化工学报 ›› 2024, Vol. 75 ›› Issue (6): 2123-2133.DOI: 10.11949/0438-1157.20240169
赵志星1( ), 姚智豪1, 于雪峰1,2, 杨游胜1, 曾英1,3, 于旭东1,3(
), 姚智豪1, 于雪峰1,2, 杨游胜1, 曾英1,3, 于旭东1,3( )
)
收稿日期:2024-02-05
修回日期:2024-03-15
出版日期:2024-06-25
发布日期:2024-07-03
通讯作者:
于旭东
作者简介:赵志星(1998—),男,硕士,zhaozhoxingc413@126.com
基金资助:
Zhixing ZHAO1( ), Zhihao YAO1, Xuefeng YU1,2, Yousheng YANG1, Ying ZENG1,3, Xudong YU1,3(
), Zhihao YAO1, Xuefeng YU1,2, Yousheng YANG1, Ying ZENG1,3, Xudong YU1,3( )
)
Received:2024-02-05
Revised:2024-03-15
Online:2024-06-25
Published:2024-07-03
Contact:
Xudong YU
摘要:
针对硫酸钠亚型盐湖卤水钠锂比高、镁锂比高带来的锂分离难题,通过开展锂钠镁共存硫酸盐体系多温相图研究,获取锂钠镁硫酸盐之间的作用关系和结晶析出规律,并应用于指导降低卤水中钠镁含量工艺设计。采用等温溶解平衡法获取了四元体系Li+,Na+,Mg2+//
中图分类号:
赵志星, 姚智豪, 于雪峰, 杨游胜, 曾英, 于旭东. 锂钠镁共存硫酸盐体系多温相图及其应用[J]. 化工学报, 2024, 75(6): 2123-2133.
Zhixing ZHAO, Zhihao YAO, Xuefeng YU, Yousheng YANG, Ying ZENG, Xudong YU. Multi-temperature phase diagram of lithium-sodium-magnesium coexistence sulfate system and its application[J]. CIESC Journal, 2024, 75(6): 2123-2133.
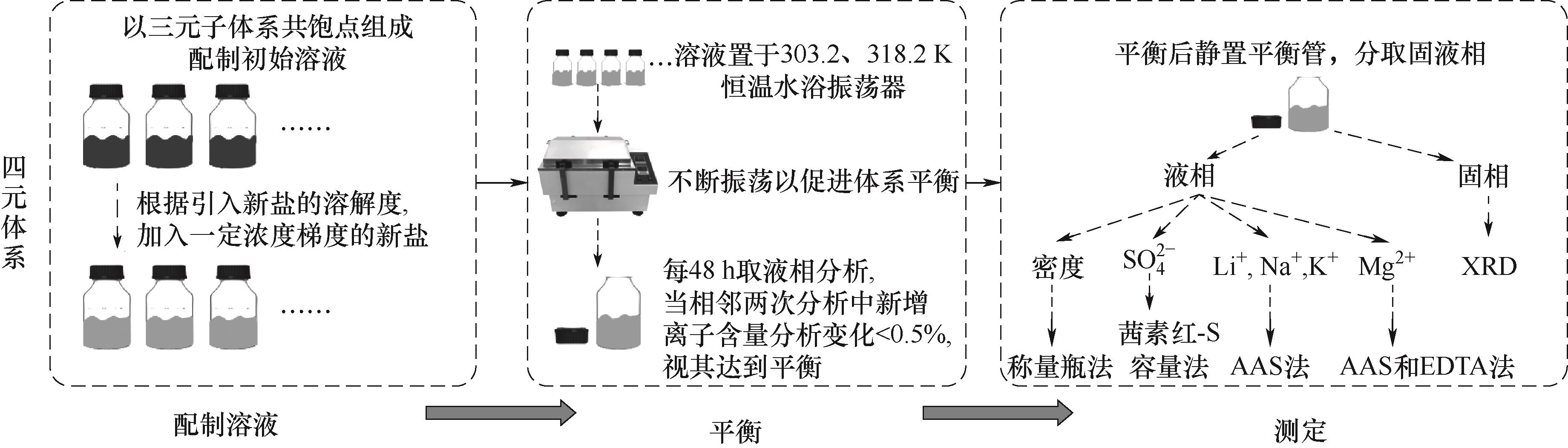
图1 四元体系Li+, Na+, Mg2+//SO42--H2O 303.2、318.2 K相平衡实验流程图
Fig.1 Flow chart of phase equilibrium experiment of quaternary systems Li+, Na+, Mg2+//SO42--H2O at 303.2 and 318.2 K
| 温度/K | 编号 | 密度/ (g/cm3) | 折射率 nD | 平衡液相组成, w(B)×102 | 干盐指数(g/100g盐) | 平衡固相 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| w(Li2SO4) | w(Na2SO4) | w(MgSO4) | w(H2O) | J(Li2SO4) | J(Na2SO4) | J(MgSO4) | J(H2O) | |||||
| 303.2 | ||||||||||||
| 1, A | 1.3134 | 1.3785 | 4.95 | 27.39 | 0 | 67.66 | 15.30 | 84.70 | 0 | 209.29 | Mir + Db1 | |
| 2 | 1.3247 | 1.3812 | 4.68 | 25.93 | 1.98 | 67.41 | 14.36 | 79.58 | 6.06 | 206.85 | Mir + Db1 | |
| 3 | 1.3317 | 1.3834 | 4.42 | 24.29 | 3.96 | 67.33 | 13.53 | 74.35 | 12.12 | 206.06 | Mir + Db1 | |
| 4 | 1.3361 | 1.3850 | 4.23 | 23.53 | 5.75 | 66.49 | 12.63 | 70.20 | 17.17 | 198.37 | Mir + Db1 | |
| 5 | 1.3431 | 1.3870 | 4.00 | 22.31 | 7.43 | 66.26 | 11.85 | 66.12 | 22.03 | 196.35 | Mir + Db1 | |
| 6 | 1.3519 | 1.3884 | 3.85 | 21.52 | 8.74 | 65.89 | 11.28 | 63.09 | 25.63 | 193.26 | Th + Db1 | |
| 7 | 1.3543 | 1.3900 | 3.67 | 20.75 | 10.43 | 65.15 | 10.53 | 59.54 | 29.93 | 186.92 | Th + Db1 | |
| 8 | 1.3669 | 1.3916 | 3.53 | 20.35 | 11.60 | 64.52 | 9.94 | 57.36 | 32.70 | 181.91 | Th + Db1 | |
| 9 | 1.3692 | 1.3925 | 3.61 | 20.05 | 12.72 | 63.62 | 9.94 | 55.09 | 34.97 | 174.85 | Th + Db1 | |
| 10, B | 1.3630 | 1.3859 | 0 | 27.40 | 8.18 | 64.42 | 0 | 77.01 | 22.99 | 181.06 | Th + Mir | |
| 11 | 1.3524 | 1.3857 | 1.58 | 26.46 | 7.91 | 64.05 | 4.39 | 73.61 | 22.00 | 178.23 | Th + Mir | |
| 12 | 1.3480 | 1.3855 | 2.39 | 25.94 | 7.84 | 63.83 | 6.62 | 71.70 | 21.68 | 176.37 | Th + Mir | |
| 13 | 1.3475 | 1.3860 | 3.61 | 24.53 | 8.40 | 63.46 | 9.89 | 67.13 | 22.98 | 173.67 | Th + Mir | |
| 14, S1 | 1.3473 | 1.3856 | 4.26 | 23.14 | 8.28 | 64.32 | 11.94 | 64.85 | 23.21 | 180.26 | Th+Mir+Db1 | |
| 15 | 1.3721 | 1.3943 | 3.14 | 18.52 | 13.48 | 64.86 | 8.95 | 52.68 | 38.37 | 184.55 | Ast + Db1 | |
| 16 | 1.3801 | 1.3954 | 3.18 | 15.98 | 16.33 | 64.51 | 8.97 | 45.02 | 46.01 | 181.78 | Ast + Db1 | |
| 17 | 1.3814 | 1.3959 | 3.31 | 15.91 | 18.04 | 62.74 | 8.88 | 42.70 | 48.42 | 168.40 | Ast + Db1 | |
| 18 | 1.3959 | 1.3991 | 3.17 | 14.55 | 20.05 | 62.23 | 8.39 | 38.52 | 53.09 | 164.78 | Ast + Db1 | |
| 19 | 1.3993 | 1.3997 | 3.07 | 13.34 | 21.44 | 62.15 | 8.11 | 35.24 | 56.65 | 164.24 | Ast + Db1 | |
| 20 | 1.3944 | 1.3997 | 3.10 | 13.04 | 22.12 | 61.74 | 8.09 | 34.08 | 57.83 | 161.39 | Ast + Db1 | |
| 21, C | 1.3756 | 1.3930 | 0 | 22.96 | 13.34 | 63.70 | 0 | 63.25 | 36.75 | 175.51 | Ast + Th | |
| 22 | 1.3802 | 1.3930 | 0.61 | 22.43 | 13.22 | 63.74 | 1.69 | 61.85 | 36.46 | 175.82 | Ast + Th | |
| 23 | 1.3874 | 1.3937 | 1.28 | 22.21 | 13.00 | 63.51 | 3.50 | 60.86 | 35.64 | 174.02 | Ast + Th | |
| 24 | 1.3917 | 1.3942 | 2.60 | 21.06 | 12.79 | 63.55 | 7.13 | 57.78 | 35.09 | 174.39 | Ast + Th | |
| 25, S2 | 1.3806 | 1.3935 | 3.33 | 19.55 | 12.88 | 64.24 | 9.31 | 54.68 | 36.01 | 179.65 | Ast+Th+Db1 | |
| 26, D | 1.3850 | 1.3976 | 0 | 12.30 | 23.10 | 64.60 | 0 | 34.74 | 65.26 | 182.51 | Ast + Eps | |
