化工学报 ›› 2023, Vol. 74 ›› Issue (2): 784-795.DOI: 10.11949/0438-1157.20221488
彭晓婉( ), 郭笑楠, 邓春(
), 郭笑楠, 邓春( ), 刘蓓, 孙长宇, 陈光进
), 刘蓓, 孙长宇, 陈光进
收稿日期:2022-11-15
修回日期:2023-01-14
出版日期:2023-02-05
发布日期:2023-03-21
通讯作者:
邓春
作者简介:彭晓婉(1995—),女,博士研究生,1836160461@qq.com
基金资助:
Xiaowan PENG( ), Xiaonan GUO, Chun DENG(
), Xiaonan GUO, Chun DENG( ), Bei LIU, Changyu SUN, Guangjin CHEN
), Bei LIU, Changyu SUN, Guangjin CHEN
Received:2022-11-15
Revised:2023-01-14
Online:2023-02-05
Published:2023-03-21
Contact:
Chun DENG
摘要:
由于ZIF-8浆液独特的可流动性,可以借鉴传统的吸收-解吸工艺,实现煤层气中甲烷的多级连续高效富集。在单吸收-吸附塔工艺的基础上,为了进一步降低能耗,提出了高低压双吸收-吸附塔新型分离工艺,并对该工艺进行了全流程建模及模拟。采用平衡级法,建立了工艺流程中各单元传质设备的数学模型,包括吸收-吸附塔、闪蒸罐、解吸塔。此外,还进行了灵敏度分析,探究了平衡级数、进料位置、气液比、解吸压力等因素对产品气中甲烷浓度以及回收率等工艺性能的影响。模拟结果表明,当产品气中甲烷浓度达到90.13%(mol)时,回收率为90.25%。并且单位原料气能耗为0.445 kW·h∙m-3(原料气),低于单塔能耗(0.510 kW·h∙m-3)。由此,改进的双塔工艺在满足甲烷纯度和回收率的同时,相较于单塔工艺进一步降低了能耗。
中图分类号:
彭晓婉, 郭笑楠, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8浆液法分离CH4/N2的双吸收-吸附塔工艺流程建模与模拟[J]. 化工学报, 2023, 74(2): 784-795.
Xiaowan PENG, Xiaonan GUO, Chun DENG, Bei LIU, Changyu SUN, Guangjin CHEN. Modeling and simulation of CH4/N2 separation process with two absorption-adsorption columns using ZIF-8 slurry[J]. CIESC Journal, 2023, 74(2): 784-795.
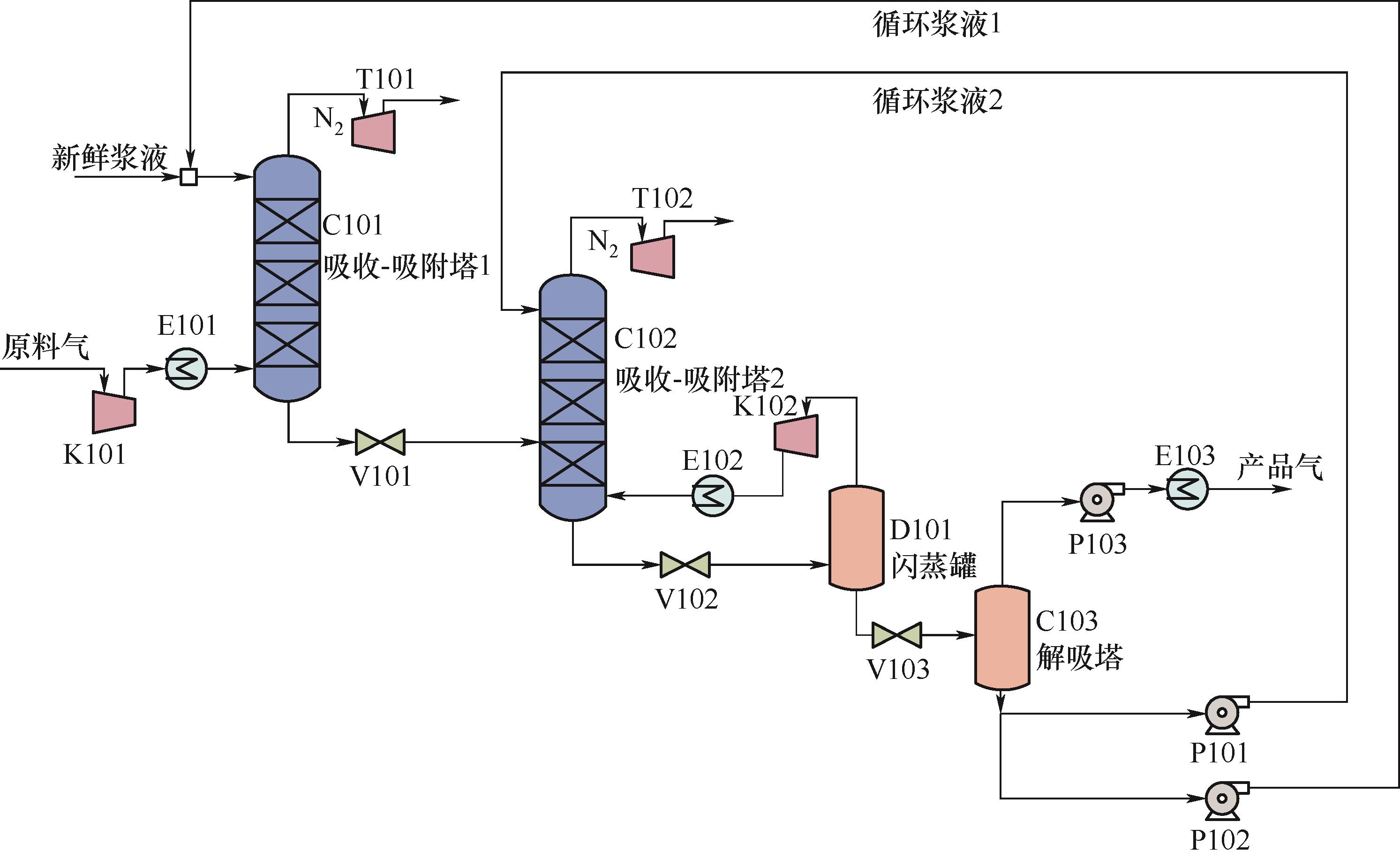
图1 ZIF-8浆液吸收-吸附分离CH4/N2的双塔工艺流程图C101、C102—吸收-吸附塔;D101—闪蒸罐;C103—解吸塔;K101、K102—气体压缩机;P101、P102—循环泵;P103—真空泵;T101、T102—透平;V101、V102、V103—阀;E101, E102, E103—换热器
Fig.1 Schematic diagram of twin columns of ZIF-8 slurry absorption-adsorption separation of CH4/N2 C101, C102—absorption-adsorption column; D101—flash tank; C103—desorber; K101, K102—gas compressor; P101, P102—circulation slurry pump; P103—vacuum pump; T101、T102—turbine; V101, V102, V103—valve; E101, E102, E103—heat exchanger
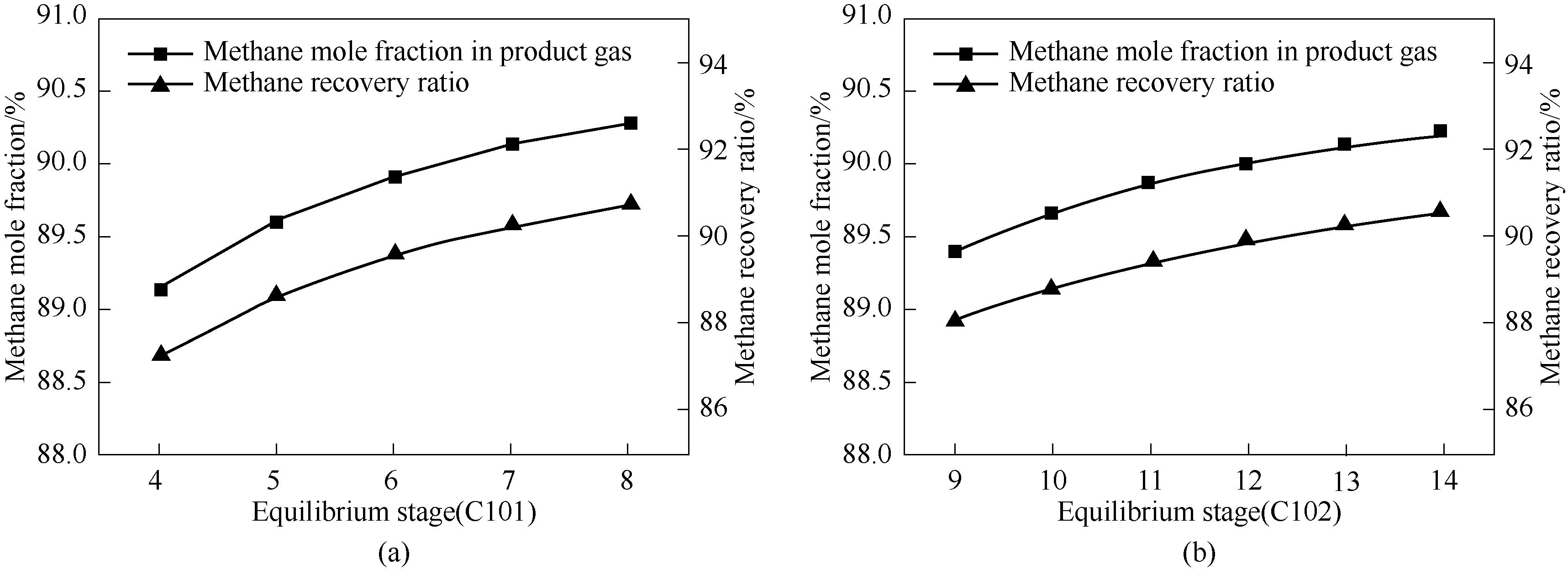
图2 吸收-吸附塔平衡级数对解吸塔塔顶气相中甲烷摩尔分数和甲烷回收率的影响
Fig.2 Effect of equilibrium stage numbers of absorption-adsorption column on methane mole fraction in the product gas stream and the methane recovery ratio
| 气液比 | 吸收-吸附塔塔顶气相流量(C102)/(kmol∙h-1) | 吸收-吸附塔塔顶气相中 甲烷摩尔分数(C102)/% | 产品气流量/ (kmol∙h-1) | 产品气中甲烷 摩尔分数/% | 甲烷回收率/% |
|---|---|---|---|---|---|
| 12 | 0.4080 | 3.520 | 0.3117 | 87.66 | 91.08 |
| 14 | 0.4190 | 3.974 | 0.3004 | 90.13 | 90.25 |
| 16 | 0.4279 | 4.511 | 0.2914 | 91.98 | 89.34 |
表1 吸收-吸附塔C102取不同气液比的模拟计算结果
Table 1 Simulation results of different gas-slurry ratios of C102
