化工学报 ›› 2022, Vol. 73 ›› Issue (1): 322-331.DOI: 10.11949/0438-1157.20211441
黄子轩1( ),陈欢1(
),陈欢1( ),李海1,2(
),李海1,2( ),王明龙1,陈光进1,刘蓓1
),王明龙1,陈光进1,刘蓓1
收稿日期:2021-10-09
修回日期:2021-11-27
出版日期:2022-01-05
发布日期:2022-01-18
通讯作者:
李海
作者简介:黄子轩(1996—),男,博士研究生,基金资助:
Zixuan HUANG1( ),Huan CHEN1(
),Huan CHEN1( ),Hai LI1,2(
),Hai LI1,2( ),Minglong WANG1,Guangjin CHEN1,Bei LIU1
),Minglong WANG1,Guangjin CHEN1,Bei LIU1
Received:2021-10-09
Revised:2021-11-27
Online:2022-01-05
Published:2022-01-18
Contact:
Hai LI
摘要:
ZIF-8/2-甲基咪唑-乙二醇-水浆液(ZIF-8浆液)可以高效低耗能地分离CO2。为了进一步评估ZIF-8浆液在填料塔中分离CO2的塔效率及能耗,使用Peng-Robinson(PR)状态方程,求出CO2和ZIF-8浆液的二元交互作用参数(kCO2),将二元交互作用参数和Aspen Plus软件进行关联,对CO2/N2多级吸收分离进行过程模拟。计算结果表明在中试填料塔中,ZIF-8浆液仅需要5块理论塔板即可将CO2浓度由20%(mol)降低至2%(mol)以下,填料塔的塔板效率为25%。对中试分离CO2/N2进行能耗计算,结果表明当解吸条件为解吸温度333 K,解吸压力0.8 MPa和空气吹扫流量200 L/h时,CO2捕集等效功最低可至0.474 GJ/t CO2。在同样条件下使用ZIF-8浆液和MEA(30%(mass))水溶液进行碳捕集时,CO2捕集等效功分别为0.507 GJ/t CO2和0.957 GJ/t CO2,ZIF-8浆液的CO2捕集等效功仅为MEA水溶液的53%。
中图分类号:
黄子轩, 陈欢, 李海, 王明龙, 陈光进, 刘蓓. ZIF-8浆液中试分离CO2/N2过程模拟及能耗分析[J]. 化工学报, 2022, 73(1): 322-331.
Zixuan HUANG, Huan CHEN, Hai LI, Minglong WANG, Guangjin CHEN, Bei LIU. Process simulation and energy consumption analysis of CO2/N2 pilot-scale separation using ZIF-8 slurry[J]. CIESC Journal, 2022, 73(1): 322-331.
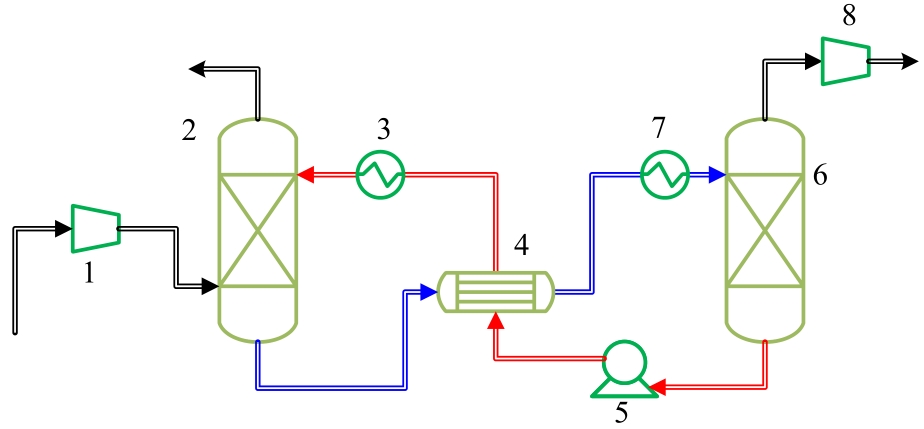
图3 ZIF-8浆液脱碳系统能耗评价流程图1—compressor; 2—absorption tower; 3—lean liquid cooler; 4—heat exchanger; 5—metering pump; 6—desorption tower; 7—rich liquid heater; 8—vacuum pump; black—gas flow; blue—rich liquid flow; red—lean liquid flow
Fig.3 The flow chart for CO2 capture equivalent work evaluation of decarbonization system
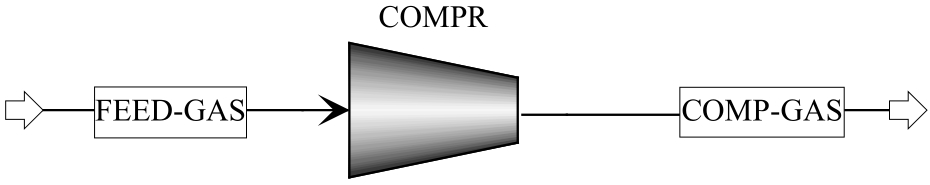
图4 Wcompr计算模板: Aspen Plus软件中的气体压缩机模块(COMPR);进入压缩机模块的原料气流(FEED-GAS);压缩后的混合气流(COMPR-GAS)
Fig.4 Computation module of Wcompr: gas compression module in the Aspen Plus (COMPR); gas stream before compression (FEED-GAS); gas stream after compression (COMP-GAS)

图5 Wmet计算模板: Aspen Plus软件中的计量泵模块(MET-PUMP);解吸塔底出来的贫液(SLU-IN);贫液增压至吸收塔操作压力(SLU-OUT)
Fig.5 Computation module of Wmet: metering pump module in the Aspen Plus (MET-PUMP); lean slurry under desorption pressure (SLU-IN); lean slurry under sorption pressure (SLU-OUT)
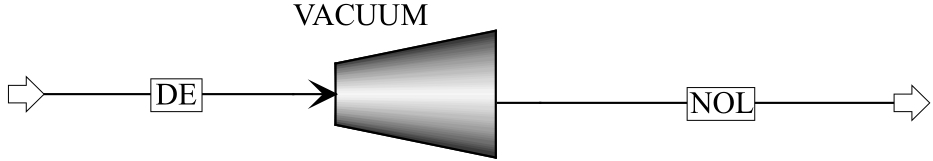
图6 Wvac计算模板: Aspen Plus软件中的真空泵模块(VACUUM);常压下气体流体(DE);解吸压力下的气体流体(NOL)
