• •
吕高颖1( ), 穆超群1, 王婷1, 何志仙1, 徐浩洋1, 张志强2, 张良1(
), 穆超群1, 王婷1, 何志仙1, 徐浩洋1, 张志强2, 张良1( )
)
收稿日期:2025-06-03
修回日期:2025-08-05
出版日期:2025-08-21
通讯作者:
张良
作者简介:吕高颖(2001—),女,硕士研究生,学生,751411153@qq.com
基金资助:
Gaoying LV1( ), Chaoqun Mu1, Ting Wang1, Zhixian He1, haoyang Zhang Zhiqiang Xu1, Liang Zhang2
), Chaoqun Mu1, Ting Wang1, Zhixian He1, haoyang Zhang Zhiqiang Xu1, Liang Zhang2
Received:2025-06-03
Revised:2025-08-05
Online:2025-08-21
摘要:
针对传统吸附材料对Ag⁺选择性差、再生效率低的问题,本研究引入谷氨酸钠(MSG)与温敏性聚N-异丙基丙烯酰胺(PNIPAM)协同改性壳聚糖(CS)微球,构建具有动态响应性的智能吸附剂(PNIPAM/MSG/CS)。利用MSG的-NH₂/-COOH双齿配位特性定向螯合Ag⁺,结合PNIPAM的相变行为(LCST=32℃)实现“吸附-解吸”的智能调控;通过一步法交联反应实现功能基团与温敏网络的精准集成。SEM表征分析显示,复合材料表面呈现多孔结构,且XPS证实Ag(I)与-NH₂(399.35 eV)、-OH(531.64 eV)发生配位作用。吸附实验表明:最优pH=5.0,最大吸附容量达90.44 mg/g;动力学符合Elovich模型(R²>0.97),表明吸附过程以化学吸附为主导;热力学拟合Freundlich模型(1/n=0.36),揭示多层非均质吸附特性。材料在竞争离子共存下对Ag⁺具有优异的选择性,且通过升温至40℃(>LCST)触发PNIPAM疏水收缩,实现绿色再生(5次循环后容量保持率82.1%),为重金属污染治理提供了“智能识别-可控释放”新策略。
中图分类号:
吕高颖, 穆超群, 王婷, 何志仙, 徐浩洋, 张志强, 张良. 温度敏感聚N-异丙基丙烯酰胺/谷氨酸钠/壳聚糖复合材料制备及吸附性能研究[J]. 化工学报, DOI: 10.11949/0438-1157.20250598.
Gaoying LV, Chaoqun Mu, Ting Wang, Zhixian He, haoyang Zhang Zhiqiang Xu, Liang Zhang. Preparation and Adsorption Properties of Temperature sensitive poly (N-isopropylacrylamide) / Monosodium glutamate /Chitosan Composites[J]. CIESC Journal, DOI: 10.11949/0438-1157.20250598.
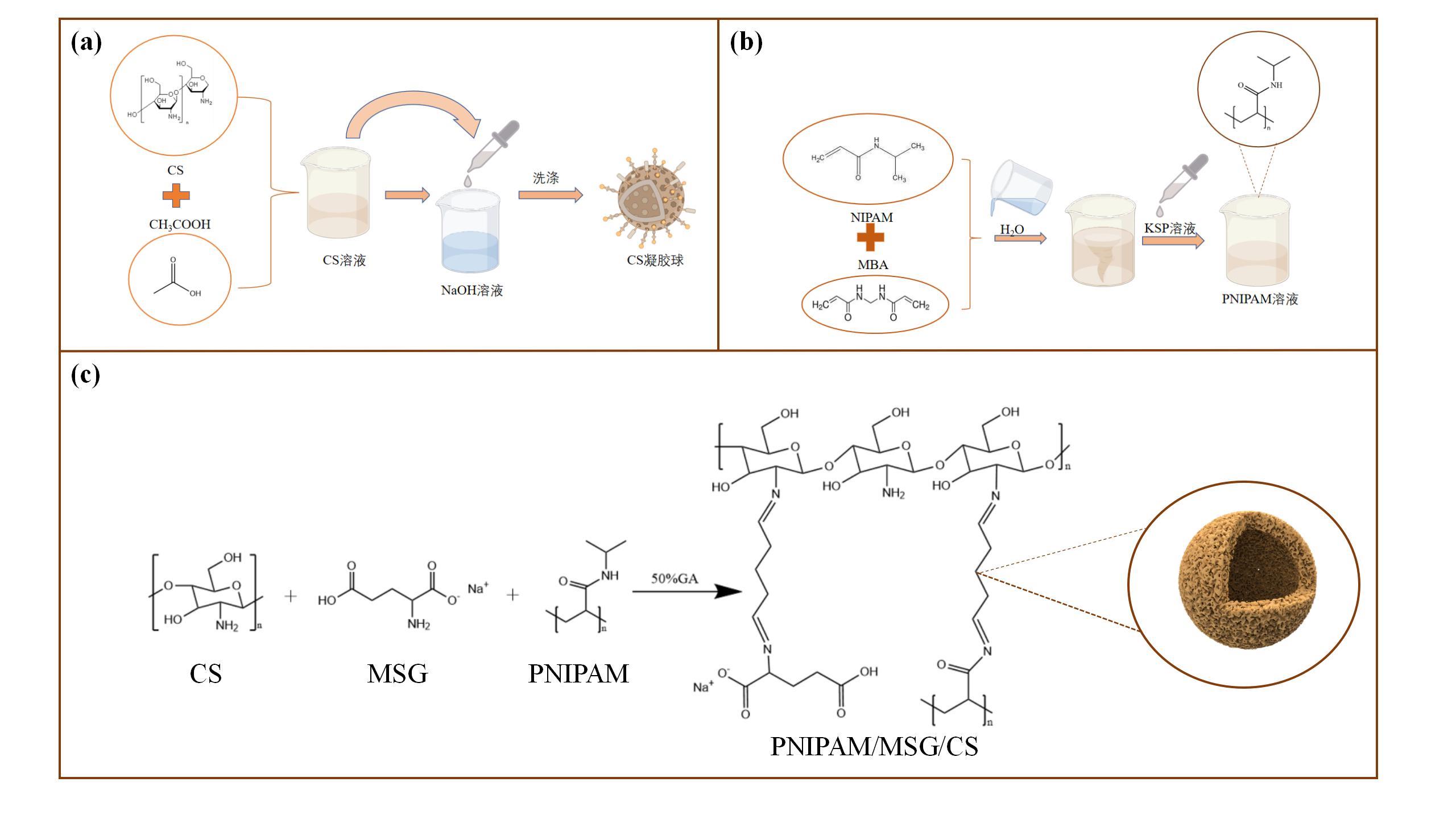
图1 CS凝胶球的制备示意图(a); 聚N-异丙基丙烯酰胺 (PNIPAM)溶液的制备示意图(b); PNIPAM/MSG/CS复合材料的合成示意图(c)
Fig.1 Schematic diagram of the preparation of CS gel beads (a); Schematic diagram of the preparation of poly(N-isopropylacrylamide) (PNIPAM) solution (b); Schematic diagram for the synthesis of PNIPAM/MSG/CS composite material (c)
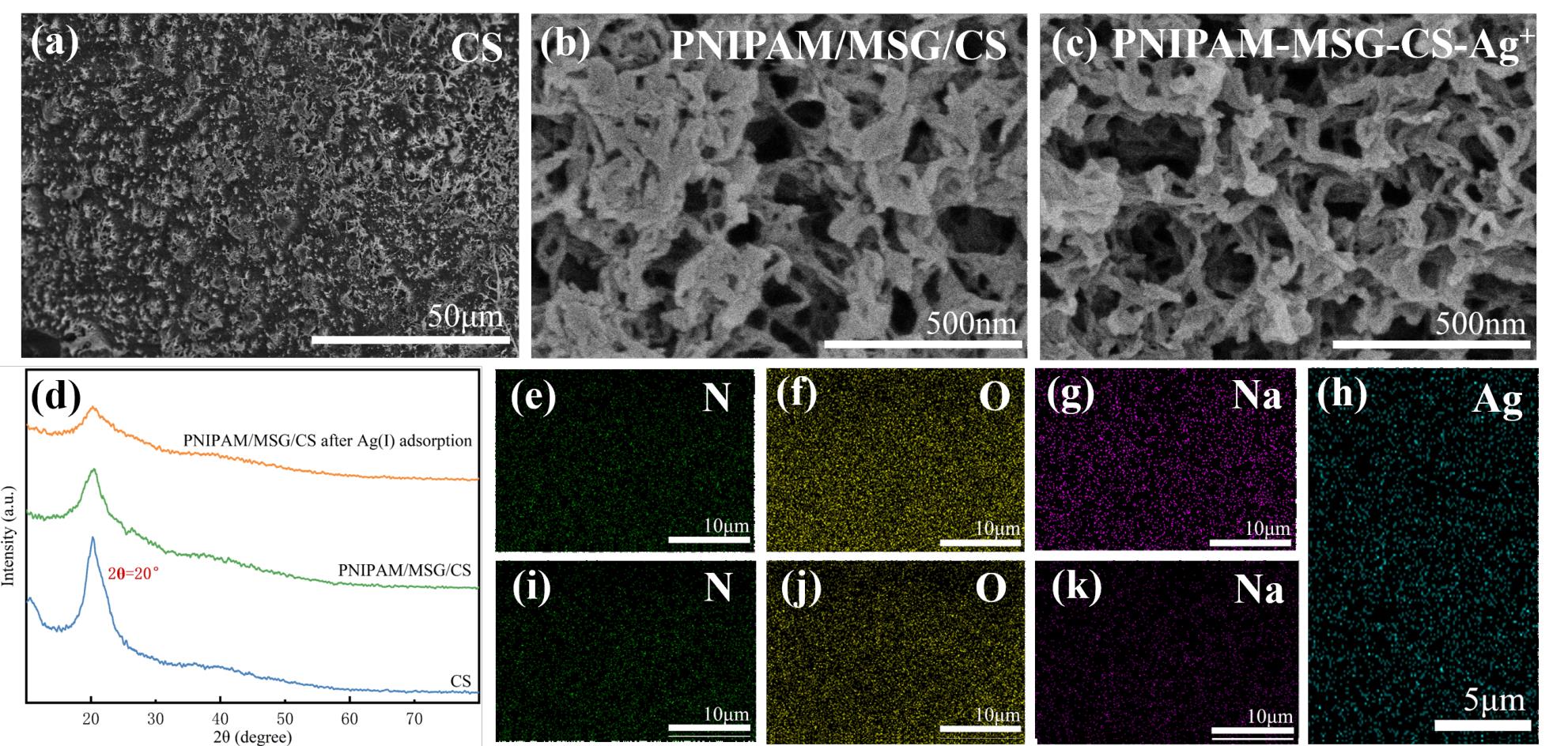
图2 CS、PNIPAM/MSG/CS和PNIPAM/MSG/CS-Ag+在不同尺度下的SEM图像(a-c); CS、PNIPAM/MSG/CS和吸附Ag(I)后的PNIPAM/MSG/CS的XRD图像(d) 吸附前后,PNIPAM/MSG/CS和PNIPAM/MSG/CS-Ag+的EDS-mapping图像(e-h);
Fig.2 SEM images of CS, PNIPAM/MSG/CS and PNIPAM/MSG/CS-Ag+ at different scales (a- c); XRD images of CS, PNIPAM/MSG/CS, and PNIPAM/MSG/CS after Ag(I) adsorption(d)EDS-mapping images of PNIPAM/MSG/CS before and after Ag(I) adsorption(e-h)
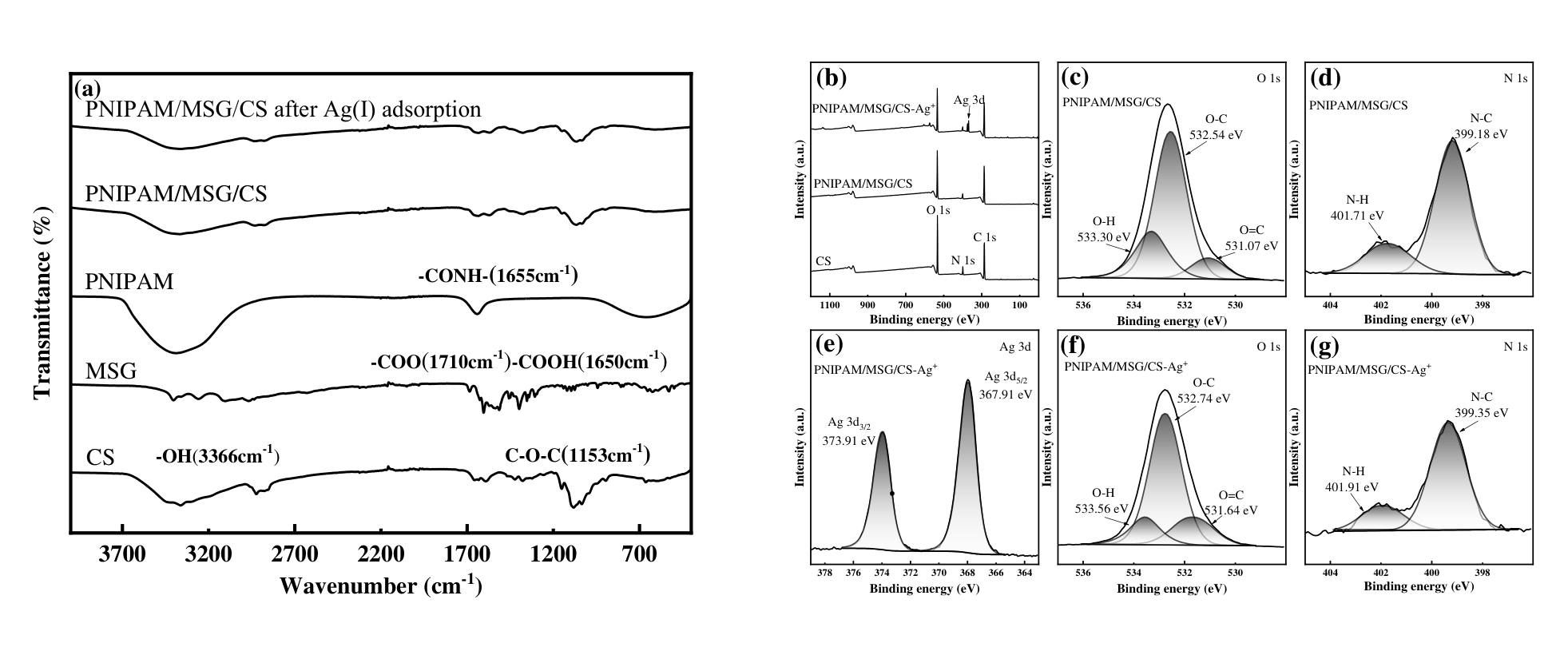
图3 CS、PNIPAM、MSG、PNIPAM/MSG/CS和吸附Ag(I)后的PNIPAM/MSG/CS的FTIR图像(a);CS、PNIPAM/MSG/CS和吸附Ag(I)后的PNIPAM/MSG/CS的全尺度XPS谱图(b),高分辨率XPS谱O 1s(c, f),N 1s(d, g)和Ag 3d(e)
