CIESC Journal ›› 2020, Vol. 71 ›› Issue (1): 177-191.DOI: 10.11949/0438-1157.20191361
• Thermodynamics • Previous Articles Next Articles
Xinxin WANG1( ),Qing ZHOU1,2,Xiaochun ZHANG1,Zhibo ZHANG1,Xingmei LYU1,2,Suojiang ZHANG1,2(
),Qing ZHOU1,2,Xiaochun ZHANG1,Zhibo ZHANG1,Xingmei LYU1,2,Suojiang ZHANG1,2( )
)
Received:2019-11-11
Revised:2019-11-21
Online:2020-01-05
Published:2020-01-05
Contact:
Suojiang ZHANG
王薪薪1( ),周清1,2,张晓春1,张志博1,吕兴梅1,2,张锁江1,2(
),周清1,2,张晓春1,张志博1,吕兴梅1,2,张锁江1,2( )
)
通讯作者:
张锁江
作者简介:王薪薪(1987—),女,硕士,基金资助:CLC Number:
Xinxin WANG, Qing ZHOU, Xiaochun ZHANG, Zhibo ZHANG, Xingmei LYU, Suojiang ZHANG. Densities and viscosities of binary system containing 1,3-dimethylimidazolium dimethylphosphate and dimethyl sulfoxide or acetonitrile[J]. CIESC Journal, 2020, 71(1): 177-191.
王薪薪, 周清, 张晓春, 张志博, 吕兴梅, 张锁江. 离子液体[Mmim][DMP]与DMSO/乙腈二元体系的密度和黏度[J]. 化工学报, 2020, 71(1): 177-191.
Add to citation manager EndNote|Ris|BibTeX
| Compound | T/K | ρ/(g·cm-3) | η/(mPa·s) | ||
|---|---|---|---|---|---|
| Exp. | Lit. | Exp. | Lit. | ||
| [Mmim][DMP] | 298.15 | 1.261314 | 1.2587[ 1.2612[ | 290.7599 | 271.86[ |
| 303.15 | 1.257795 | 1.2559[ 1.2441[ | 210.0863 | 188.30[ | |
| 308.15 | 1.254402 | 1.2530[ | 155.5793 | 139.49[ | |
| 313.15 | 1.250993 | 1.2491[ 1.2505[ 1.2368[ | 119.1838 | 110.25[ | |
| 318.15 | 1.247590 | 1.2452[ | 92.7692 | 84.44[ | |
| 323.15 | 1.244193 | 1.2415[ 1.2274[ | 74.2418 | 69.99[ | |
| DMSO | 293.15 | 1.100318 | 1.10076[ | 1.9937 | 2.210[ |
| 298.15 | 1.095326 | 1.0958[ | 1.8027 | 1.964[ | |
| 1.09574[ | |||||
| 303.15 | 1.090312 | 1.09073[ | 1.6362 | 1.787[ | |
| 1.0900[ | |||||
| 308.15 | 1.085265 | 1.0839[ | 1.4961 | 1.566[ | |
| 313.15 | 1.080264 | 1.08069[ | 1.3746 | 1.490[ | |
| 1.0783[ | |||||
| 318.15 | 1.075234 | 1.0731[ | 1.2682 | 1.3173[ | |
| 323.15 | 1.070217 | 1.07066[ | 1.1756 | 1.285[ | |
| 1.0693[ | |||||
| 乙腈 | 293.15 | 0.782374 | 0.7820[ | 0.3548 | 0.3645[ |
| 298.15 | 0.776984 | 0.7760[ | 0.3440 | 0.344[ | |
| 303.15 | 0.771554 | 0.7712[ | 0.3323 | 0.3307[ | |
| 308.15 | 0.766094 | 0.7663[ | 0.3220 | 0.313[ | |
| 313.15 | 0.760602 | 0.7603[ | 0.3124 | 0.3005[ | |
| 318.15 | 0.755076 | 0.7550[ | 0.3037 | 0.289[ | |
