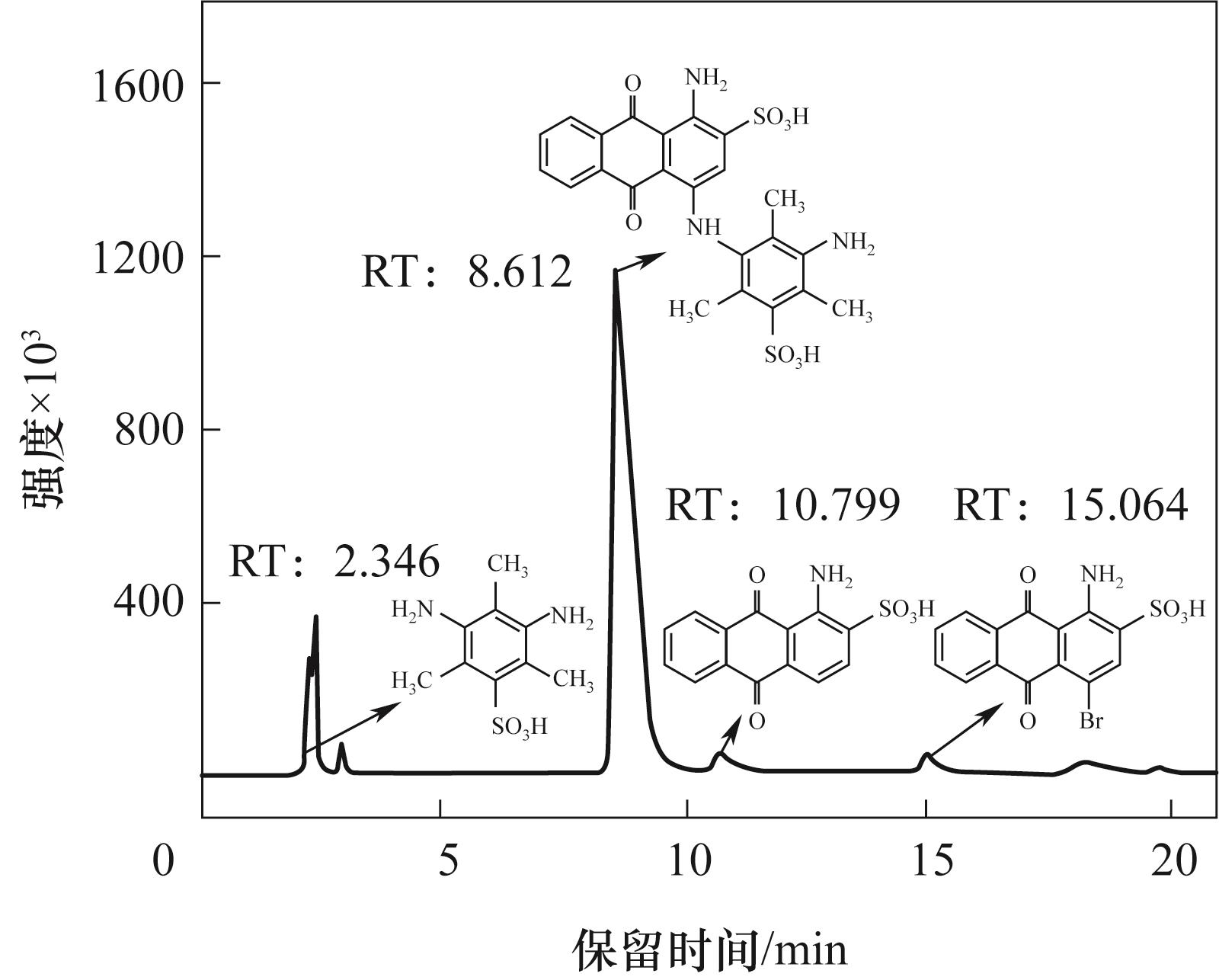
图10 溴氨酸与M酸芳胺化放大反应的反应液LC-MS图
Fig.10 Liquid chromatography mass spectrum of reaction solution in enlarged scale of amination of bromaminic acid with M acid
| [1] | 穆振义. 乌尔曼反应在染料合成中的应用[J]. 染料工业, 1984, 21(3): 1-7. |
| Mu Z Y. Application of Ullman reaction in dye synthesis[J]. Dyestuffs and Coloration, 1984, 21(3): 1-7. | |
| [2] | 李光辉, 孔祥文, 王玲艳. Ullmann反应的绿色化研究进展[J]. 染料与染色, 2021, 58(3): 25-35. |
| Li G H, Kong X W, Wang L Y. Development of green chemical research on Ullmann reaction[J]. Dyestuffs and Coloration, 2021, 58(3): 25-35. | |
| [3] | Simon M S. Spectral shifts in anthraquinone dyes caused by non-conjugated substituents[J]. Journal of the American Chemical Society, 1963, 85(13): 1974-1977. |
| [4] | Baqi Y. Anthraquinone dyes: a synthetic and chemical characterization protocol for an industrial chemistry laboratory course[J]. Journal of Chemical Education, 2022, 99(3): 1441-1447. |
| [5] | 顾高炜, 邬伟国, 李建昌, 等. C. I. 酸性蓝324的乌尔曼缩合反应研究[J]. 染料与染色, 2020, 57(3): 8-9. |
| Gu G W, Wu W G, Li J C, et al. Study on Ullmann condensation by synthesis of C. I. acid blue 324[J]. Dyestuffs and Coloration, 2020, 57(3): 8-9. | |
| [6] | 刘慧, 陈煌煌, 高爱芹, 等. 纳米双核催化剂在溴氨蓝合成反应中的催化性能研究[J]. 染料与染色, 2018, 55(6): 20-24, 49. |
| Liu H, Chen H H, Gao A Q, et al. Catalytic performance of nano-sized catalyst on synthesis of bromine ammonia blue[J]. Dyestuffs and Coloration, 2018, 55(6): 20-24, 49. | |
| [7] | 姚蒙正, 王军宽. 溴氨酸的芳胺化条件研究[J]. 染料工业, 1984, 21(3):15-19. |
| Yao M Z, Wang J K. Study on the arylamination conditions of bromamic acid[J]. Dyestuffs and Coloration, 1984, 21(3): 15-19. | |
| [8] | Chen X, Ding K, Jun L. Synthesis, identification and application of aldehyde reactive dyes[J]. Dyes and Pigments, 2015, 123: 404-412. |
| [9] | Sperotto E, van Klink G P M, van Koten G, et al. The mechanism of the modified Ullmann reaction[J]. Dalton Transactions, 2010, 39(43): 10338-10351. |
| [10] | Ma Z D, Li Y X, Jin M M, et al. Fabrication of adsorbents with enhanced CuI stability: creating a superhydrophobic microenvironment through grafting octadecylamine[J]. Chinese Journal of Chemical Engineering, 2023, 55: 41-48. |
