• •
徐烨1( ), 惠庆雪2, 方超越1, 丁琦1(
), 惠庆雪2, 方超越1, 丁琦1( ), 周建成1, 张照强2(
), 周建成1, 张照强2( )
)
收稿日期:2025-11-18
修回日期:2026-01-11
出版日期:2026-01-19
通讯作者:
丁琦,张照强
作者简介:徐烨(2002—),男,硕士研究生,220245230@seu.edu.cn
基金资助:
Ye XU1( ), Qingxue HUI2, Chaoyue FANG1, Qi DING1(
), Qingxue HUI2, Chaoyue FANG1, Qi DING1( ), Jiancheng ZHOU1, Zhaoqiang ZHANG2(
), Jiancheng ZHOU1, Zhaoqiang ZHANG2( )
)
Received:2025-11-18
Revised:2026-01-11
Online:2026-01-19
Contact:
Qi DING, Zhaoqiang ZHANG
摘要:
金属有机框架凝胶(metal–organic framework gels, MOF凝胶)是一类由MOF纳米颗粒通过交联或物理相互作用自组装形成的具有三维连续网络结构的凝胶态材料,具有框架结构易调控、无粘结剂自成型、传质性能优越等优势。通过与石墨烯、天然高分子等基底材料复合,不仅可显著提升其结构稳定性,还可赋予材料独特的功能性。综述了MOF凝胶及其复合材料近年在能源气体存储、气体吸附分离及水污染物去除等领域的研究进展,并探讨了其面向工业化应用所面临的关键问题与未来发展方向。
中图分类号:
徐烨, 惠庆雪, 方超越, 丁琦, 周建成, 张照强. 金属—有机框架凝胶及其复合材料在吸附分离领域的研究进展[J]. 化工学报, DOI: 10.11949/0438-1157.20251277.
Ye XU, Qingxue HUI, Chaoyue FANG, Qi DING, Jiancheng ZHOU, Zhaoqiang ZHANG. Recent progress in metal–organic framework gels and their composites for adsorption separation[J]. CIESC Journal, DOI: 10.11949/0438-1157.20251277.
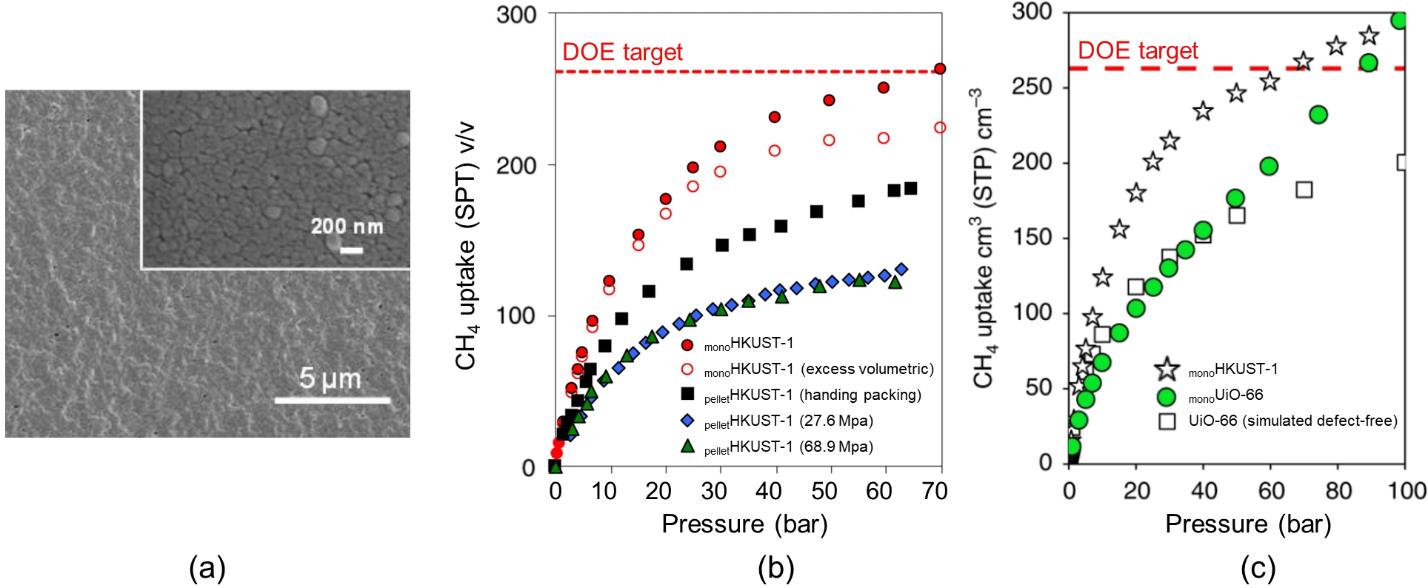
图1 monoHKUST-1的SEM图像(a)[27];monoHKUST-1和机械成型的HKUST-1吸附剂在298 K下对CH4的吸附等温线(b)[27];monoUiO-66和monoHKUST-1在298 K下对CH4的吸附等温线(c)[29]
Fig.1 SEM image ofmonoHKUST-1 (a)[27]; CH4 adsorption isotherms ofmonoHKUST-1 and HKUST-1 pellets at 298 K (b)[27]; CH4 adsorption isotherms ofmonoHKUST-1 andmonoUiO-66 at 298 K (c)[29]
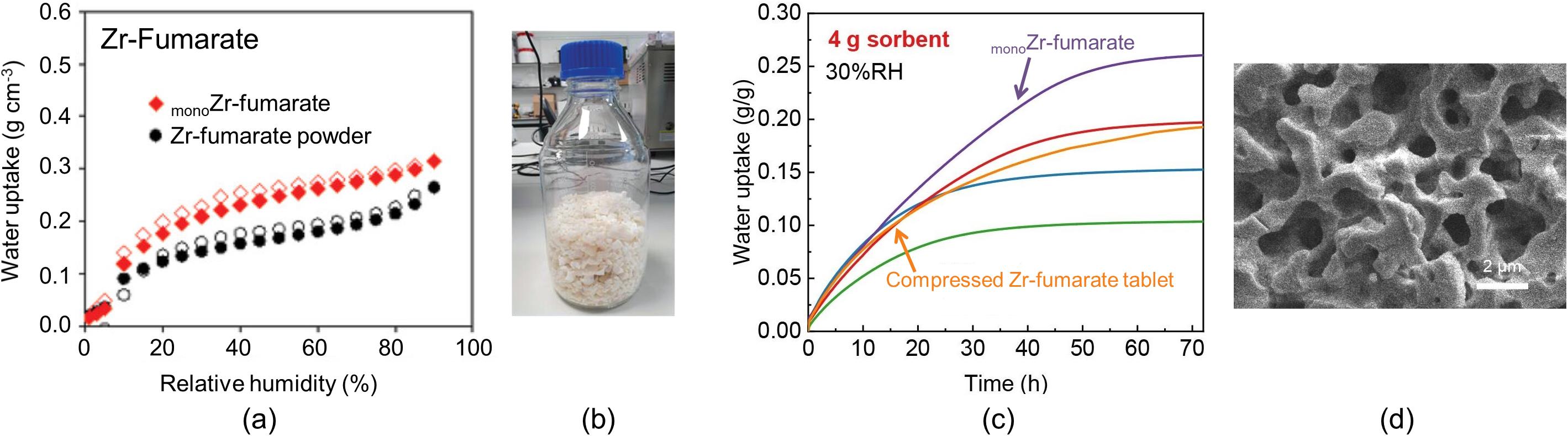
图2 monoZr-fumarate及Zr-fumarate晶体粉末在298 K下对水蒸气的吸附等温线(a)[37];批量合成的monoZr-fumarate,质量为335 g (b)[37];相分离诱导策略合成的monoZr-fumarate及挤压成型的Zr-fumarate吸附剂在298 K、30%相对湿度下对水蒸气的动力学吸附曲线(c)[38];相分离诱导策略合成的monoZr-fumarate的SEM图像(d)[38]
Fig.2 Water vapor adsorption isotherms ofmonoZr-fumarate and Zr-fumarate crystalline powders at 298 K (a)[37]; batch-synthesizedmonoZr-fumarate with a mass of 335 g (b)[37]; kinetic water vapor adsorption curves of the phase-separation-inducedmonoZr-fumarate and compressed Zr-fumarate tablet at 298 K and 30% relative humidity (c)[38]; SEM image of the phase-separation-inducedmonoZr-fumarate (d)[38]
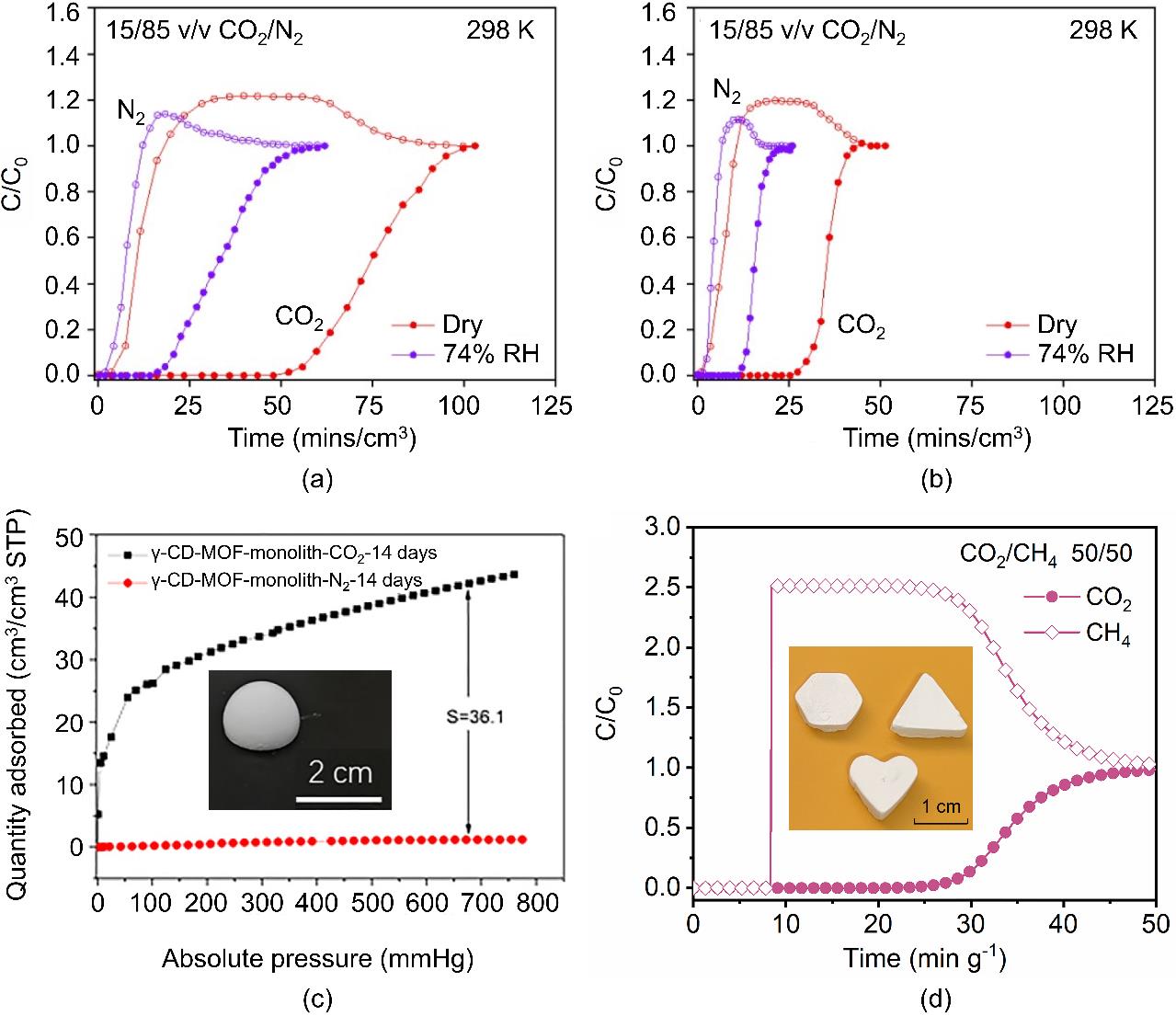
图3 monoHKUST-1 (a)和HKUST-1晶体粉末(b)对CO2/N2 (15/85)混合气的固定床穿透曲线[41];γ-CD-MOF-monolith暴露于60%相对湿度环境中14天后在273 K下对CO2和N2的吸附等温线(c)[42];ZnATA凝胶吸附剂在298 K下对CO2/CH4混合气的固定床穿透曲线(d)[28]
Fig.3 Breakthrough curves ofmonoHKUST-1 (a) and HKUST-1 crystalline powder (b) for CO2/N2 (15/85) gas mixture[41]; CO2 and N2 adsorption isotherms of γ-CD-MOF-monolith at 273 K after exposure to 60% relative humidity for 14 days (c)[42]; breakthrough curves of ZnATA gel adsorbent for CO2/CH4 (50/50) gas mixture at 298 K (d)[28]
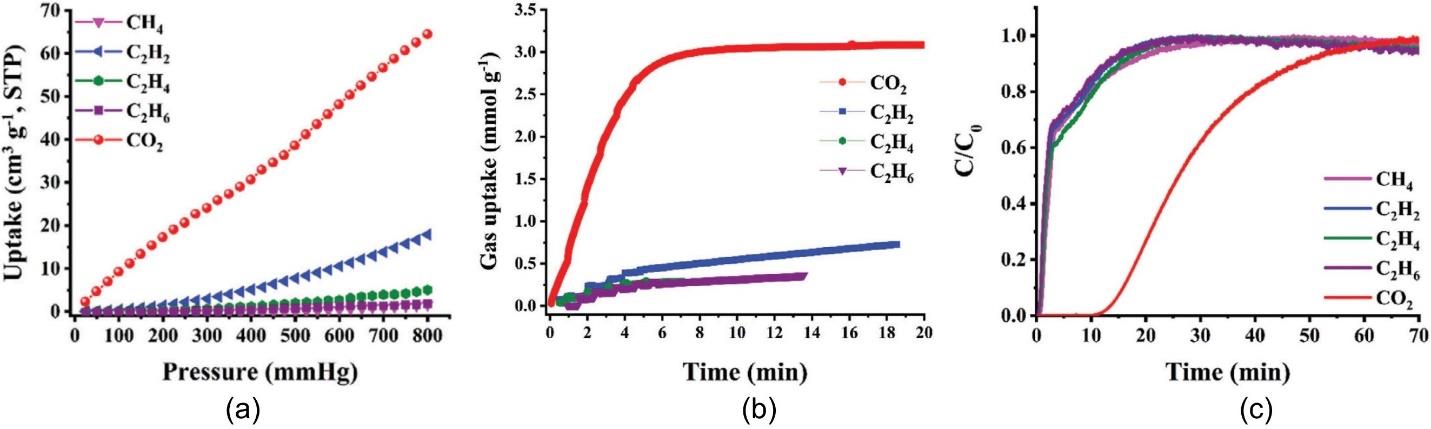
图4 经过强化活化的CuBTC凝胶吸附剂在298 K下对不同气体的吸附等温线(a)和动力学吸附曲线(b);经过强化活化的CuBTC凝胶吸附剂对含CO2五元混合气的固定床穿透曲线(c)[48]