Fig.6 Computation module of Wvac: vacuum pump module in the Aspen Plus (VACUUM); gas stream under normal pressure (DE); gas stream under desorption pressure (NOL)

图7 Qheat计算模板: Aspen Plus软件中的热交换器模块(HEATX);AspenPlus软件中的加热器模块(RICH-H);从解吸塔塔底流入热交换器的贫液(LEAN-IN);从热交换器流向吸收塔塔顶的贫液(LEAN-OUT);从吸收塔塔底流向热交换器的富液(RICH-IN);从热交换器流向加热器模块的富液(RICH-OUT);从加热器流向解吸塔塔顶的富液(RICH)
Fig.7 Computation module of Qheat: heat exchanger module in the Aspen Plus (HEATX); lean slurry from desorption tower to heat exchanger (LEAN-IN); lean slurry from heat exchanger to sorption tower (LEAN-OUT); rich slurry from the sorption tower to heat exchanger (RICH-IN); rich slurry from heat exchanger to heater (RICH-OUT); rich slurry from heater to desorption tower (RICH)

图8 CO2在新鲜ZIF-8浆液(a)和从中试解吸塔底(解吸温度、解吸压力和空气吹扫流速分别设定为333.15 K、0.08 MPa和200 L/h)获得的ZIF-8浆液(b)中的溶解度曲线(303.15 K);N2在新鲜ZIF-8浆液中的溶解度曲线(303.15 K)(c)[33]
Fig.8 Sorption isotherms of CO2 at 303.15 K in fresh ZIF-8 slurry (a), the ZIF-8 slurry obtained from the desorption packed tower(desorption condition: the desorption temperature, pressure, and air-purge flow rate were fixed at 333.15 K, 0.08 MPa, and 200 L/h)(b); Sorption isotherm of N2 at 303.15 K in fresh ZIF-8 slurry (c)[33]
| ZIF-8浆液 | kCO2=(mT+n)p+MT+N | |||
|---|---|---|---|---|
| m | n | M | N | |
| 新鲜ZIF-8浆液 | -0.01148 | 4.20695 | 0.00029 | -0.54962 |
| 从中试解吸塔底获得的ZIF-8浆液① | -0.01776 | 5.82127 | 0.00156 | -0.88403 |
表1 CO2和ZIF-8浆液的二元交互作用参数kCO2与温度、压力的函数关系
Table 1 The relationship between temperature, pressure, and the binary interaction parameter kCO2 of CO2 and ZIF-8 slurry
| ZIF-8浆液 | kCO2=(mT+n)p+MT+N | |||
|---|---|---|---|---|
| m | n | M | N | |
| 新鲜ZIF-8浆液 | -0.01148 | 4.20695 | 0.00029 | -0.54962 |
| 从中试解吸塔底获得的ZIF-8浆液① | -0.01776 | 5.82127 | 0.00156 | -0.88403 |
| T/K | kCO2 | pE/MPa | xcal | xexp | AADx/% |
|---|---|---|---|---|---|
| 293.15 | -0.4596 | 0.007 | 0.0067 | 0.0078 | 13.40 |
| 293.15 | -0.4503 | 0.018 | 0.0145 | 0.0137 | 5.24 |
| 293.15 | -0.4352 | 0.036 | 0.0223 | 0.0203 | 9.66 |
| 293.15 | -0.4032 | 0.074 | 0.0281 | 0.0265 | 6.15 |
| 303.15 | -0.4575 | 0.007 | 0.0046 | 0.0054 | 13.52 |
| 303.15 | -0.4495 | 0.018 | 0.0104 | 0.0103 | 0.55 |
| 303.15 | -0.4292 | 0.046 | 0.0191 | 0.0179 | 6.82 |
| 303.15 | -0.4045 | 0.08 | 0.0233 | 0.0230 | 1.30 |
| 303.15 | -0.3870 | 0.104 | 0.0237 | 0.0262 | 9.40 |