| [11] | Xin Y Y, Zhou L, Ma K K, et al. Removal of bromoamine acid in dye wastewater by gas-liquid plasma: the role of ozone and hydroxyl radical[J]. Journal of Water Process Engineering, 2020, 37: 101457. |
| [12] | Jedinák L, Zátopková R, Zemánková H, et al. The Suzuki-Miyaura cross-coupling reaction of halogenated aminopyrazoles: method development, scope, and mechanism of dehalogenation side reaction[J]. The Journal of Organic Chemistry, 2017, 82(1): 157-169. |
| [13] | Yang Q, Zhao Y S, Ma D W. Cu-mediated Ullmann-type cross-coupling and industrial applications in route design, process development, and scale-up of pharmaceutical and agrochemical processes[J]. Organic Process Research & Development, 2022, 26(6): 1690-1750. |
| [14] | Chen W, Wu Y D, Jiang Y M, et al. Catalyst selection over an electrochemical reductive coupling reaction toward direct electrosynthesis of oxime from NO x and aldehyde[J]. Journal of the American Chemical Society, 2024, 146(9): 6294-6306. |
| [15] | Hu J Y, Ma R, Hu J C, et al. Electrochemical oxidative dehydrogenation aromatization of cyclohex-2-enone and amines to 1,4-phenylenediamine[J]. Green Chemistry, 2024, 26(8): 4684-4690. |
| [16] | 卢林德, 孟胜锋, 上官开泰, 等. 溴氨酸类染料合成的机理及生产实践[J]. 染料与染色, 2024, 61(1): 25-29, 39. |
| Lu L D, Meng S F, Shangguan K T, et al. Mechanism and manufacture of dyes synthesized from 1-amino-4-bromoanthraquinone-2-sulfonic acid[J]. Dyestuffs and Coloration, 2024, 61(1): 25-29, 39. | |
| [17] | He J B, Lu D Y, Jin G P. Potential dependence of cuprous/cupric duplex film growth on copper electrode in alkaline media[J]. Applied Surface Science, 2006, 253(2): 689-697. |
| [18] | Fan H H, Weng W L, Lee C Y, et al. Electrochemical cycling-induced spiky Cu x O/Cu nanowire array for glucose sensing[J]. ACS Omega, 2019, 4(7): 12222-12229. |
| [19] | Tromans D, Sun R H. Anodic behavior of copper in weakly alkaline solutions[J]. Journal of Electrochemical Society, 1992, 139(7): 1945-1951. |
| [20] | Liu F L, Gao X T, Guo Z X, et al. Sustainable adipic acid production via paired electrolysis of lignin-derived phenolic compounds with water as hydrogen and oxygen sources[J]. Journal of the American Chemical Society, 2024, 146(22): 15275-15285. |