| 323.15 | 0.749510 | 0.7492[ | 0.2956 | 0.2746[ | |
Table 1 Comparison of measured with literature values of densities and viscosities of pure compounds
| Compound | T/K | ρ/(g·cm-3) | η/(mPa·s) | ||
|---|---|---|---|---|---|
| Exp. | Lit. | Exp. | Lit. | ||
| [Mmim][DMP] | 298.15 | 1.261314 | 1.2587[ 1.2612[ | 290.7599 | 271.86[ |
| 303.15 | 1.257795 | 1.2559[ 1.2441[ | 210.0863 | 188.30[ | |
| 308.15 | 1.254402 | 1.2530[ | 155.5793 | 139.49[ | |
| 313.15 | 1.250993 | 1.2491[ 1.2505[ 1.2368[ | 119.1838 | 110.25[ | |
| 318.15 | 1.247590 | 1.2452[ | 92.7692 | 84.44[ | |
| 323.15 | 1.244193 | 1.2415[ 1.2274[ | 74.2418 | 69.99[ | |
| DMSO | 293.15 | 1.100318 | 1.10076[ | 1.9937 | 2.210[ |
| 298.15 | 1.095326 | 1.0958[ | 1.8027 | 1.964[ | |
| 1.09574[ | |||||
| 303.15 | 1.090312 | 1.09073[ | 1.6362 | 1.787[ | |
| 1.0900[ | |||||
| 308.15 | 1.085265 | 1.0839[ | 1.4961 | 1.566[ | |
| 313.15 | 1.080264 | 1.08069[ | 1.3746 | 1.490[ | |
| 1.0783[ | |||||
| 318.15 | 1.075234 | 1.0731[ | 1.2682 | 1.3173[ | |
| 323.15 | 1.070217 | 1.07066[ | 1.1756 | 1.285[ | |
| 1.0693[ | |||||
| 乙腈 | 293.15 | 0.782374 | 0.7820[ | 0.3548 | 0.3645[ |
| 298.15 | 0.776984 | 0.7760[ | 0.3440 | 0.344[ | |
| 303.15 | 0.771554 | 0.7712[ | 0.3323 | 0.3307[ | |
| 308.15 | 0.766094 | 0.7663[ | 0.3220 | 0.313[ | |
| 313.15 | 0.760602 | 0.7603[ | 0.3124 | 0.3005[ | |
| 318.15 | 0.755076 | 0.7550[ | 0.3037 | 0.289[ | |
| 323.15 | 0.749510 | 0.7492[ | 0.2956 | 0.2746[ | |
| x 1 | A/(g·cm-3) | B×10-4/(g·cm-3·K-1) | R 2 |
|---|---|---|---|
| [Mmim][DMP] (1) + DMSO (2) | |||
| 0 | 1.394 | -10.000 | 1 |
| 0.1002 | 1.412 | -9.248 | 1 |
| 0.1997 | 1.426 | -8.732 | 1 |
| 0.2998 | 1.436 | -8.333 | 0.99999 |
| 0.4000 | 1.447 | -8.124 | 0.99988 |
| 0.5008 | 1.450 | -7.746 | 0.99999 |
| 0.6023 | 1.456 | -7.553 | 0.99995 |
| 0.7014 | 1.458 | -7.324 | 0.99999 |
| 0.7996 | 1.460 | -7.127 | 1 |
| 0.8964 | 1.463 | -6.984 | 0.99997 |
| 1.0000 | 1.466 | -6.879 | 0.9999 |
| [Mmim][DMP] (1) + 乙腈 (2) | |||
| 0 | 1.103 | -11.000 | 0.99997 |
| 0.0901 | 1.203 | -9.771 | 0.99998 |
| 0.2010 | 1.285 | -8.998 | 0.99976 |
| 0.3001 | 1.329 | -8.316 | 1 |
| 0.4013 | 1.365 | -7.940 | 0.99998 |