Fig.3 FTIR images of CS, PNIPAM, MSG, PNIPAM/MSG/CS, and PNIPAM/MSG/CS after Ag(I) adsorption(a);Full-scale XPS spectra of CS, PNIPAM/MSG/CS and PNIPAM/MSG/CS after adsorption of Ag(I) (b), high-resolution XPS spectra of O 1s (c, f), N 1s (d, g) and Ag 3d (e)
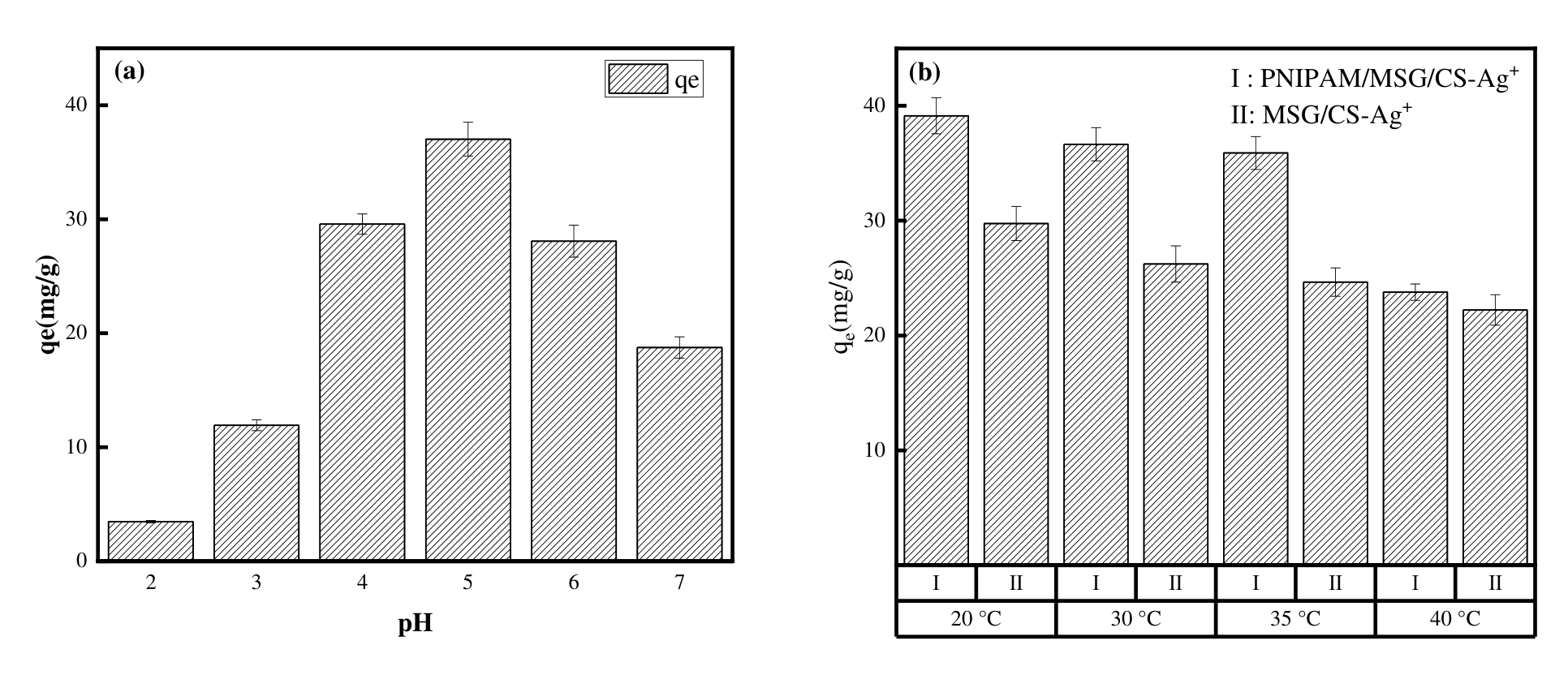
图4 (a)不同pH下PNIPAM/MSG/CS的吸附容量(T=303.15K, C0=100mg/L)和(b)不同温度下PNIPAM/MSG/CS和MSG/CS的吸附容量(pH=5, C0=100mg/L)
Fig.4 (a)The adsorption capacity of PNIPAM/MSG/CS under different pH (T=303.15K, C0=100mg/L)and(b) the adsorption capacity of PNIPAM/MSG/CS and MSG/CS at different temperatures (pH=5, C0=100mg/L)
| Adsorbent | m0/g | m20°C/g | m40°C/g | 吸水率(20°C) | 吸水率(40°C) |
|---|---|---|---|---|---|
| PNIPAM/MSG/CS | 0.0996 | 0.3418 | 0.2696 | 2.577 | 1.707 |
| MSG/CS | 0.0995 | 0.3563 | 0.3556 | 2.581 | 2.574 |
表1 PNIPAM/MSG/CS和MSG/CS在20°C和40°C下的吸水率比较
Table 1 Comparison of water absorption between PNIPAM/MSG/CS and MSG/CS at 20°C and 40°C
| Adsorbent | m0/g | m20°C/g | m40°C/g | 吸水率(20°C) | 吸水率(40°C) |
|---|---|---|---|---|---|
| PNIPAM/MSG/CS | 0.0996 | 0.3418 | 0.2696 | 2.577 | 1.707 |
| MSG/CS | 0.0995 | 0.3563 | 0.3556 | 2.581 | 2.574 |
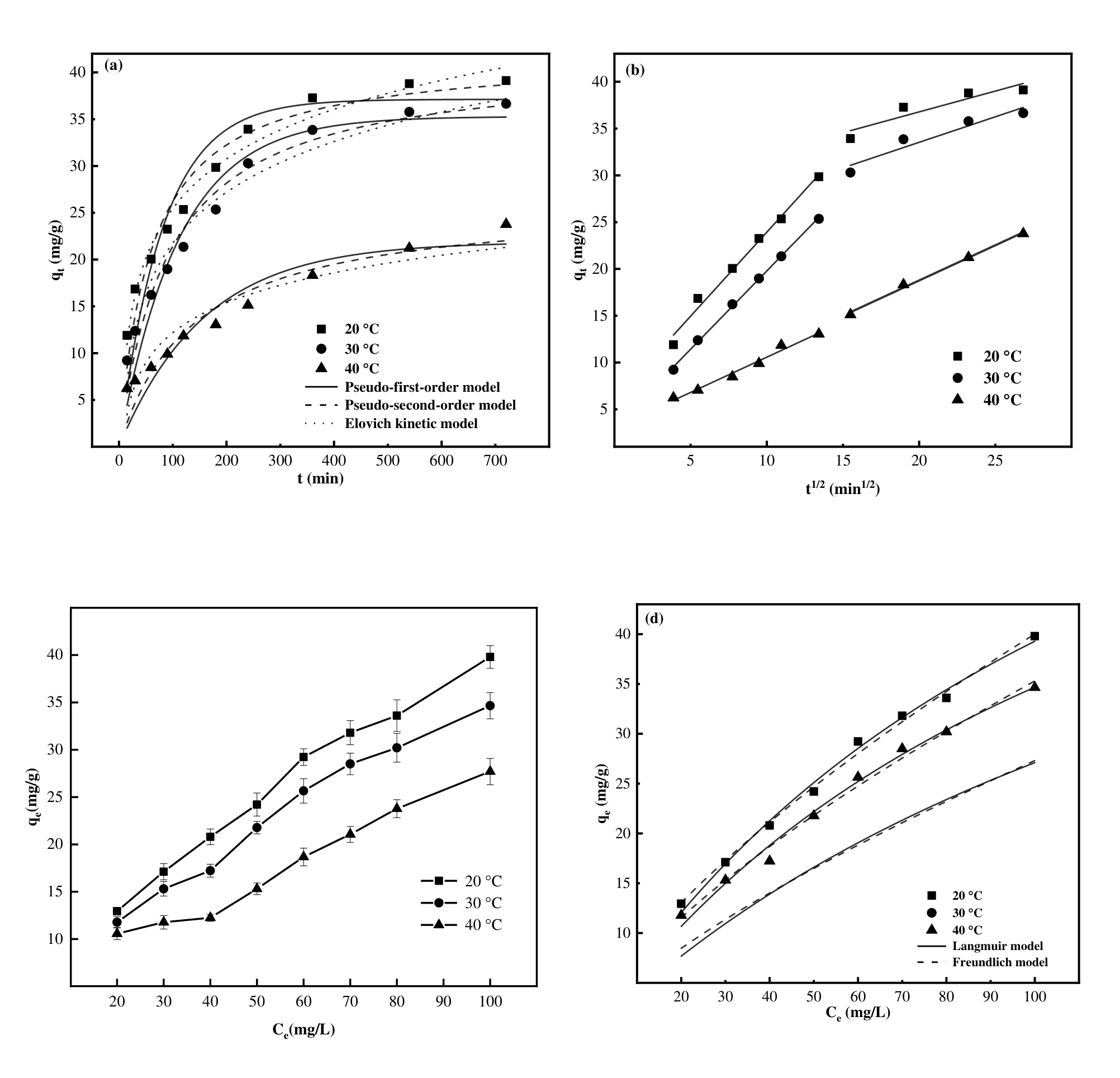
图5 PNIPAM/MSG/CS的吸附动力学拟合图(a),颗粒内扩散模型(b),初始浓度对PNIPAM/MSG/CS吸附容量的影响(c)和PNIPAM/MSG/CS的吸附热力学拟合图(d)(pH=5)
Fig. 5 The adsorption kinetics fitting diagram of PNIPAM/MSG/CS (a), the intraparticle diffusion model (b), the effect of initial concentration on the adsorption capacity of PNIPAM/MSG/CS (c) and the adsorption thermodynamics fitting diagram of PNIPAM/MSG/CS (d) (pH=5)
| Adsorbent | T(°C) | Pseudo-first-order model | Pseudo-second-order model | Elovich kinetic model | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| k1 (min-1) | R2 | k2 | R2 | a | b(g/mg) | R2 | |||||
| (mg/g) | (mg/g) | (g/mg min) | (mg/g min) | ||||||||
| PNIPAM/MSG/CS | 20.00 | 39.13 | 37.12 | 0.0122 | 0.8740 | 41.90 | 3.984 | 0.9496 | 2.121 | 0.1306 | 0.9770 |
| 30.00 | 36.65 | 35.28 | 0.0089 | 0.9268 | 41.18 | 2.629 | 0.9642 | 1.262 | 0.1278 | 0.9694 | |
| 40.00 | 23.78 | 21.88 | 0.0063 | 0.8459 | 26.26 | 2.718 | 0.9011 | 0.6429 | 0.2162 | 0.9076 | |
表2 准一级、准二级和Elovich模型的动力学参数
Table 2 Kinetic parameters for Pseudo-first-order, Pseudo-second-order and Elovich models