Fig.4 Gas adsorption isotherms (a) and kinetic adsorption curves (b) of the rigorously activated CuBTC gel adsorbent at 298 K; breakthrough curves for a CO2-containing five-component gas mixture on the rigorously activated CuBTC gel adsorbent (c)[48]
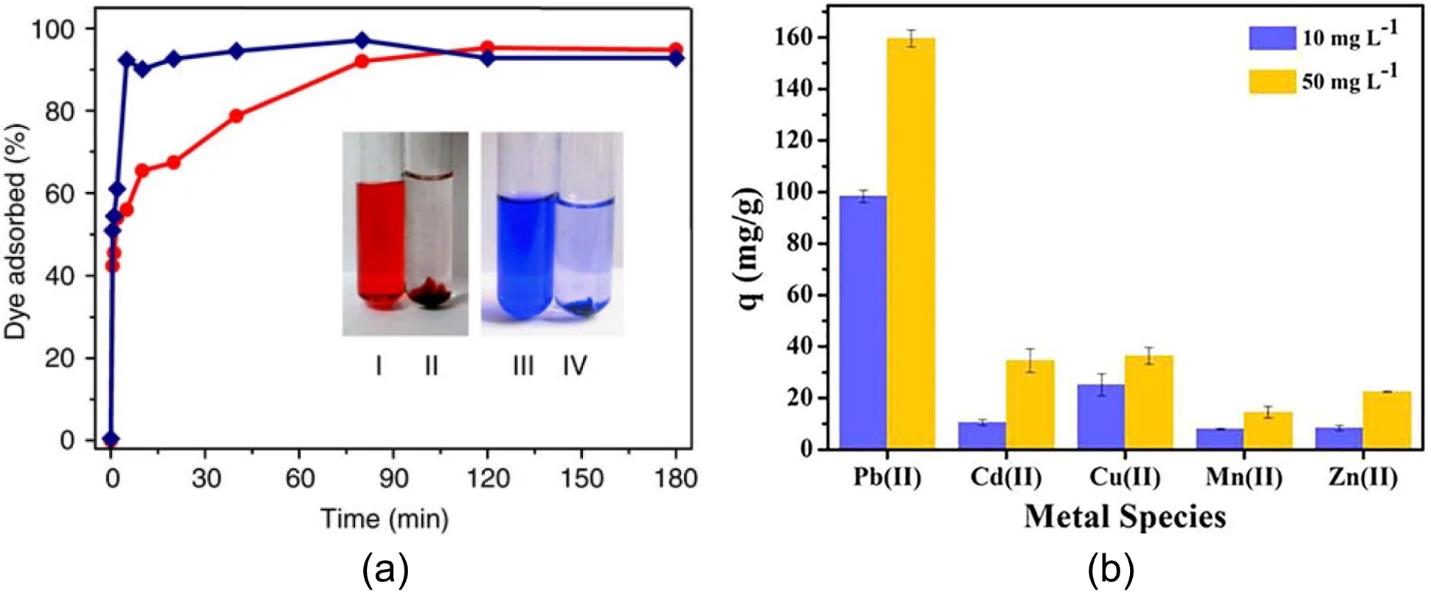
图5 AlBDC-3:2-0.15凝胶吸附剂(7.5 mg)在50 mL、浓度为100 mg·L-1的染料溶液中对刚果红和亮蓝R-250(分别用圆圈和菱形表示)的动力学吸附曲线(a)[33];Zr-MOG-12在含有多种竞争金属离子的溶液中对Pb2+的选择性吸附(b)[52]
Fig.5 The kinetic curves of AlBDC-3:2-0.15 gel adsorbent (7.5 mg) in 50 ml of dyes solution (100 mg L-1) for congo red (circle) and brilliant blue R-250 (diamond) (a)[33]; selective adsorption of Zr-MOG-12 toward Pb2+ in solutions containing various competing metal ions (b)[52]
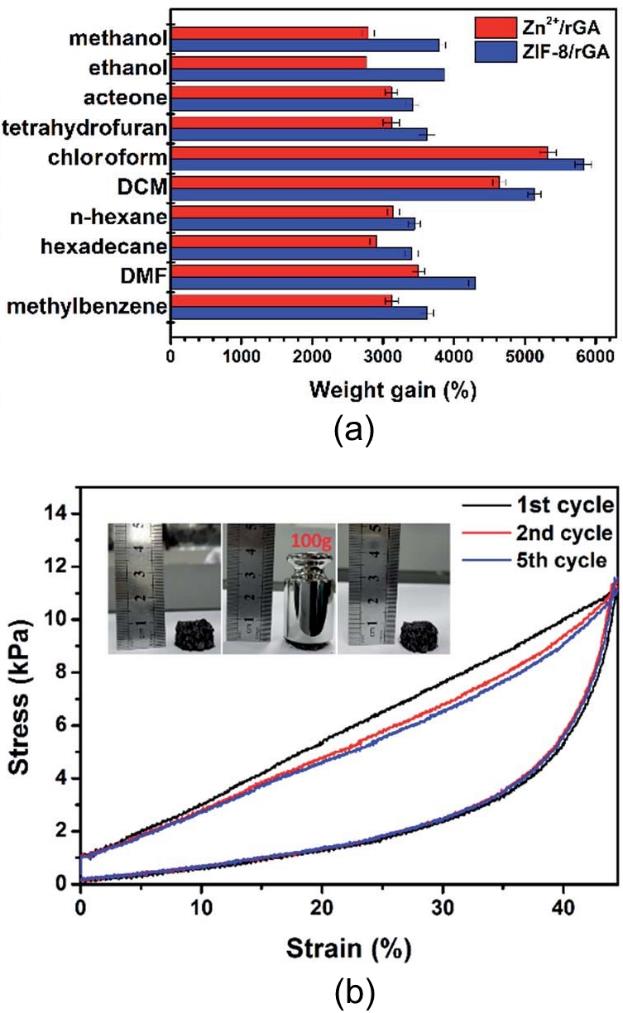
图6 ZIF-8/rGO气凝胶对多种有机液体的吸附容量(a);ZIF-8/rGO气凝胶的循环压缩应力–应变曲线,插图为其在承载100 g砝码前后的形貌(b)[56]
Fig.6 Absorption capacities of ZIF-8/rGO aerogel for various organic liquids (a); cyclic compressive stress–strain curves of ZIF-8/rGO, with the inset showing its appearance before and after supporting a 100 g load (b)[56]
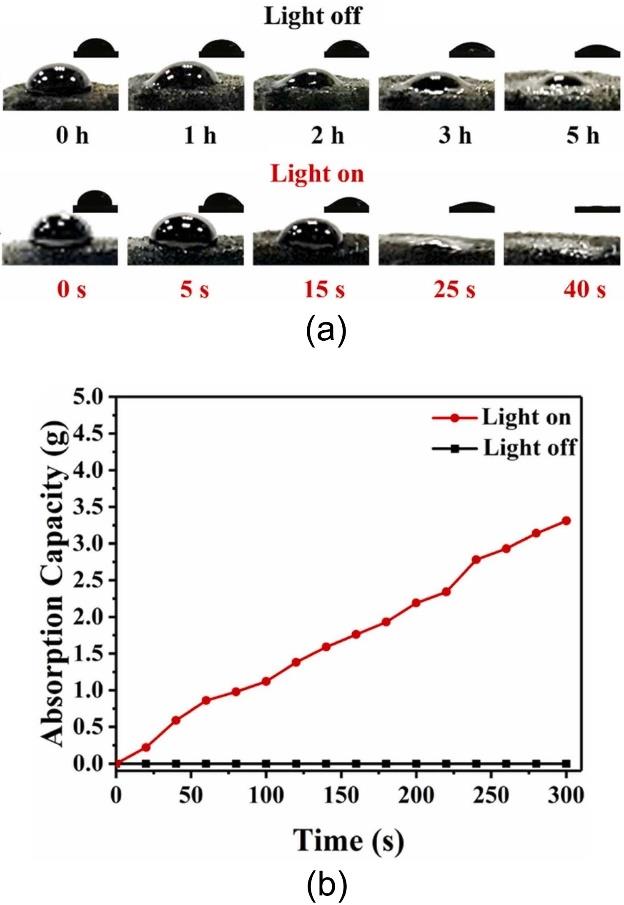
图7 在有无模拟阳光照射条件下,原油在HKUST-1/rGO气凝胶表面的渗透行为(a),以及HKUST-1/rGO气凝胶对原油的动态吸附曲线(b)[58]
Fig.7 Permeation behavior of crude oil on the surface of the HKUST-1/rGO aerogel with and without simulated sunlight (a); kinetic curves of crude-oil adsorption by the HKUST-1/rGO aerogel under simulated sunlight and in darkness (b)[58]
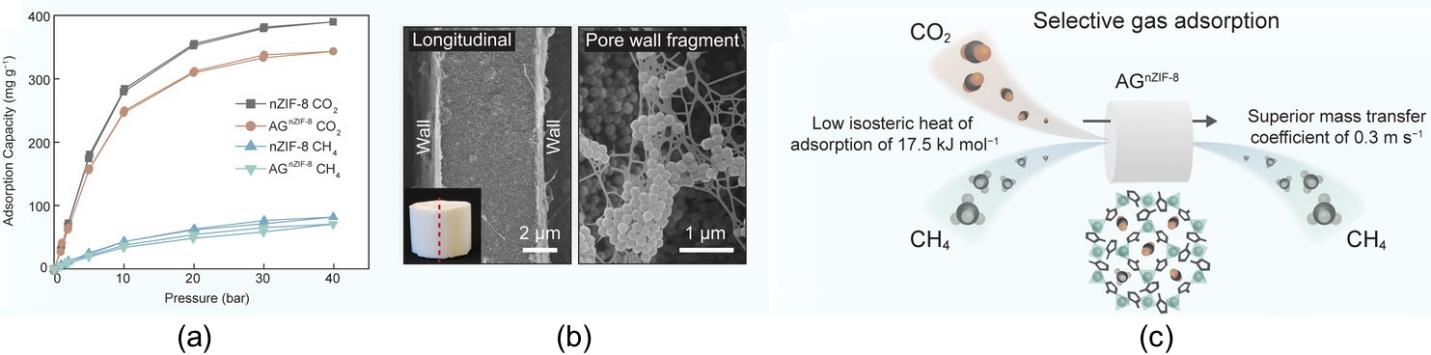
图8 AGnZIF-8和nZIF-8在293 K下对CO2和CH4的吸附等温线(a);圆柱状的AGnZIF-8吸附剂沿轴向方向的形貌SEM图(b);通过调控AGnZIF-8气凝胶堆积取向以实现快速、高选择性CO2吸附的示意图(c)[62]
Fig.8 Adsorption isotherms of AGnZIF-8 and nZIF-8 for CO2 and CH4 at 293 K (a); SEM images of the cylindrical AGnZIF-8 showing the longitudinal morphology and a porous section of the pore wall (b); schematic illustration of the rapid and selective CO2 adsorption process in AGnZIF-8 achieved by adjusting the packing orientation of the adsorbent (c)[62]
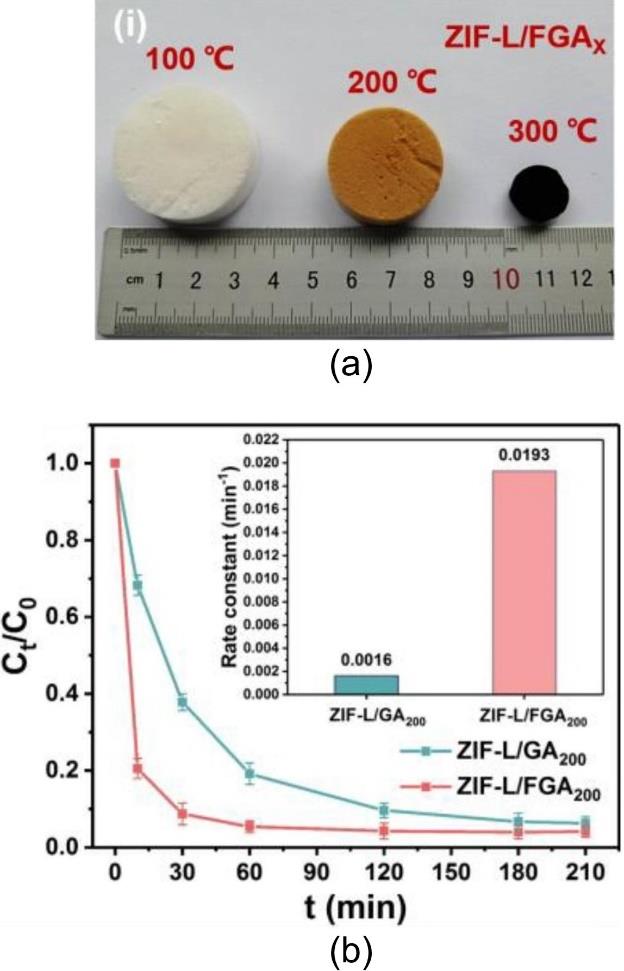
图9 经过不同温度碳化处理得到的ZIF-L/FGAx气凝胶(a);加入发泡剂制备得到的ZIF-L/FGA200气凝胶与未加发泡剂的ZIF-L/GA200气凝胶在298 K、pH = 4.4条件下对四环素(300 mg L-1)的动力学吸附曲线(b)[66]
Fig.9 ZIF-L/FGAx aerogels obtained after carbonization at different temperatures (a); kinetic adsorption curves for tetracycline (300 mg·L-1) on the foaming-agent-assisted ZIF-L/FGA200 aerogel and the non-foamed ZIF-L/GA200 aerogel at 298 K and pH of 4.4 (b)[66]
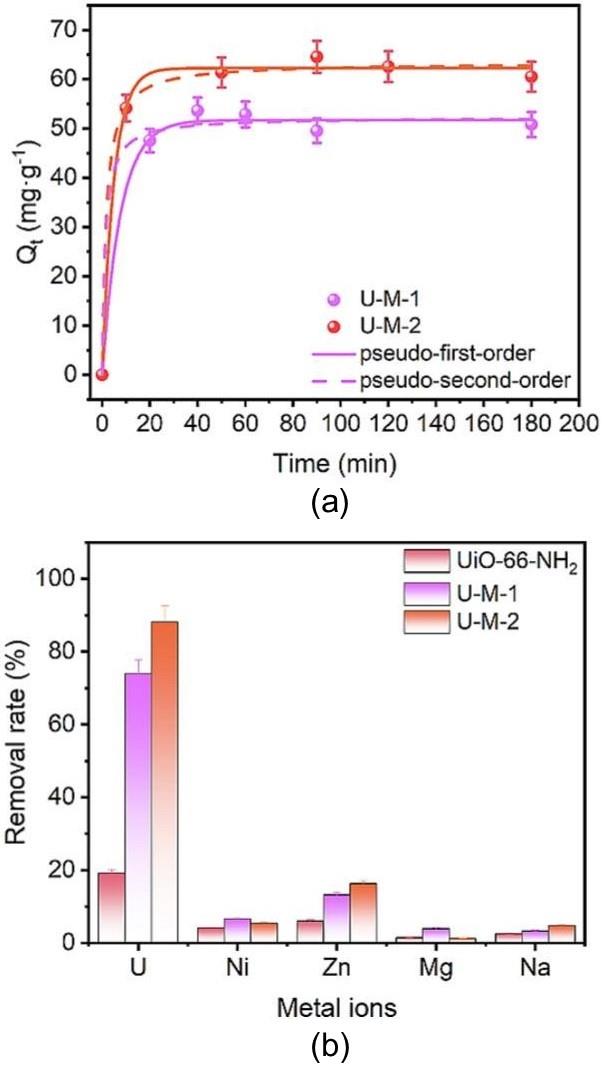
图10 在298 K、U(VI)初始浓度为100 mg·L-1、pH = 6.0条件下,U-M-x的动力学吸附曲线(a);在含有竞争离子的溶液中,U-M-x对U(VI)的去除效率,并以MOF材料UiO-66-NH2作为对比(b)[68]
Fig.10 The kinetic adsorption curves of U-M-x at 298 K with an initial U(VI) concentration of 100 mg L-1 and pH of 6.0 (a); U(VI) removal rates of U-M-x in solutions containing competing ions, with pristine UiO-66-NH2 as a comparison (b)[68]