| 0.5005 | 1.396 | -7.792 | 0.99994 |
| 0.5984 | 1.414 | -7.487 | 0.99999 |
| 0.6978 | 1.430 | -7.270 | 1 |
| 0.8007 | 1.444 | -7.097 | 0.99999 |
| 0.8995 | 1.462 | -7.184 | 0.99993 |
| 1.0000 | 1.466 | -6.879 | 0.9999 |
Table 2 Fitted values of empirical parameters A and B for densities of binary mixtures based on Eq. (1)
| x 1 | A/(g·cm-3) | B×10-4/(g·cm-3·K-1) | R 2 |
|---|---|---|---|
| [Mmim][DMP] (1) + DMSO (2) | |||
| 0 | 1.394 | -10.000 | 1 |
| 0.1002 | 1.412 | -9.248 | 1 |
| 0.1997 | 1.426 | -8.732 | 1 |
| 0.2998 | 1.436 | -8.333 | 0.99999 |
| 0.4000 | 1.447 | -8.124 | 0.99988 |
| 0.5008 | 1.450 | -7.746 | 0.99999 |
| 0.6023 | 1.456 | -7.553 | 0.99995 |
| 0.7014 | 1.458 | -7.324 | 0.99999 |
| 0.7996 | 1.460 | -7.127 | 1 |
| 0.8964 | 1.463 | -6.984 | 0.99997 |
| 1.0000 | 1.466 | -6.879 | 0.9999 |
| [Mmim][DMP] (1) + 乙腈 (2) | |||
| 0 | 1.103 | -11.000 | 0.99997 |
| 0.0901 | 1.203 | -9.771 | 0.99998 |
| 0.2010 | 1.285 | -8.998 | 0.99976 |
| 0.3001 | 1.329 | -8.316 | 1 |
| 0.4013 | 1.365 | -7.940 | 0.99998 |
| 0.5005 | 1.396 | -7.792 | 0.99994 |
| 0.5984 | 1.414 | -7.487 | 0.99999 |
| 0.6978 | 1.430 | -7.270 | 1 |
| 0.8007 | 1.444 | -7.097 | 0.99999 |
| 0.8995 | 1.462 | -7.184 | 0.99993 |
| 1.0000 | 1.466 | -6.879 | 0.9999 |

Fig.2 Densities for [Mmim][DMP] binary systems as a function of temperature at different mole fractions of IL ■ x 1 = 0; □ x 1 = 0.1002; ▲ x 1 = 0.1997; △ x 1 = 0.2998; ▼ x 1 = 0.4000; ▽ x 1 = 0.5008; ◆ x 1 = 0.6023; ◇ x 1 = 0.7014; ● x 1 = 0.7996; ○ x 1 = 0.8964; × x 1 = 1.0000

Fig.3 Densities for [Mmim][DMP] binary system as a function of mole fractions of IL at different temperatures ■ 293.15 K; □ 298.15 K; ▲ 303.15 K; △ 308.15 K; ▼ 313.15 K; ▽ 318.15 K; ◆ 323.15 K
| T/K | | | | |
|---|---|---|---|---|
| [Mmim][DMP] (1) + DMSO (2) | ||||
| 293.15 | 170.85 | 39.10 | -76.28 | 0.11 |
| 298.15 | 171.22 | 40.07 | -78.19 | 0.12 |
| 303.15 | 171.59 | 41.16 | -80.62 | 0.12 |
| 308.15 | 171.93 | 42.51 | -83.45 | 0.13 |
| 313.15 | 172.28 | 43.62 | -85.68 | 0.13 |
| 318.15 | 172.62 | 44.97 | -88.46 | 0.14 |
| 323.15 | 172.95 | 46.30 | -91.13 | 0.14 |
| [Mmim][DMP] (1) + 乙腈 (2) | ||||
| 293.15 | 162.96 | 85.85 | -122.79 | 0.54 |