| 313.15 | -0.4530 | 0.011 | 0.0050 | 0.0050 | 1.15 |
| 313.15 | -0.4444 | 0.025 | 0.0098 | 0.0098 | 0.18 |
| 313.15 | -0.4334 | 0.043 | 0.0143 | 0.0144 | 0.75 |
| 313.15 | -0.4114 | 0.079 | 0.0193 | 0.0187 | 3.01 |
| 313.15 | -0.3906 | 0.113 | 0.0209 | 0.0215 | 3.03 |
| 总AADx | 5.30 |
表2 新鲜ZIF-8浆液吸收CO2气体相平衡实验数据与模拟结果
Table 2 Experimental and simulated phase equilibrium data of CO2 absorption by fresh ZIF-8 slurry
| T/K | kCO2 | pE/MPa | xcal | xexp | AADx/% |
|---|---|---|---|---|---|
| 293.15 | -0.4596 | 0.007 | 0.0067 | 0.0078 | 13.40 |
| 293.15 | -0.4503 | 0.018 | 0.0145 | 0.0137 | 5.24 |
| 293.15 | -0.4352 | 0.036 | 0.0223 | 0.0203 | 9.66 |
| 293.15 | -0.4032 | 0.074 | 0.0281 | 0.0265 | 6.15 |
| 303.15 | -0.4575 | 0.007 | 0.0046 | 0.0054 | 13.52 |
| 303.15 | -0.4495 | 0.018 | 0.0104 | 0.0103 | 0.55 |
| 303.15 | -0.4292 | 0.046 | 0.0191 | 0.0179 | 6.82 |
| 303.15 | -0.4045 | 0.08 | 0.0233 | 0.0230 | 1.30 |
| 303.15 | -0.3870 | 0.104 | 0.0237 | 0.0262 | 9.40 |
| 313.15 | -0.4530 | 0.011 | 0.0050 | 0.0050 | 1.15 |
| 313.15 | -0.4444 | 0.025 | 0.0098 | 0.0098 | 0.18 |
| 313.15 | -0.4334 | 0.043 | 0.0143 | 0.0144 | 0.75 |
| 313.15 | -0.4114 | 0.079 | 0.0193 | 0.0187 | 3.01 |
| 313.15 | -0.3906 | 0.113 | 0.0209 | 0.0215 | 3.03 |
| 总AADx | 5.30 |
| T/K | kCO2 | pE/MPa | xcal | xexp | AADx/% |
|---|---|---|---|---|---|
| 293.15 | -0.4224 | 0.007 | 0.0039 | 0.0044 | 12.75 |
| 293.15 | -0.4113 | 0.025 | 0.0113 | 0.0096 | 17.46 |
| 293.15 | -0.3990 | 0.045 | 0.0166 | 0.0154 | 8.32 |
| 293.15 | -0.3751 | 0.084 | 0.0215 | 0.0207 | 3.65 |
| 293.15 | -0.3615 | 0.106 | 0.0222 | 0.0227 | 2.38 |
| 303.15 | -0.4067 | 0.01 | 0.0032 | 0.0035 | 7.90 |
| 303.15 | -0.3997 | 0.026 | 0.0074 | 0.0072 | 1.95 |
| 303.15 | -0.3932 | 0.041 | 0.0105 | 0.0112 | 5.88 |
| 303.15 | -0.3809 | 0.069 | 0.0147 | 0.0157 | 6.37 |
| 303.15 | -0.3648 | 0.106 | 0.0179 | 0.0190 | 5.77 |
| 313.15 | -0.3911 | 0.017 | 0.0033 | 0.0033 | 1.10 |
| 313.15 | -0.3875 | 0.031 | 0.0057 | 0.0054 | 4.66 |
| 313.15 | -0.3838 | 0.045 | 0.0078 | 0.0076 | 2.91 |
| 313.15 | -0.3797 | 0.061 | 0.0099 | 0.0098 | 1.65 |
| 313.15 | -0.3724 | 0.089 | 0.0131 | 0.0127 | 3.19 |
| 313.15 | -0.3690 | 0.102 | 0.0143 | 0.0141 | 1.56 |
| 总AADx | 5.47 |
表3 从中试解吸塔底获得的ZIF-8浆液吸收CO2气体相平衡实验数据与模拟结果