| Adsorbent | T(°C) | Pseudo-first-order model | Pseudo-second-order model | Elovich kinetic model | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| k1 (min-1) | R2 | k2 | R2 | a | b(g/mg) | R2 | |||||
| (mg/g) | (mg/g) | (g/mg min) | (mg/g min) | ||||||||
| PNIPAM/MSG/CS | 20.00 | 39.13 | 37.12 | 0.0122 | 0.8740 | 41.90 | 3.984 | 0.9496 | 2.121 | 0.1306 | 0.9770 |
| 30.00 | 36.65 | 35.28 | 0.0089 | 0.9268 | 41.18 | 2.629 | 0.9642 | 1.262 | 0.1278 | 0.9694 | |
| 40.00 | 23.78 | 21.88 | 0.0063 | 0.8459 | 26.26 | 2.718 | 0.9011 | 0.6429 | 0.2162 | 0.9076 | |
| Adsorbent | T(°C) | intra-particle diffusion model | |||||
|---|---|---|---|---|---|---|---|
| ki.1 | c (mg/g) | R | ki.2 | c (mg/g) | R | ||
| (mg/g min1/2) | (mg/g min1/2) | ||||||
| PNIPAM/MSG/CS | 20.00 | 1.790 | 5.967 | 0.9855 | 0.440 | 27.88 | 0.7838 |
| 30.00 | 1.670 | 3.039 | 0.9986 | 0.550 | 22.62 | 0.8761 | |
| 40.00 | 0.750 | 3.024 | 0.9776 | 0.750 | 3.715 | 0.9932 | |
表3 颗粒内扩散模型的动力学参数
Table 3 Kinetic parameters for intra-particle diffusion model
| Adsorbent | T(°C) | intra-particle diffusion model | |||||
|---|---|---|---|---|---|---|---|
| ki.1 | c (mg/g) | R | ki.2 | c (mg/g) | R | ||
| (mg/g min1/2) | (mg/g min1/2) | ||||||
| PNIPAM/MSG/CS | 20.00 | 1.790 | 5.967 | 0.9855 | 0.440 | 27.88 | 0.7838 |
| 30.00 | 1.670 | 3.039 | 0.9986 | 0.550 | 22.62 | 0.8761 | |
| 40.00 | 0.750 | 3.024 | 0.9776 | 0.750 | 3.715 | 0.9932 | |
| Adsorbent | T(°C) | Langmuir models | Freundlich models | ||||
|---|---|---|---|---|---|---|---|
| qm(mg/g) | KL (L/mg) | R2 | KF (mg/g) | 1/n | R2 | ||
| PNIPAM/MSG/CS | 20.00 | 90.44 | 0.0077 | 0.9933 | 1.612 | 0.6975 | 0.9947 |
| 30.00 | 79.38 | 0.0078 | 0.9881 | 1.440 | 0.6948 | 0.9887 | |
| 40.00 | 73.88 | 0.0058 | 0.9387 | 0.9551 | 0.7281 | 0.9585 | |
表4 PNIPAM/MSG/CS上Ag(I)吸附的Langmuir和Freundlich等温模型常数
Table 4 Langmuir and Freundlich isotherm models for Ag (Ⅰ) adsorption on PNIPAM/MSG/CS
| Adsorbent | T(°C) | Langmuir models | Freundlich models | ||||
|---|---|---|---|---|---|---|---|
| qm(mg/g) | KL (L/mg) | R2 | KF (mg/g) | 1/n | R2 | ||
| PNIPAM/MSG/CS | 20.00 | 90.44 | 0.0077 | 0.9933 | 1.612 | 0.6975 | 0.9947 |
| 30.00 | 79.38 | 0.0078 | 0.9881 | 1.440 | 0.6948 | 0.9887 | |
| 40.00 | 73.88 | 0.0058 | 0.9387 | 0.9551 | 0.7281 | 0.9585 | |
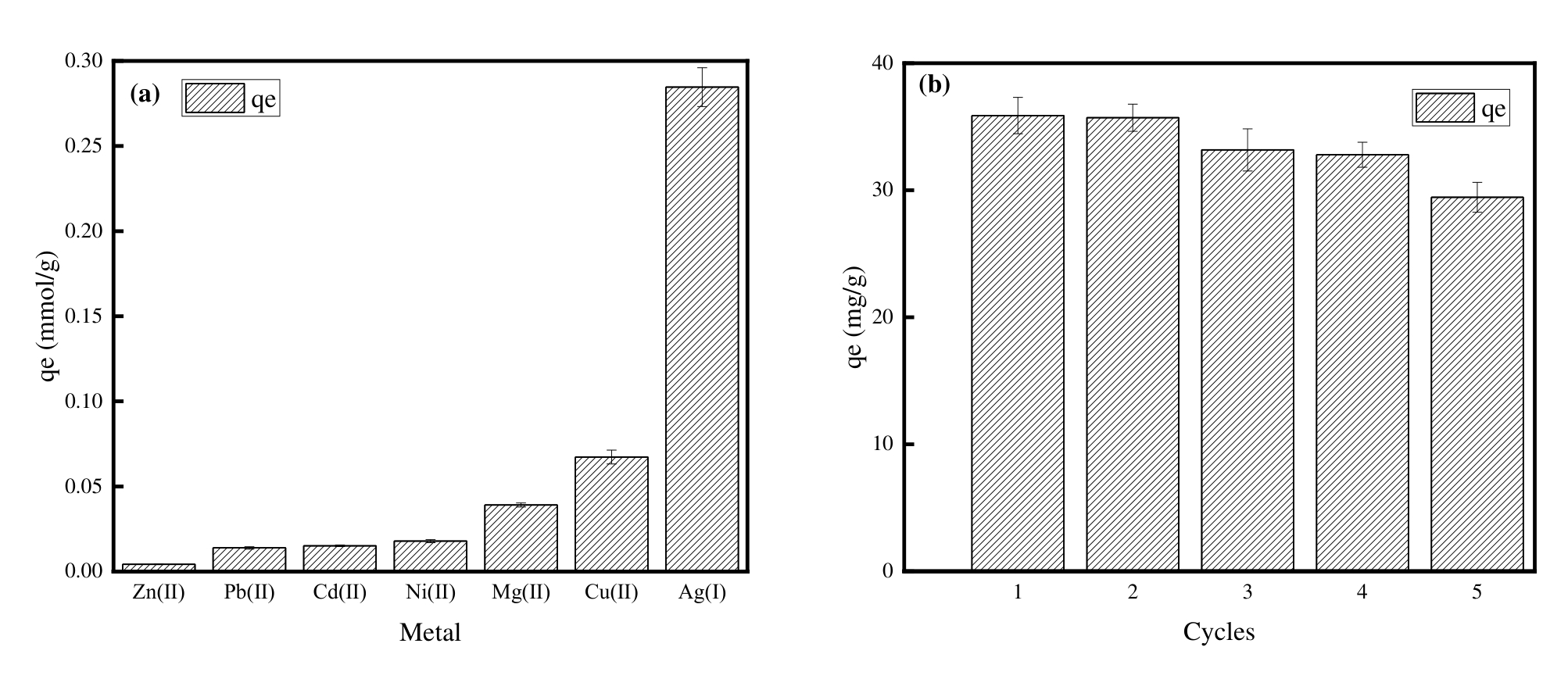
图6 (a) PNIPAM/MSG/CS在七元金属离子溶液中的吸附选择性,(b) PNIPAM/MSG/CS的吸附再生性(在5个吸附-解吸循中的吸附容量变化)(pH=5, T=303.15K, C0=100mg/L)