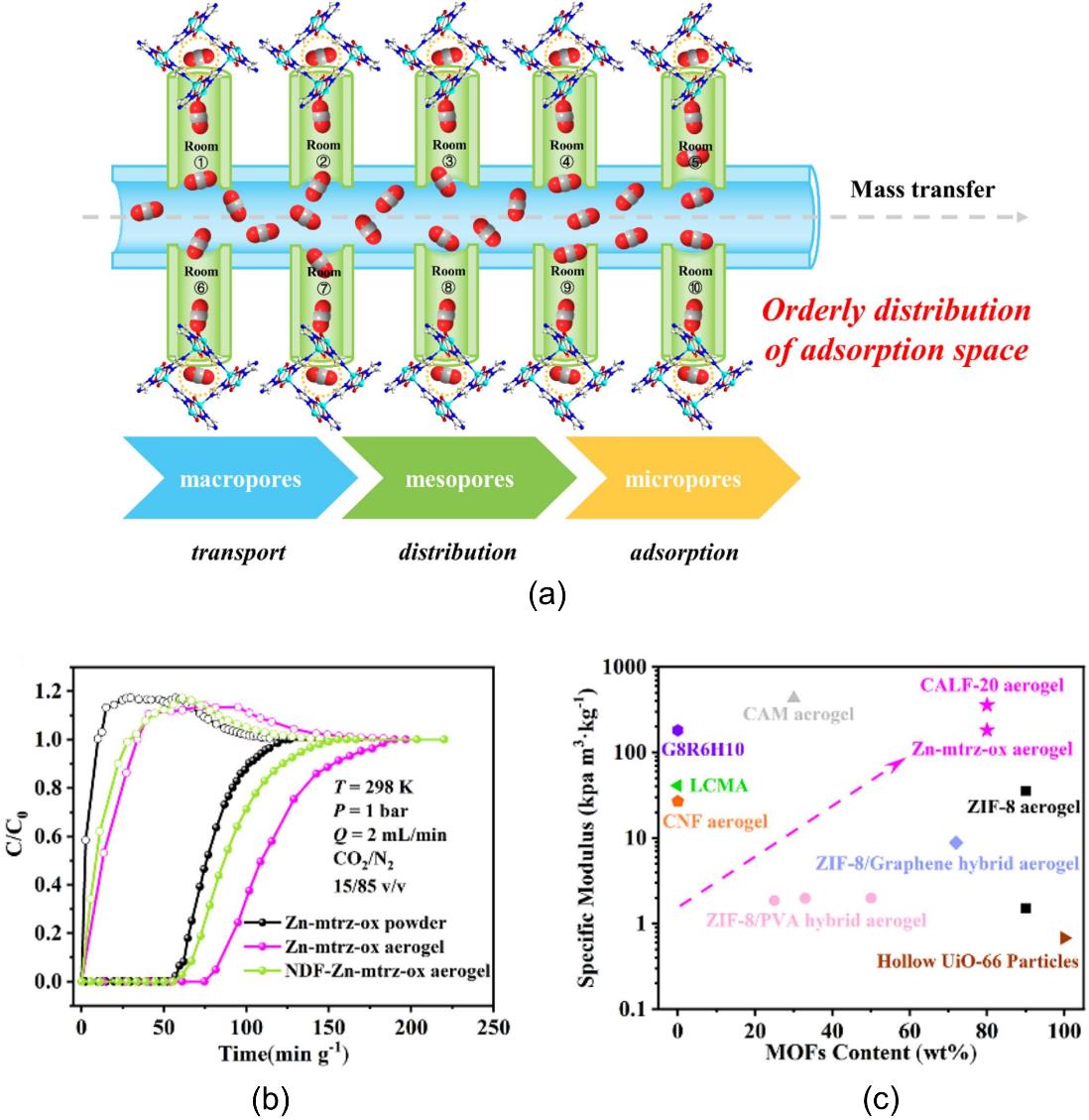
图11 复合气凝胶中大孔、介孔和微孔的功能及其在CO2传质过程中的协同机制(a);Zn-mtrz-ox复合气凝胶及晶体粉末对CO2/N2混合气的固定床穿透曲线(b);不同MOF复合凝胶吸附剂的比压缩模量及MOF含量对比(c)[69]
Fig.11 The respective roles of macropores, mesopores and micropores in aerogel and their synergistic mechanism during CO2 mass transfer (a); dynamic breakthrough curves in columns packed with Zn-mtrz-ox powder and Zn-mtrz-ox aerogel, respectively (b); comparison of the specific compression modulus and MOF content of different MOF composite gel adsorbents (c)[69]
| [1] | Wang T, Lin E, Peng Y L, et al. Rational design and synthesis of ultramicroporous metal-organic frameworks for gas separation[J]. Coordination Chemistry Reviews, 2020, 423: 213485. |
| [2] | Li L B, Lin R B, Krishna R, et al. Ethane/ethylene separation in a metal-organic framework with iron-peroxo sites[J]. Science, 2018, 362(6413): 443-446. |
| [3] | Chen Z J, Kirlikovali K O, Li P, et al. Reticular chemistry for highly porous metal–organic frameworks: the chemistry and applications[J]. Accounts of Chemical Research, 2022, 55(4): 579-591. |
| [4] | Xie X J, Zhou M Y, Zeng H, et al. Pore engineering in metal–organic frameworks for enhanced hydrocarbon adsorption and separation[J]. Accounts of Materials Research, 2025, 6(2): 195-209. |
| [5] | Pang J D, Jiang W T, Zhang X W, et al. Recent progress in metal-organic frameworks (Part II: material application)[J]. Science China Chemistry, 2025, 68(5): 1642-1702 |
| [6] | Hu P, Hu J L, Zhu M, et al. Induced-fit-identification in a rigid metal-organic framework for ppm-level CO2 removal and ultra-pure CO enrichment[J]. Angewandte Chemie (International Ed. in English), 2023, 62(40): e202305944. |
| [7] | Ye Z M, Xie Y, Kirlikovali K O, et al. Architecting metal–organic frameworks at molecular level toward direct air capture[J]. Journal of the American Chemical Society, 2025, 147(7): 5495-5514. |
| [8] | Rohde R C, Carsch K M, Dods M N, et al. High-temperature carbon dioxide capture in a porous material with terminal zinc hydride sites[J]. Science, 2024, 386(6723): 814-819. |
| [9] | Mukherjee S, Sikdar N, O'Nolan D, et al. Trace CO2 capture by an ultramicroporous physisorbent with low water affinity[J]. Science Advances, 2019, 5(11): eaax9171. |
| [10] | Cui J Y, Zhang Z Q, Yang L F, et al. A molecular sieve with ultrafast adsorption kinetics for propylene separation[J]. Science, 2024, 383(6679): 179-183. |
| [11] | Yang R, Wang Y, Cao J W, et al. Hydrogen bond unlocking-driven pore structure control for shifting multi-component gas separation function[J]. Nature Communications, 2024, 15: 804. |
| [12] | Zhang Y B, Han Y, Luan B Q, et al. Metal-organic framework with space-partition pores by fluorinated anions for benchmark C2H2/CO2 separation[J]. Journal of the American Chemical Society, 2024, 146(25): 17220-17229. |