Table 3 Experimental and simulated phase equilibrium data of CO2 absorption by ZIF-8 slurry obtained from the desorption packed tower
| T/K | kCO2 | pE/MPa | xcal | xexp | AADx/% |
|---|---|---|---|---|---|
| 293.15 | -0.4224 | 0.007 | 0.0039 | 0.0044 | 12.75 |
| 293.15 | -0.4113 | 0.025 | 0.0113 | 0.0096 | 17.46 |
| 293.15 | -0.3990 | 0.045 | 0.0166 | 0.0154 | 8.32 |
| 293.15 | -0.3751 | 0.084 | 0.0215 | 0.0207 | 3.65 |
| 293.15 | -0.3615 | 0.106 | 0.0222 | 0.0227 | 2.38 |
| 303.15 | -0.4067 | 0.01 | 0.0032 | 0.0035 | 7.90 |
| 303.15 | -0.3997 | 0.026 | 0.0074 | 0.0072 | 1.95 |
| 303.15 | -0.3932 | 0.041 | 0.0105 | 0.0112 | 5.88 |
| 303.15 | -0.3809 | 0.069 | 0.0147 | 0.0157 | 6.37 |
| 303.15 | -0.3648 | 0.106 | 0.0179 | 0.0190 | 5.77 |
| 313.15 | -0.3911 | 0.017 | 0.0033 | 0.0033 | 1.10 |
| 313.15 | -0.3875 | 0.031 | 0.0057 | 0.0054 | 4.66 |
| 313.15 | -0.3838 | 0.045 | 0.0078 | 0.0076 | 2.91 |
| 313.15 | -0.3797 | 0.061 | 0.0099 | 0.0098 | 1.65 |
| 313.15 | -0.3724 | 0.089 | 0.0131 | 0.0127 | 3.19 |
| 313.15 | -0.3690 | 0.102 | 0.0143 | 0.0141 | 1.56 |
| 总AADx | 5.47 |

图10 ZIF-8浆液的解吸热:新鲜ZIF-8浆液(a);从中试解吸塔底获得的ZIF-8浆液(解吸温度333.15 K、解吸压力0.08 MPa、空气吹扫流速200 L/h)(b)
Fig.10 Sorption heat of ZIF-8 slurry in fresh ZIF-8 slurry (a) and the ZIF-8 slurry obtained from the desorption packed tower (the desorption temperature, pressure, and air-purge flow rate were fixed at 333.15 K, 0.08 MPa, and 200 L/h) (b)
| 理论塔板级数 | 气相中CO2浓度,y/% | 液相中CO2浓度,x/% | 气相中CO2分压,p/MPa | 平衡级温度,T/K | CO2-浆液二元交互作用参数,kCO2 |
|---|---|---|---|---|---|
| 塔顶(净化气/贫液) | 0 | 0 | 0 | 303.15 | -0.4626 |
| 2 | 0.0001 | 0 | 0 | 303.15 | -0.4626 |
| 3 | 0.0005 | 0 | 0 | 303.15 | -0.4626 |
| 4 | 0.0034 | 0.0002 | 0 | 303.15 | -0.4626 |
| 5 | 0.0235 | 0.0015 | 0.0001 | 303.16 | -0.4625 |
| 6 | 0.1612 | 0.0105 | 0.001 | 303.22 | -0.4619 |
| 7 | 1.078 | 0.0706 | 0.0065 | 303.6 | -0.4578 |
| 8 | 6.2328 | 0.4297 | 0.0374 | 305.9 | -0.4358 |
| 塔底(原料气/富液) | 20 | 1.5859 | 0.12 | 313.3 | -0.3865 |
表4 新鲜ZIF-8浆液多级吸收CO2的过程模拟结果
Table 4 The simulation results of multi-stage CO2 absorption by fresh ZIF-8 slurry
| 理论塔板级数 | 气相中CO2浓度,y/% | 液相中CO2浓度,x/% | 气相中CO2分压,p/MPa | 平衡级温度,T/K | CO2-浆液二元交互作用参数,kCO2 |
|---|---|---|---|---|---|
| 塔顶(净化气/贫液) | 0 | 0 | 0 | 303.15 | -0.4626 |
| 2 | 0.0001 | 0 | 0 | 303.15 | -0.4626 |
| 3 | 0.0005 | 0 | 0 | 303.15 | -0.4626 |
| 4 | 0.0034 | 0.0002 | 0 | 303.15 | -0.4626 |