| 气液比 | 吸收-吸附塔塔顶气相流量(C102)/(kmol∙h-1) | 吸收-吸附塔塔顶气相中 甲烷摩尔分数(C102)/% | 产品气流量/ (kmol∙h-1) | 产品气中甲烷 摩尔分数/% | 甲烷回收率/% |
|---|---|---|---|---|---|
| 12 | 0.4080 | 3.520 | 0.3117 | 87.66 | 91.08 |
| 14 | 0.4190 | 3.974 | 0.3004 | 90.13 | 90.25 |
| 16 | 0.4279 | 4.511 | 0.2914 | 91.98 | 89.34 |
| 气液比 | 吸收-吸附塔塔顶气相流量 (C101)/(kmol∙h-1) | 吸收-吸附塔塔顶气相中 甲烷摩尔分数(C101)/% | 产品气流量/ (kmol∙h-1) | 产品气中甲烷 摩尔分数/% | 甲烷回收率/% |
|---|---|---|---|---|---|
| 17 | 0.1706 | 3.409 | 0.3075 | 88.10 | 90.30 |
| 20 | 0.2806 | 4.486 | 0.3004 | 90.13 | 90.25 |
| 23 | 0.3624 | 5.867 | 0.2917 | 91.19 | 88.67 |
表2 吸收-吸附塔C101取不同气液比的模拟计算结果
Table 2 Simulation results of different gas-slurry ratios of C101
| 气液比 | 吸收-吸附塔塔顶气相流量 (C101)/(kmol∙h-1) | 吸收-吸附塔塔顶气相中 甲烷摩尔分数(C101)/% | 产品气流量/ (kmol∙h-1) | 产品气中甲烷 摩尔分数/% | 甲烷回收率/% |
|---|---|---|---|---|---|
| 17 | 0.1706 | 3.409 | 0.3075 | 88.10 | 90.30 |
| 20 | 0.2806 | 4.486 | 0.3004 | 90.13 | 90.25 |
| 23 | 0.3624 | 5.867 | 0.2917 | 91.19 | 88.67 |
内蒸 压力/MPa | 吸收-吸附塔(C101)塔顶LV1中甲烷 吸附量/ (mol∙L-1) | 吸收-吸附塔(C102)塔顶 吸附量/ (mol∙L-1) | 贫液 吸附量/(mol∙L-1) | 贫液 流量/ (kmol∙h-1) | 贫液 摩尔 分数/% | 浆液 | 吸收- 吸附塔(C101) 塔顶气流流量/(kmol∙h-1) | 吸收- 吸附塔(C101) 塔顶气流中甲烷摩尔分数/% | 吸收- 吸附塔(C102) 塔顶气流流量/(kmol∙h-1) | 吸收- 吸附塔(C102)塔顶气流中甲烷摩尔分数/% | 产品气流量/(kmol∙h-1) | 产品气中甲烷摩尔 分数/% | 甲烷 回收率/ % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.01 | 0.0459 | 0.0326 | 0.0100 | 0.0279 | 97.56 | 90.76 | 0.2806 | 4.486 | 0.4190 | 3.973 | 0.3004 | 90.13 | 90.25 |
| 0.03 | 0.0808 | 0.0686 | 0.0303 | 0.0842 | 97.95 | 93.13 | 0.2986 | 7.618 | 0.4368 | 7.980 | 0.2646 | 91.59 | 80.78 |
| 0.05 | 0.1148 | 0.1032 | 0.0513 | 0.1417 | 98.43 | 95.34 | 0.3168 | 10.48 | 0.4536 | 11.52 | 0.2296 | 93.43 | 71.51 |
表3 不同解吸压力下的模拟计算结果
Table 3 Simulation results under different desorption pressures
内蒸 压力/MPa | 吸收-吸附塔(C101)塔顶LV1中甲烷 吸附量/ (mol∙L-1) | 吸收-吸附塔(C102)塔顶 吸附量/ (mol∙L-1) | 贫液 吸附量/(mol∙L-1) | 贫液 流量/ (kmol∙h-1) | 贫液 摩尔 分数/% | 浆液 | 吸收- 吸附塔(C101) 塔顶气流流量/(kmol∙h-1) | 吸收- 吸附塔(C101) 塔顶气流中甲烷摩尔分数/% | 吸收- 吸附塔(C102) 塔顶气流流量/(kmol∙h-1) | 吸收- 吸附塔(C102)塔顶气流中甲烷摩尔分数/% | 产品气流量/(kmol∙h-1) | 产品气中甲烷摩尔 分数/% | 甲烷 回收率/ % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.01 | 0.0459 | 0.0326 | 0.0100 | 0.0279 | 97.56 | 90.76 | 0.2806 | 4.486 | 0.4190 | 3.973 | 0.3004 | 90.13 | 90.25 |
| 0.03 | 0.0808 | 0.0686 | 0.0303 | 0.0842 | 97.95 | 93.13 | 0.2986 | 7.618 | 0.4368 | 7.980 | 0.2646 | 91.59 | 80.78 |
| 0.05 | 0.1148 | 0.1032 | 0.0513 | 0.1417 | 98.43 | 95.34 | 0.3168 | 10.48 | 0.4536 | 11.52 | 0.2296 | 93.43 | 71.51 |