| 27 | 1.3802 | 1.3974 | 1.48 | 11.65 | 22.44 | 64.43 | 4.15 | 32.76 | 63.09 | 181.11 | Ast + Eps | |
| 28 | 1.3816 | 1.3975 | 2.43 | 11.94 | 22.65 | 62.98 | 6.57 | 32.26 | 61.17 | 170.07 | Ast + Eps | |
| 29, S3 | 1.3880 | 1.3977 | 2.98 | 11.28 | 21.51 | 64.23 | 8.33 | 31.53 | 60.14 | 179.53 | Ast+Eps+Db1 | |
| 30 | 1.3809 | 1.3980 | 3.69 | 11.18 | 21.18 | 63.95 | 10.23 | 31.02 | 58.75 | 177.42 | Eps + Db1 | |
| 31 | 1.3816 | 1.3982 | 4.61 | 10.49 | 20.85 | 64.05 | 12.82 | 29.19 | 57.99 | 178.11 | Eps + Db1 | |
| 32 | 1.3828 | 1.3986 | 6.06 | 9.36 | 20.46 | 64.12 | 16.89 | 26.09 | 57.02 | 178.69 | Eps + Db1 | |
| 33 | 1.3881 | 1.3998 | 8.75 | 8.95 | 19.54 | 62.76 | 23.49 | 24.04 | 52.47 | 168.54 | Eps + Db1 | |
| 34 | 1.3960 | 1.4003 | 10.40 | 9.08 | 17.85 | 62.67 | 27.86 | 24.32 | 47.82 | 167.85 | Eps + Db1 | |
| 35 | 1.3951 | 1.4005 | 11.50 | 8.90 | 17.32 | 62.27 | 30.50 | 23.59 | 45.91 | 165.07 | Eps + Db1 | |
| 36, S4 | 1.3986 | 1.4007 | 12.21 | 8.15 | 17.36 | 62.28 | 32.36 | 21.60 | 46.04 | 165.14 | Eps+Db1+Ls | |
| 37, E | 1.3451 | 1.3970 | 13.60 | 0 | 20.91 | 65.49 | 39.40 | 0 | 60.60 | 189.80 | Eps + Ls | |
| 38 | 1.3467 | 1.3972 | 13.53 | 2.16 | 19.44 | 64.87 | 38.52 | 6.16 | 55.32 | 184.59 | Eps + Ls | |
| 39 | 1.3560 | 1.3976 | 13.36 | 3.19 | 19.02 | 64.43 | 37.56 | 8.98 | 53.46 | 181.08 | Eps + Ls | |
| 40 | 1.3598 | 1.3983 | 13.18 | 4.29 | 18.88 | 63.65 | 36.27 | 11.79 | 51.94 | 175.06 | Eps + Ls | |
| 41 | 1.3657 | 1.3988 | 12.94 | 5.26 | 18.67 | 63.13 | 35.10 | 14.26 | 50.64 | 171.17 | Eps + Ls | |
| 42 | 1.3717 | 1.3996 | 12.59 | 6.67 | 18.54 | 62.20 | 33.32 | 17.65 | 49.03 | 164.53 | Eps + Ls | |
| 43 | 1.3885 | 1.4003 | 12.28 | 7.73 | 17.88 | 62.11 | 32.40 | 20.41 | 47.19 | 163.93 | Eps + Ls | |
| 44, F | 1.3051 | 1.3842 | 20.63 | 12.64 | 0 | 66.73 | 62.01 | 37.99 | 0 | 200.50 | Db1 + Ls | |
| 45 | 1.3119 | 1.3861 | 19.12 | 11.73 | 2.33 | 66.82 | 57.63 | 35.36 | 7.01 | 201.46 | Db1 + Ls | |
| 46 | 1.3235 | 1.3880 | 17.92 | 11.11 | 4.49 | 66.48 | 53.46 | 33.13 | 13.41 | 198.32 | Db1 + Ls | |
| 47 | 1.3375 | 1.3910 | 16.26 | 10.67 | 7.93 | 65.14 | 46.64 | 30.60 | 22.76 | 186.88 | Db1 + Ls | |
| 48 | 1.3529 | 1.3939 | 14.52 | 9.85 | 11.08 | 64.55 | 40.96 | 27.79 | 31.25 | 182.14 | Db1 + Ls | |
| 49 | 1.3583 | 1.3947 | 13.78 | 9.52 | 12.39 | 64.31 | 38.60 | 26.69 | 34.71 | 180.22 | Db1 + Ls | |
| 50 | 1.3757 | 1.3974 | 12.17 | 9.02 | 15.76 | 63.05 | 32.94 | 24.40 | 42.66 | 170.61 | Db1 + Ls | |
| 318.2 | ||||||||||||
| 51, G | 1.3300 | 1.3830 | 13.36 | 21.83 | 0 | 64.81 | 37.97 | 62.03 | 0 | 184.14 | Db1 + Db2 | |
| 52 | 1.3636 | 1.3852 | 12.99 | 21.03 | 3.13 | 62.85 | 34.98 | 56.61 | 8.41 | 169.16 | Db1 + Db2 | |
| 53 | 1.3676 | 1.3878 | 11.78 | 20.37 | 6.06 | 61.79 | 30.84 | 53.31 | 15.85 | 161.75 | Db1 + Db2 | |
| 54 | 1.3730 | 1.3906 | 11.03 | 18.75 | 8.11 | 62.11 | 29.11 | 49.49 | 21.40 | 163.94 | Db1 + Db2 | |
| 55 | 1.3808 | 1.3918 | 10.47 | 17.75 | 10.20 | 61.58 | 27.25 | 46.20 | 26.55 | 160.28 | Db1 + Db2 | |
| 56, T1 | 1.3928 | 1.3931 | 9.75 | 17.34 | 10.64 | 62.27 | 25.84 | 45.96 | 28.20 | 165.11 | Db1+Db2+Ast | |
| 57 | 1.3641 | 1.3921 | 9.49 | 18.70 | 10.12 | 61.69 | 24.77 | 48.81 | 26.42 | 160.95 | Db1 + Ast | |
| 58 | 1.3708 | 1.3922 | 9.61 | 17.63 | 10.16 | 62.60 | 25.70 | 47.15 | 27.15 | 167.36 | Db1 + Ast | |
| 59, H | 1.3331 | 1.3812 | 9.68 | 24.67 | 0 | 65.65 | 28.18 | 71.82 | 0 | 191.16 | Db1 + Th | |
| 60 | 1.3443 | 1.3845 | 9.32 | 23.39 | 2.08 | 65.21 | 26.80 | 67.23 | 5.97 | 187.48 | Db1 + Th | |
| 61 | 1.3636 | 1.3880 | 9.31 | 22.38 | 4.54 | 63.77 | 25.70 | 61.78 | 12.52 | 176.00 | Db1 + Th | |
| 62 | 1.3755 | 1.3901 | 8.50 | 20.24 | 7.07 | 64.19 | 23.73 | 56.52 | 19.75 | 179.29 | Db1 + Th | |
| 63 | 1.3847 | 1.3924 | 8.47 | 19.97 | 9.23 | 62.33 | 22.49 | 53.00 | 24.51 | 165.44 | Db1 + Th | |
| 64, I | 1.3492 | 1.3886 | 0 | 22.93 | 12.74 | 64.33 | 0 | 64.28 | 35.72 | 180.28 | Ast + Th | |
| 65 | 1.3568 | 1.3888 | 2.60 | 21.97 | 12.05 | 63.38 | 7.09 | 60.01 | 32.90 | 173.07 | Ast + Th | |
| 66 | 1.3579 | 1.3897 | 5.13 | 21.07 | 11.16 | 62.64 | 13.74 | 56.40 | 29.86 | 167.71 | Ast + Th | |
