化工学报 ›› 2023, Vol. 74 ›› Issue (6): 2589-2598.DOI: 10.11949/0438-1157.20230175
收稿日期:2023-03-01
修回日期:2023-04-14
出版日期:2023-06-05
发布日期:2023-07-27
通讯作者:
张光亚
作者简介:毛磊(1998—),男,硕士研究生,ml13547940286@163.com
基金资助:
Lei MAO( ), Guanzhang LIU, Hang YUAN, Guangya ZHANG(
), Guanzhang LIU, Hang YUAN, Guangya ZHANG( )
)
Received:2023-03-01
Revised:2023-04-14
Online:2023-06-05
Published:2023-07-27
Contact:
Guangya ZHANG
摘要:
利用碳酸酐酶(CAs)捕集CO2更符合可持续发展的理念,但亟需降低其分离纯化的成本和增强在复杂环境的生存能力。以铁蛋白(Ferritin)为标签,经linker把CAs与之相连,在胞内表达形成微米级难溶活性聚集体,经低速离心实现酶高效分离,酶活回收率达84.8%,活性聚集体超声30 min后,50℃孵育50 d酶活基本不变,在pH=9.0的缓冲液中半衰期为150 d。难溶活性CAs聚集体可转变为可溶性纳米CAs,其活力提升10倍以上,80℃时半衰期为(211±22)h。在15%(质量)离子液体[N1111][Gly](pH=11.64)中半衰期达(40.8±2.2)h,可用于后续离子液体和重组CAs联合吸收和再生CO2。静电作用是难溶活性CAs聚集体形成的重要原因之一。研究结果表明,CAs融合Ferritin后,既能极大简化制备过程,又能大幅提高稳定性,为酶法捕集CO2奠定了基础。
中图分类号:
毛磊, 刘冠章, 袁航, 张光亚. 可捕集CO2的纳米碳酸酐酶粒子的高效制备及性能研究[J]. 化工学报, 2023, 74(6): 2589-2598.
Lei MAO, Guanzhang LIU, Hang YUAN, Guangya ZHANG. Efficient preparation of carbon anhydrase nanoparticles capable of capturing CO2 and their characteristics[J]. CIESC Journal, 2023, 74(6): 2589-2598.
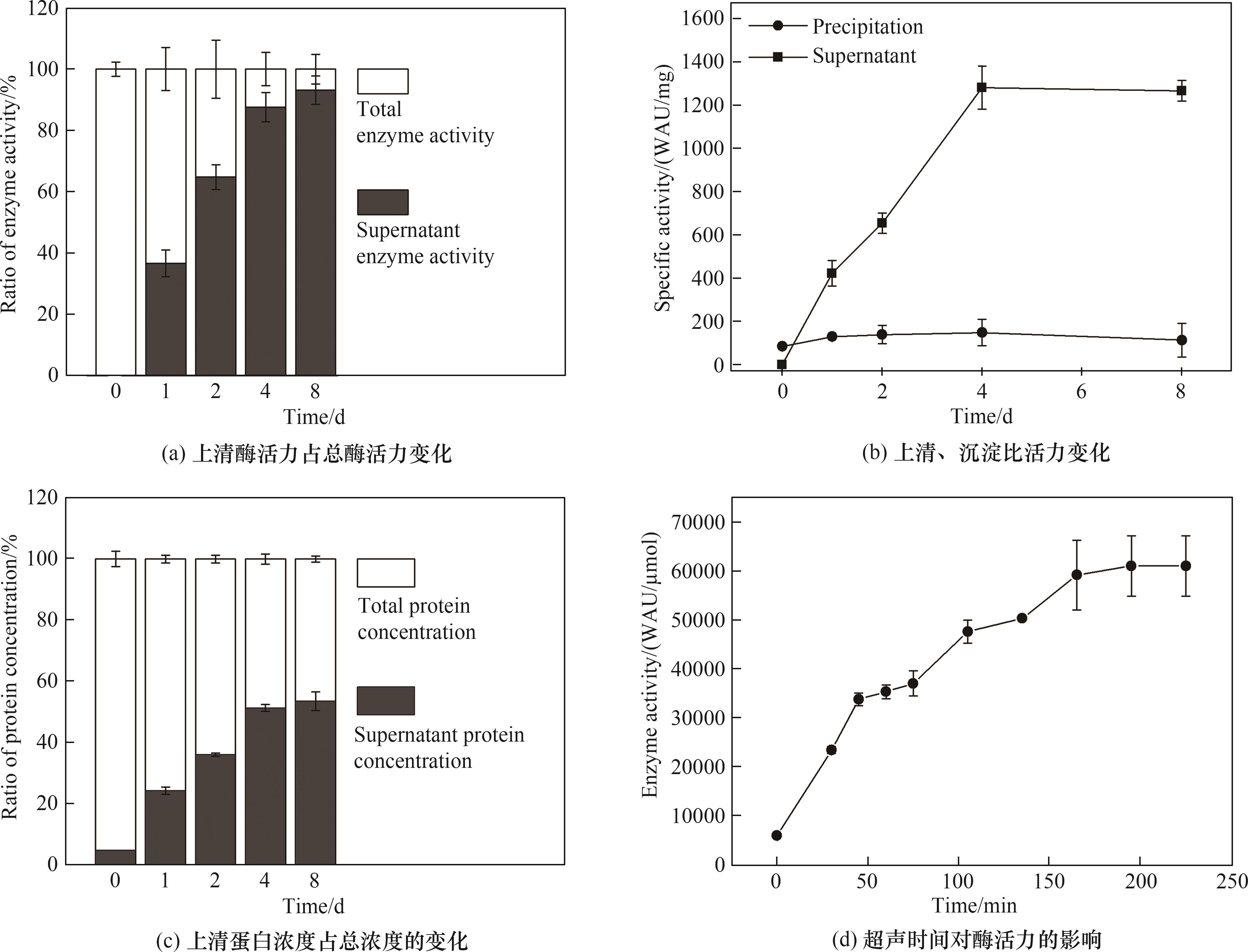
图2 难溶活性CAs聚集体自发复溶和超声加速形成可溶性纳米CAs
Fig.2 The difficultly soluble CAs aggregates spontaneously redissolved and transformed into soluble nano-CAS through ultrasound acceleration
| Category | pH=7.0 | pH=8.0 | pH=9.0 | pH=11.0 |
|---|---|---|---|---|
| SazCA-Ferritin monomer | -1.46 | -8.51 | -12.55 | -34.69 |
| SazCA monomer | 15.08 | 9.07 | 5.03 | -12.72 |
| Ferritin monomer | -2.69 | -6.19 | -8.1 | -17.64 |
表1 三种不同的单体在不同pH下的净表面电荷
Table 1 Net surface charges of three different monomers at different pH
| Category | pH=7.0 | pH=8.0 | pH=9.0 | pH=11.0 |
|---|---|---|---|---|
| SazCA-Ferritin monomer | -1.46 | -8.51 | -12.55 | -34.69 |
| SazCA monomer | 15.08 | 9.07 | 5.03 | -12.72 |
| Ferritin monomer | -2.69 | -6.19 | -8.1 | -17.64 |
| 1 | Zhang Y M, Zhu J Y, Hou J W, et al. Carbonic anhydrase membranes for carbon capture and storage[J]. Journal of Membrane Science Letters, 2022, 2(2): 100031. |
| 2 | Talekar S, Jo B H, Dordick J S, et al. Carbonic anhydrase for CO2 capture, conversion and utilization[J]. Current Opinion in Biotechnology, 2022, 74: 230-240. |
| 3 | Effendi S S W, Ng I. The prospective and potential of carbonic anhydrase for carbon dioxide sequestration: a critical review[J]. Process Biochemistry, 2019, 87: 55-65. |
| 4 | Patel H A, Byun J, Yavuz C T. Carbon dioxide capture adsorbents: chemistry and methods[J]. ChemSusChem, 2017, 10(7): 1303-1317. |
| 5 | Al-Mamoori A, Krishnamurthy A, Rownaghi A A, et al. Carbon capture and utilization update[J]. Energy Technology, 2017, 5(6): 834-849. |