| 项目 | 入口压力/MPa | 出口压力/MPa | 流量 | 有效功率/kW | 电功率/kW | |
|---|---|---|---|---|---|---|
| 合计 | 9.9786 | |||||
| 压缩机 | 原料气 | 0.12 | 2 | 1 kmol·h-1 | 2.3827 | 3.5299 |
| 闪蒸循环气 | 0.15 | 1.5 | 1.5191 kmol·h-1 | 2.6058 | 3.8604 | |
| 产品气 | 0.01 | 0.11 | 0.3004 kmol·h-1 | 0.5418 | 0.8027 | |
| 压缩机电功率合计 | 8.1930 | |||||
| 浆液循环泵P101 | 0.01 | 2 | 1.12 m3·h-1 | 0.8258 | 0.9176 | |
| 浆液循环泵P102 | 0.01 | 1.5 | 1.60 m3·h-1 | 0.8834 | 0.9815 | |
| 泵电功率合计 | 1.8991 | |||||
| 氮气透平T101 | 2 | 0.1 | 0.281 kmol·h-1 | -0.4559 | -0.3077 | |
| 氮气透平T102 | 1.5 | 0.1 | 0.419 kmol·h-1 | -0.6246 | -0.4216 | |
| 透平回收膨胀功合计 | -0.7293 | |||||
| 入口温度/K | 出口温度/K | 流量 | 热负荷/kW | 折合电功率/kW | ||
| 冷却器 | 原料气 | 323.15 | 273.15 | 1 kmol·h-1 | 0.4320 | 0.2160 |
| 闪蒸循环气 | 323.15 | 273.15 | 1.5191 kmol·h-1 | 0.7108 | 0.3554 | |
| 产品气 | 323.15 | 293.15 | 0.3004 kmol·h-1 | 0.0888 | 0.0444 | |
| 冷却器折合电功率合计 | 0.6158 | |||||
表4 能耗计算结果汇总
Table 4 Summary of energy consumption calculation results
| 项目 | 入口压力/MPa | 出口压力/MPa | 流量 | 有效功率/kW | 电功率/kW | |
|---|---|---|---|---|---|---|
| 合计 | 9.9786 | |||||
| 压缩机 | 原料气 | 0.12 | 2 | 1 kmol·h-1 | 2.3827 | 3.5299 |
| 闪蒸循环气 | 0.15 | 1.5 | 1.5191 kmol·h-1 | 2.6058 | 3.8604 | |
| 产品气 | 0.01 | 0.11 | 0.3004 kmol·h-1 | 0.5418 | 0.8027 | |
| 压缩机电功率合计 | 8.1930 | |||||
| 浆液循环泵P101 | 0.01 | 2 | 1.12 m3·h-1 | 0.8258 | 0.9176 | |
| 浆液循环泵P102 | 0.01 | 1.5 | 1.60 m3·h-1 | 0.8834 | 0.9815 | |
| 泵电功率合计 | 1.8991 | |||||
| 氮气透平T101 | 2 | 0.1 | 0.281 kmol·h-1 | -0.4559 | -0.3077 | |
| 氮气透平T102 | 1.5 | 0.1 | 0.419 kmol·h-1 | -0.6246 | -0.4216 | |
| 透平回收膨胀功合计 | -0.7293 | |||||
| 入口温度/K | 出口温度/K | 流量 | 热负荷/kW | 折合电功率/kW | ||
| 冷却器 | 原料气 | 323.15 | 273.15 | 1 kmol·h-1 | 0.4320 | 0.2160 |
| 闪蒸循环气 | 323.15 | 273.15 | 1.5191 kmol·h-1 | 0.7108 | 0.3554 | |
| 产品气 | 323.15 | 293.15 | 0.3004 kmol·h-1 | 0.0888 | 0.0444 | |
| 冷却器折合电功率合计 | 0.6158 | |||||
| 项目 | 单吸收-吸附塔工艺 | 双吸收-吸附塔工艺(C101/C102) | |
|---|---|---|---|
| 吸收-吸附塔操作条件 | 平衡级数 | 13 | 7/13 |
| 进料级 | 11 | 7/11 | |
| 全塔温度/K | 273.15 | 273.15/273.15 | |
| 压力/MPa | 2 | 2/1.5 | |
| 闪蒸罐操作条件 | 温度/K | 273.15 | 273.15 |
| 压力/MPa | 0.15 | 0.15 | |
| 解吸塔操作条件 | 温度/K | 273.15 | 273.15 |
| 压力/MPa | 0.01 | 0.01 | |
| 原料气流量/(kmol∙h-1) | 1 | 1 | |
| 原料气中甲烷的摩尔分数/% | 30 | 30 | |
| 循环浆液量/(m3·h-1) | 2.24 | 2.72 | |
| 产品气摩尔分数/% | 95.46 | 90.13 | |
| 甲烷回收率/% | 90.74 | 90.25 | |
表5 不同工艺流程下模拟计算结果对比
Table 5 Comparison of simulation results under different processes