| 67 | 1.3618 | 1.3908 | 7.50 | 20.35 | 10.94 | 61.21 | 19.34 | 52.46 | 28.20 | 157.83 | Ast + Th | |
| 68, T2 | 1.3625 | 1.3913 | 8.69 | 19.85 | 10.01 | 61.45 | 22.54 | 51.49 | 25.97 | 159.47 | Ast+Th+Db1 | |
| 69 | 1.3829 | 1.3935 | 9.84 | 17.23 | 11.93 | 61.00 | 25.24 | 44.18 | 30.58 | 156.43 | Db2 + Ast | |
| 70 | 1.3842 | 1.3938 | 9.35 | 15.41 | 13.08 | 62.16 | 24.70 | 40.73 | 34.57 | 164.28 | Db2 + Ast | |
| 71 | 1.3827 | 1.3954 | 9.00 | 12.59 | 16.20 | 62.21 | 23.82 | 33.32 | 42.86 | 164.60 | Db2 + Ast | |
| 72 | 1.3829 | 1.3958 | 8.67 | 10.65 | 18.47 | 62.21 | 22.95 | 28.18 | 48.87 | 164.62 | Db2 + Ast | |
| 73 | 1.3906 | 1.3979 | 8.17 | 8.86 | 20.98 | 61.99 | 21.50 | 23.31 | 55.19 | 163.12 | Db2 + Ast | |
| 74 | 1.4000 | 1.3992 | 7.77 | 7.55 | 22.69 | 61.99 | 20.44 | 19.87 | 59.69 | 163.09 | Db2 + Ast | |
| 75 | 1.4012 | 1.3996 | 7.28 | 7.07 | 24.86 | 60.79 | 18.57 | 18.04 | 63.39 | 154.99 | Db2 + Ast | |
| 76 | 1.4053 | 1.4004 | 6.59 | 6.32 | 25.98 | 61.11 | 16.94 | 16.26 | 66.80 | 157.12 | Db2 + Ast | |
| 77, T3 | 1.4083 | 1.4020 | 6.30 | 5.72 | 27.09 | 60.89 | 16.10 | 14.62 | 69.28 | 155.69 | Db2+Ast+Eps | |
| 78, J | 1.4044 | 1.4005 | 0 | 7.75 | 29.13 | 63.12 | 0 | 21.02 | 78.98 | 171.14 | Ast + Eps | |
| 79 | 1.4030 | 1.3994 | 1.69 | 7.30 | 27.61 | 63.40 | 4.62 | 19.94 | 75.44 | 173.25 | Ast + Eps | |
| 80 | 1.4076 | 1.4008 | 3.39 | 6.62 | 26.39 | 63.60 | 9.31 | 18.20 | 72.49 | 174.70 | Ast + Eps | |
| 81 | 1.4141 | 1.4014 | 5.19 | 5.61 | 25.34 | 63.86 | 14.36 | 15.53 | 70.11 | 176.66 | Ast + Eps | |
| 82 | 1.4196 | 1.4033 | 6.83 | 4.70 | 26.65 | 61.82 | 17.90 | 12.31 | 69.79 | 161.93 | Db2 + Eps | |
| 83 | 1.4088 | 1.4028 | 8.04 | 4.44 | 26.03 | 61.49 | 20.88 | 11.52 | 67.60 | 159.71 | Db2 + Eps | |
| 84, K | 1.3924 | 1.4030 | 10.35 | 0 | 26.34 | 63.31 | 28.20 | 0 | 71.80 | 172.54 | Ls + Eps | |
| 85 | 1.4008 | 1.4038 | 10.09 | 1.45 | 27.43 | 61.03 | 25.88 | 3.72 | 70.40 | 156.64 | Ls + Eps | |
| 86 | 1.4138 | 1.4052 | 9.68 | 3.22 | 26.92 | 60.18 | 24.31 | 8.08 | 67.61 | 151.15 | Ls + Eps | |
| 87, T4 | 1.4315 | 1.4058 | 8.70 | 4.59 | 26.49 | 60.22 | 21.86 | 11.53 | 66.61 | 151.40 | Ls+Eps+Db2 | |
| 88, L | 1.2933 | 1.3820 | 20.98 | 11.60 | 0 | 67.42 | 64.39 | 35.61 | 0 | 206.87 | Ls + Db2 | |
| 89 | 1.3062 | 1.3840 | 19.48 | 11.00 | 2.17 | 67.35 | 59.67 | 33.70 | 6.63 | 206.31 | Ls + Db2 | |
| 90 | 1.3173 | 1.3860 | 18.53 | 10.82 | 4.68 | 65.97 | 54.46 | 31.79 | 13.75 | 193.89 | Ls + Db2 | |
| 91 | 1.3314 | 1.3896 | 17.16 | 10.15 | 8.70 | 63.99 | 47.65 | 28.19 | 24.16 | 177.71 | Ls + Db2 | |
| 92 | 1.3500 | 1.3921 | 15.11 | 9.31 | 11.94 | 63.64 | 41.57 | 25.60 | 32.83 | 175.06 | Ls + Db2 | |
| 93 | 1.3675 | 1.3950 | 14.23 | 8.63 | 13.75 | 63.39 | 38.87 | 23.57 | 37.56 | 173.20 | Ls + Db2 | |
| 94 | 1.3868 | 1.3979 | 12.64 | 7.83 | 18.98 | 60.55 | 32.03 | 19.84 | 48.13 | 153.52 | Ls + Db2 | |
| 95 | 1.4061 | 1.4008 | 11.30 | 7.17 | 21.67 | 59.86 | 28.16 | 17.85 | 53.99 | 149.10 | Ls + Db2 | |
| 96 | 1.4111 | 1.4028 | 10.16 | 6.22 | 22.85 | 60.77 | 25.89 | 15.86 | 58.25 | 154.87 | Ls + Db2 | |
| 97 | 1.4233 | 1.4050 | 9.54 | 4.84 | 27.01 | 58.61 | 23.06 | 11.68 | 65.26 | 141.57 | Ls + Db2 | |
表1 四元体系Li+, Na+, Mg2+//SO42--H2O 303.2、318.2 K固液相平衡数据
Table 1 Phase equilibria data of quaternary systems Li+, Na+, Mg2+//SO42--H2O at 303.2 and 318.2 K
| 温度/K | 编号 | 密度/ (g/cm3) | 折射率 nD | 平衡液相组成, w(B)×102 | 干盐指数(g/100g盐) | 平衡固相 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| w(Li2SO4) | w(Na2SO4) | w(MgSO4) | w(H2O) | J(Li2SO4) | J(Na2SO4) | J(MgSO4) | J(H2O) | |||||
| 303.2 | ||||||||||||
| 1, A | 1.3134 | 1.3785 | 4.95 | 27.39 | 0 | 67.66 | 15.30 | 84.70 | 0 | 209.29 | Mir + Db1 | |
| 2 | 1.3247 | 1.3812 | 4.68 | 25.93 | 1.98 | 67.41 | 14.36 | 79.58 | 6.06 | 206.85 | Mir + Db1 | |
| 3 | 1.3317 | 1.3834 | 4.42 | 24.29 | 3.96 | 67.33 | 13.53 | 74.35 | 12.12 | 206.06 | Mir + Db1 | |
| 4 | 1.3361 | 1.3850 | 4.23 | 23.53 | 5.75 | 66.49 | 12.63 | 70.20 | 17.17 | 198.37 | Mir + Db1 | |
| 5 | 1.3431 | 1.3870 | 4.00 | 22.31 | 7.43 | 66.26 | 11.85 | 66.12 | 22.03 | 196.35 | Mir + Db1 | |
| 6 | 1.3519 | 1.3884 | 3.85 | 21.52 | 8.74 | 65.89 | 11.28 | 63.09 | 25.63 | 193.26 | Th + Db1 | |