| [13] | Yu L, Ullah S, Zhou K, et al. A microporous metal–organic framework incorporating both primary and secondary building units for splitting alkane isomers[J]. Journal of the American Chemical Society, 2022, 144(9): 3766-3770. |
| [14] | Zhang X, Chen Q C, Bai X F, et al. Achieving record C2H2 packing density for highly efficient C2H2/C2H4 separation with a metal-organic framework prepared by a scalable synthesis in water[J]. Angewandte Chemie (International Ed. in English), 2024, 63(45): e202411744. |
| [15] | Shang S Q, Zhou Z W, Wang H, et al. A rigid, stable, and scalable aliphatic MOF adsorbent for efficient C2H2/CO2 separation with record acetylene packing density[J]. Angewandte Chemie (International Ed. in English), 2025, 64(23): e202503317. |
| [16] | Shi Y S, Xie Y, Cui H, et al. Highly selective adsorption of carbon dioxide over acetylene in an ultramicroporous metal-organic framework[J]. Advanced Materials, 2021, 33(45): 2105880. |
| [17] | Xie W P, Fu Q J, Yang L Z, et al. Methane storage and purification of natural gas in metal-organic frameworks[J]. ChemSusChem, 2025, 18(3): e202401382. |
| [18] | Dou Y B, Grande C, Kaiser A, et al. Highly structured metal-organic framework nanofibers for methane storage[J]. Science China Materials, 2021, 64(7): 1742-1750. |
| [19] | Ahmadijokani F, Ghaffarkhah A, Molavi H, et al. COF and MOF hybrids: advanced materials for wastewater treatment[J]. Advanced Functional Materials, 2024, 34(43): 2305527. |
| [20] | Rego R M, Kuriya G, Kurkuri M D, et al. MOF based engineered materials in water remediation: Recent trends[J]. Journal of Hazardous Materials, 2021, 403: 123605. |
| [21] | Bueken B, Van Velthoven N, Willhammar T, et al. Gel-based morphological design of zirconium metal–organic frameworks[J]. Chemical Science, 2017, 8(5): 3939-3948. |
| [22] | Ferreira A F P, Santos J C, Plaza M G, et al. Suitability of Cu-BTC extrudates for propane–propylene separation by adsorption processes[J]. Chemical Engineering Journal, 2011, 167(1): 1-12. |
| [23] | Zheng J Y, Cui X L, Yang Q W, et al. Shaping of ultrahigh-loading MOF pellet with a strongly anti-tearing binder for gas separation and storage[J]. Chemical Engineering Journal, 2018, 354: 1075-1082. |
| [24] | Tan C, Lee M C, Arshadi M, et al. A spiderweb-like metal-organic framework multifunctional foam[J]. Angewandte Chemie (International Ed. in English), 2020, 59(24): 9506-9513. |
| [25] | Ding Q, Zhang Z Q, Zhang P X, et al. Control of intracrystalline diffusion in a bilayered metal-organic framework for efficient kinetic separation of propylene from propane[J]. Chemical Engineering Journal, 2022, 434: 134784. |