| 6 | Farrelly D J, Everard C D, Fagan C C, et al. Carbon sequestration and the role of biological carbon mitigation: a review[J]. Renewable and Sustainable Energy Reviews, 2013, 21: 712-727. |
| 7 | Pu X, Han Y J. Promotion of carbon dioxide biofixation through metabolic and enzyme engineering[J]. Catalysts, 2022, 12(4): 399. |
| 8 | Yoshimoto M, Schweizer T, Rathlef M, et al. Immobilization of carbonic anhydrase in glass micropipettes and glass fiber filters for flow-through reactor applications[J]. ACS Omega, 2018, 3(8): 10391-10405. |
| 9 | Kanth B K, Lee J, Pack S P. Carbonic anhydrase: its biocatalytic mechanisms and functional properties for efficient CO2 capture process development[J]. Engineering in Life Sciences, 2013, 13(5): 422-431. |
| 10 | Yong J K J, Stevens G W, Caruso F, et al. The use of carbonic anhydrase to accelerate carbon dioxide capture processes[J]. Journal of Chemical Technology & Biotechnology, 2015, 90(1): 3-10. |
| 11 | Zaidi S, Srivastava N, Khare S K. Microbial carbonic anhydrase mediated carbon capture, sequestration & utilization: a sustainable approach to delivering bio-renewables[J]. Bioresource Technology, 2022, 365: 128174. |
| 12 | Yadav R R, Krishnamurthi K, Mudliar S N, et al. Carbonic anhydrase mediated carbon dioxide sequestration: promises, challenges and future prospects[J]. Journal of Basic Microbiology, 2014, 54(6): 472-481. |
| 13 | Di Fiore A, Alterio V, Monti S M, et al. Thermostable carbonic anhydrases in biotechnological applications[J]. International Journal of Molecular Sciences, 2015, 16(7): 15456-15480. |
| 14 | 张雷, 雷林超, 张光亚, 等. 基于foldon介导的寡聚化以提高阿魏酸酯酶催化效率[J]. 生物工程学报, 2019, 35(5): 816-826. |
| Zhang L, Lei L C, Zhang G Y, et al. Oligomerization triggered by foldon to enhance the catalytic efficiency of feruloyl esterase[J]. Chinese Journal of Biotechnology, 2019, 35(5): 816-826. | |
| 15 | Wang X Z, Ge H H, Zhang D D, et al. Oligomerization triggered by foldon: a simple method to enhance the catalytic efficiency of lichenase and xylanase[J]. BMC Biotechnology, 2017,17(1): 57. |
| 16 | Yang X F, Huang A, Peng J Z, et al. Self-assembly amphipathic peptides induce active enzyme aggregation that dramatically increases the operational stability of nitrilase[J]. RSC Advances, 2014, 4(105): 60675-60684. |
| 17 | Wen H, Zhang L, Du Y J, et al. Bimetal based inorganic-carbonic anhydrase hybrid hydrogel membrane for CO2 capture[J]. Journal of CO2 Utilization, 2020, 39: 101171. |
| 18 | Xv J, Zhang Z Y, Pang S Z, et al. Accelerated CO2 capture using immobilized carbonic anhydrase on polyethyleneimine/dopamine co-deposited MOFs[J]. Biochemical Engineering Journal, 2022, 189: 108719. |