| 7 | 1.3543 | 1.3900 | 3.67 | 20.75 | 10.43 | 65.15 | 10.53 | 59.54 | 29.93 | 186.92 | Th + Db1 | |
| 8 | 1.3669 | 1.3916 | 3.53 | 20.35 | 11.60 | 64.52 | 9.94 | 57.36 | 32.70 | 181.91 | Th + Db1 | |
| 9 | 1.3692 | 1.3925 | 3.61 | 20.05 | 12.72 | 63.62 | 9.94 | 55.09 | 34.97 | 174.85 | Th + Db1 | |
| 10, B | 1.3630 | 1.3859 | 0 | 27.40 | 8.18 | 64.42 | 0 | 77.01 | 22.99 | 181.06 | Th + Mir | |
| 11 | 1.3524 | 1.3857 | 1.58 | 26.46 | 7.91 | 64.05 | 4.39 | 73.61 | 22.00 | 178.23 | Th + Mir | |
| 12 | 1.3480 | 1.3855 | 2.39 | 25.94 | 7.84 | 63.83 | 6.62 | 71.70 | 21.68 | 176.37 | Th + Mir | |
| 13 | 1.3475 | 1.3860 | 3.61 | 24.53 | 8.40 | 63.46 | 9.89 | 67.13 | 22.98 | 173.67 | Th + Mir | |
| 14, S1 | 1.3473 | 1.3856 | 4.26 | 23.14 | 8.28 | 64.32 | 11.94 | 64.85 | 23.21 | 180.26 | Th+Mir+Db1 | |
| 15 | 1.3721 | 1.3943 | 3.14 | 18.52 | 13.48 | 64.86 | 8.95 | 52.68 | 38.37 | 184.55 | Ast + Db1 | |
| 16 | 1.3801 | 1.3954 | 3.18 | 15.98 | 16.33 | 64.51 | 8.97 | 45.02 | 46.01 | 181.78 | Ast + Db1 | |
| 17 | 1.3814 | 1.3959 | 3.31 | 15.91 | 18.04 | 62.74 | 8.88 | 42.70 | 48.42 | 168.40 | Ast + Db1 | |
| 18 | 1.3959 | 1.3991 | 3.17 | 14.55 | 20.05 | 62.23 | 8.39 | 38.52 | 53.09 | 164.78 | Ast + Db1 | |
| 19 | 1.3993 | 1.3997 | 3.07 | 13.34 | 21.44 | 62.15 | 8.11 | 35.24 | 56.65 | 164.24 | Ast + Db1 | |
| 20 | 1.3944 | 1.3997 | 3.10 | 13.04 | 22.12 | 61.74 | 8.09 | 34.08 | 57.83 | 161.39 | Ast + Db1 | |
| 21, C | 1.3756 | 1.3930 | 0 | 22.96 | 13.34 | 63.70 | 0 | 63.25 | 36.75 | 175.51 | Ast + Th | |
| 22 | 1.3802 | 1.3930 | 0.61 | 22.43 | 13.22 | 63.74 | 1.69 | 61.85 | 36.46 | 175.82 | Ast + Th | |
| 23 | 1.3874 | 1.3937 | 1.28 | 22.21 | 13.00 | 63.51 | 3.50 | 60.86 | 35.64 | 174.02 | Ast + Th | |
| 24 | 1.3917 | 1.3942 | 2.60 | 21.06 | 12.79 | 63.55 | 7.13 | 57.78 | 35.09 | 174.39 | Ast + Th | |
| 25, S2 | 1.3806 | 1.3935 | 3.33 | 19.55 | 12.88 | 64.24 | 9.31 | 54.68 | 36.01 | 179.65 | Ast+Th+Db1 | |
| 26, D | 1.3850 | 1.3976 | 0 | 12.30 | 23.10 | 64.60 | 0 | 34.74 | 65.26 | 182.51 | Ast + Eps | |
| 27 | 1.3802 | 1.3974 | 1.48 | 11.65 | 22.44 | 64.43 | 4.15 | 32.76 | 63.09 | 181.11 | Ast + Eps | |
| 28 | 1.3816 | 1.3975 | 2.43 | 11.94 | 22.65 | 62.98 | 6.57 | 32.26 | 61.17 | 170.07 | Ast + Eps | |
| 29, S3 | 1.3880 | 1.3977 | 2.98 | 11.28 | 21.51 | 64.23 | 8.33 | 31.53 | 60.14 | 179.53 | Ast+Eps+Db1 | |
| 30 | 1.3809 | 1.3980 | 3.69 | 11.18 | 21.18 | 63.95 | 10.23 | 31.02 | 58.75 | 177.42 | Eps + Db1 | |
| 31 | 1.3816 | 1.3982 | 4.61 | 10.49 | 20.85 | 64.05 | 12.82 | 29.19 | 57.99 | 178.11 | Eps + Db1 | |
| 32 | 1.3828 | 1.3986 | 6.06 | 9.36 | 20.46 | 64.12 | 16.89 | 26.09 | 57.02 | 178.69 | Eps + Db1 | |
| 33 | 1.3881 | 1.3998 | 8.75 | 8.95 | 19.54 | 62.76 | 23.49 | 24.04 | 52.47 | 168.54 | Eps + Db1 | |
| 34 | 1.3960 | 1.4003 | 10.40 | 9.08 | 17.85 | 62.67 | 27.86 | 24.32 | 47.82 | 167.85 | Eps + Db1 | |
| 35 | 1.3951 | 1.4005 | 11.50 | 8.90 | 17.32 | 62.27 | 30.50 | 23.59 | 45.91 | 165.07 | Eps + Db1 | |
| 36, S4 | 1.3986 | 1.4007 | 12.21 | 8.15 | 17.36 | 62.28 | 32.36 | 21.60 | 46.04 | 165.14 | Eps+Db1+Ls | |
| 37, E | 1.3451 | 1.3970 | 13.60 | 0 | 20.91 | 65.49 | 39.40 | 0 | 60.60 | 189.80 | Eps + Ls | |
| 38 | 1.3467 | 1.3972 | 13.53 | 2.16 | 19.44 | 64.87 | 38.52 | 6.16 | 55.32 | 184.59 | Eps + Ls | |
| 39 | 1.3560 | 1.3976 | 13.36 | 3.19 | 19.02 | 64.43 | 37.56 | 8.98 | 53.46 | 181.08 | Eps + Ls | |
| 40 | 1.3598 | 1.3983 | 13.18 | 4.29 | 18.88 | 63.65 | 36.27 | 11.79 | 51.94 | 175.06 | Eps + Ls | |
| 41 | 1.3657 | 1.3988 | 12.94 | 5.26 | 18.67 | 63.13 | 35.10 | 14.26 | 50.64 | 171.17 | Eps + Ls | |
| 42 | 1.3717 | 1.3996 | 12.59 | 6.67 | 18.54 | 62.20 | 33.32 | 17.65 | 49.03 | 164.53 | Eps + Ls | |
| 43 | 1.3885 | 1.4003 | 12.28 | 7.73 | 17.88 | 62.11 | 32.40 | 20.41 | 47.19 | 163.93 | Eps + Ls | |
| 44, F | 1.3051 | 1.3842 | 20.63 | 12.64 | 0 | 66.73 | 62.01 | 37.99 | 0 | 200.50 | Db1 + Ls | |
| 45 | 1.3119 | 1.3861 | 19.12 | 11.73 | 2.33 | 66.82 | 57.63 | 35.36 | 7.01 | 201.46 | Db1 + Ls | |
| 46 | 1.3235 | 1.3880 | 17.92 | 11.11 | 4.49 | 66.48 | 53.46 | 33.13 | 13.41 | 198.32 | Db1 + Ls | |