| [26] | Zhuang Z Y, Mai Z H, Wang T Y, et al. Strategies for conversion between metal–organic frameworks and gels[J]. Coordination Chemistry Reviews, 2020, 421: 213461. |
| [27] | Tian T, Zeng Z X, Vulpe D, et al. A sol–gel monolithic metal–organic framework with enhanced methane uptake[J]. Nature Materials, 2018, 17(2): 174-179. |
| [28] | Ding Q, Liu Y L, Liu J, et al. Ultrafast synthesis and binder-free fabrication of a monolithic metal–organic framework for efficient carbon capture[J]. AIChE Journal, 2025, 71(3): e18673. |
| [29] | Connolly B M, Aragones-Anglada M, Gandara-Loe J, et al. Tuning porosity in macroscopic monolithic metal-organic frameworks for exceptional natural gas storage[J]. Nature Communications, 2019, 10: 2345. |
| [30] | Rosado A, Borrás A, Fraile J, et al. HKUST-1 metal–organic framework nanoparticle/graphene oxide nanocomposite aerogels for CO2 and CH4 adsorption and separation[J]. ACS Applied Nano Materials, 2021, 4(11): 12712-12725. |
| [31] | Guo T Y, Mashhadimoslem H, Choopani L, et al. Recent progress in MOF-aerogel fabrication and applications[J]. Small, 2024, 20(43): 2402942. |
| [32] | Albacete P, Asgari M, Yang Y, et al. Self-shaping monolithic reticular materials: ingredients for success[J]. Advanced Functional Materials, 2024, 34(43): 2305979. |
| [33] | Li L, Xiang S L, Cao S Q, et al. A synthetic route to ultralight hierarchically micro/mesoporous Al(III)-carboxylate metal-organic aerogels[J]. Nature Communications, 2013, 4: 1774. |
| [34] | Liu L P, Zhang J Y, Fang H B, et al. Metal–organic gel material based on UiO-66-NH2 nanoparticles for improved adsorption and conversion of carbon dioxide[J]. Chemistry – An Asian Journal, 2016, 11(16): 2278-2283. |
| [35] | Zhao T, Nie S Q, Luo M L, et al. Research progress in structural regulation and applications of HKUST-1 and HKUST-1 based materials[J]. Journal of Alloys and Compounds, 2024, 974: 172897. |
| [36] | Madden D G, O'Nolan D, Rampal N, et al. Densified HKUST-1 monoliths as a route to high volumetric and gravimetric hydrogen storage capacity[J]. Journal of the American Chemical Society, 2022, 144(30): 13729-13739. |
| [37] | Çamur C, Babu R, Suárez del Pino J A, et al. Monolithic zirconium-based metal–organic frameworks for energy-efficient water adsorption applications[J]. Advanced Materials, 2023, 35(23): 2209104. |
| [38] | He Y, Fu T, Wang L J, et al. Self-assembly of MOF-801 into robust hierarchically porous monoliths for scale-up atmospheric water harvesting[J]. Chemical Engineering Journal, 2023, 472: 144786. |
| [39] | Rehman S, Zheng X M, Zhang P Y. Green synthesis of a hydrophobic metal-organic gel for the capture of trace odorous hexanal from humid air[J]. Journal of Hazardous Materials, 2023, 441: 129852. |