| 项目 | 单吸收-吸附塔工艺 | 双吸收-吸附塔工艺(C101/C102) | |
|---|---|---|---|
| 吸收-吸附塔操作条件 | 平衡级数 | 13 | 7/13 |
| 进料级 | 11 | 7/11 | |
| 全塔温度/K | 273.15 | 273.15/273.15 | |
| 压力/MPa | 2 | 2/1.5 | |
| 闪蒸罐操作条件 | 温度/K | 273.15 | 273.15 |
| 压力/MPa | 0.15 | 0.15 | |
| 解吸塔操作条件 | 温度/K | 273.15 | 273.15 |
| 压力/MPa | 0.01 | 0.01 | |
| 原料气流量/(kmol∙h-1) | 1 | 1 | |
| 原料气中甲烷的摩尔分数/% | 30 | 30 | |
| 循环浆液量/(m3·h-1) | 2.24 | 2.72 | |
| 产品气摩尔分数/% | 95.46 | 90.13 | |
| 甲烷回收率/% | 90.74 | 90.25 | |
| 项目 | 单吸收-吸附塔工艺 | 双吸收-吸附塔工艺(C101/C102) | |
|---|---|---|---|
| 压缩机/kW | 原料气 | 3.5299 | 3.5299 |
| 闪蒸循环气 | 5.3728 | 3.8604 | |
| 产品气 | 0.7621 | 0.8027 | |
| 浆液循环泵合计/kW | 1.8353 | 1.8991 | |
| 冷却器/kW | 原料气 | 0.2160 | 0.2160 |
| 闪蒸循环气 | 0.4412 | 0.3554 | |
| 产品气 | 0.0418 | 0.0444 | |
| 氮气回收合计/kW | -0.7840 | -0.7293 | |
| 合计/kW | 11.4151 | 9.9786 | |
| 单位原料气能耗/(kW·h∙m-3) | 0.510 | 0.445 | |
表6 不同工艺流程下能耗计算结果对比
Table 6 Comparison of energy consumption under different processes
| 项目 | 单吸收-吸附塔工艺 | 双吸收-吸附塔工艺(C101/C102) | |
|---|---|---|---|
| 压缩机/kW | 原料气 | 3.5299 | 3.5299 |
| 闪蒸循环气 | 5.3728 | 3.8604 | |
| 产品气 | 0.7621 | 0.8027 | |
| 浆液循环泵合计/kW | 1.8353 | 1.8991 | |
| 冷却器/kW | 原料气 | 0.2160 | 0.2160 |
| 闪蒸循环气 | 0.4412 | 0.3554 | |
| 产品气 | 0.0418 | 0.0444 | |
| 氮气回收合计/kW | -0.7840 | -0.7293 | |
| 合计/kW | 11.4151 | 9.9786 | |
| 单位原料气能耗/(kW·h∙m-3) | 0.510 | 0.445 | |
| 1 | Xue C L, Cheng W P, Hao W M, et al. CH4/N2 adsorptive separation on zeolite X/AC composites[J]. Journal of Chemistry, 2019, 2019: 1-9. |
| 2 | Li Q Z, Yuan C C, Zhang G Y, et al. Effects of doping Mg2+ on the pore structure of MIL-101 and its adsorption selectivity for CH4/N2 gas mixtures[J]. Fuel, 2019, 240: 206-218. |
| 3 | Yang B, Xu E L, Li M. Purification of coal mine methane on carbon molecular sieve by vacuum pressure swing adsorption[J]. Separation Science and Technology, 2016, 51(6): 909-916. |
| 4 | Li Q Y, Wang L, Ju Y L. Analysis of flammability limits for the liquefaction process of oxygen-bearing coal-bed methane[J]. Applied Energy, 2011, 88(9): 2934-2939. |
| 5 | Anna H R S, Barreto Jr A G, Tavares F W, et al. Methane/nitrogen separation through pressure swing adsorption process from nitrogen-rich streams[J]. Chemical Engineering and Processing: Process Intensification, 2016, 103: 70-79. |
| 6 | Niu Z, Cui X L, Pham T, et al. A metal-organic framework based methane nano-trap for the capture of coal-mine methane[J]. Angewandte Chemie International Edition, 2019, 58(30): 10138-10141. |