| 19 | Ölçücü G, Klaus O, Jaeger K E, et al. Emerging solutions for in vivo biocatalyst immobilization: tailor-made catalysts for industrial biocatalysis[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(27): 8919-8945. |
| 20 | Xu T W, Huang X L, Li Z, et al. Enhanced purification efficiency and thermal tolerance of Thermoanaerobacterium aotearoense β-xylosidase through aggregation triggered by short peptides[J]. Journal of Agricultural and Food Chemistry, 2018, 66(16): 4182-4188. |
| 21 | Bulos J A, Guo R, Wang Z H, et al. Design of a superpositively charged enzyme: human carbonic anhydrase Ⅱ variant with ferritin encapsulation and immobilization[J]. Biochemistry, 2021, 60(47): 3596-3609. |
| 22 | Zhang J L, Chen X H, Hong J J, et al. Biochemistry of mammalian ferritins in the regulation of cellular iron homeostasis and oxidative responses[J]. Science China Life Sciences, 2021, 64(3): 352-362. |
| 23 | Yao H L, Soldano A, Fontenot L, et al. Pseudomonas aeruginosa bacterioferritin is assembled from FtnA and BfrB subunits with the relative proportions dependent on the environmental oxygen availability[J]. Biomolecules, 2022, 12(3): 366. |
| 24 | Honarmand E K, Hagedoorn P L, Hagen W R. Unity in the biochemistry of the iron-storage proteins ferritin and bacterioferritin[J]. Chemical Reviews, 2015, 115(1): 295-326. |
| 25 | Chen H, Tan X Y, Han X E, et al. Ferritin nanocage based delivery vehicles: from single-, co- to compartmentalized- encapsulation of bioactive or nutraceutical compounds[J]. Biotechnology Advances, 2022, 61: 108037. |
| 26 | Li H, Tan X Y, Xia X Y, et al. Improvement of thermal stability of oyster (Crassostrea gigas) ferritin by point mutation[J]. Food Chemistry, 2021, 346: 128879. |
| 27 | Liu G Z, Yuan H, Li X B, et al. Tailoring the properties of self-assembled carbonic anhydrase supraparticles for CO2 capture[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(37): 12374-12385. |
| 28 | Lin Z L, Jing Y Y, Huang Y, et al. A cleavable self-aggregating tag scheme for the expression and purification of disulfide bonded proteins and peptides[J]. Chemical Engineering Science, 2022, 262: 118052. |
| 29 | Shanbhag B K, Liu B Y, Fu J, et al. Self-assembled enzyme nanoparticles for carbon dioxide capture[J]. Nano Letters, 2016, 16(5): 3379-3384. |
| 30 | 葛慧华, 葛钟琪, 毛磊,等. 铁蛋白介导的地衣多糖酶胞内自发聚集及其高效制备[J]. 生物工程学报, 2022, 38(4): 1602-1611. |
| Ge H H, Ge Z Q, Mao L, et al. In vivo self-aggregation and efficient preparation of recombinant lichenase based on ferritin[J]. Chinese Journal of Biotechnology, 2022, 38(4): 1602-1611. | |