Fig.6 (a) adsorption selectivity of PNIPAM/MSG/CS in seven-membered metal ion solutions, (b) adsorption regeneration of PNIPAM/MSG/CS (change in adsorption capacity over five adsorption-desorption cycles) (pH=5, T=303.15K, C0=100mg/L)
| Adsorbent | Substrate | C(mg/L) | pH | qe(mg/g) | Kd (mL/g) | k |
|---|---|---|---|---|---|---|
| PNIPAM/MSG/CS | Zn(II) | 100.0 | 5.000 | 0.2800 | 0.0024 | |
| Mg(II) | 100.0 | 5.000 | 0.9500 | 0.0089 | ||
| Ni(II) | 100.0 | 5.000 | 1.050 | 0.0093 | ||
| Cd(II) | 100.0 | 5.000 | 1.700 | 0.0129 | ||
| Pb(II) | 100.0 | 5.000 | 2.880 | 0.0220 | ||
| Cu(II) | 100.0 | 5.000 | 4.280 | 0.0398 | ||
| Ag(I) | 100.0 | 5.000 | 30.70 | 0.4384 | — |
表5 PNIPAM/MSG/CS吸附不同金属离子的选择性参数
Table 5 Selectivity parameters for adsorption of different metal ions by PNIPAM/MSG/CS
| Adsorbent | Substrate | C(mg/L) | pH | qe(mg/g) | Kd (mL/g) | k |
|---|---|---|---|---|---|---|
| PNIPAM/MSG/CS | Zn(II) | 100.0 | 5.000 | 0.2800 | 0.0024 | |
| Mg(II) | 100.0 | 5.000 | 0.9500 | 0.0089 | ||
| Ni(II) | 100.0 | 5.000 | 1.050 | 0.0093 | ||
| Cd(II) | 100.0 | 5.000 | 1.700 | 0.0129 | ||
| Pb(II) | 100.0 | 5.000 | 2.880 | 0.0220 | ||
| Cu(II) | 100.0 | 5.000 | 4.280 | 0.0398 | ||
| Ag(I) | 100.0 | 5.000 | 30.70 | 0.4384 | — |
| 吸附剂 | 吸附容量(mg/g) | 循环稳定性 | k(相对异离子的选择性系数) | 材料结构变化 (吸附/解析时) |
|---|---|---|---|---|
| 壳聚糖/聚多巴胺@ C @磁性粉煤灰[ | 57.02 | 95.70%(5次循环) | Cu(II) 8.660 Zn(II) 14.15 Ni(II) 37.66 | 否 |
| 多硫基聚合物(PDMTD)[ | 127.9 | 52.00%(5次循环) | Zn(II) 28.80 Pb(II) 24.50 Ni(II) 26.00 Mn(II) 192.7 | 否 |
| 丙烯酸接枝羧甲基壳聚糖/双醛淀粉[ | 404.8 | 91.29%(5次循环) | Co(II) 3.200 Cr(III) 1.540 Ni(II) 4.95 | 否 |
| [n-BBIM]9PW9O34[ | 141.6 | 52.22%(5次循环) | Zn(II) 18.10 Pb(II) 46.20 | 否 |
| 聚苯胺包被聚苯乙烯(PS/PANI)颗粒[ | 330.0 | 96.00%(4次循环) | / | 否 |
| 本研究 | 90.44 | 82.10%(5次循环) | 见 | 是 |
表6 PNIPAM/MSG/CS 与其他吸附剂的性能比较
Table 6 Comparison of properties between PNIPAM/MSG/CS and other adsorbents.
| 吸附剂 | 吸附容量(mg/g) | 循环稳定性 | k(相对异离子的选择性系数) | 材料结构变化 (吸附/解析时) |
|---|---|---|---|---|
| 壳聚糖/聚多巴胺@ C @磁性粉煤灰[ | 57.02 | 95.70%(5次循环) | Cu(II) 8.660 Zn(II) 14.15 Ni(II) 37.66 | 否 |
| 多硫基聚合物(PDMTD)[ | 127.9 | 52.00%(5次循环) | Zn(II) 28.80 Pb(II) 24.50 Ni(II) 26.00 Mn(II) 192.7 | 否 |
| 丙烯酸接枝羧甲基壳聚糖/双醛淀粉[ | 404.8 | 91.29%(5次循环) | Co(II) 3.200 Cr(III) 1.540 Ni(II) 4.95 | 否 |