| 7 | Lavoie T N, Shepson P B, Gore C A, et al. Assessing the methane emissions from natural gas-fired power plants and oil refineries[J]. Environmental Science & Technology, 2017, 51(6): 3373-3381. |
| 8 | 郑德志. 我国煤层气产业政策评价研究[J]. 煤炭经济研究, 2019, 39(1): 62-65. |
| Zheng D Z. Research on China's coalbed methane industry policy evaluation[J]. Coal Economic Research, 2019, 39(1): 62-65. | |
| 9 | Qadir S, Li D F, Gu Y M, et al. Experimental and numerical investigations on the separation performance of [Cu(INA)2] adsorbent for CH4 recovery by VPSA from oxygen-bearing coal mine methane[J]. Chemical Engineering Journal, 2021, 408: 127238. |
| 10 | Yang R T. Adsorbents: Fundamentals and Applications[M]. Hoboken, NJ, USA: John Wiley & Sons, Inc., 2003. |
| 11 | Gao T, Lin W S, Gu A Z, et al. Coalbed methane liquefaction adopting a nitrogen expansion process with propane pre-cooling[J]. Applied Energy, 2010, 87(7): 2142-2147. |
| 12 | Baker R W, Lokhandwala K. Natural gas processing with membranes: an overview[J]. Industrial & Engineering Chemistry Research, 2008, 47(7): 2109-2121. |
| 13 | Yang J F, Bai H H, Shang H, et al. Experimental and simulation study on efficient CH4/N2 separation by pressure swing adsorption on silicalite-1 pellets[J]. Chemical Engineering Journal, 2020, 388: 124222. |
| 14 | Feng W R, Wu H, Jin J S, et al. Transformation of Al-CDC from 3D crystals to 2D nanosheets in macroporous polyacrylates with enhanced CH4/N2 separation efficiency and stability[J]. Chemical Engineering Journal, 2022, 429: 132285 |
| 15 | Yousef S, Tuckute S, Tonkonogovas A, et al. Ultra-permeable CNTs/PES membranes with a very low CNTs content and high H2/N2 and CH4/N2 selectivity for clean energy extraction applications[J]. Journal of Materials Research and Technology, 2021, 15: 5114-5127. |
| 16 | Yu H J, Shin J H, Lee A S, et al. Tailoring selective pores of carbon molecular sieve membranes towards enhanced N2/CH4 separation efficiency[J]. Journal of Membrane Science, 2021, 620: 118814. |
| 17 | Gu Z J, Yang Z B, Guo X Y, et al. Vacuum resistance treated ZIF-8 mixed matrix membrane for effective CH4/N2 separation[J]. Separation and Purification Technology, 2021, 272: 118845. |
| 18 | Gu Z J, Yang Z B, Sun Y X, et al. Large-area vacuum-treated ZIF-8 mixed-matrix membrane for highly efficient methane/nitrogen separation[J]. AIChE Journal, 2022, 68(9): e17749. |
