化工学报 ›› 2021, Vol. 72 ›› Issue (7): 3680-3685.DOI: 10.11949/0438-1157.20201838
洪燕珍( ),王笛,李卓昱,徐亚南,王宏涛,苏玉忠,彭丽,李军(
),王笛,李卓昱,徐亚南,王宏涛,苏玉忠,彭丽,李军( )
)
收稿日期:2020-12-16
修回日期:2021-01-21
出版日期:2021-07-05
发布日期:2021-07-05
通讯作者:
李军
作者简介:洪燕珍(1981—),女,硕士研究生,工程师,基金资助:
HONG Yanzhen( ),WANG Di,LI Zhuoyu,XU Yanan,WANG Hongtao,SU Yuzhong,PENG Li,LI Jun(
),WANG Di,LI Zhuoyu,XU Yanan,WANG Hongtao,SU Yuzhong,PENG Li,LI Jun( )
)
Received:2020-12-16
Revised:2021-01-21
Online:2021-07-05
Published:2021-07-05
Contact:
LI Jun
摘要:
提出超临界二氧化碳介入α-松油醇异构合成1,8-桉叶素的方法,并采用二极管阵列检测器测量超临界二氧化碳下α-松油醇/环己烷体系的最大吸收波长研究二氧化碳加入量与极性的关系。考察了溶剂体系极性、溶剂量和二氧化碳压力对磷钨酸/聚离子液体(PW/PIL)催化剂催化α-松油醇异构合成1,8-桉叶素的影响,并探讨了超临界二氧化碳介质中,PW/PIL催化剂催化α-松油醇异构合成1,8-桉叶素可能的反应机理。开发了一种绿色高效的1,8-桉叶素合成工艺,当环己烷/α-松油醇的质量比10∶1,PW/PIL催化剂/α-松油醇的摩尔比0.0163∶1,CO2压力19.0 MPa,50℃反应8 h时,α-松油醇的转化率为89.3%,1,8-桉叶素的选择性为54.6%。研究表明,超临界二氧化碳起到共溶剂、膨胀溶剂、降低溶剂体系极性的三重作用,从而改善了催化剂的团聚现象,提高了1,8-桉叶素的选择性。
中图分类号:
洪燕珍, 王笛, 李卓昱, 徐亚南, 王宏涛, 苏玉忠, 彭丽, 李军. 超临界二氧化碳介入的α-松油醇催化合成1,8-桉叶素[J]. 化工学报, 2021, 72(7): 3680-3685.
HONG Yanzhen, WANG Di, LI Zhuoyu, XU Yanan, WANG Hongtao, SU Yuzhong, PENG Li, LI Jun. Catalytic isomerization of α-terpineol to 1,8-cineole in supercritical carbon dioxide[J]. CIESC Journal, 2021, 72(7): 3680-3685.
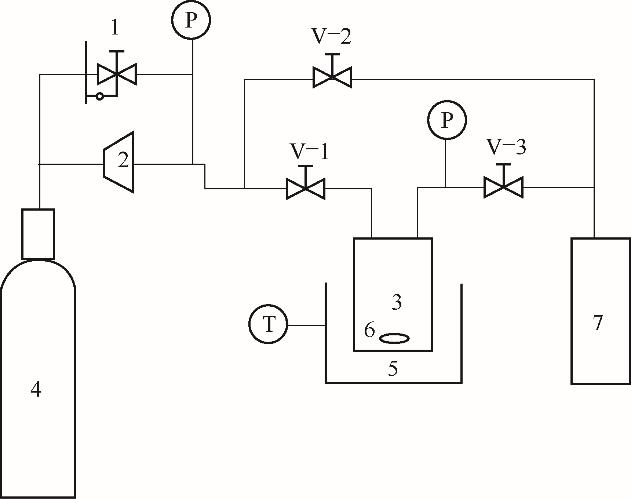
图1 超临界CO2反应装置图1—背压阀;2—压缩机;3—高压釜;4—CO2气瓶;5—水浴锅;6—磁子;7—缓冲釜;V-1~V-3—截止阀;T—控温系统;P—压力表
Fig.1 The schematic diagram for reaction in supercritical CO21—back pressure regulator; 2—compressor; 3—high pressure reactor; 4—cylinder; 5—water bath; 6—magnetic stirrer; 7—buffer tank; V-1—V-3—valves; T—temperature controller; P—pressure gage
| 压力/MPa | 最大吸收波长 /nm | 仪器 | |
|---|---|---|---|
| 9 | 9∶1 | 211 | SFC Pre30 |
| 9 | 8∶2 | 215 | SFC Pre30 |
| 9 | 7∶3 | 222 | SFC Pre30 |
| 0.1 | — | 224 | 常压UV-780 |
表1 α-松油醇/环己烷体系的最大吸收波长
Table 1 The maximum absorption wavelength of the α-terpineol/cyclohexane mixture
| 压力/MPa | 最大吸收波长 /nm | 仪器 | |
|---|---|---|---|
| 9 | 9∶1 | 211 | SFC Pre30 |
| 9 | 8∶2 | 215 | SFC Pre30 |
| 9 | 7∶3 | 222 | SFC Pre30 |
| 0.1 | — | 224 | 常压UV-780 |
| 1 | 尹晓燕, 王燕燕. 1, 8-桉叶素药理作用及其机制研究进展[J]. 生命的化学, 2020, 40(11): 2026-2034. |
| Yin X Y, Wang Y Y. Research progress on pharmacological activities and mechanism of 1, 8-cineole[J]. Chemistry of Life, 2020, 40(11): 2026-2034. | |
| 2 | Greiner J F W, Müller J, Zeuner M T, et al. 1, 8-Cineol inhibits nuclear translocation of NF-κB p65 and NF-κB-dependent transcriptional activity[J]. Biochimica et Biophysica Acta, 2013, 1833(12): 2866-2878. |
