化工学报 ›› 2021, Vol. 72 ›› Issue (6): 3014-3021.DOI: 10.11949/0438-1157.20201711
李恩泽1( ),叶培远1,王亚欣1,康锦1,阴彩霞2,程芳琴1(
),叶培远1,王亚欣1,康锦1,阴彩霞2,程芳琴1( )
)
收稿日期:2020-11-30
修回日期:2021-03-05
出版日期:2021-06-05
发布日期:2021-06-05
通讯作者:
程芳琴
作者简介:李恩泽(1988—),男,博士,副教授,基金资助:
LI Enze1( ),YE Peiyuan1,WANG Yaxin1,KANG Jin1,YIN Caixia2,CHENG Fangqin1(
),YE Peiyuan1,WANG Yaxin1,KANG Jin1,YIN Caixia2,CHENG Fangqin1( )
)
Received:2020-11-30
Revised:2021-03-05
Online:2021-06-05
Published:2021-06-05
Contact:
CHENG Fangqin
摘要:
盐湖卤水提锂已逐渐成为我国锂及锂产品的生产途径之一,而我国盐湖卤水高镁锂比的特点导致锂离子提取难度大。传统溶剂萃取提锂过程中需使用大量协萃剂和高浓度酸,产品纯度低、危险度高。设计合成了一种具有溶剂极性响应性分子结构“异构互变”的丁基-环四联吡啶提锂分子,实现极性条件下“络合”锂,非极性条件下“释放”锂。核磁共振氢谱和高分辨质谱证实了目标分子结构的准确性,通过对比Li+和Mg2+存在时目标分子的光谱性质表明丁基-环四联吡啶分子对锂离子具有较强的选择性。此外,支撑液膜的离子跨膜传输结果进一步表明,丁基-环四联吡啶分子在不同极性溶剂条件下可以实现对锂离子的高选择性提取。
中图分类号:
李恩泽, 叶培远, 王亚欣, 康锦, 阴彩霞, 程芳琴. 溶剂极性响应型丁基-环四联吡啶提锂分子的合成及性能研究[J]. 化工学报, 2021, 72(6): 3014-3021.
LI Enze, YE Peiyuan, WANG Yaxin, KANG Jin, YIN Caixia, CHENG Fangqin. Synthesis and properties of solvent polar responsive butyl-cyclic tetrapyridine molecule for lithium extraction[J]. CIESC Journal, 2021, 72(6): 3014-3021.

图1 6-丁基-3,6-二氰基-环四联吡啶分子的合成路线及不同溶剂极性下分子结构的可逆变化和络合锂离子示意图
Fig.1 The synthetic route of butyl tetrapyridine, reversible changes of molecular structure under different solvent polarity and schematic complexion of lithium ion
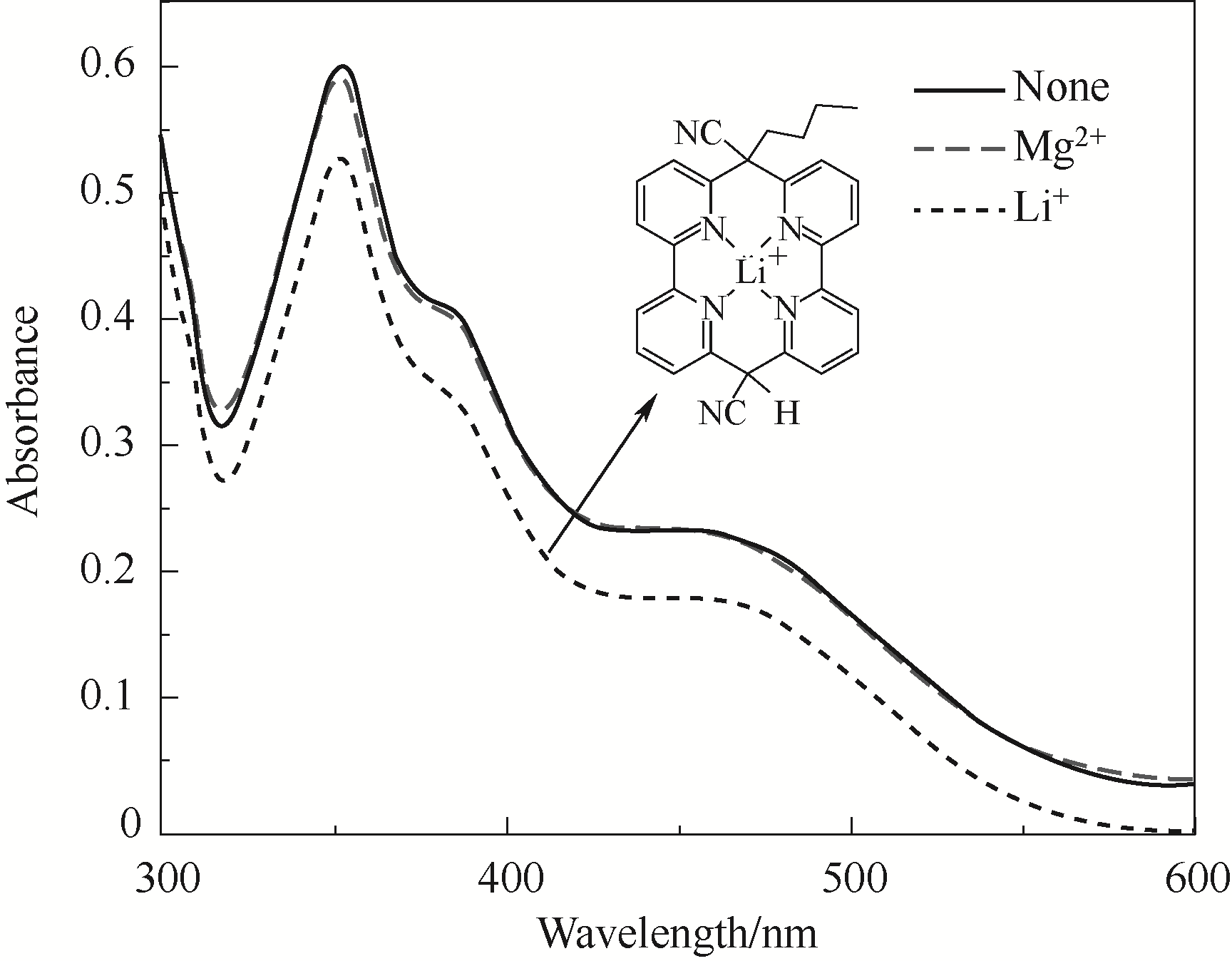
图7 Li+和Mg2+存在时化合物C在1,4-二氧六环-水溶剂中的紫外-可见光谱图(实验中采用LiCl和MgCl2,Li+和Mg2+的浓度均为6×10-2 mol/L,1,4-二氧六环和水的体积比为1∶1)
Fig.7 The UV-Vis absorption spectra of compound C in the presence of Li+ or Mg2+ in 1,4-dioxan aqueous solution (LiCl and MgCl2 were used as salts, both the concentrations of Li+ and Mg2+ were 6×10-2 mol/L, the volume ratio of 1,4-dioxane to H2O was 1∶1)

图8 Li+或Mg2+存在时化合物C在1,4-二氧六环-水溶液中的高分辨质谱图(化合物C的浓度为1×10-6 mol/L,1,4-二氧六环和水的体积比为1∶1)
Fig.8 HRMS spectrum of compound C in the presence of Li+ or Mg2+ in 1,4-dioxan aqueous solution (the concentration of compound C was 1×10-6 mol/L, the volume ratio of 1,4-dioxane to H2O was 1∶1)
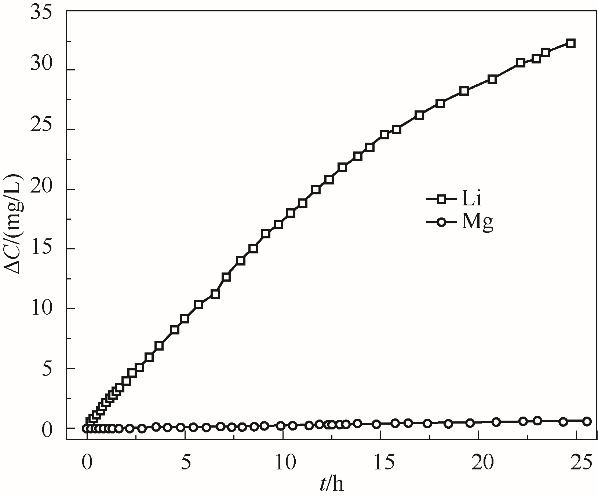
图9 Li+和Mg2+在跨膜传输过程中接收相阳离子浓度增加量随时间的变化