| 5 | 0.0235 | 0.0015 | 0.0001 | 303.16 | -0.4625 |
| 6 | 0.1612 | 0.0105 | 0.001 | 303.22 | -0.4619 |
| 7 | 1.078 | 0.0706 | 0.0065 | 303.6 | -0.4578 |
| 8 | 6.2328 | 0.4297 | 0.0374 | 305.9 | -0.4358 |
| 塔底(原料气/富液) | 20 | 1.5859 | 0.12 | 313.3 | -0.3865 |
| 理论塔板级数 | 气相中CO2浓度,y/% | 液相中CO2浓度,x/% | 气相中CO2分压,p/MPa | 平衡级温度,T/K | CO2-浆液二元交互作用参数,kCO2 |
|---|---|---|---|---|---|
| 塔顶(净化气/贫液) | 0.0429 | 0 | 0.0003 | 303.15 | -0.411 |
| 2 | 0.1794 | 0.0089 | 0.0011 | 303.2 | -0.4106 |
| 3 | 0.6083 | 0.0369 | 0.0036 | 303.38 | -0.4092 |
| 4 | 1.894 | 0.1223 | 0.0114 | 303.93 | -0.4051 |
| 5 | 5.2582 | 0.3561 | 0.0315 | 305.42 | -0.395 |
| 6 | 11.503 | 0.8351 | 0.069 | 308.49 | -0.3791 |
| 7 | 17.4369 | 1.3552 | 0.1046 | 311.81 | -0.3679 |
| 8 | 19.5741 | 1.5612 | 0.1174 | 313.13 | -0.365 |
| 塔底(原料气/富液) | 20 | 1.5832 | 0.12 | 313.27 | -0.3644 |
表5 中试解吸后的ZIF-8浆液多级吸收CO2的过程模拟结果
Table 5 The simulation results of multi-stage CO2 absorption by ZIF-8 slurry obtained from the desorption packed tower
| 理论塔板级数 | 气相中CO2浓度,y/% | 液相中CO2浓度,x/% | 气相中CO2分压,p/MPa | 平衡级温度,T/K | CO2-浆液二元交互作用参数,kCO2 |
|---|---|---|---|---|---|
| 塔顶(净化气/贫液) | 0.0429 | 0 | 0.0003 | 303.15 | -0.411 |
| 2 | 0.1794 | 0.0089 | 0.0011 | 303.2 | -0.4106 |
| 3 | 0.6083 | 0.0369 | 0.0036 | 303.38 | -0.4092 |
| 4 | 1.894 | 0.1223 | 0.0114 | 303.93 | -0.4051 |
| 5 | 5.2582 | 0.3561 | 0.0315 | 305.42 | -0.395 |
| 6 | 11.503 | 0.8351 | 0.069 | 308.49 | -0.3791 |
| 7 | 17.4369 | 1.3552 | 0.1046 | 311.81 | -0.3679 |
| 8 | 19.5741 | 1.5612 | 0.1174 | 313.13 | -0.365 |
| 塔底(原料气/富液) | 20 | 1.5832 | 0.12 | 313.27 | -0.3644 |
| Test No. | Tde/K | pde/MPa | Vde-air/(L/h) | pab/MPa | Vin-mixgas/(L/h) | φ | Cout-CO2/% (mol) | ΔSV/(mol/L) | ηCO2/% | wtotal/(GJ/t CO2) |
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 298 | 0.08 | 200 | 0.6 | 300 | 18 | 3.07 | 0.12 | 84.6 | 0.680 |
| S2 | 298 | 0.08 | 600 | 0.6 | 720 | 42 | 5.82 | 0.26 | 69.1 | 0.609 |
| S3 | 313 | 0.08 | 600 | 0.6 | 720 | 42 | 3.08 | 0.32 | 84.1 | 0.649 |
| S4 | 333 | 0.08 | 200 | 0.6 | 720 | 90 | 1.42 | 0.75 | 93.0 | 0.507 |
| S5 | 333 | 0.08 | 200 | 0.6 | 720 | 80 | 0.92 | 0.68 | 95.0 | 0.509 |
| S6 | 333 | 0.08 | 200 | 0.5 | 720 | 80 | 2.02 | 0.64 | 89.9 | 0.474 |
| SMEA | 333 | 0.08 | 200 | 0.6 | 720 | 90 | 3.39 | 0.66 | 82.5 | 0.957 |
表6 ZIF-8浆液在中试填料塔中运行时不同操作条件对CO2捕集等效功的影响