| 298.15 | 162.83 | 89.72 | -128.38 | 0.55 |
| 303.15 | 162.70 | 93.54 | -133.90 | 0.57 |
| 308.15 | 162.54 | 97.57 | -139.64 | 0.58 |
| 313.15 | 163.21 | 75.33 | -107.73 | 0.70 |
| 318.15 | 163.11 | 78.95 | -112.86 | 0.71 |
| 323.15 | 163.05 | 82.34 | -117.73 | 0.73 |
Table 3 Apparent molar volume at infinite dilution ( V ? ∞ ) of [Mmim][DMP] at temperatures from 293.15 to 323.15 K
| T/K | | | | |
|---|---|---|---|---|
| [Mmim][DMP] (1) + DMSO (2) | ||||
| 293.15 | 170.85 | 39.10 | -76.28 | 0.11 |
| 298.15 | 171.22 | 40.07 | -78.19 | 0.12 |
| 303.15 | 171.59 | 41.16 | -80.62 | 0.12 |
| 308.15 | 171.93 | 42.51 | -83.45 | 0.13 |
| 313.15 | 172.28 | 43.62 | -85.68 | 0.13 |
| 318.15 | 172.62 | 44.97 | -88.46 | 0.14 |
| 323.15 | 172.95 | 46.30 | -91.13 | 0.14 |
| [Mmim][DMP] (1) + 乙腈 (2) | ||||
| 293.15 | 162.96 | 85.85 | -122.79 | 0.54 |
| 298.15 | 162.83 | 89.72 | -128.38 | 0.55 |
| 303.15 | 162.70 | 93.54 | -133.90 | 0.57 |
| 308.15 | 162.54 | 97.57 | -139.64 | 0.58 |
| 313.15 | 163.21 | 75.33 | -107.73 | 0.70 |
| 318.15 | 163.11 | 78.95 | -112.86 | 0.71 |
| 323.15 | 163.05 | 82.34 | -117.73 | 0.73 |
| x 1 | | | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 293.15 K | 298.15 K | 303.15 K | 308.15 K | 313.15 K | 318.15 K | 323.15 K | 293.15 K | 298.15 K | 303.15 K | 308.15 K | 313.15 K | 318.15 K | 323.15 K | |
| [Mmim][DMP] (1) + DMSO (2) | ||||||||||||||
| 0 | 201.93 | 202.85 | 203.78 | 204.73 | 205.68 | 206.64 | 207.61 | 70.63 | 70.94 | 71.26 | 71.58 | 71.90 | 72.23 | 72.55 |
| 0.1002 | 195.17 | 195.49 | 196.29 | 197.09 | 197.90 | 198.72 | 199.55 | 68.31 | 68.58 | 68.86 | 69.14 | 69.42 | 69.71 | 69.99 |
| 0.1997 | 190.64 | 190.62 | 191.34 | 192.07 | 192.79 | 193.52 | 194.26 | 66.72 | 66.97 | 67.23 | 67.48 | 67.74 | 67.99 | 68.25 |
| 0.2998 | 187.30 | 187.08 | 187.74 | 188.40 | 189.07 | 189.74 | 190.41 | 65.58 | 65.81 | 66.07 | 66.30 | 66.54 | 66.78 | 67.02 |
| 0.4000 | 184.77 | 184.42 | 185.05 | 185.70 | 186.32 | 186.95 | 187.58 | 64.66 | 64.88 | 65.08 | 65.30 | 65.52 | 65.74 | 65.96 |
| 0.5008 | 182.71 | 182.26 | 182.85 | 183.42 | 184.01 | 184.60 | 185.20 | 63.97 | 64.18 | 64.39 | 64.60 | 64.81 | 65.02 | 65.23 |