Fig.9 The increment of the receiving phase cation concentration varies against time during the transmembrane transmission of Li+ and Mg2+
| 离子 | 接收相增加质量(m接)/mg | 原料相剩余质量(m原)/mg | 分配系数(Kd) | 选择性分离因子(α) |
|---|---|---|---|---|
| Li+ | 3.553 | 63.946 | 0.056 | 56 |
| Mg2+ | 0.069 | 67.430 | 0.001 |
表1 跨膜传输后Li+和Mg2+的分配系数和选择性分离因子
Table 1 The partition coefficient and selective separation factor of Li+ and Mg2+ after membrane transport
| 离子 | 接收相增加质量(m接)/mg | 原料相剩余质量(m原)/mg | 分配系数(Kd) | 选择性分离因子(α) |
|---|---|---|---|---|
| Li+ | 3.553 | 63.946 | 0.056 | 56 |
| Mg2+ | 0.069 | 67.430 | 0.001 |
| 1 | Liu X H, Chen X Y, He L H, et al. Study on extraction of lithium from salt lake brine by membrane electrolysis[J]. Desalination, 2015, 376: 35-40. |
| 2 | Swain B. Recovery and recycling of lithium: a review[J]. Separation and Purification Technology, 2017, 172: 388-403. |
| 3 | Li H F, Li L J, Peng X W, et al. Extraction kinetics of lithium from salt lake brine by N, N-bis(2-ethylhexyl) acetamide using Lewis cell[J]. Hydrometallurgy, 2018, 178: 84-87. |
| 4 | Shi D, Zhang L C, Peng X W, et al. Extraction of lithium from salt lake brine containing boron using multistage centrifuge extractors[J]. Desalination, 2018, 441: 44-51. |
| 5 | Song J F, Nghiem L D, Li X M, et al. Lithium extraction from Chinese salt-lake brines: opportunities, challenges, and future outlook[J]. Environmental Science: Water Research & Technology, 2017, 3(4): 593-597. |
| 6 | Song J F, Huang T, Qiu H B, et al. Recovery of lithium from salt lake brine of high Mg/Li ratio using Na[FeCl4*2TBP] as extractant: thermodynamics, kinetics and processes[J]. Hydrometallurgy, 2017, 173: 63-70. |
| 7 | Sun Y, Wang Q, Wang Y H, et al. Recent advances in magnesium/lithium separation and lithium extraction technologies from salt lake brine[J]. Separation and Purification Technology, 2021, 256: 117807. |
| 8 | Xiang W, Liang S K, Zhou Z Y, et al. Extraction of lithium from salt lake brine containing borate anion and high concentration of magnesium[J]. Hydrometallurgy, 2016, 166: 9-15. |