| 3 | Santos F A, Rao V S N. Antiinflammatory and antinociceptive effects of 1, 8-cineole a terpenoid oxide present in many plant essential oils[J]. Phytotherapy Research, 2000, 14(4): 240-244. |
| 4 | Southwell I. Eucalyptus leaf oils: use, chemistry, distillation and marketing[J]. Journal of Chromatography A, 1992, 598(2): 316. |
| 5 | 田玉红, 张祥民, 黄泰松, 等. 桉叶油的研究进展[J]. 食品与发酵工业, 2007, 33(10): 139-143. |
| Tian Y H, Zhang X M, Huang T S, et al. Research advances on the essential oils from leaves of eucalyptus[J]. Food and Fermentation Industries, 2007, 33(10): 139-143. | |
| 6 | 李士雨, 王静康. 桉叶素应用与制备综述[J]. 精细化工, 1995, 12(5): 12-15. |
| Li S Y, Wang J K. A review about the application and refinement of 1,8-cineole[J]. Fine Chemicals, 1995, 12(5): 12-15. | |
| 7 | Wu H W, Hendrawinata W, Yu Y, et al. Effect of hydrodistillation on 1, 8-cineole extraction from mallee leaf and the fuel properties of spent biomass[J]. Industrial & Engineering Chemistry Research, 2011, 50(19): 11280-11287. |
| 8 | 田玉红. 广西桉叶挥发性成分分析及抗菌抗氧化性能研究[D]. 南宁: 广西大学, 2006. |
| Tian Y H. Chemical composition, antimicrobial and antioxidative properties of volatile components from leaves of eucalyptus growing in Guangxi[D]. Nanning: Guangxi University, 2006. | |
| 9 | 田玉红, 刘雄民, 周永红, 等. 圆角桉叶精油的化学成分[J]. 食品科学, 2007, 28(1): 36-38. |
| Tian Y H, Liu X M, Zhou Y H, et al. Chemical compositions of essential oil of eucalyptus umbellate growing in Guangxi[J]. Food Science, 2007, 28(1): 36-38. | |
| 10 | 王健英. 1,8-桉叶油素的提取与提纯[D]. 天津: 天津大学, 2004. |
| Wang J Y. The extraction and purify of 1, 8-cineole[D]. Tianjin: Tianjin University, 2004. | |
| 11 | 应安国, 许松林, 徐世民. 间歇真空精馏提纯桉叶油的研究[J]. 林产工业, 2005, 32(2): 29-31. |
| 31 | Nishimura T, Okuhara T, Misono M. High catalytic activities of pseudoliquid phase of dodecatungstophosphoric acid for reactions of polar molecules[J]. Chemistry Letters, 1991, 20(10): 1695-1698. |
| 32 | Lee K Y, Arai T, Nakata S, et al. Catalysis by heteropoly compounds(20): an NMR study of ethanol dehydration in the pseudoliquid phase of 12-tungstophosphoric acid[J]. Journal of the American Chemical Society, 1992, 114(8): 2836-2842. |
| 11 | Ying A G, Xu S L, Xu S M. Purification of 1, 8-cineol by vacuum batch distillation[J]. China Forest Products Industry, 2005, 32(2): 29-31. |
| 12 | 李士雨. 桉叶素的纯化[J]. 精细化工, 2006, 23(1): 35-37. |
| Li S Y. Purification process of 1,8-cineole[J]. Fine Chemicals, 2006, 23(1): 35-37. | |
| 13 | 胡翔飞, 叶志恒. 松油醇合成1,8-桉叶素的方法: 108558902A[P]. 2018-09-21. |