| 0.6023 | 181.05 | 180.55 | 181.12 | 181.67 | 182.23 | 182.79 | 183.36 | 63.41 | 63.59 | 63.79 | 63.99 | 64.19 | 64.39 | 64.59 |
| 0.7014 | 179.69 | 179.19 | 179.72 | 180.24 | 180.78 | 181.32 | 181.86 | 62.96 | 63.14 | 63.32 | 63.51 | 63.71 | 63.90 | 64.09 |
| 0.7996 | 178.55 | 178.07 | 178.57 | 179.07 | 179.59 | 180.11 | 180.63 | 62.58 | 62.77 | 62.95 | 63.13 | 63.31 | 63.50 | 63.68 |
| 0.8964 | 177.57 | 177.12 | 177.62 | 178.10 | 178.60 | 179.10 | 179.60 | 62.17 | 62.36 | 62.55 | 62.72 | 62.90 | 63.08 | 63.26 |
| 1.0000 | 176.65 | 176.15 | 176.64 | 177.12 | 177.60 | 178.09 | 178.57 | 61.77 | 61.94 | 62.12 | 62.28 | 62.45 | 62.62 | 62.80 |
| [Mmim][DMP] (1) + 乙腈 (2) | ||||||||||||||
| 0 | 283.98 | 285.95 | 287.96 | 290.02 | 292.11 | 294.25 | 296.43 | 51.50 | 51.81 | 52.14 | 52.48 | 52.82 | 53.16 | 53.51 |
| 0.0901 | 243.49 | 243.74 | 245.05 | 246.39 | 247.74 | 249.10 | 250.48 | 44.40 | 44.62 | 44.85 | 45.07 | 45.30 | 45.56 | 45.81 |
| 0.2010 | 219.19 | 218.63 | 219.59 | 220.55 | 221.53 | 222.55 | 223.61 | 40.07 | 40.24 | 40.41 | 40.59 | 40.76 | 40.93 | 41.10 |
| 0.3001 | 206.58 | 205.63 | 206.43 | 207.23 | 208.04 | 208.85 | 209.67 | 37.88 | 38.02 | 38.18 | 38.33 | 38.48 | 38.64 | 38.79 |
| 0.4013 | 198.07 | 196.96 | 197.66 | 198.36 | 199.07 | 199.78 | 200.50 | 36.38 | 36.52 | 36.65 | 36.79 | 36.94 | 37.10 | 37.25 |
| 0.5005 | 192.11 | 190.97 | 191.60 | 192.23 | 192.88 | 193.55 | 194.22 | 35.35 | 35.48 | 35.61 | 35.74 | 35.86 | 35.98 | 36.11 |
| 0.5984 | 187.65 | 186.56 | 187.16 | 187.74 | 188.34 | 188.93 | 189.54 | 34.65 | 34.76 | 34.88 | 35.00 | 35.12 | 35.24 | 35.36 |
| 0.6978 | 184.11 | 183.17 | 183.72 | 184.26 | 184.82 | 185.38 | 185.95 | 34.06 | 34.17 | 34.28 | 34.39 | 34.50 | 34.62 | 34.74 |
| 0.8007 | 181.12 | 180.37 | 180.88 | 181.40 | 181.92 | 182.45 | 182.99 | 33.48 | 33.63 | 33.74 | 33.86 | 33.98 | 34.11 | 34.24 |
| 0.8995 | 178.72 | 178.11 | 178.64 | 179.14 | 179.65 | 180.17 | 180.70 | 33.05 | 33.14 | 33.25 | 33.34 | 33.44 | 33.53 | 33.62 |
| 1.0000 | 176.65 | 176.15 | 176.64 | 177.12 | 177.60 | 178.09 | 178.57 | 32.45 | 32.55 | 32.64 | 32.72 | 32.81 | 32.90 | 32.99 |
Table 4 Partial molar volumes( V ? ) of mixtures of [Mmim][DMP] with DMSO and acetonitrile at temperatures from 293.15 to 323.15 K