| 9 | Wang Y, Liu H T, Fan J H, et al. Recovery of lithium ions from salt lake brine with a high magnesium/lithium ratio using heteropolyacid ionic liquid[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(3): 3062-3072. |
| 10 | 赵旭, 张琦, 武海虹, 等. 盐湖卤水提锂[J]. 化学进展, 2017, 29(7): 796-808. |
| Zhao X, Zhang Q, Wu H H, et al. Extraction of lithium from salt lake brine[J]. Progress in Chemistry, 2017, 29(7): 796-808. | |
| 11 | Nie X Y, Sun S Y, Song X F, et al. Further investigation into lithium recovery from salt lake brines with different feed characteristics by electrodialysis[J]. Journal of Membrane Science, 2017, 530: 185-191. |
| 12 | Zhang C Y, Mu Y X, Zhang W, et al. PVC-based hybrid membranes containing metal-organic frameworks for Li+/Mg2+ separation[J]. Journal of Membrane Science, 2020, 596: 117724. |
| 13 | Zhou Z Y, Qin W, Liu Y, et al. Extraction equilibria of lithium with tributyl phosphate in kerosene and FeCl3[J]. Journal of Chemical & Engineering Data, 2012, 57(1): 82-86. |
| 14 | Zhou Z Y, Liu H T, Fan J H, et al. Selective extraction of lithium ion from aqueous solution with sodium phosphomolybdate as a coextraction agent[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(9): 8885-8892. |
| 15 | Masmoudi A, Zante G, Trébouet D, et al. Solvent extraction of lithium ions using benzoyltrifluoroacetone in new solvents[J]. Separation and Purification Technology, 2021, 255: 117653. |
| 16 | Yu X P, Fan X B, Guo Y F, et al. Recovery of lithium from underground brine by multistage centrifugal extraction using tri-isobutyl phosphate[J]. Separation and Purification Technology, 2019, 211: 790-798. |
| 17 | Zhou Z Y, Fan J H, Liu X T, et al. Recovery of lithium from salt-lake brines using solvent extraction with TBP as extractant and FeCl3 as co-extraction agent[J]. Hydrometallurgy, 2020, 191: 105244. |
| 18 | 张燕辉, 李丽娟, 李晋峰, 等. 磷酸三丁酯从盐湖卤水中萃取锂的机理研究[J]. 无机盐工业, 2012, 44(3): 12-15, 24. |
| Zhang Y H, Li L J, Li J F, et al. Study on mechanism of extraction of lithium from salt lake brine by tributylphosphate[J]. Inorganic Chemicals Industry, 2012, 44(3): 12-15, 24. | |