Table 6 Effect of different operating conditions of ZIF-8 slurry on the CO2 capture equivalent work in the packed tower
| Test No. | Tde/K | pde/MPa | Vde-air/(L/h) | pab/MPa | Vin-mixgas/(L/h) | φ | Cout-CO2/% (mol) | ΔSV/(mol/L) | ηCO2/% | wtotal/(GJ/t CO2) |
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 298 | 0.08 | 200 | 0.6 | 300 | 18 | 3.07 | 0.12 | 84.6 | 0.680 |
| S2 | 298 | 0.08 | 600 | 0.6 | 720 | 42 | 5.82 | 0.26 | 69.1 | 0.609 |
| S3 | 313 | 0.08 | 600 | 0.6 | 720 | 42 | 3.08 | 0.32 | 84.1 | 0.649 |
| S4 | 333 | 0.08 | 200 | 0.6 | 720 | 90 | 1.42 | 0.75 | 93.0 | 0.507 |
| S5 | 333 | 0.08 | 200 | 0.6 | 720 | 80 | 0.92 | 0.68 | 95.0 | 0.509 |
| S6 | 333 | 0.08 | 200 | 0.5 | 720 | 80 | 2.02 | 0.64 | 89.9 | 0.474 |
| SMEA | 333 | 0.08 | 200 | 0.6 | 720 | 90 | 3.39 | 0.66 | 82.5 | 0.957 |
| Test No. | wcompr/(GJ/t CO2) | wheat/(GJ/t CO2) | wpump/(GJ/t CO2) | wtotal/(GJ/t CO2) |
|---|---|---|---|---|
| S1 | 0.477 | 0.000 | 0.203 | 0.680 |
| S2 | 0.478 | 0.000 | 0.131 | 0.609 |
| S3 | 0.412 | 0.119 | 0.117 | 0.649 |
| S4 | 0.382 | 0.081 | 0.045 | 0.507 |
| S5 | 0.374 | 0.084 | 0.051 | 0.509 |
| S6 | 0.343 | 0.086 | 0.045 | 0.474 |
| SMEA | 0.418 | 0.177 | 0.052 | 0.957 |
表7 不同操作条件下CO2捕集等效功的构成
Table 7 The composition of CO2 capture equivalent work in different operating conditions
| Test No. | wcompr/(GJ/t CO2) | wheat/(GJ/t CO2) | wpump/(GJ/t CO2) | wtotal/(GJ/t CO2) |
|---|---|---|---|---|
| S1 | 0.477 | 0.000 | 0.203 | 0.680 |
| S2 | 0.478 | 0.000 | 0.131 | 0.609 |
| S3 | 0.412 | 0.119 | 0.117 | 0.649 |
| S4 | 0.382 | 0.081 | 0.045 | 0.507 |
| S5 | 0.374 | 0.084 | 0.051 | 0.509 |
| S6 | 0.343 | 0.086 | 0.045 | 0.474 |
| SMEA | 0.418 | 0.177 | 0.052 | 0.957 |
| 1 | Shafiee S, Topal E. When will fossil fuel reserves be diminished? [J]. Energy Policy, 2009, 37(1): 181-189. |
| 2 | Raupach M R, Marland G, Ciais P, et al. Global and regional drivers of accelerating CO2 emissions[J]. PNAS, 2007, 104(24): 10288-10293. |
| 3 | Renfrew S E, Starr D E, Strasser P. Electrochemical approaches toward CO2 capture and concentration[J]. ACS Catalysis, 2020, 10(21): 13058-13074. |
| 4 |
Chen Y, Liu C, Guo S, et al. CO2 capture and conversion to value-added products promoted by MXene-based materials[J]. Green Energy & Environment, 2020. doi: 10.1016/j.gee.2020.11.008.