| 47 | 1.3375 | 1.3910 | 16.26 | 10.67 | 7.93 | 65.14 | 46.64 | 30.60 | 22.76 | 186.88 | Db1 + Ls | |
| 48 | 1.3529 | 1.3939 | 14.52 | 9.85 | 11.08 | 64.55 | 40.96 | 27.79 | 31.25 | 182.14 | Db1 + Ls | |
| 49 | 1.3583 | 1.3947 | 13.78 | 9.52 | 12.39 | 64.31 | 38.60 | 26.69 | 34.71 | 180.22 | Db1 + Ls | |
| 50 | 1.3757 | 1.3974 | 12.17 | 9.02 | 15.76 | 63.05 | 32.94 | 24.40 | 42.66 | 170.61 | Db1 + Ls | |
| 318.2 | ||||||||||||
| 51, G | 1.3300 | 1.3830 | 13.36 | 21.83 | 0 | 64.81 | 37.97 | 62.03 | 0 | 184.14 | Db1 + Db2 | |
| 52 | 1.3636 | 1.3852 | 12.99 | 21.03 | 3.13 | 62.85 | 34.98 | 56.61 | 8.41 | 169.16 | Db1 + Db2 | |
| 53 | 1.3676 | 1.3878 | 11.78 | 20.37 | 6.06 | 61.79 | 30.84 | 53.31 | 15.85 | 161.75 | Db1 + Db2 | |
| 54 | 1.3730 | 1.3906 | 11.03 | 18.75 | 8.11 | 62.11 | 29.11 | 49.49 | 21.40 | 163.94 | Db1 + Db2 | |
| 55 | 1.3808 | 1.3918 | 10.47 | 17.75 | 10.20 | 61.58 | 27.25 | 46.20 | 26.55 | 160.28 | Db1 + Db2 | |
| 56, T1 | 1.3928 | 1.3931 | 9.75 | 17.34 | 10.64 | 62.27 | 25.84 | 45.96 | 28.20 | 165.11 | Db1+Db2+Ast | |
| 57 | 1.3641 | 1.3921 | 9.49 | 18.70 | 10.12 | 61.69 | 24.77 | 48.81 | 26.42 | 160.95 | Db1 + Ast | |
| 58 | 1.3708 | 1.3922 | 9.61 | 17.63 | 10.16 | 62.60 | 25.70 | 47.15 | 27.15 | 167.36 | Db1 + Ast | |
| 59, H | 1.3331 | 1.3812 | 9.68 | 24.67 | 0 | 65.65 | 28.18 | 71.82 | 0 | 191.16 | Db1 + Th | |
| 60 | 1.3443 | 1.3845 | 9.32 | 23.39 | 2.08 | 65.21 | 26.80 | 67.23 | 5.97 | 187.48 | Db1 + Th | |
| 61 | 1.3636 | 1.3880 | 9.31 | 22.38 | 4.54 | 63.77 | 25.70 | 61.78 | 12.52 | 176.00 | Db1 + Th | |
| 62 | 1.3755 | 1.3901 | 8.50 | 20.24 | 7.07 | 64.19 | 23.73 | 56.52 | 19.75 | 179.29 | Db1 + Th | |
| 63 | 1.3847 | 1.3924 | 8.47 | 19.97 | 9.23 | 62.33 | 22.49 | 53.00 | 24.51 | 165.44 | Db1 + Th | |
| 64, I | 1.3492 | 1.3886 | 0 | 22.93 | 12.74 | 64.33 | 0 | 64.28 | 35.72 | 180.28 | Ast + Th | |
| 65 | 1.3568 | 1.3888 | 2.60 | 21.97 | 12.05 | 63.38 | 7.09 | 60.01 | 32.90 | 173.07 | Ast + Th | |
| 66 | 1.3579 | 1.3897 | 5.13 | 21.07 | 11.16 | 62.64 | 13.74 | 56.40 | 29.86 | 167.71 | Ast + Th | |
| 67 | 1.3618 | 1.3908 | 7.50 | 20.35 | 10.94 | 61.21 | 19.34 | 52.46 | 28.20 | 157.83 | Ast + Th | |
| 68, T2 | 1.3625 | 1.3913 | 8.69 | 19.85 | 10.01 | 61.45 | 22.54 | 51.49 | 25.97 | 159.47 | Ast+Th+Db1 | |
| 69 | 1.3829 | 1.3935 | 9.84 | 17.23 | 11.93 | 61.00 | 25.24 | 44.18 | 30.58 | 156.43 | Db2 + Ast | |
| 70 | 1.3842 | 1.3938 | 9.35 | 15.41 | 13.08 | 62.16 | 24.70 | 40.73 | 34.57 | 164.28 | Db2 + Ast | |
| 71 | 1.3827 | 1.3954 | 9.00 | 12.59 | 16.20 | 62.21 | 23.82 | 33.32 | 42.86 | 164.60 | Db2 + Ast | |
| 72 | 1.3829 | 1.3958 | 8.67 | 10.65 | 18.47 | 62.21 | 22.95 | 28.18 | 48.87 | 164.62 | Db2 + Ast | |
| 73 | 1.3906 | 1.3979 | 8.17 | 8.86 | 20.98 | 61.99 | 21.50 | 23.31 | 55.19 | 163.12 | Db2 + Ast | |
| 74 | 1.4000 | 1.3992 | 7.77 | 7.55 | 22.69 | 61.99 | 20.44 | 19.87 | 59.69 | 163.09 | Db2 + Ast | |
| 75 | 1.4012 | 1.3996 | 7.28 | 7.07 | 24.86 | 60.79 | 18.57 | 18.04 | 63.39 | 154.99 | Db2 + Ast | |
| 76 | 1.4053 | 1.4004 | 6.59 | 6.32 | 25.98 | 61.11 | 16.94 | 16.26 | 66.80 | 157.12 | Db2 + Ast | |
| 77, T3 | 1.4083 | 1.4020 | 6.30 | 5.72 | 27.09 | 60.89 | 16.10 | 14.62 | 69.28 | 155.69 | Db2+Ast+Eps | |
| 78, J | 1.4044 | 1.4005 | 0 | 7.75 | 29.13 | 63.12 | 0 | 21.02 | 78.98 | 171.14 | Ast + Eps | |
| 79 | 1.4030 | 1.3994 | 1.69 | 7.30 | 27.61 | 63.40 | 4.62 | 19.94 | 75.44 | 173.25 | Ast + Eps | |
| 80 | 1.4076 | 1.4008 | 3.39 | 6.62 | 26.39 | 63.60 | 9.31 | 18.20 | 72.49 | 174.70 | Ast + Eps | |
| 81 | 1.4141 | 1.4014 | 5.19 | 5.61 | 25.34 | 63.86 | 14.36 | 15.53 | 70.11 | 176.66 | Ast + Eps | |
| 82 | 1.4196 | 1.4033 | 6.83 | 4.70 | 26.65 | 61.82 | 17.90 | 12.31 | 69.79 | 161.93 | Db2 + Eps | |
| 83 | 1.4088 | 1.4028 | 8.04 | 4.44 | 26.03 | 61.49 | 20.88 | 11.52 | 67.60 | 159.71 | Db2 + Eps | |
| 84, K | 1.3924 | 1.4030 | 10.35 | 0 | 26.34 | 63.31 | 28.20 | 0 | 71.80 | 172.54 | Ls + Eps | |
| 85 | 1.4008 | 1.4038 | 10.09 | 1.45 | 27.43 | 61.03 | 25.88 | 3.72 | 70.40 | 156.64 | Ls + Eps | |
| 86 | 1.4138 | 1.4052 | 9.68 | 3.22 | 26.92 | 60.18 | 24.31 | 8.08 | 67.61 | 151.15 | Ls + Eps | |