| 19 | Zhou Z Y, Qin W, Chu Y F, et al. Elucidation of the structures of tributyl phosphate/Li complexes in the presence of FeCl3via UV-visible, Raman and IR spectroscopy and the method of continuous variation[J]. Chemical Engineering Science, 2013, 101: 577-585. |
| 20 | Shi D, Cui B, Li L J, et al. Lithium extraction from low-grade salt lake brine with ultrahigh Mg/Li ratio using TBP - kerosene - FeCl3 system[J]. Separation and Purification Technology, 2019, 211: 303-309. |
| 21 | Li E Z, Kang J, Ye P Y, et al. A prospective material for the highly selective extraction of lithium ions based on a photochromic crowned spirobenzopyran[J]. Journal of Materials Chemistry B, 2019, 7(6): 903-907. |
| 22 | Li E Z, Ye P Y, Kang J, et al. Facile fabrication of photochromic microspheres with multimodal hierarchically porous for selective extraction of lithium ions[J]. Materials Letters, 2020, 270: 127670. |
| 23 | Takano K, Furuhama A, Ogawa S, et al. A molecular orbital study on a tetra-aza macrocycle containing 2, 2'-bipyridines and its lithium complex[J]. Journal of the Chemical Society, Perkin Transactions 2, 1999, (6): 1063-1068. |
| 24 | Joliat E, Schnidrig S, Probst B, et al. Cobalt complexes of tetradentate, bipyridine-based macrocycles: their structures, properties and photocatalytic proton reduction[J]. Dalton Transactions, 2016, 45(4): 1737-1745. |
| 25 | Furuhama A, Takano K, Ogawa S, et al. Theoretical study of a conformational change occurring with lithium complexation to a tetra-aza macrocycle containing 2, 2'-bipyridines[J]. Bulletin of the Chemical Society of Japan, 2001, 74(7): 1241-1249. |
| 26 | Iyoda M, Yamakawa J, Rahman M J. Conjugated macrocycles: concepts and applications[J]. Angewandte Chemie International Edition, 2011, 50(45): 10522-10553. |
| 27 | Ogawa S, Narushima R, Arai Y. Aza macrocycle that selectively binds lithium ion with color change[J]. Journal of the American Chemical Society, 1984, 106(19): 5760-5762. |