DOI |
| 5 | Nourouzi-Lavasani S, Larachi F, Benali M. Energy and hydrogen coproduction from (athabasca bitumen) coke gasification with CO2 capture[J]. Industrial & Engineering Chemistry Research, 2008, 47(18): 7118-7129. |
| 6 | Gui X, Tang Z G, Fei W Y. CO2 capture with physical solvent dimethyl carbonate at high pressures[J]. Journal of Chemical & Engineering Data, 2010, 55(9): 3736-3741. |
| 7 | Rayer A V, Henni A, Tontiwachwuthikul P. High pressure physical solubility of carbon dioxide (CO2) in mixed polyethylene glycol dimethyl ethers (Genosorb 1753)[J]. The Canadian Journal of Chemical Engineering, 2012, 90(3): 576-583. |
| 8 | Sun L, Smith R. Rectisol wash process simulation and analysis[J]. Journal of Cleaner Production, 2013, 39: 321-328. |
| 9 | Fauth D J, Frommell E A, Hoffman J S, et al. Eutectic salt promoted lithium zirconate: novel high temperature sorbent for CO2 capture[J]. Fuel Processing Technology, 2005, 86(14/15): 1503-1521. |
| 10 | Yeh J T, Resnik K P, Rygle K, et al. Semi-batch absorption and regeneration studies for CO2 capture by aqueous ammonia[J]. Fuel Processing Technology, 2005, 86(14/15): 1533-1546. |
| 11 | Oyevaar M H, Morssinkhof R W J, Westerterp K R. The kinetics of the reaction between CO2 and diethanolamine in aqueous ethyleneglycol at 298 K: a viscous gas-liquid reaction system for the determination of interfacial areas in gas-liquid contactors[J]. Chemical Engineering Science, 1990, 45(11): 3283-3298. |
| 12 | Chowdhury F A, Yamada H, Higashii T, et al. CO2 capture by tertiary amine absorbents: a performance comparison study[J]. Industrial & Engineering Chemistry Research, 2013, 52(24): 8323-8331. |
| 13 | Clausse M, Merel J, Meunier F. Numerical parametric study on CO2 capture by indirect thermal swing adsorption[J]. International Journal of Greenhouse Gas Control, 2011, 5(5): 1206-1213. |
| 14 | Wang L, Liu Z, Li P, et al. Experimental and modeling investigation on post-combustion carbon dioxide capture using zeolite 13X-APG by hybrid VTSA process[J]. Chemical Engineering Journal, 2012, 197: 151-161. |
| 15 | Das M, Koros W J. Performance of 6FDA-6FpDA polyimide for propylene/propane separations[J]. Journal of Membrane Science, 2010, 365(1/2): 399-408. |
| 16 | Liu Q, Wang N Y, Caro J, et al. Bio-inspired polydopamine: a versatile and powerful platform for covalent synthesis of molecular sieve membranes[J]. Journal of the American Chemical Society, 2013, 135(47): 17679-17682. |
| 17 | Kwon H T, Jeong H K, Lee A S, et al. Heteroepitaxially grown zeolitic imidazolate framework membranes with unprecedented propylene/propane separation performances[J]. Journal of the American Chemical Society, 2015, 137(38): 12304-12311. |
| 18 | Hart A, Gnanendran N. Cryogenic CO2 capture in natural gas[J]. Energy Procedia, 2009, 1(1): 697-706. |
| 19 | Liu H, Wang J, Chen G J, et al. High-efficiency separation of a CO2/H2 mixture via hydrate formation in W/O emulsions in the presence of cyclopentane and TBAB[J]. International Journal of Hydrogen Energy, 2014, 39(15): 7910-7918. |
| 20 | O'Reilly N, Giri N, James S. Porous liquids[J]. Chemistry-A European Journal, 2007, 13(11): 3020-3025. |
| 21 | Zhang J S, Chai S H, Qiao Z A, et al. Porous liquids: a promising class of media for gas separation[J]. Angewandte Chemie, 2015, 54(3): 932-936. |
| 22 | Knebel A, Bavykina A, Datta S J, et al. Solution processable metal-organic frameworks for mixed matrix membranes using porous liquids[J]. Nature Materials, 2020, 19(12): 1346-1353. |
| 23 | Banerjee R, Phan A, Wang B, et al. High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture[J]. Science, 2008, 319(5865): 939-943. |
| 24 | Wang B, Côté A P, Furukawa H, et al. Colossal cages in zeolitic imidazolate frameworks as selective carbon dioxide reservoirs[J]. Nature, 2008, 453(7192): 207-211. |