| x 1 | | | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 293.15 K | 298.15 K | 303.15 K | 308.15 K | 313.15 K | 318.15 K | 323.15 K | 293.15 K | 298.15 K | 303.15 K | 308.15 K | 313.15 K | 318.15 K | 323.15 K | |
| [Mmim][DMP] (1) + DMSO (2) | ||||||||||||||
| 0 | 201.93 | 202.85 | 203.78 | 204.73 | 205.68 | 206.64 | 207.61 | 70.63 | 70.94 | 71.26 | 71.58 | 71.90 | 72.23 | 72.55 |
| 0.1002 | 195.17 | 195.49 | 196.29 | 197.09 | 197.90 | 198.72 | 199.55 | 68.31 | 68.58 | 68.86 | 69.14 | 69.42 | 69.71 | 69.99 |
| 0.1997 | 190.64 | 190.62 | 191.34 | 192.07 | 192.79 | 193.52 | 194.26 | 66.72 | 66.97 | 67.23 | 67.48 | 67.74 | 67.99 | 68.25 |
| 0.2998 | 187.30 | 187.08 | 187.74 | 188.40 | 189.07 | 189.74 | 190.41 | 65.58 | 65.81 | 66.07 | 66.30 | 66.54 | 66.78 | 67.02 |
| 0.4000 | 184.77 | 184.42 | 185.05 | 185.70 | 186.32 | 186.95 | 187.58 | 64.66 | 64.88 | 65.08 | 65.30 | 65.52 | 65.74 | 65.96 |
| 0.5008 | 182.71 | 182.26 | 182.85 | 183.42 | 184.01 | 184.60 | 185.20 | 63.97 | 64.18 | 64.39 | 64.60 | 64.81 | 65.02 | 65.23 |
| 0.6023 | 181.05 | 180.55 | 181.12 | 181.67 | 182.23 | 182.79 | 183.36 | 63.41 | 63.59 | 63.79 | 63.99 | 64.19 | 64.39 | 64.59 |
| 0.7014 | 179.69 | 179.19 | 179.72 | 180.24 | 180.78 | 181.32 | 181.86 | 62.96 | 63.14 | 63.32 | 63.51 | 63.71 | 63.90 | 64.09 |
| 0.7996 | 178.55 | 178.07 | 178.57 | 179.07 | 179.59 | 180.11 | 180.63 | 62.58 | 62.77 | 62.95 | 63.13 | 63.31 | 63.50 | 63.68 |
| 0.8964 | 177.57 | 177.12 | 177.62 | 178.10 | 178.60 | 179.10 | 179.60 | 62.17 | 62.36 | 62.55 | 62.72 | 62.90 | 63.08 | 63.26 |
| 1.0000 | 176.65 | 176.15 | 176.64 | 177.12 | 177.60 | 178.09 | 178.57 | 61.77 | 61.94 | 62.12 | 62.28 | 62.45 | 62.62 | 62.80 |
| [Mmim][DMP] (1) + 乙腈 (2) | ||||||||||||||
| 0 | 283.98 | 285.95 | 287.96 | 290.02 | 292.11 | 294.25 | 296.43 | 51.50 | 51.81 | 52.14 | 52.48 | 52.82 | 53.16 | 53.51 |
| 0.0901 | 243.49 | 243.74 | 245.05 | 246.39 | 247.74 | 249.10 | 250.48 | 44.40 | 44.62 | 44.85 | 45.07 | 45.30 | 45.56 | 45.81 |
| 0.2010 | 219.19 | 218.63 | 219.59 | 220.55 | 221.53 | 222.55 | 223.61 | 40.07 | 40.24 | 40.41 | 40.59 | 40.76 | 40.93 | 41.10 |