| 87, T4 | 1.4315 | 1.4058 | 8.70 | 4.59 | 26.49 | 60.22 | 21.86 | 11.53 | 66.61 | 151.40 | Ls+Eps+Db2 | |
| 88, L | 1.2933 | 1.3820 | 20.98 | 11.60 | 0 | 67.42 | 64.39 | 35.61 | 0 | 206.87 | Ls + Db2 | |
| 89 | 1.3062 | 1.3840 | 19.48 | 11.00 | 2.17 | 67.35 | 59.67 | 33.70 | 6.63 | 206.31 | Ls + Db2 | |
| 90 | 1.3173 | 1.3860 | 18.53 | 10.82 | 4.68 | 65.97 | 54.46 | 31.79 | 13.75 | 193.89 | Ls + Db2 | |
| 91 | 1.3314 | 1.3896 | 17.16 | 10.15 | 8.70 | 63.99 | 47.65 | 28.19 | 24.16 | 177.71 | Ls + Db2 | |
| 92 | 1.3500 | 1.3921 | 15.11 | 9.31 | 11.94 | 63.64 | 41.57 | 25.60 | 32.83 | 175.06 | Ls + Db2 | |
| 93 | 1.3675 | 1.3950 | 14.23 | 8.63 | 13.75 | 63.39 | 38.87 | 23.57 | 37.56 | 173.20 | Ls + Db2 | |
| 94 | 1.3868 | 1.3979 | 12.64 | 7.83 | 18.98 | 60.55 | 32.03 | 19.84 | 48.13 | 153.52 | Ls + Db2 | |
| 95 | 1.4061 | 1.4008 | 11.30 | 7.17 | 21.67 | 59.86 | 28.16 | 17.85 | 53.99 | 149.10 | Ls + Db2 | |
| 96 | 1.4111 | 1.4028 | 10.16 | 6.22 | 22.85 | 60.77 | 25.89 | 15.86 | 58.25 | 154.87 | Ls + Db2 | |
| 97 | 1.4233 | 1.4050 | 9.54 | 4.84 | 27.01 | 58.61 | 23.06 | 11.68 | 65.26 | 141.57 | Ls + Db2 | |
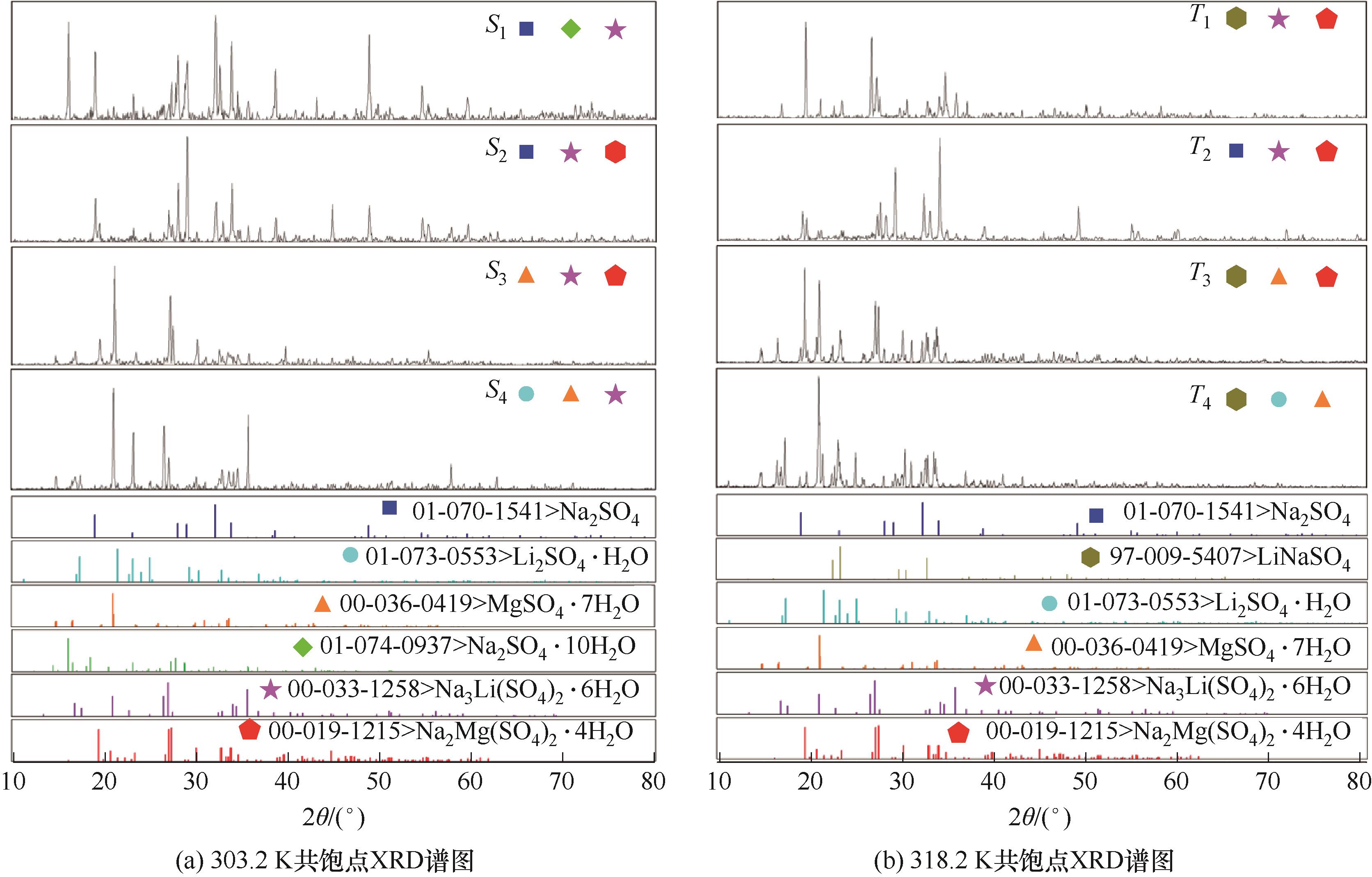
图3 四元体系Li+, Na+, Mg2+//SO42--H2O 303.2、318.2 K共饱点XRD谱图
Fig.3 The X-ray diffraction patterns of invariant points of quaternary system Li+, Na+, Mg2+//SO42--H2O at 303.2 and 318.2 K
| 1 | 郑绵平, 邢恩袁, 张雪飞, 等. 全球锂矿床的分类、外生锂矿成矿作用与提取技术[J]. 中国地质, 2023, 50(6): 1599-1620. |
| Zheng M P, Xing E Y, Zhang X F, et al. Classification and mineralization of global lithium deposits and lithium extraction technologies for exogenetic lithium deposits[J]. Geology in China, 2023, 50(6): 1599-1620. | |
| 2 | 中华人民共和国自然资源部. 2022年全国矿产资源储量统计表[EB/OL]. [2024-02-05].. |
| Ministry of Natural Resources of the People's Republic of China. Statistical table of national mineral resources reserves in 2022[EB/OL]. [2024-02-05]. . | |
| 3 | 蒋晨啸, 陈秉伦, 张东钰, 等. 我国盐湖锂资源分离提取进展[J]. 化工学报, 2022, 73(2): 481-503. |
| Jiang C X, Chen B L, Zhang D Y, et al. Progress in isolating lithium resources from China salt lake brine[J]. CIESC Journal, 2022, 73(2): 481-503. | |
| 4 | 王琪, 赵有璟, 刘洋, 等. 高镁锂比盐湖镁锂分离与锂提取技术研究进展[J]. 化工学报, 2021, 72(6): 2905-2921, 3433. |
| Wang Q, Zhao Y J, Liu Y, et al. Recent advances in magnesium/lithium separation and lithium extraction technologies from salt lake brine with high magnesium/lithium ratio[J]. CIESC Journal, 2021, 72(6): 2905-2921, 3433. | |
| 5 | 杨游胜, 姚智豪, 赵志星, 等. 富锂硫酸盐型盐湖卤水蒸发实验研究进展[J]. 无机盐工业, 2024, 56(4): 1-7. |
| Yang Y S, Yao Z H, Zhao Z X, et al. Research progress of lithium-rich sulfate type salt lake brine evaporation experiment[J]. Inorganic Chemicals Industry, 2024, 56(4): 1-7. | |
| 6 | Yu J J, Zheng M P, Wu Q, et al. Extracting lithium from Tibetan Dangxiong Tso Salt Lake of carbonate type by using geothermal salinity-gradient solar pond[J]. Solar Energy, 2015, 115: 133-144. |
| 7 | Krumgalz B S. Temperature dependence of mineral solubility in water(part 3): Alkaline and alkaline earth sulfates[J]. Journal of Physical and Chemical Reference Data, 2018, 47(2): 043101. |
| 8 | Li D D, Zeng D W, Yin X, et al. Phase diagrams and thermochemical modeling of salt lake brine systems(Ⅲ): Li2SO4 + H2O, Na2SO4 + H2O, K2SO4 + H2O, MgSO4 + H2O, and CaSO4 + H2O systems[J]. Calphad, 2018, 60: 163-176. |
| 9 | Guo Y F, Liu Y H, Wang Q, et al. Phase equilibria and phase diagrams for the aqueous ternary system (Na2SO4 + Li2SO4 + H2O) at (288 and 308) K[J]. Journal of Chemical & Engineering Data, 2013, 58(10): 2763-2767. |
| 10 | Yu X D, Yao Z H, Zhao Z X, et al. Phase equilibria of aqueous ternary systems Li2SO4 + Na2SO4 + H2O and Na2SO4 + K2SO4 + H2O at 303.2 K[J]. Journal of Chemical & Engineering Data, 2023, 68(2): 474-482. |
| 11 | Ji Z Y, Peng J L, Yuan J S, et al. Stable phase equilibria in the ternary system (Na2SO4+Li2SO4+H2O) at 308.15 K and 313.15 K[J]. Fluid Phase Equilibria, 2015, 397: 81-86. |
| 12 | Khu K Y. Politerma rastvorimosti v sisteme Li2SO4-Na2SO4-H2O[J]. Russian Journal of Inorganic Chemistry, 1959, 4: 1909-1911. |
| 13 | 张永明, 张志宏, 崔瑞芝, 等. 三元体系LiCl-Li2SO4-H2O和Li2SO4-Na2SO4-H2O 333.15 K相平衡研究[J]. 盐湖研究, 2022, 30(3): 34-41. |