| [21] | Dong L, Ge W X, Fan Y, et al. Surfactant‐modified electrode-electrolyte interface for steering CO2 electrolysis on Cu electrodes[J]. American Institute of Chemical Engineers Journal, 2024, 70(1): e18271. |
| [22] | Shao B B, Du H Y, Hao X Y, et al. Ligand assisted copper-catalyzed Ullmann cross coupling reaction of bromaminic acid with amines[J]. Chinese Journal of Chemical Engineering, 2016, 24(8): 1000-1006. |
| [23] | 舒余德, 孟爱东. 碱性NaCl溶液中铜阳极生成Cu2O的机理[J]. 有色金属, 1996, 48(4): 58-62. |
| Shu Y D, Meng A D. Mechanism of forming Cu2O on copper anode in alkaline[J]. Nonferrous Metals Engineering, 1996, 48(4): 58-62. | |
| [24] | 雷惊雷, 李凌杰, 张胜涛, 等. 铜电极在弱碱性介质中腐蚀行为的研究[J]. 化学学报, 2001, 59(8): 1216-1221. |
| Lei J L, Li L J, Zhang S T, et al. Studies on corrosion behavior of copper electrode in weak alkaline solution[J]. Acta Chimica Sinica, 2001, 59(8): 1216-1221. | |
| [25] | Chen F Y, Elgazzar A, Pecaut S, et al. Electrochemical nitrate reduction to ammonia with cation shuttling in a solid electrolyte reactor[J]. Nature Catalysis, 2024, 7(9): 1032-1043. |
| [26] | Thanh N T K, Maclean N, Mahiddine S. Mechanisms of nucleation and growth of nanoparticles in solution[J]. Chemical Reviews, 2014, 114(15): 7610-7630. |
| [27] | Candal R J, Regazzoni A E, Blesa M A. Precipitation of copper(Ⅱ) hydrous oxides and copper(Ⅱ) basic salts[J]. Journal of Materials Chemistry, 1992, 2(6): 657-661. |
| [28] | Grujicic D, Pesic B. Electrodeposition of copper: the nucleation mechanisms[J]. Electrochimica Acta, 2002, 47(18): 2901-2912. |
| [29] | Lazanas A C, Prodromidis M I. Electrochemical impedance spectroscopy—a tutorial[J]. ACS Measurement Science Au, 2023, 3(3): 162-193. |
| [30] | Sagar P, Arun Kumar N S, Shreenivasa L, et al. Citric acid assisted one-pot approach to synthesize CuO, CuO/Cu2O, Cu/Cu2O, and metallic Cu: potential electrocatalyst for enhanced OER[J]. Ionics, 2023, 29(2): 711-719. |
| [31] | Huang J. Diffusion impedance of electroactive materials, electrolytic solutions and porous electrodes: Warburg impedance and beyond[J]. Electrochimica Acta, 2018, 281: 170-188. |
| [32] | He H, Huang C, Luo C W, et al. Dynamic study of Li intercalation into graphite by in situ high energy synchrotron XRD[J]. Electrochimica Acta, 2013, 92: 148-152. |