| 0.3001 | 206.58 | 205.63 | 206.43 | 207.23 | 208.04 | 208.85 | 209.67 | 37.88 | 38.02 | 38.18 | 38.33 | 38.48 | 38.64 | 38.79 |
| 0.4013 | 198.07 | 196.96 | 197.66 | 198.36 | 199.07 | 199.78 | 200.50 | 36.38 | 36.52 | 36.65 | 36.79 | 36.94 | 37.10 | 37.25 |
| 0.5005 | 192.11 | 190.97 | 191.60 | 192.23 | 192.88 | 193.55 | 194.22 | 35.35 | 35.48 | 35.61 | 35.74 | 35.86 | 35.98 | 36.11 |
| 0.5984 | 187.65 | 186.56 | 187.16 | 187.74 | 188.34 | 188.93 | 189.54 | 34.65 | 34.76 | 34.88 | 35.00 | 35.12 | 35.24 | 35.36 |
| 0.6978 | 184.11 | 183.17 | 183.72 | 184.26 | 184.82 | 185.38 | 185.95 | 34.06 | 34.17 | 34.28 | 34.39 | 34.50 | 34.62 | 34.74 |
| 0.8007 | 181.12 | 180.37 | 180.88 | 181.40 | 181.92 | 182.45 | 182.99 | 33.48 | 33.63 | 33.74 | 33.86 | 33.98 | 34.11 | 34.24 |
| 0.8995 | 178.72 | 178.11 | 178.64 | 179.14 | 179.65 | 180.17 | 180.70 | 33.05 | 33.14 | 33.25 | 33.34 | 33.44 | 33.53 | 33.62 |
| 1.0000 | 176.65 | 176.15 | 176.64 | 177.12 | 177.60 | 178.09 | 178.57 | 32.45 | 32.55 | 32.64 | 32.72 | 32.81 | 32.90 | 32.99 |
| x 1 | A/(mPa·s) | B/K | T 0/K | R 2 |
|---|---|---|---|---|
| [Mmim][DMP] (1) + DMSO (2) | ||||
| 0 | 0.074 | 518.8 | 135.9 | 0.99999 |
| 0.1002 | 0.102 | 521.2 | 154.7 | 1.00000 |
| 0.1997 | 0.132 | 589.7 | 157.4 | 0.99992 |
| 0.2998 | 0.246 | 477.2 | 183.1 | 0.99999 |
| 0.4000 | 0.024 | 1264.1 | 115.9 | 0.99970 |
| 0.5008 | 0.324 | 514.3 | 192.9 | 1.00000 |
| 0.6023 | 0.336 | 536.0 | 196.6 | 1.00000 |
| 0.7014 | 0.294 | 594.8 | 195.7 | 1.00000 |
| 0.7996 | 0.418 | 579.2 | 200.0 | 0.99994 |
| 0.8964 | 0.031 | 1295.5 | 150.8 | 0.99971 |
| 1.0000 | 0.287 | 701.9 | 196.7 | 0.99999 |
| [Mmim][DMP] (1) + 乙腈 (2) | ||||
| 0 | 0.069 | 386.2 | 56.85 | 0.99963 |
| 0.0901 | 0.080 | 419.7 | 123.3 | 0.99999 |
| 0.2010 | 0.043 | 889.3 | 79.73 | 0.99992 |
| 0.3001 | 0.032 | 1055.9 | 94.83 | 0.99484 |
| 0.4013 | 0.223 | 515.1 | 172.1 | 0.99999 |
| 0.5005 | 0.032 | 1229.1 | 111.9 | 0.99860 |
| 0.5984 | 0.192 | 633.3 | 180.1 | 0.99998 |
| 0.6978 | 0.341 | 547.0 | 195.7 | 1.00000 |
| 0.8007 | 0.064 | 1134.4 | 144.4 | 0.99878 |
| 0.8995 | 0.038 | 1240.9 | 152.1 | 0.99971 |
| 1.0000 | 0.287 | 701.9 | 196.7 | 0.99999 |