| [n-BBIM]9PW9O34[ | 141.6 | 52.22%(5次循环) | Zn(II) 18.10 Pb(II) 46.20 | 否 |
| 聚苯胺包被聚苯乙烯(PS/PANI)颗粒[ | 330.0 | 96.00%(4次循环) | / | 否 |
| 本研究 | 90.44 | 82.10%(5次循环) | 见 | 是 |
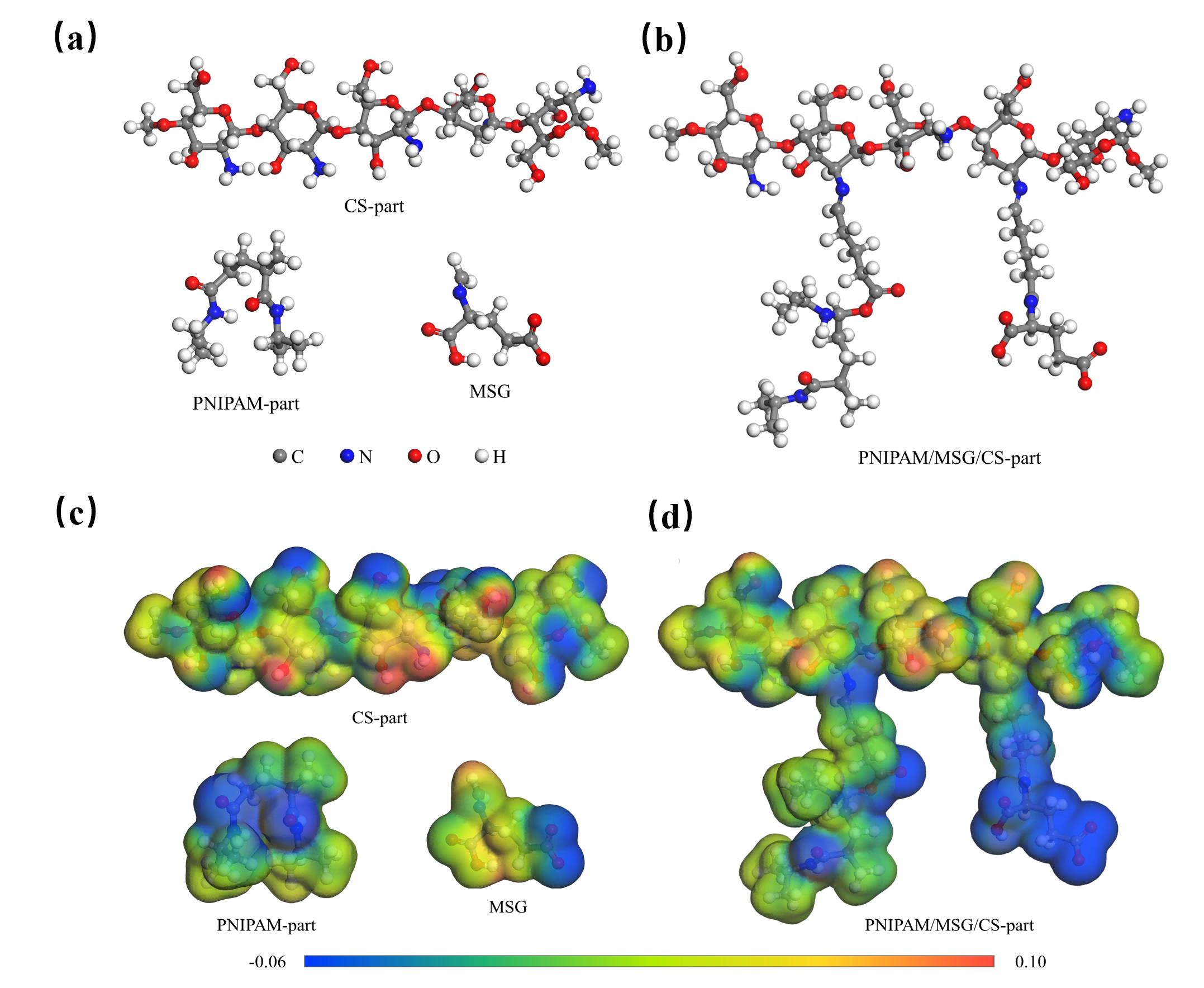
图7 各单体(a)和PNIPAM/MSG/CS(b)几何优化后构型图;各单体(c)和PNIPAM/MSG/CS(d)的静电势分布图
Fig.7 Conformations of each monomer (a) and PNIPAM/MSG/CS (b) after geometry optimization;Electrostatic potential distribution of each monomer (c) and PNIPAM/MSG/CS (d)
| EHOMO/eV | ELUMO/eV | Eg/eV | |
|---|---|---|---|
| PNIPAM-part | -5.785 | -0.6941 | 5.091 |
| MSG | -4.831 | -1.609 | 3.222 |
| CS-part | -5.456 | 0.1800 | 5.636 |
| PNIPAM/MSG/CS-part | -4.689 | -1.457 | 3.233 |
表7 PNIPAM/MSG/CS的HOMO和LUMO轨道参数
Table 7 HOMO and LUMO orbital parameters for PNIPAM/MSG/CS
| EHOMO/eV | ELUMO/eV | Eg/eV | |
|---|---|---|---|
| PNIPAM-part | -5.785 | -0.6941 | 5.091 |
| MSG | -4.831 | -1.609 | 3.222 |
| CS-part | -5.456 | 0.1800 | 5.636 |
| PNIPAM/MSG/CS-part | -4.689 | -1.457 | 3.233 |
| [1] | Fu F L, Xie L P, Tang B, et al. Application of a novel strategy: Advanced Fenton-chemical precipitation to the treatment of strong stability chelated heavy metal containing wastewater[J]. Chemical Engineering Journal, 2012, 189/190: 283-287. |
| [2] | Grimshaw P, Calo J M, Hradil G. Cyclic electrowinning/precipitation (CEP) system for the removal of heavy metal mixtures from aqueous solutions[J]. Chemical Engineering Journal, 2011, 175: 103-109. |
| [3] | Ahmed Basha C, Bhadrinarayana N S, Anantharaman N, et al. Heavy metal removal from copper smelting effluent using electrochemical cylindrical flow reactor[J]. Journal of Hazardous Materials, 2008, 152(1): 71-78. |
| [4] | Gao S, Wang Y, Wang Z Q, et al. Removal behavior and mechanisms of cadmium and lead by coupled ethylenediaminetetraacetic acid washing and electrochemical reduction: influence of current conditions[J]. Environmental Science and Pollution Research, 2022, 29(20): 29818-29829. |
| [5] | Aziz F F A, Jalil A A, Hassan N S, et al. A review on synergistic coexisting pollutants for efficient photocatalytic reaction in wastewater remediation[J]. Environmental Research, 2022, 209: 112748. |
| [6] | Wang Z Y, Sim A, Urban J J, et al. Removal and recovery of heavy metal ions by two-dimensional MoS2 nanosheets: performance and mechanisms[J]. Environmental Science & Technology, 2018, 52(17): 9741-9748. |