| [33] | Poulston S, Parlett P M, Stone P, et al. Surface oxidation and reduction of CuO and Cu2O studied using XPS and XAES[J]. Surface and Interface Analysis, 1996, 24(12): 811-820. |
| [34] | Biesinger M C, Lau L W M, Gerson A R, et al. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn[J]. Applied Surface Science, 2010, 257(3): 887-898. |
| [35] | Malik E M, Rashed M, Wingen L, et al. Ullmann reactions of 1-amino-4-bromoanthraquinones bearing various 2-substituents furnishing novel dyes[J]. Dyes and Pigments, 2016, 131: 33-40. |
| [36] | Beletskaya I P, Cheprakov A V. The complementary competitors: palladium and copper in C—N cross-coupling reactions[J]. Organometallics, 2012, 31(22): 7753-7808. |
| [1] | 胡国祥, 朱忆魁, 龙华, 刘晓雯, 熊勤钢. 组分配比影响氯化胆碱-乳酸低共熔溶剂碱木质素溶解度的底层机理研究[J]. 化工学报, 2025, 76(9): 4449-4461. |
| [2] | 张荟钦, 赵泓竣, 付正军, 庄力, 董凯, 贾添智, 曹雪丽, 孙世鹏. 纳滤膜在离子型稀土浸出液提浓中的应用研究[J]. 化工学报, 2025, 76(8): 4095-4107. |
| [3] | 马谌睿, 王翔, 宋民航, 敬军, 吴琼, 黄云. 氯化法钛白氧化反应器内颗粒碰撞行为及生成演化过程理论分析[J]. 化工学报, 2025, 76(7): 3316-3324. |
| [4] | 林纬, 杜建, 姚晨, 朱家豪, 汪威, 郑小涛, 徐建民, 喻九阳. 电化学水软化过程中离子输运与成核机理研究[J]. 化工学报, 2025, 76(4): 1788-1799. |
| [5] | 胡岩松, 杨昭, 高磊, 张步健. R513A与PVE润滑油的溶解度及黏度研究[J]. 化工学报, 2025, 76(10): 5015-5023. |
| [6] | 杜宇鹏, 葛春亮, 丁垒琳, 张力, 胡家俊, 俞峰苹, 林益, 王峰, 蒋师, 郭燏. 尿素均匀沉淀法合成CeO2-Al2O3载体负载Pt催化剂的CO氧化性能[J]. 化工学报, 2025, 76(10): 5114-5127. |
| [7] | 李玲玉, 胡鑫, 成怀刚, 赵云, 安东, 马玉军, 金家豪, 于旭东, 张卫东. K+(Mg2+), Ca2+//Cl--H2O三元水盐体系的等温蒸发成盐相区[J]. 化工学报, 2025, 76(1): 120-130. |
| [8] | 王新月, 徐小虎, 张海洋, 尹春华. 维生素A醋酸酯/环糊精包合及性质研究[J]. 化工学报, 2024, 75(S1): 321-328. |
| [9] | 秦思宇, 刘艺佳, 杨佳成, 佟薇, 金立文, 孟祥兆. 受限蒸汽腔内气液两相传热特性研究[J]. 化工学报, 2024, 75(S1): 47-55. |
| [10] | 杨明军, 宋维, 张磊, 凌铮, 陈兵兵, 宋永臣. CO2-海水水合物生成强化方法研究[J]. 化工学报, 2024, 75(8): 2939-2948. |
| [11] | 齐琪, 郭利平, 石李明, 郑映, 潘鹏举. 山梨醇类成核剂改性聚丙烯及其共聚物的结晶行为与性能[J]. 化工学报, 2024, 75(7): 2688-2699. |
| [12] | 赵志星, 姚智豪, 于雪峰, 杨游胜, 曾英, 于旭东. 锂钠镁共存硫酸盐体系多温相图及其应用[J]. 化工学报, 2024, 75(6): 2123-2133. |
| [13] | 司友明, 郑凌峰, 陈鹏忠, 樊江莉, 彭孝军. 新型锑氧簇光刻胶的性能与机理研究[J]. 化工学报, 2024, 75(4): 1705-1717. |
| [14] | 杨百玉, 寇悦, 姜峻韬, 詹亚力, 王庆宏, 陈春茂. 炼化碱渣湿式氧化预处理过程DOM的化学转化特征[J]. 化工学报, 2023, 74(9): 3912-3920. |
| [15] | 于旭东, 李琪, 陈念粗, 杜理, 任思颖, 曾英. 三元体系KCl + CaCl2 + H2O 298.2、323.2及348.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(8): 3256-3265. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号