| [40] | Zheng X M, Rehman S, Zhang P Y. Room temperature synthesis of monolithic MIL-100(Fe) in aqueous solution for energy-efficient removal and recovery of aromatic volatile organic compounds[J]. Journal of Hazardous Materials, 2023, 442: 129998. |
| [41] | Madden D G, Babu R, Çamur C, et al. Monolithic metal–organic frameworks for carbon dioxide separation[J]. Faraday Discussions, 2021, 231: 51-65. |
| [42] | Fan S T, Chen Z H, Yang Z, et al. Facile preparation of humidity stable green γ-cyclodextrin metal–organic framework monolith for CO2 capture[J]. AIChE Journal, 2022, 68(12): e17872. |
| [43] | Yang L Z, Yan L T, Wang Y, et al. Adsorption site selective occupation strategy within a metal-organic framework for highly efficient sieving acetylene from carbon dioxide[J]. Angewandte Chemie (International Ed. in English), 2021, 60(9): 4570-4574. |
| [44] | Zhang L, Jiang K, Yang L F, et al. Benchmark C2H2/CO2 separation in an ultra-microporous metal–organic framework via copper(I)-alkynyl chemistry[J]. Angewandte Chemie (International Ed. in English), 2021, 60(29): 15995-16002. |
| [45] | Ye Y X, Xian S K, Cui H, et al. Metal–organic framework based hydrogen-bonding nanotrap for efficient acetylene storage and separation[J]. Journal of the American Chemical Society, 2022, 144(4): 1681-1689. |
| [46] | Zheng F, Chen R D, Ding Z X, et al. Interlayer symmetry control in flexible-robust layered metal-organic frameworks for highly efficient C2H2/CO2 separation[J]. Journal of the American Chemical Society, 2023, 145(36): 19903-19911. |
| [47] | Zhang Z Q, Peh S B, Krishna R, et al. Optimal pore chemistry in an ultramicroporous metal–organic framework for benchmark inverse CO2/C2H2 separation[J]. Angewandte Chemie (International Ed. in English), 2021, 60(31): 17198-17204. |
| [48] | Mohamed M H, Elzeny I, Samuel J, et al. Turning normal to abnormal: reversing CO2/C2-hydrocarbon selectivity in HKUST-1[J]. Advanced Functional Materials, 2024, 34(19): 2312280. |
| [49] | Şevik M, Sezdi S M, Kavak E, et al. Stable two-fold interpenetrated Cd(II)-MOF for selective dye adsorption and luminescence detection of hydroxyl-substituted nitroaromatic compounds[J]. Crystal Growth & Design, 2023, 23(7): 5163-5172. |
| [50] | El-Sewify I M, Radwan A, Shahat A, et al. Superior adsorption and removal of aquaculture and bio-staining dye from industrial wastewater using microporous nanocubic Zn-MOFs[J]. Microporous and Mesoporous Materials, 2022, 329: 111506. |
| [51] | Lu H, Yang Q, Huang B W, et al. Removal performance and adsorption kinetics of dyes by a Co-based metal organic framework[J]. Microporous and Mesoporous Materials, 2023, 360: 112665. |