| 28 | Ibrahim R, Tsuchiya S, Ogawa S. A color-switching molecule: specific properties of new tetraaza macrocycle zinc complex with a facile hydrogen atom[J]. Journal of the American Chemical Society, 2000, 122(49): 12174-12185. |
| 29 | Sakamoto H, Kimura K, Shono T. Lithium separation and enrichment by proton-driven cation transport through liquid membranes of lipophilic crown nitrophenols[J]. Analytical Chemistry, 1987, 59(11): 1513-1517. |
| 30 | Kocherginsky N M, Yang Q, Seelam L. Recent advances in supported liquid membrane technology[J]. Separation and Purification Technology, 2007, 53(2): 171-177. |
| 31 | Parhi P K, Das N N, Sarangi K. Extraction of cadmium from dilute solution using supported liquid membrane[J]. Journal of Hazardous Materials, 2009, 172(2/3): 773-779. |
| [1] | 邵苛苛, 宋孟杰, 江正勇, 张旋, 张龙, 高润淼, 甄泽康. 水平方向上冰中受陷气泡形成和分布实验研究[J]. 化工学报, 2023, 74(S1): 161-164. |
| [2] | 王志国, 薛孟, 董芋双, 张田震, 秦晓凯, 韩强. 基于裂隙粗糙性表征方法的地热岩体热流耦合数值模拟与分析[J]. 化工学报, 2023, 74(S1): 223-234. |
| [3] | 王琪, 张斌, 张晓昕, 武虎建, 战海涛, 王涛. 氯铝酸-三乙胺离子液体/P2O5催化合成伊索克酸和2-乙基蒽醌[J]. 化工学报, 2023, 74(S1): 245-249. |
| [4] | 吴延鹏, 李晓宇, 钟乔洋. 静电纺丝纳米纤维双疏膜油性细颗粒物过滤性能实验分析[J]. 化工学报, 2023, 74(S1): 259-264. |
| [5] | 胡建波, 刘洪超, 胡齐, 黄美英, 宋先雨, 赵双良. 有机笼跨细胞膜易位行为的分子动力学模拟研究[J]. 化工学报, 2023, 74(9): 3756-3765. |
| [6] | 齐聪, 丁子, 余杰, 汤茂清, 梁林. 基于选择吸收纳米薄膜的太阳能温差发电特性研究[J]. 化工学报, 2023, 74(9): 3921-3930. |
| [7] | 何松, 刘乔迈, 谢广烁, 王斯民, 肖娟. 高浓度水煤浆管道气膜减阻两相流模拟及代理辅助优化[J]. 化工学报, 2023, 74(9): 3766-3774. |
| [8] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [9] | 张佳怡, 何佳莉, 谢江鹏, 王健, 赵鹬, 张栋强. 渗透汽化技术用于锂电池生产中N-甲基吡咯烷酮回收的研究进展[J]. 化工学报, 2023, 74(8): 3203-3215. |
| [10] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [11] | 胡亚丽, 胡军勇, 马素霞, 孙禹坤, 谭学诣, 黄佳欣, 杨奉源. 逆电渗析热机新型工质开发及电化学特性研究[J]. 化工学报, 2023, 74(8): 3513-3521. |
| [12] | 涂玉明, 邵高燕, 陈健杰, 刘凤, 田世超, 周智勇, 任钟旗. 钙基催化剂的设计合成及应用研究进展[J]. 化工学报, 2023, 74(7): 2717-2734. |
| [13] | 葛加丽, 管图祥, 邱新民, 吴健, 沈丽明, 暴宁钟. 垂直多孔碳包覆的FeF3正极的构筑及储锂性能研究[J]. 化工学报, 2023, 74(7): 3058-3067. |
| [14] | 张贲, 王松柏, 魏子亚, 郝婷婷, 马学虎, 温荣福. 超亲水多孔金属结构驱动的毛细液膜冷凝及传热强化[J]. 化工学报, 2023, 74(7): 2824-2835. |
| [15] | 王光, 单发顺, 钱禹丞, 焦建芳. 基于集成学习传递熵的化工过程微小故障检测方法[J]. 化工学报, 2023, 74(7): 2967-2978. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号