| 25 | Li J R, Sculley J, Zhou H C. Metal-organic frameworks for separations[J]. Chemical Reviews, 2012, 112(2): 869-932. |
| 26 | Lei Z G, Dai C N, Song W J. Adsorptive absorption: a preliminary experimental and modeling study on CO2 solubility[J]. Chemical Engineering Science, 2015, 127: 260-268. |
| 27 | Liu H, Liu B, Lin L C, et al. A hybrid absorption-adsorption method to efficiently capture carbon[J]. Nat. Commun., 2014, 5: 5147. |
| 28 | Pan Y, Li H, Zhang X X, et al. Large-scale synthesis of ZIF-67 and highly efficient carbon capture using a ZIF-67/glycol-2-methylimidazole slurry[J]. Chemical Engineering Science, 2015, 137: 504-514. |
| 29 | Yang M K, Han Y, Zou E B, et al. Separation of IGCC syngas by using ZIF-8/dimethylacetamide slurry with high CO2 sorption capacity and sorption speed but low sorption heat[J]. Energy, 2020, 201: 117605. |
| 30 | Chen W, Zou E B, Zuo J Y, et al. Separation of ethane from natural gas using porous ZIF-8/water-glycol slurry[J]. Industrial & Engineering Chemistry Research, 2019, 58(23): 9997-10006. |
| 31 | Li H, Gao X T, Jia C Z, et al. Enrichment of hydrogen from a hydrogen/propylene gas mixture using ZIF-8/water-glycol slurry[J]. Energies, 2018, 11(7): 1890. |
| 32 | Chen W, Guo X N, Zou E B, et al. A continuous and high-efficiency process to separate coal bed methane with porous ZIF-8 slurry: experimental study and mathematical modelling[J]. Green Energy & Environment, 2020, 5(3): 347-363. |
| 33 | Li H, Liu B, Yang M K, et al. CO2 separation performance of zeolitic imidazolate framework-8 porous slurry in a pilot-scale packed tower[J]. Industrial & Engineering Chemistry Research, 2020, 59(13): 6154-6163. |
| 34 | Peng D Y, Robinson D B. A new two-constant equation of state[J]. Industrial & Engineering Chemistry Fundamentals, 1976, 15(1): 59-64. |
| 35 | Wang Z Q, Tanabe K, Cohen S. Tuning hydrogen sorption properties of metal-organic frameworks by postsynthetic covalent modification[J]. Chemistry-A European Journal, 2010, 16(1): 212-217. |
| 36 | House K Z, Harvey C F, Aziz M J, et al. The energy penalty of post-combustion CO2 capture & storage and its implications for retrofitting the US installed base[J]. Energy & Environmental Science, 2009, 2(2): 193. |
| [1] | 毛磊, 刘冠章, 袁航, 张光亚. 可捕集CO2的纳米碳酸酐酶粒子的高效制备及性能研究[J]. 化工学报, 2023, 74(6): 2589-2598. |
| [2] | 王皓, 唐思扬, 钟山, 梁斌. MEA吸收CO2富液解吸过程中固体颗粒表面的强化作用分析[J]. 化工学报, 2023, 74(4): 1539-1548. |
| [3] | 彭晓婉, 郭笑楠, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8浆液法分离CH4/N2的双吸收-吸附塔工艺流程建模与模拟[J]. 化工学报, 2023, 74(2): 784-795. |
| [4] | 王煦清, 严圣林, 朱礼涛, 张希宝, 罗正鸿. 填料塔中有机胺吸收CO2气液传质的研究进展[J]. 化工学报, 2023, 74(1): 237-256. |
| [5] | 张劢, 田瑶, 郭之旗, 王叶, 窦广进, 宋浩. 光催化-生物杂合系统设计优化用于燃料和化学品绿色合成[J]. 化工学报, 2022, 73(7): 2774-2789. |
| [6] | 李淼, 赵虹, 姜标, 陈思远, 闫龙. 煤制乙炔关键中间体BaC2合成的热力学分析[J]. 化工学报, 2022, 73(5): 1908-1919. |
| [7] | 刘潜, 张香兰, 李志平, 栗卓琦, 喻红. 油酚分离过程离子液体萃取溶剂的多尺度筛选[J]. 化工学报, 2022, 73(11): 5011-5024. |
| [8] | 李贵贤, 王可, 王健, 孟文亮, 李婧玮, 杨勇, 范宗良, 王东亮, 周怀荣. 膜分离捕集燃煤电厂烟气CO2过程优化设计[J]. 化工学报, 2022, 73(11): 5065-5077. |
| [9] | 刘立, 蒋鹏, 王伟, 张同桓, 穆立文, 陆小华, 朱家华. 基于过程模拟和随机森林模型的生物质制氢过程因素分析与预测[J]. 化工学报, 2022, 73(11): 5230-5239. |
| [10] | 赵旭, 卜昌盛, 王昕晔, 张鑫, 程晓磊, 王乃继, 朴桂林. 铁基载氧体辅助无烟煤焦富氧燃烧动力学分析[J]. 化工学报, 2022, 73(1): 384-392. |
| [11] | 方远鑫, 肖武, 姜晓滨, 李祥村, 贺高红, 吴雪梅. 膜分离耦合CO2电催化加氢制甲酸工艺的设计及模拟[J]. 化工学报, 2021, 72(9): 4740-4749. |
| [12] | 平甜甜, 尹鑫, 董玉, 申淑锋. 有机胺非水溶液吸收CO2的动力学研究进展[J]. 化工学报, 2021, 72(8): 3968-3983. |
| [13] | 宋振兴, 崔现宝, 张缨, 张雪梅, 何杰, 冯天扬, 王纪孝. 混合离子液体催化反应精馏合成乙酸正己酯[J]. 化工学报, 2021, 72(8): 4155-4165. |
| [14] | 曹健, 冯新, 吉晓燕, 陆小华. 基于混合工质的多级蒸发ORC理论极限性能研究[J]. 化工学报, 2021, 72(7): 3780-3787. |
| [15] | 于雪菲, 张帅, 刘琳琳, 都健. 电厂和碳捕集装置同步集成与调度优化研究[J]. 化工学报, 2021, 72(3): 1447-1456. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号