| [52] | Zhao F, Yang W X, Han Y, et al. A straightforward strategy to synthesize supramolecular amorphous zirconium metal-organic gel for efficient Pb(II) removal[J]. Chemical Engineering Journal, 2021, 407: 126744. |
| [53] | Di Palma G, Banerjee P, Enemark-Rasmussen K, et al. Highly efficient removal of perfluorooctanoic acid from water using zirconium terephthalate (UiO-66) gel[J]. Advanced Materials Interfaces, 2025, 12(17): 2500166. |
| [54] | Li C X, Yang J, Pachfule P, et al. Ultralight covalent organic framework/graphene aerogels with hierarchical porosity[J]. Nature Communications, 2020, 11: 4712. |
| [55] | Li Z, Liu C, Frick J J, et al. Synthesis and characterization of UiO-66-NH2 incorporated graphene aerogel composites and their utilization for absorption of organic liquids[J]. Carbon, 2023, 201: 561-567. |
| [56] | Mao J J, Ge M Z, Huang J Y, et al. Constructing multifunctional MOF@rGO hydro-/ aerogels by the self-assembly process for customized water remediation[J]. Journal of Materials Chemistry A, 2017, 5(23): 11873-11881. |
| [57] | Sun T C, Hao S E, Fan R Q, et al. Hydrophobicity-adjustable MOF constructs superhydrophobic MOF-rGO aerogel for efficient oil–water separation[J]. ACS Applied Materials & Interfaces, 2020, 12(50): 56435-56444. |
| [58] | Hu Y W, Jiang Y J, Ni L Y, et al. An elastic MOF/graphene aerogel with high photothermal efficiency for rapid removal of crude oil[J]. Journal of Hazardous Materials, 2023, 443: 130339. |
| [59] | Zhu H, Yang X, Cranston E D, et al. Flexible and porous nanocellulose aerogels with high loadings of metal–organic-framework particles for separations applications[J]. Advanced Materials, 2016, 28(35): 7652-7657. |
| [60] | Nasser Abdelhamid H, Sultan S, Mathew A P. Binder-free Three-dimensional (3D) printing of Cellulose-ZIF8 (CelloZIF-8) for water treatment and carbon dioxide (CO2) adsorption[J]. Chemical Engineering Journal, 2023, 468: 143567. |
| [61] | Li C H, Wang F L, Xu X, et al. A high-capacity malleable cellulose aerogel with layered double hydroxide decorating ZIF-8 for efficient adsorption of ciprofloxacin[J]. Chemical Engineering Journal, 2023, 455: 140841. |
| [62] | Rostami J, Benselfelt T, Maddalena L, et al. Shaping 90 wt% NanoMOFs into robust multifunctional aerogels using tailored bio-based nanofibrils[J]. Advanced Materials, 2022, 34(38): 2204800. |
| [63] | Hammi N, Bonneau M, El Kadib A, et al. Enhanced gas adsorption in HKUST-1@Chitosan aerogels, cryogels, and xerogels: an evaluation study[J]. ACS Applied Materials & Interfaces, 2023, 15(46): 53395-53404. |
| [64] | Gong D, Zhu W L, Wu M Z, et al. A chitosan/MOF hybrid monolith with improved stability and enhanced adsorption performances via a pre-frozen crosslinking route[J]. Environmental Research, 2025, 271: 121095. |