| Zhang Y M, Zhang Z H, Cui R Z, et al. Phase equilibria of LiCl-Li2SO4-H2O and Li2SO4-Na2SO4-H2O ternary systems at 333.15 K[J]. Journal of Salt Lake Research, 2022, 30(3): 34-41. | |
| 14 | Wang S Q, Guo Y F, Li D C, et al. Solid-liquid phase equilibria in the ternary systems (LiCl + MgCl2 + H2O) and (Li2SO4 + MgSO4 + H2O) at 288.15 K[J]. Journal of Chemical & Engineering Data, 2015, 60(3): 821-827. |
| 15 | Li B, Li J, Fang C H, et al. Study on phase diagrams and properties of solutions in ternary systems Li+, K+(Mg2+)/ S O 4 2 - -H2O at 25℃[J]. Chinese Journal of Chemistry, 1995, 13(2): 112-117. |
| 16 | Aravamùdan G. The system lithium sulphate-magnesium sulphate-water at 30℃[J]. Canadian Journal of Chemistry, 1962, 40(5): 1035-1037. |
| 17 | Li H X, Guo L J, Zhu F Y. Solubility phase diagram of the ternary system LiCl-MgCl2-H2O and Li2SO4-MgSO4-H2O at 348.15 K[J]. Journal of Chemical & Engineering Data, 2021, 66(1): 640-645. |
| 18 | Sohr J, Voigt W, Zeng D W. IUPAC-NIST solubility data series.104. Lithium sulfate and its double salts in aqueous solutions[J]. Journal of Physical and Chemical Reference Data, 2017, 46(2): 023101. |
| 19 | 余杰, 陈美珍. Na2SO4-MgSO4-H2O体系的相图与两盐分离方法的研究[J]. 福建化工, 1994, (2): 16-20. |
| Yu J, Chen M Z. Study on phase diagram of Na2SO4-MgSO4-H2O system and the separation method of two salts[J]. Fujian Chemical Industry, 1994, (2): 16-20. | |
| 20 | 刘宝树, 何岩, 孙华, 等. 45℃ Na2SO4-MgSO4-H2O三元水盐体系相平衡研究[J]. 河北科技大学学报, 2013, 34(1): 36-39. |
| Liu B S, He Y, Sun H, et al. Study on phase equilibrium of Na2SO4-MgSO4-H2O ternary system at 45℃[J]. Journal of Hebei University of Science and Technology, 2013, 34(1): 36-39. | |
| 21 | 牛自得, 程芳琴. 水盐体系相图及其应用[M]. 天津: 天津大学出版社, 2002. |
| Niu Z D, Cheng F Q. Phase Diagram of Water-Salt System and Its Application[M]. Tianjin: Tianjin University Press, 2002. | |
| 22 | Wang S Q, Gu P, Yuan F, et al. Solid-liquid phase equilibria of the quaternary system (Li2SO4 + Na2SO4 + MgSO4 + H2O) at 288.15 K: experimental and model simulation[J]. Journal of Chemical & Engineering Data, 2020, 65(5): 2597-2602. |
| 23 | Zuo W Z, Zeng Y, Yu X D, et al. Stable phase diagram of the quaternary water-salt system Li+, Na+, Mg2+ // S O 4 2 - -H2O at T = 323 K[J]. Journal of Chemical & Engineering Data, 2020, 65(1): 133-139. |
| 24 | 周红艳, 曾德文, 韩海军, 等. Pitzer模型对盐湖卤水体系Li+-K+-Mg2+- S O 4 2 - -H2O 273.15 K时溶解度的预测[J]. 盐湖研究, 2013, 21(3): 49-55. |
| Zhou H Y, Zeng D W, Han H J, et al. Prediction of solubilities for the salt lake system Li+-K+-Mg2+- S O 4 2 - -H2O at 273.15 K using Pitzer model[J]. Journal of Salt Lake Research, 2013, 21(3): 49-55. | |
| 25 | Wang S Q, Shi C C, Yuan F, et al. Experimental and thermodynamic modeling study of the quaternary system containing lithium, potassium, magnesium, and sulfate at 288.15 K[J]. Journal of Chemical & Engineering Data, 2020, 65(1): 49-55. |
| 26 | Cui R Z, Li W, Dong Y P, et al. Mineral solubilities of salts in the three quaternary systems: LiCl-NaCl-MgCl2-H2O, LiCl-KCl-MgCl2-H2O and Li2SO4-K2SO4-MgSO4-H2O at 288.15 K[J]. The Journal of Chemical Thermodynamics, 2019, 138: 127-139. |
| 27 | 房春晖, 李冰. 四元体系Li+, K+, Mg2+/ S O 4 2 - -H2O 25℃相关系和溶液物化性质的研究[J]. 化学学报, 1994, 52(10): 954-959. |
| Fang C H, Li B. Studies on the phase diagram and solution properties for the quarternary system Li+, K+, Mg2+/ S O 4 2 - -H2O at 25℃[J]. Acta Chimica Sinica, 1994, 52(10): 954-959. | |
| 28 | Zeng Y, Lin X F, Yu X D. Study on the solubility of the aqueous quaternary system Li2SO4 + Na2SO4 + K2SO4 + H2O at 273.15 K[J]. Journal of Chemical & Engineering Data, 2012, 57(12): 3672-3676. |
| 29 | Cui R Z, Yang L, Wang W, et al. Measurements and calculations of solid-liquid equilibria in quaternary system Li2SO4-Na2SO4-K2SO4-H2O at 288 K[J]. Chemical Research in Chinese Universities, 2017, 33(3): 460-465. |
| 30 | 赵志星, 姚智豪, 黄琴, 等. 四元体系Li2SO4 + Na2SO4 + K2SO4 + H2O 298.2 K相平衡研究[J]. 盐湖研究, 2022, 30(4): 41-49. |
| Zhao Z X, Yao Z H, Huang Q, et al. Phase equilibria of aqueous quaternary system Li2SO4 + Na2SO4 + K2SO4 + H2O at 298.2 K[J]. Journal of Salt Lake Research, 2022, 30(4): 41-49. | |