| [7] | Bashir A, Ahmad Malik L, Ahad S, et al. Removal of heavy metal ions from aqueous system by ion-exchange and biosorption methods[J]. Environmental Chemistry Letters, 2019, 17(2): 729-754. |
| [8] | Da̧browski A, Hubicki Z, Podkościelny P, et al. Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method[J]. Chemosphere, 2004, 56(2): 91-106. |
| [9] | Peng H, Guo J. Removal of chromium from wastewater by membrane filtration, chemical precipitation, ion exchange, adsorption electrocoagulation, electrochemical reduction, electrodialysis, electrodeionization, photocatalysis and nanotechnology: a review[J]. Environmental Chemistry Letters, 2020, 18(6): 2055-2068. |
| [10] | Castro-Muñoz R, González-Melgoza L L, García-Depraect O. Ongoing progress on novel nanocomposite membranes for the separation of heavy metals from contaminated water[J]. Chemosphere, 2021, 270: 129421. |
| [11] | Xiang H R, Min X B, Tang C J, et al. Recent advances in membrane filtration for heavy metal removal from wastewater: a mini review[J]. Journal of Water Process Engineering, 2022, 49: 103023. |
| [12] | Abdollahi N, Moussavi G, Giannakis S. A review of heavy metals' removal from aqueous matrices by Metal-Organic Frameworks (MOFs): State-of-the art and recent advances[J]. Journal of Environmental Chemical Engineering, 2022, 10(3): 107394. |
| [13] | Mu C Q, Zhang L, Zhang X M, et al. Selective adsorption of Ag (Ⅰ) from aqueous solutions using Chitosan/polydopamine@C@magnetic fly ash adsorbent beads[J]. Journal of Hazardous Materials, 2020, 381: 120943. |
| [14] | Zhang L, Yang F, Zhao Y C, et al. Preparation of thiosemicarbazide-modified polyvinyl alcohol and its selective adsorption of Cu(II)[J]. Colloid and Interface Science Communications, 2021, 43: 100377. |
| [15] | Sonker S, Fulke A B, Monga A. Recent trends on bioremediation of heavy metals; an insight with reference to the potential of marine microbes[J]. International Journal of Environmental Science and Technology, 2024, 21(15): 9763-9774. |
| [16] | Leong Y K, Chang J S. Bioremediation of heavy metals using microalgae: Recent advances and mechanisms[J]. Bioresource Technology, 2020, 303: 122886. |
| [17] | Kayan G Ö, Kayan A. Composite of natural polymers and their adsorbent properties on the dyes and heavy metal ions[J]. Journal of Polymers and the Environment, 2021, 29(11): 3477-3496. |