| Hu X F, Ye Z H. Method for synthesizing 1,8-cineole from terpilenol: 108558902A[P]. 2018-09-21. | |
| 14 | Mitchell P. Method of preparing cineoles: EP0300117 (B1) [P]. 1989-01-25. |
| 15 | Fariña L, Boido E, Carrau F, et al. Terpene compounds as possible precursors of 1, 8-cineole in red grapes and wines[J]. Journal of Agricultural and Food Chemistry, 2005, 53(5): 1633-1636. |
| 16 | 崔军涛, 汪锦航, 黄金龙, 等. α-松油醇合成1,8-桉叶素的方法: 106366090A[P]. 2017-02-01. |
| Cui J T, Wang J H, Huang J L, et al. Method of synthesizing1, 8-cineole from α-terpilenol: 106366090A[P]. 2017-02-01. | |
| 17 | Leão Lana E J, da Silva Rocha K A, Kozhevnikov I V, et al. Synthesis of 1, 8-cineole and 1, 4-cineole by isomerization of α-terpineol catalyzed by heteropoly acid[J]. Journal of Molecular Catalysis A: Chemical, 2006, 259(1/2): 99-102. |
| 18 | Goussevskaia E V, Rocha K A D S, Lana E J L. Process for the preparation of 1,4- and 1,8-cineols via polyhetero acid catalyzed isomerization of α-terpineol: BRPI0605089A[P]. 2008-05-20. |
| 19 | 张东丽. 负载型磷钨酸催化合成1,8-桉叶素的研究[D]. 厦门: 厦门大学, 2014. |
| Zhang D L. Study of 1,8-cineole synthesis by supported phosphotungstic acids catalyst[D]. Xiamen: Xiamen University, 2014. | |
| 20 | 王宏涛, 张东丽, 郑哲楠, 等. 一种合成1,8-桉叶素的固体杂多酸催化剂的制备方法: 103785468A[P]. 2014-05-14. |
| Wang H T, Zhang D L, Zheng Z N, et al. Preparation method for solid heteropolyacid catalyst for synthesizing 1,8-cineole: 103785468A[P]. 2014-05-14. | |
| 21 | Wu S S, Wang J, Zhang W H, et al. Preparation of keggin and preyssler heteropolyacid catalysts on amine-modified SBA-15 and their catalytic performances in esterification of n-butanol with acetic acid[J]. Catalysis Letters, 2008, 125(3/4): 308-314. |
| 22 | Wu S S, Liu P, Leng Y, et al. Heteropolyacids anchored on amino-functionalized MCM-41 via condensation as reusable catalysts for esterification[J]. Catalysis Letters, 2009, 132(3/4): 500-505. |
| 23 | Wu R J, Song L, Ma G F, et al. Phosphotungstic acid immobilized on imidazole poly(ionic liquid) for isomerization of α-terpineol to 1, 8-cineole[J]. Catalysis Letters, 2017, 147(11): 2736-2744. |
| 24 | 吴榕君. 咪唑类聚离子液体固载磷钨酸及其催化α-松油醇异构合成1, 8-桉叶素的研究[D]. 厦门: 厦门大学, 2017. |
| Wu R J. Study of phosphotungstic acid immobilized on imidazolium-based poly (ionic liquid)and its application for isomerization of α-terpineol to 1, 8-cineole[D]. Xiamen: Xiamen University, 2017. | |
| 25 | 王宏涛, 吴榕君, 李军, 等. 一种负载型杂多酸催化剂及其制备方法与应用: 106732779B[P]. 2019-07-09. |