| 31 | De Luca V, Vullo D, Scozzafava A, et al. An α-carbonic anhydrase from the thermophilic bacterium Sulphurihydrogenibium azorense is the fastest enzyme known for the CO2 hydration reaction[J]. Bioorganic & Medicinal Chemistry, 2013, 21(6): 1465-1469. |
| 32 | Kumari M, Lee J, Lee D W, et al. High-level production in a plant system of a thermostable carbonic anhydrase and its immobilization on microcrystalline cellulose beads for CO2 capture[J]. Plant Cell Reports, 2020, 39(10): 1317-1329. |
| 33 | Zhu Y Z, Liu Y R, Ai M M, et al. Surface display of carbonic anhydrase on Escherichia coli for CO2 capture and mineralization[J]. Synthetic and Systems Biotechnology, 2022, 7(1): 460-473. |
| 34 | Hsieh C J, Cheng J C, Hu C J, et al. Entrapment of the fastest known carbonic anhydrase with biomimetic silica and its application for CO2 sequestration[J]. Polymers, 2021, 13(15): 2452. |
| 35 | Kim S, Joo K I, Jo B H, et al. Stability-controllable self-immobilization of carbonic anhydrase fused with a silica-binding tag onto diatom biosilica for enzymatic CO2 capture and utilization[J]. ACS Applied Materials & Interfaces, 2020, 12(24): 27055-27063. |
| 36 | Shin S, Kim H S, Kim M I, et al. Crowding and confinement effects on enzyme stability in mesoporous silicas[J]. International Journal of Biological Macromolecules, 2020, 144: 118-126. |
| 37 | Rios N S, Arana-Peña S, Mendez-Sanchez C, et al. Increasing the enzyme loading capacity of porous supports by a layer-by-layer immobilization strategy using PEI as glue[J]. Catalysts, 2019, 9(7): 576. |
| 38 | Tan S I, Han Y L, Yu Y J, et al. Efficient carbon dioxide sequestration by using recombinant carbonic anhydrase[J]. Process Biochemistry, 2018, 73: 38-46. |
| 39 | Steger F, Reich J, Fuchs W, et al. Comparison of carbonic anhydrases for CO2 sequestration[J]. International Journal of Molecular Sciences, 2022, 23(2): 957. |
| 40 | Song N N, Zhang J L, Zhai J, et al. Ferritin: a multifunctional nanoplatform for biological detection, imaging diagnosis, and drug delivery[J]. Accounts of Chemical Research, 2021, 54(17): 3313-3325. |
| 41 | Alam P, Siddiqi K, Chturvedi S K, et al. Protein aggregation: from background to inhibition strategies[J]. International Journal of Biological Macromolecules, 2017, 103: 208-219. |
| 42 | Wang W, Nema S, Teagarden D. Protein aggregation—pathways and influencing factors[J]. International Journal of Pharmaceutics, 2010, 390(2): 89-99. |
| 43 | Minton A P. Influence of macromolecular crowding upon the stability and state of association of proteins: predictions and observations[J]. Journal of Pharmaceutical Sciences, 2005, 94(8): 1668-1675. |