| [18] | Wang T, Yang F, Zhang L, et al. Fluorescence quenching and highly selective adsorption of Ag+ using N-doped graphene quantum dots/poly(vinyl alcohol) composite membrane[J]. Industrial & Engineering Chemistry Research, 2022, 61(49): 18090-18099. |
| [19] | Kumar M N V R, Muzzarelli R A A, Muzzarelli C, et al. Chitosan chemistry and pharmaceutical perspectives[J]. ChemInform, 2005, 36(11): ■-■. |
| [20] | Negm N A, Hefni H H H, Abd-Elaal A A A, et al. Advancement on modification of chitosan biopolymer and its potential applications[J]. International Journal of Biological Macromolecules, 2020, 152: 681-702. |
| [21] | Liu X W, Hu Q Y, Fang Z, et al. Magnetic chitosan nanocomposites: a useful recyclable tool for heavy metal ion removal[J]. Langmuir, 2009, 25(1): 3-8. |
| [22] | Zhang Y Z, Zhao M W, Cheng Q, et al. Research progress of adsorption and removal of heavy metals by chitosan and its derivatives: a review[J]. Chemosphere, 2021, 279: 130927. |
| [23] | Begum S, Yuhana N Y, Md Saleh N, et al. Review of chitosan composite as a heavy metal adsorbent: Material preparation and properties[J]. Carbohydrate Polymers, 2021, 259: 117613. |
| [24] | Wang J L, Chen C. Chitosan-based biosorbents: Modification and application for biosorption of heavy metals and radionuclides[J]. Bioresource Technology, 2014, 160: 129-141. |
| [25] | Xu C Z, Xu Y L, Zhong D J, et al. Zr4+ cross-linked chitosan-thiourea composite for efficient detoxification of Cr(VI) ions in aqueous solution[J]. Carbohydrate Polymers, 2022, 296: 119872. |
| [26] | Morcos G S, Ibrahim A A, El-Sayed M M H, et al. High performance functionalized UiO metal organic frameworks for the efficient and selective adsorption of Pb (II) ions in concentrated multi-ion systems[J]. Journal of Environmental Chemical Engineering, 2021, 9(3): 105191. |
| [27] | Xiao P Y, Xu J Q, Shi H L, et al. Simultaneous Cr(VI) reduction and Cr(III) sequestration in a wide pH range by using magnetic chitosan-based biopolymer[J]. International Journal of Biological Macromolecules, 2023, 253: 127398. |
| [28] | Largitte L, Pasquier R. A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon[J]. Chemical Engineering Research and Design, 2016, 109: 495-504. |