| Wang H T, Wu R J, Li J, et al. Supported heteropolyacid catalyst, and preparation method and application thereof: 106732779B[P]. 2019-07-09. | |
| 26 | 王宏涛, 王笛, 李军, 等. 一种α-松油醇合成1,8-桉叶素的方法: 109810118B[P]. 2020-09-22. |
| Wang H T, Wang D, Li J, et al. Method for synthetizing1,8-eudesmin from α-terpilenol: 109810118B[P]. 2020-09-22. | |
| 27 | Kelley S P, Lemert R M. Solvatochromic characterization of the liquid phase in liquid-supercritical CO2 mixtures[J]. AIChE Journal, 1996, 42(7): 2047-2056. |
| 28 | Hua D, Hong J D, Hu X H, et al. Solid-liquid-gas equilibrium of the naphthalene-biphenyl-CO2 system: measurement and modeling[J]. Fluid Phase Equilibria, 2010, 299(1): 109-115. |
| 29 | 花丹, 洪燕珍, 苏玉忠, 等. 高压等温结晶研究装置的建立及初步应用[J]. 化工进展, 2011, 30(S1): 59-61. |
| Hua D, Hong Y Z, Su Y Z, et al. An apparatus for high-pressure isothermal crystallization study and its tentative application[J]. Chemical Industry and Engineering Progress, 2011, 30(S1): 59-61. | |
| 30 | 王恩波, 赵世良, 郑汝骊. 杂多酸型催化剂[J]. 石油化工, 1985, 14(10): 615-625. |
| Wang E B, Zhao S L, Zheng R L. Heteropolyacids catalyst[J]. Petrochemical Technology, 1985, 14(10): 615-625. |
| [1] | 张义飞, 刘舫辰, 张双星, 杜文静. 超临界二氧化碳用印刷电路板式换热器性能分析[J]. 化工学报, 2023, 74(S1): 183-190. |
| [2] | 洪瑞, 袁宝强, 杜文静. 垂直上升管内超临界二氧化碳传热恶化机理分析[J]. 化工学报, 2023, 74(8): 3309-3319. |
| [3] | 朱兵国, 何吉祥, 徐进良, 彭斌. 冷却条件下渐扩/渐缩管内超临界压力二氧化碳的传热特性[J]. 化工学报, 2023, 74(3): 1062-1072. |
| [4] | 许婉婷, 许波, 王鑫, 陈振乾. 方形微通道内超临界CO2流动换热特性研究[J]. 化工学报, 2022, 73(4): 1534-1545. |
| [5] | 孙铭泽, 马宁, 李浩然, 姜海峰, 洪文鹏, 牛晓娟. 中低温超临界CO2及其混合工质布雷顿循环热力学分析[J]. 化工学报, 2022, 73(3): 1379-1388. |
| [6] | 汪森林, 李照志, 邵应娟, 钟文琪. 超临界二氧化碳垂直管内传热恶化数值模拟研究[J]. 化工学报, 2022, 73(3): 1072-1082. |
| [7] | 尤红运, 林景骏, 黄凯越, 舒日洋, 田志鹏, 王超, 陈颖. 溶剂效应对木质素酚类化合物加氢反应的影响机理[J]. 化工学报, 2022, 73(10): 4498-4506. |
| [8] | 颜建国, 郑书闽, 郭鹏程, 张博, 毛振凯. 基于GA-BP神经网络的超临界CO2传热特性预测研究[J]. 化工学报, 2021, 72(9): 4649-4657. |
| [9] | 严如奇, 丁雪兴, 徐洁, 洪先志, 包鑫. 基于湍流模型的S-CO2干气密封流场与稳态性能分析[J]. 化工学报, 2021, 72(8): 4292-4303. |
| [10] | 李恩泽, 叶培远, 王亚欣, 康锦, 阴彩霞, 程芳琴. 溶剂极性响应型丁基-环四联吡啶提锂分子的合成及性能研究[J]. 化工学报, 2021, 72(6): 3014-3021. |
| [11] | 江锦波, 滕黎明, 孟祥铠, 李纪云, 彭旭东. 基于多变量摄动的超临界CO2干气密封动态特性[J]. 化工学报, 2021, 72(4): 2190-2202. |
| [12] | 严如奇, 洪先志, 包鑫, 徐洁, 丁雪兴. 超临界二氧化碳干气密封相态分布规律与密封性能研究[J]. 化工学报, 2020, 71(8): 3681-3690. |
| [13] | 蒋瑞, 胡冬冬, 刘涛, 赵玲. 热塑性聚醚酯弹性体硬段含量对其超临界CO 2发泡行为的影响[J]. 化工学报, 2020, 71(2): 871-878. |
| [14] | 李子辉,蒋晶,金章勇,蔡泊志,曹永俊,李倩. 生物可降解PCL/PLA开孔发泡材料制备及吸油性能[J]. 化工学报, 2020, 71(12): 5842-5853. |
| [15] | 郭晓璐,喻健良,闫兴清,徐鹏,徐双庆. 超临界CO2管道泄漏特性研究进展[J]. 化工学报, 2020, 71(12): 5430-5442. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号