| 44 | Munishkina L A, Henriques J, Uversky V N, et al. Role of protein-water interactions and electrostatics in α-synuclein fibril formation[J]. Biochemistry, 2004, 43(11): 3289-3300. |
| 45 | Jackson G S, Hosszu L L P, Power A, et al. Reversible conversion of monomeric human prion protein between native and fibrilogenic conformations[J]. Science, 1999, 283(5409): 1935-1937. |
| 46 | Holst J V, Versteeg G F, Brilman D W F, et al. Kinetic study of CO2 with various amino acid salts in aqueous solution[J]. Chemical Engineering Science, 2009, 64(1): 59-68. |
| 47 | Portugal A F, Sousa J M, Magalhães F D, et al. Solubility of carbon dioxide in aqueous solutions of amino acid salts[J]. Chemical Engineering Science, 2009, 64(9): 1993-2002. |
| 48 | He L, Xie H G, Zong Y, et al. Enhancing CO2 absorption with amino acid ionic liquid [N1111][Gly] aqueous solution by twin-liquid film flow: experimental and numerical study[J]. Chemical Engineering Science, 2022, 256: 117691. |
| 49 | 周兰娟. 氨基酸离子液体[N1111][Gly]溶液吸收CO2的研究[D]. 泉州:华侨大学,2012. |
| Zhou L J. Study on absorption of carbon dioxide by amino acid ionic liquid [N1111][Gly] solution[D]. Quanzhou: Huaqiao University, 2012. | |
| 50 | Alvizo O, Nguyen L J, Savile C K, et al. Directed evolution of an ultrastable carbonic anhydrase for highly efficient carbon capture from flue gas[J]. Proceedings of the National Academy of Sciences, 2014, 111(46): 16436-16441. |
| [1] | 王琪, 张斌, 张晓昕, 武虎建, 战海涛, 王涛. 氯铝酸-三乙胺离子液体/P2O5催化合成伊索克酸和2-乙基蒽醌[J]. 化工学报, 2023, 74(S1): 245-249. |
| [2] | 车睿敏, 郑文秋, 王小宇, 李鑫, 许凤. 基于离子液体的纤维素均相加工研究进展[J]. 化工学报, 2023, 74(9): 3615-3627. |
| [3] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [4] | 陆俊凤, 孙怀宇, 王艳磊, 何宏艳. 离子液体界面极化及其调控氢键性质的分子机理[J]. 化工学报, 2023, 74(9): 3665-3680. |
| [5] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [6] | 宋明昊, 赵霏, 刘淑晴, 李国选, 杨声, 雷志刚. 离子液体脱除模拟油中挥发酚的多尺度模拟与研究[J]. 化工学报, 2023, 74(9): 3654-3664. |
| [7] | 杨绍旗, 赵淑蘅, 陈伦刚, 王晨光, 胡建军, 周清, 马隆龙. Raney镍-质子型离子液体体系催化木质素平台分子加氢脱氧制备烷烃[J]. 化工学报, 2023, 74(9): 3697-3707. |
| [8] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [9] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [10] | 王俐智, 杭钱程, 郑叶玲, 丁延, 陈家继, 叶青, 李进龙. 离子液体萃取剂萃取精馏分离丙酸甲酯+甲醇共沸物[J]. 化工学报, 2023, 74(9): 3731-3741. |
| [11] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [12] | 孟令玎, 崇汝青, 孙菲雪, 孟子晖, 刘文芳. 改性聚乙烯膜和氧化硅固定化碳酸酐酶[J]. 化工学报, 2023, 74(8): 3472-3484. |
| [13] | 陈雅鑫, 袁航, 刘冠章, 毛磊, 杨纯, 张瑞芳, 张光亚. 蛋白质纳米笼介导的酶自固定化研究进展[J]. 化工学报, 2023, 74(7): 2773-2782. |
| [14] | 汤晓玲, 王嘉瑞, 朱玄烨, 郑仁朝. 基于Pickering乳液的卤醇脱卤酶催化合成手性环氧氯丙烷[J]. 化工学报, 2023, 74(7): 2926-2934. |
| [15] | 张缘良, 栾昕奇, 苏伟格, 李畅浩, 赵钟兴, 周利琴, 陈健民, 黄艳, 赵祯霞. 离子液体复合萃取剂选择性萃取尼古丁的研究及DFT计算[J]. 化工学报, 2023, 74(7): 2947-2956. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号