| 19 | Sun Q, Zhao Y Y, Liu A X, et al. Continuous separation of CH4/N2 mixture via hydrates formation in the presence of TBAB[J]. Chemical Engineering and Processing: Process Intensification, 2015, 95: 284-288. |
| 20 | Ning X, Koros W J. Carbon molecular sieve membranes derived from Matrimid® polyimide for nitrogen/methane separation[J]. Carbon, 2014, 66: 511-522. |
| 21 | Zhong D L, Lu Y Y, Sun D J, et al. Performance evaluation of methane separation from coal mine gas by gas hydrate formation in a stirred reactor and in a fixed bed of silica sand[J]. Fuel, 2015, 143: 586-594. |
| 22 | 刘佳奇, 尚华, 唐轩, 等. 分子筛基CH4-N2分离材料的研究进展[J]. 化工进展, 2019, 38(1): 449-456. |
| Liu J Q, Shang H, Tang X, et al. Zeolite based materials for CH4-N2 separation[J]. Chemical Industry and Engineering Progress, 2019, 38(1): 449-456. | |
| 23 | Lu B, Shen Y H, Tang Z L, et al. Vacuum pressure swing adsorption process for coalbed methane enrichment[J]. Chinese Journal of Chemical Engineering, 2021, 32: 264-280. |
| 24 | 陈高飞, 汪亚燕, 张东辉, 等. 基于平衡效应的多种吸附剂对CH4/N2分离性能研究[J]. 天然气化工(C1化学与化工), 2020, 45(2): 17-23, 37. |
| Chen G F, Wang Y Y, Zhang D H, et al. Separation performance of CH4/N2 on various adsorbents based on equilibrium effect[J]. Natural Gas Chemical Industry, 2020, 45(2): 17-23, 37. | |
| 25 | 尚华, 白洪灏, 刘佳奇, 等. CH4-N2在自支撑颗粒型Silicalite-1上的吸附分离及PSA模拟[J]. 化工学报, 2020, 71(5): 2088-2098. |
| Shang H, Bai H H, Liu J Q, et al. PSA simulation and adsorption separation of CH4-N2 by self-supporting pellets Silicalite-1[J]. CIESC Journal, 2020, 71(5): 2088-2098. | |
| 26 | Li T, Jia X X, Chen H, et al. Tuning the pore environment of MOFs toward efficient CH4/N2 separation under humid conditions[J]. ACS Applied Materials & Interfaces, 2022, 14(13): 15830-15839. |
| 27 | Chang M, Zhao Y J, Liu D H, et al. Methane-trapping metal-organic frameworks with an aliphatic ligand for efficient CH4/N2 separation[J]. Sustainable Energy & Fuels, 2020, 4(1): 138-142. |
| 28 | Wang S M, Shivanna M, Yang Q Y. Nickel-based metal-organic frameworks for coal-bed methane purification with record CH4/N2 selectivity[J]. Angewandte Chemie International Edition, 2022, 61(15): e202201017. |
| 29 | 杨江峰, 赵强, 于秋红, 等. 煤层气回收及CH4/N2分离PSA材料的研究进展[J]. 化工进展, 2011, 30(4): 793-801. |