| [65] | Wang C H, Kim J, Tang J, et al. Large-scale synthesis of MOF-derived superporous carbon aerogels with extraordinary adsorption capacity for organic solvents[J]. Angewandte Chemie (International Ed. in English), 2020, 59(5): 2066-2070. |
| [66] | Peng H H, Xiong W P, Yang Z H, et al. Facile fabrication of three-dimensional hierarchical porous ZIF-L/gelatin aerogel: Highly efficient adsorbent with excellent recyclability towards antibiotics[J]. Chemical Engineering Journal, 2021, 426: 130798. |
| [67] | Baimenov A, Daulbayev C, Poulopoulos S G, et al. MXene filled hydrogel and aerogel composites[J]. Materials Today, 2024, 78: 75-91. |
| [68] | Li W T, Kang W, Ye C G, et al. Zirconium-based MOF/MXene aerogel composite for highly stable and selective capture of uranium from aqueous solution[J]. Applied Surface Science, 2025, 702: 163323. |
| [69] | Che Y H, Wang C Q, Cai Y L, et al. Hierarchically structured MOF aerogels with tandem pores for high-performance CO2 capture and separation[J]. Chemical Engineering Journal, 2025, 515: 163538. |
| [70] | Zhao G D, Zhao H J, Shi L, et al. A highly efficient adsorbent constructed by the in situ assembly of Zeolitic imidazole framework-67 on 3D aramid nanofiber aerogel scaffold[J]. Separation and Purification Technology, 2021, 274: 119054. |
| [1] | 臧子晴, 李修真, 谈莹莹, 刘晓庆. 分凝器对两级分离自复叠制冷循环特性影响研究[J]. 化工学报, 2025, 76(S1): 17-25. |
| [2] | 裴星亮, 叶翠平, 裴赢丽, 李文英. 碱改性MIL-53(Cr)选择性吸附分离二甲苯异构体[J]. 化工学报, 2025, 76(S1): 258-267. |
| [3] | 李银龙, 刘国强, 晏刚. 分馏与闪蒸分离耦合自复叠制冷循环性能分析[J]. 化工学报, 2025, 76(S1): 26-35. |
| [4] | 吴梓航, 徐震原, 游锦方, 潘权稳, 王如竹. 基于吸附式储冷技术的深井钻探设备冷却系统[J]. 化工学报, 2025, 76(S1): 309-317. |
| [5] | 黄国瑞, 赵耀, 谢明熹, 陈尔健, 代彦军. 一种新型数据中心余热回收系统实验与分析[J]. 化工学报, 2025, 76(S1): 409-417. |
| [6] | 张建民, 何美贵, 贾万鑫, 赵静, 金万勤. 聚氧化乙烯/冠醚共混膜及其二氧化碳分离性能[J]. 化工学报, 2025, 76(9): 4862-4871. |
| [7] | 赵维, 邢文乐, 韩朝旭, 袁兴中, 蒋龙波. g-C3N4基非金属异质结光催化降解水中有机污染物的研究进展[J]. 化工学报, 2025, 76(9): 4752-4769. |
| [8] | 郭旭, 贾继宁, 姚克俭. 基于优化CNN-BiLSTM神经网络的间歇精馏过程建模[J]. 化工学报, 2025, 76(9): 4613-4629. |
| [9] | 王杰, 林渠成, 张先明. 基于分解算法的混合气体多级膜分离系统全局优化[J]. 化工学报, 2025, 76(9): 4670-4682. |
| [10] | 李文龙, 常程, 吴小林, 姬忠礼. 油水聚结过滤材料中的液体分布特性及过程压降演化研究[J]. 化工学报, 2025, 76(9): 4850-4861. |
| [11] | 田宇红, 杜壮壮, 徐慧芳, 祝自强, 王宇聪. ZIF-8基多孔液体制备及其SO2吸附性能[J]. 化工学报, 2025, 76(8): 4284-4296. |
| [12] | 陈治宏, 吴佳伟, 楼小玲, 贠军贤. 化学品生物制造过程机器学习的研究进展[J]. 化工学报, 2025, 76(8): 3789-3804. |
| [13] | 张荟钦, 赵泓竣, 付正军, 庄力, 董凯, 贾添智, 曹雪丽, 孙世鹏. 纳滤膜在离子型稀土浸出液提浓中的应用研究[J]. 化工学报, 2025, 76(8): 4095-4107. |
| [14] | 史松伟, 赵诚, 刘帅, 应雨轩, 严密. 富铁飞灰耦合Fe-Zn/Al2O3脱除沼气H2S研究[J]. 化工学报, 2025, 76(8): 4239-4247. |
| [15] | 郭铮铮, 赵一丹, 王辅强, 裴璐, 靳彦岭, 任芳, 任鹏刚. 异质结构MoS2/RGO/NiFe2O4复合材料的构筑及电磁波吸收性能研究[J]. 化工学报, 2025, 76(7): 3719-3732. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号