| [29] | Zhang B, Wang S X, Fu L K, et al. Selective adsorption of silver ions from highly acidic aqueous solutions by polymers containing multiple sulfur groups[J]. Water, Air, & Soil Pollution, 2018, 229(6): 199. |
| [30] | Ouyang J Y, Zhan L Q, Luo Q C, et al. Adsorption performance of silver ion on acrylic grafted carboxymethyl chitosan/dialdehyde starch[J]. Adsorption, 2024, 30(6): 1225-1237. |
| [31] | Zhang H X, Zhang J, Cui M L, et al. Construction of adsorptive nanorods from polyoxometalates and ionic liquid and their adsorption properties for silver ion from AMD[J]. Water Science and Technology, 2016, 74(4): 1005-1015. |
| [32] | Bhattarai S, Kim J S, Yun Y S, et al. Preparation of polyaniline-coated polystyrene nanoparticles for the sorption of silver ions[J]. Reactive and Functional Polymers, 2016, 105: 52-59. |
| [1] | 吴馨, 龚建英, 李祥宇, 王宇涛, 杨小龙, 蒋震. 超声波激励疏水表面液滴运动的实验研究[J]. 化工学报, 2025, 76(S1): 133-139. |
| [2] | 曹庆泰, 郭松源, 李建强, 蒋赞, 汪彬, 耑锐, 吴静怡, 杨光. 负过载下多孔隔板对液氧贮箱蓄液性能的影响研究[J]. 化工学报, 2025, 76(S1): 217-229. |
| [3] | 裴星亮, 叶翠平, 裴赢丽, 李文英. 碱改性MIL-53(Cr)选择性吸附分离二甲苯异构体[J]. 化工学报, 2025, 76(S1): 258-267. |
| [4] | 吴梓航, 徐震原, 游锦方, 潘权稳, 王如竹. 基于吸附式储冷技术的深井钻探设备冷却系统[J]. 化工学报, 2025, 76(S1): 309-317. |
| [5] | 黄国瑞, 赵耀, 谢明熹, 陈尔健, 代彦军. 一种新型数据中心余热回收系统实验与分析[J]. 化工学报, 2025, 76(S1): 409-417. |
| [6] | 徐鹏国, 孟子衡, 朱干宇, 李会泉, 王晨晔, 孙振华, 田国才. 粗碳酸锂CO2微气泡深度碳化工艺与动力学研究[J]. 化工学报, 2025, 76(7): 3325-3338. |
| [7] | 唐羽丰, 陶春珲, 王永正, 李印辉, 段然, 赵泽一, 马和平. 超高比表面积碳基多孔吸附剂制备及其Kr气存储性能研究[J]. 化工学报, 2025, 76(7): 3339-3349. |
| [8] | 卢煦旸, 徐强, 康浩鹏, 史健, 曹泽水, 郭烈锦. 化学链制氢系统中磁铁矿氧载体的CO还原特性研究[J]. 化工学报, 2025, 76(7): 3286-3294. |
| [9] | 高凤凤, 程慧峰, 杨博, 郝晓刚. 电驱动NiFeMn LDH/CNTs/PVDF膜电极选择性提取钨酸根离子[J]. 化工学报, 2025, 76(7): 3350-3360. |
| [10] | 乔亮, 李尚, 刘新亮, 王明, 张沛, 侯影飞. 三元共聚物稠油降黏剂的合成及分子模拟研究[J]. 化工学报, 2025, 76(7): 3686-3695. |
| [11] | 何军, 李勇, 赵楠, 何孝军. 碳负载硒掺杂硫化钴在锂硫电池中的性能研究[J]. 化工学报, 2025, 76(6): 2995-3008. |
| [12] | 刘峰, 韩春硕, 张益, 刘彦成, 郁林军, 申家伟, 高晓泉, 杨凯. 高温高盐环境下单烃链和双烃链表面活性剂对油水界面性质影响的微观机理研究[J]. 化工学报, 2025, 76(6): 2939-2957. |
| [13] | 赵清萍, 张敏, 赵辉, 王刚, 邱永福. 乙烯氢甲酯化合成丙酸甲酯的氢键作用机制及反应动力学研究[J]. 化工学报, 2025, 76(6): 2701-2713. |
| [14] | 麦棹铭, 武颖韬, 王维, 穆海宝, 黄佐华, 汤成龙. 正十二烷-甲烷双燃料非线性着火特性及稀释气体效应研究[J]. 化工学报, 2025, 76(6): 3115-3124. |
| [15] | 彭新艳, 刘云鸿, 陈凌宇, 韦跃兰, 陈淑琴, 胡柱东. 小分子外交联法制备超高交联聚苯乙烯血液灌流吸附剂[J]. 化工学报, 2025, 76(6): 3093-3103. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号