| 31 | Lepeshkov I N, Bodaleva N V, Kotova L T. Solubility studies in the system Li2SO4-Na2SO4-K2SO4-H2O at 25℃[J]. Zhurnal Neorganicheskoi Khimii, 1958, 3(12): 2781-2785. |
| 32 | 中国科学院青海盐湖研究所分析室. 卤水和盐的分析方法[M]. 2版. 北京: 科学出版社, 1988. |
| Qinghai Salt Lake Research Institute, Chinese Academy of Sciences. Analysis Method of Brine and Salt[M]. 2nd ed. Beijing: Science Press, 1988. | |
| 33 | 国家市场监督管理总局, 国家标准化管理委员会. 铝及铝合金化学分析方法第9部分:锂含量的测定 火焰原子吸收光谱法: [S]. 北京: 中国标准出版社, 2020. |
| General Administration for National Market Supervision, Standardization Administration of the People's Republic of China. Methods for chemical analysis of aluminium and aluminium alloys(part 9): Determination of lithium content—Flame atomic absorption spectrometry: [S]. Beijing: Standards Press of China, 2020. | |
| 34 | 国家市场监督管理总局, 国家标准化管理委员会. 铝及铝合金化学分析方法 第34部分: 钠含量的测定 火焰原子吸收光谱法: [S]. 北京: 中国标准出版社, 2020. |
| General Administration for National Market Supervision, Standardization Administration of the People's Republic of China. Methods for chemical analysis of aluminium and aluminium alloys(part 34): Determination of sodium content—Flame atomic absorption spectrometric method: [S]. Beijing: Standards Press of China, 2020. | |
| 35 | 国家市场监督管理总局, 国家标准化管理委员会. 铝及铝合金化学分析方法 第16部分: 镁含量的测定: [S]. 北京: 中国标准出版社, 2020. |
| General Administration for National Market Supervision, Standardization Administration of the People's Republic of China. Methods for chemical analysis of aluminium and aluminium alloys(part 16): Determination of magnesium content: [S]. Beijing: Standards Press of China, 2020. | |
| 36 | 倪晓丽. 化学分析测量不确定度评定指南[M]. 北京: 中国计量出版社, 2008. |
| Ni X L. Guide for Evaluation of Uncertainty in Chemical Analysis Measurement[M]. Beijing: China Metrology Publishing House, 2008. | |
| 37 | Ellison S L, Williams A. EURACHEM/CITAC Guide Quantifying Uncertainty in Analytical Measurement[M]. 3rd ed. London: A Focus for Analytical Chemistry in Europe, 2011. |
| 38 | 王慧玲, 李明慧, 方小敏, 等. 柴达木盆地白钠镁矾的矿物学和稳定同位素特征[J]. 矿物岩石, 2018, 38(4): 12-20 |
| Wang H L, Li M H, Fang X M, et al. Mineralogical and isotopic characteristics of bloedite in Qaidam Basin[J]. Journal of Mineralogy and Petrology, 2018, 38(4): 12-20. | |
| 39 | 曲懿华. 盐矿物鉴定手册[M]. 北京: 地质出版社, 1979. |
| Qu Y H. Handbook of Salt Mineral Identification[M]. Beijing: Geological Publishing House, 1979. | |
| 40 | Haynes W M, Lide D R, Bruno T J. CRC Handbook of Chemistry and Physics[M]. 97th ed. Boca Raton: CRC Press, 2016. |
| [1] | 司友明, 郑凌峰, 陈鹏忠, 樊江莉, 彭孝军. 新型锑氧簇光刻胶的性能与机理研究[J]. 化工学报, 2024, 75(4): 1705-1717. |
| [2] | 陈好奇, 史博会, 彭琪, 康琦, 宋尚飞, 姚海元, 陈海宏, 吴海浩, 宫敬. 基于稳定性分析的含酸/醇烃水体系相平衡计算[J]. 化工学报, 2024, 75(3): 789-800. |
| [3] | 王林, 江荣鼎, 张春晓, 李修真, 谈莹莹. 含R1234yf混合工质汽液相平衡的混合规则评估与预测研究[J]. 化工学报, 2024, 75(2): 475-483. |
| [4] | 常明慧, 王林, 苑佳佳, 曹艺飞. 盐溶液蓄能型热泵循环特性研究[J]. 化工学报, 2023, 74(S1): 329-337. |
| [5] | 金正浩, 封立杰, 李舒宏. 氨水溶液交叉型再吸收式热泵的能量及 分析[J]. 化工学报, 2023, 74(S1): 53-63. 分析[J]. 化工学报, 2023, 74(S1): 53-63. |
| [6] | 杨百玉, 寇悦, 姜峻韬, 詹亚力, 王庆宏, 陈春茂. 炼化碱渣湿式氧化预处理过程DOM的化学转化特征[J]. 化工学报, 2023, 74(9): 3912-3920. |
| [7] | 于旭东, 李琪, 陈念粗, 杜理, 任思颖, 曾英. 三元体系KCl + CaCl2 + H2O 298.2、323.2及348.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(8): 3256-3265. |
| [8] | 程小松, 殷勇高, 车春文. 不同工质在溶液除湿真空再生系统中的性能对比[J]. 化工学报, 2023, 74(8): 3494-3501. |
| [9] | 陈科, 杜理, 曾英, 任思颖, 于旭东. 四元体系LiCl+MgCl2+CaCl2+H2O 323.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(5): 1896-1903. |
| [10] | 周璇, 李孟亚, 孙杰, 岑振凯, 吕强三, 周立山, 王海涛, 韩丹丹, 龚俊波. 添加剂对氨基酸晶体生长的影响[J]. 化工学报, 2023, 74(2): 500-510. |
| [11] | 高靖博, 孙强, 李青, 王逸伟, 郭绪强. 考虑水合物结构转变的含氢气体水合物相平衡模型[J]. 化工学报, 2023, 74(2): 666-673. |
| [12] | 程文婷, 李杰, 徐丽, 程芳琴, 刘国际. AlCl3·6H2O在FeCl3、CaCl2、KCl及KCl–FeCl3溶液中溶解度的实验及预测[J]. 化工学报, 2023, 74(2): 642-652. |
| [13] | 桑世华, 刘佳, 杨晓军, 冯振华, 谭啸天, 岑雨秋, 唐彬彬. 三元体系KCl-K2B4O7-H2O在318.15 K下热力学活度系数及相平衡实验和预测[J]. 化工学报, 2023, 74(10): 4051-4062. |
| [14] | 蔡进, 王晓辉, 汤涵, 陈光进, 孙长宇. TBAB水溶液体系中半笼型水合物的相平衡预测模型[J]. 化工学报, 2023, 74(1): 408-415. |
| [15] | 周桓, 张梦丽, 郝晴, 吴思, 李杰, 徐存兵. 硫酸镁型光卤石转化钾盐镁矾的过程机制与动态规律[J]. 化工学报, 2022, 73(9): 3841-3850. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号