| Yang J F, Zhao Q, Yu Q H, et al. Progress of recovery of coal bed methane and adsorption materials for separation of CH4/N2 by pressure swing adsorption[J]. Chemical Industry and Engineering Progress, 2011, 30(4): 793-801. | |
| 30 | Yang Z X, Hussain M Z, MarÃn P, et al. Enrichment of low concentration methane: an overview of ventilation air methane[J]. Journal of Materials Chemistry A, 2022, 10(12): 6397-6413. |
| 31 | 郭武杰, 李媛, 李世帅, 等. 高硅沸石分子筛ZSM-11用于CH4与N2吸附分离性能的研究[J]. 天然气化工—C1化学与化工, 2022, 47(1): 67-72. |
| Guo W J, Li Y, Li S S, et al. Study on adsorption and separation performance of CH4/N2 by high-silica ZSM-11 zeolite[J]. Natural Gas Chemical Industry, 2022, 47(1): 67-72. | |
| 32 | 田军鹏, 沈圆辉, 张东辉, 等. 规整复合吸附剂真空变压吸附分离CH4/N2工艺模拟与分析[J]. 化工学报, 2021, 72(11): 5675-5685. |
| Tian J P, Shen Y H, Zhang D H, et al. Simulation and analysis of CH4/N2 separation by vacuum pressure swing adsorption with structured composite adsorption media[J]. CIESC Journal, 2021, 72(11): 5675-5685. | |
| 33 | Liu H, Liu B, Lin L C, et al. A hybrid absorption–adsorption method to efficiently capture carbon[J]. Nature Communications, 2014, 5: 5147. |
| 34 | Liu H, Pan Y, Liu B, et al. Tunable integration of absorption-membrane-adsorption for efficiently separating low boiling gas mixtures near normal temperature[J]. Scientific Reports, 2016, 6: 21114. |
| 35 | Yan S R, Xiao P, Zhu D, et al. A large-scale experimental study on CO2 capture utilizing slurry-based ab-adsorption approach[J]. Chinese Journal of Chemical Engineering, 2021, 31: 56-66. |
| 36 | Li H, Liu B, Yang M K, et al. CO2 separation performance of zeolitic imidazolate framework-8 porous slurry in a pilot-scale packed tower[J]. Industrial & Engineering Chemistry Research, 2020, 59(13): 6154-6163. |
| 37 | Yan S R, Zhu D, Zhang Z Y, et al. A pilot-scale experimental study on CO2 capture using zeolitic imidazolate framework-8 slurry under normal pressure[J]. Applied Energy, 2019, 248: 104-114. |
| 38 | Li H, Chen W, Liu B, et al. CO2 capture using ZIF-8/water-glycol-2-methylimidazole slurry with high capacity and low desorption heat[J]. Chemical Engineering Science, 2018, 182: 189-199. |
| 39 | Yang M K, Han Y, Zou E B, et al. Separation of IGCC syngas by using ZIF-8/dimethylacetamide slurry with high CO2 sorption capacity and sorption speed but low sorption heat[J]. Energy, 2020, 201: 117605. |
| 40 | Chen W, Zou E B, Zuo J Y, et al. Separation of ethane from natural gas using porous ZIF-8/water-glycol slurry[J]. Industrial & Engineering Chemistry Research, 2019, 58(23): 9997-10006. |
| 41 | Li H, Gao X T, Jia C Z, et al. Enrichment of hydrogen from a hydrogen/propylene gas mixture using ZIF-8/water-glycol slurry[J]. Energies, 2018, 11(7): 1890. |
| 42 | Peng X W, Jia C Z, Qiao Z C, et al. A new energy efficient process for hydrogen purification using ZIF-8/glycol-water slurry: experimental study and process modeling[J]. International Journal of Hydrogen Energy, 2021, 46(63): 32081-32098. |
| 43 | Chen W, Wang M L, Yang S W, et al. Experimental study on breakthrough separation for hydrogen recovery from coke oven gas using ZIF-8 slurry[J]. Energies, 2022, 15(4): 1487. |
| 44 | Li H, Chen W, Liu B, et al. A purely green approach to low-cost mass production of zeolitic imidazolate frameworks[J]. Green Energy & Environment, 2021, DOI:10.1016/j.gee.2021.09.003 . |
| 45 | Chen W, Guo X N, Zou E B, et al. A continuous and high-efficiency process to separate coal bed methane with porous ZIF-8 slurry: experimental study and mathematical modelling[J]. Green Energy & Environment, 2020, 5(3): 347-363. |
| [1] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [2] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [3] | 高燕, 伍鹏, 尚超, 胡泽君, 陈晓东. 基于双流体喷嘴的磁性琼脂糖微球的制备及其蛋白吸附性能探究[J]. 化工学报, 2023, 74(8): 3457-3471. |
| [4] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [5] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [6] | 陈吉, 洪泽, 雷昭, 凌强, 赵志刚, 彭陈辉, 崔平. 基于分子动力学的焦炭溶损反应及其机理研究[J]. 化工学报, 2023, 74(7): 2935-2946. |
| [7] | 牛超, 沈胜强, 杨艳, 潘泊年, 李熠桥. 甲烷BOG喷射器流动过程计算与性能分析[J]. 化工学报, 2023, 74(7): 2858-2868. |
| [8] | 刘晓洋, 喻健良, 侯玉洁, 闫兴清, 张振华, 吕先舒. 螺旋微通道对掺氢甲烷爆轰传播的影响[J]. 化工学报, 2023, 74(7): 3139-3148. |
| [9] | 王杰, 丘晓琳, 赵烨, 刘鑫洋, 韩忠强, 许雍, 蒋文瀚. 聚电解质静电沉积改性PHBV抗氧化膜的制备与性能研究[J]. 化工学报, 2023, 74(7): 3068-3078. |
| [10] | 周小文, 杜杰, 张战国, 许光文. 基于甲烷脉冲法的Fe2O3-Al2O3载氧体还原特性研究[J]. 化工学报, 2023, 74(6): 2611-2623. |
| [11] | 王新悦, 王俊杰, 曹思贤, 王翠, 李灵坤, 吴宏宇, 韩静, 吴昊. 玻璃内包材界面修饰对机械应力诱导的单克隆抗体聚集体形成的影响[J]. 化工学报, 2023, 74(6): 2580-2588. |
| [12] | 陈韶云, 徐东, 陈龙, 张禹, 张远方, 尤庆亮, 胡成龙, 陈建. 单层聚苯胺微球阵列结构的制备及其吸附性能[J]. 化工学报, 2023, 74(5): 2228-2238. |
| [13] | 王蕾, 王磊, 白云龙, 何柳柳. SA膜状锂离子筛的制备及其锂吸附性能[J]. 化工学报, 2023, 74(5): 2046-2056. |
| [14] | 蔺彩虹, 王丽, 吴瑜, 刘鹏, 杨江峰, 李晋平. 沸石中碱金属阳离子对CO2/N2O吸附分离性能的影响[J]. 化工学报, 2023, 74(5): 2013-2021. |
| [15] | 李辰鑫, 潘艳秋, 何流, 牛亚宾, 俞路. 基于碳微晶结构的炭膜模型及其气体分离模拟[J]. 化工学报, 2023, 74(5): 2057-2066. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号