Table 5 Fitted values of empirical parameters, A, B and T 0 for viscosity based on VFT equation
| x 1 | A/(mPa·s) | B/K | T 0/K | R 2 |
|---|---|---|---|---|
| [Mmim][DMP] (1) + DMSO (2) | ||||
| 0 | 0.074 | 518.8 | 135.9 | 0.99999 |
| 0.1002 | 0.102 | 521.2 | 154.7 | 1.00000 |
| 0.1997 | 0.132 | 589.7 | 157.4 | 0.99992 |
| 0.2998 | 0.246 | 477.2 | 183.1 | 0.99999 |
| 0.4000 | 0.024 | 1264.1 | 115.9 | 0.99970 |
| 0.5008 | 0.324 | 514.3 | 192.9 | 1.00000 |
| 0.6023 | 0.336 | 536.0 | 196.6 | 1.00000 |
| 0.7014 | 0.294 | 594.8 | 195.7 | 1.00000 |
| 0.7996 | 0.418 | 579.2 | 200.0 | 0.99994 |
| 0.8964 | 0.031 | 1295.5 | 150.8 | 0.99971 |
| 1.0000 | 0.287 | 701.9 | 196.7 | 0.99999 |
| [Mmim][DMP] (1) + 乙腈 (2) | ||||
| 0 | 0.069 | 386.2 | 56.85 | 0.99963 |
| 0.0901 | 0.080 | 419.7 | 123.3 | 0.99999 |
| 0.2010 | 0.043 | 889.3 | 79.73 | 0.99992 |
| 0.3001 | 0.032 | 1055.9 | 94.83 | 0.99484 |
| 0.4013 | 0.223 | 515.1 | 172.1 | 0.99999 |
| 0.5005 | 0.032 | 1229.1 | 111.9 | 0.99860 |
| 0.5984 | 0.192 | 633.3 | 180.1 | 0.99998 |
| 0.6978 | 0.341 | 547.0 | 195.7 | 1.00000 |
| 0.8007 | 0.064 | 1134.4 | 144.4 | 0.99878 |
| 0.8995 | 0.038 | 1240.9 | 152.1 | 0.99971 |
| 1.0000 | 0.287 | 701.9 | 196.7 | 0.99999 |
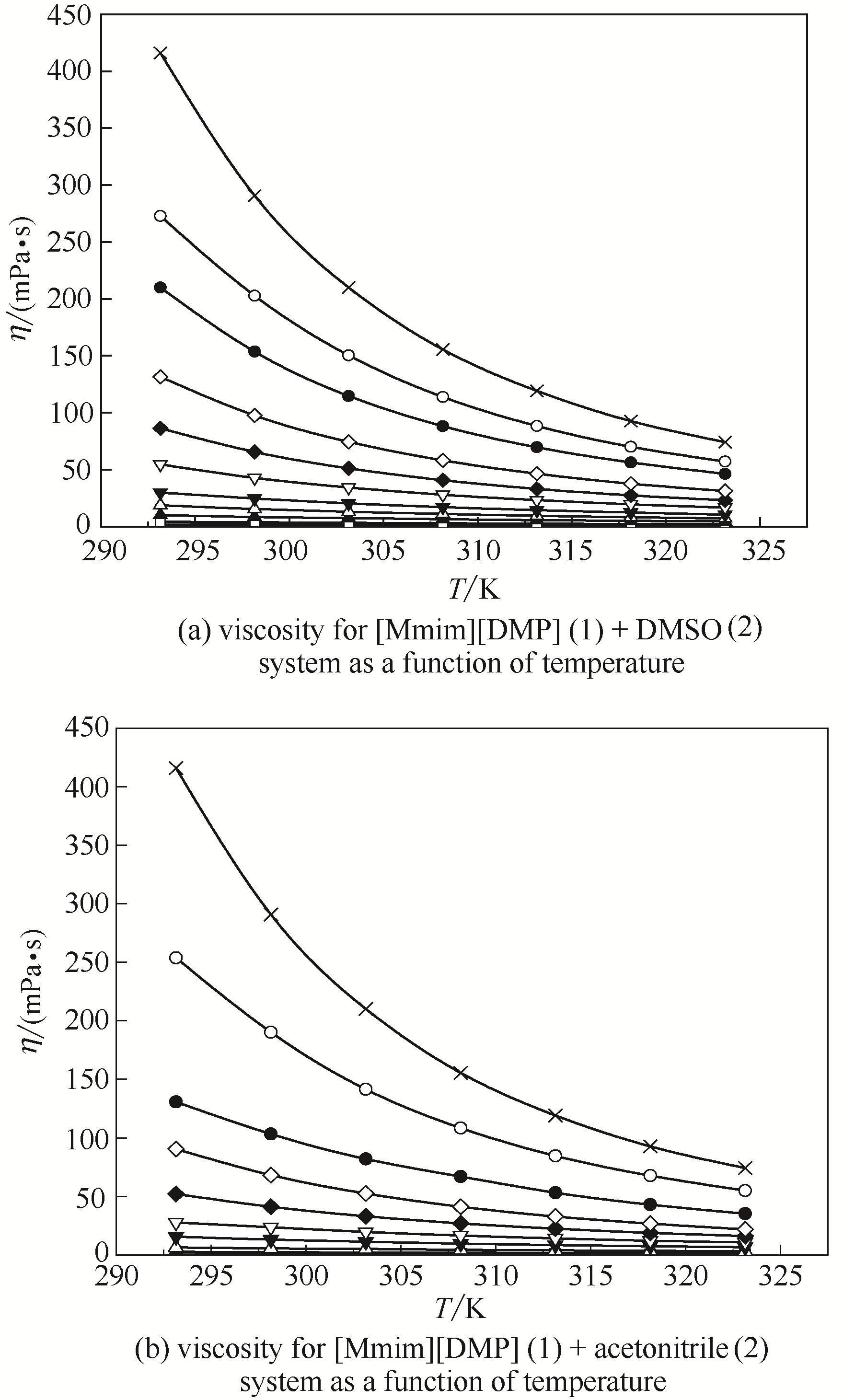
Fig.5 Viscosity for [Mmim][DMP] binary system as a function of temperature at different mole fractions of IL(The composition x 1 was the mole fraction of [Mmim][DMP] in DMSO and acetonitrile, respectively) ■ x 1 = 0; □ x 1 = 0.1002; ▲ x 1 = 0.1997; △ x 1 = 0.2998; ▼ x 1 = 0.4000; ▽ x 1 = 0.5008; ◆ x 1 = 0.6023; ◇ x 1 = 0.7014; ● x 1 = 0.7996; ○ x 1 = 0.8964; × x 1 = 1.0000

Fig.6 Viscosity for [Mmim][DMP] (1) + DMSO binary system as a function of mole fractions of IL at different temperatures ■ 293.15 K; □ 298.15 K; ▲ 303.15 K; △ 308.15 K; ▼ 313.15 K; ▽ 318.15 K; ◆ 323.15 K

Fig.7 Optimized structures of [Mmim]-acetonitrile and [Mmim]-DMSO obtained at the B3LYP/6-311++G**level (Hydrogen bonds are indicated by dotted lines, and distances are in angstroms)

Fig.8 Optimized structures of [DMP]- acetonitrile and [DMP]- DMSO obtained at B3LYP/6-311++G**level(Hydrogen bonds are indicated by dotted lines, and distances are in angstroms)
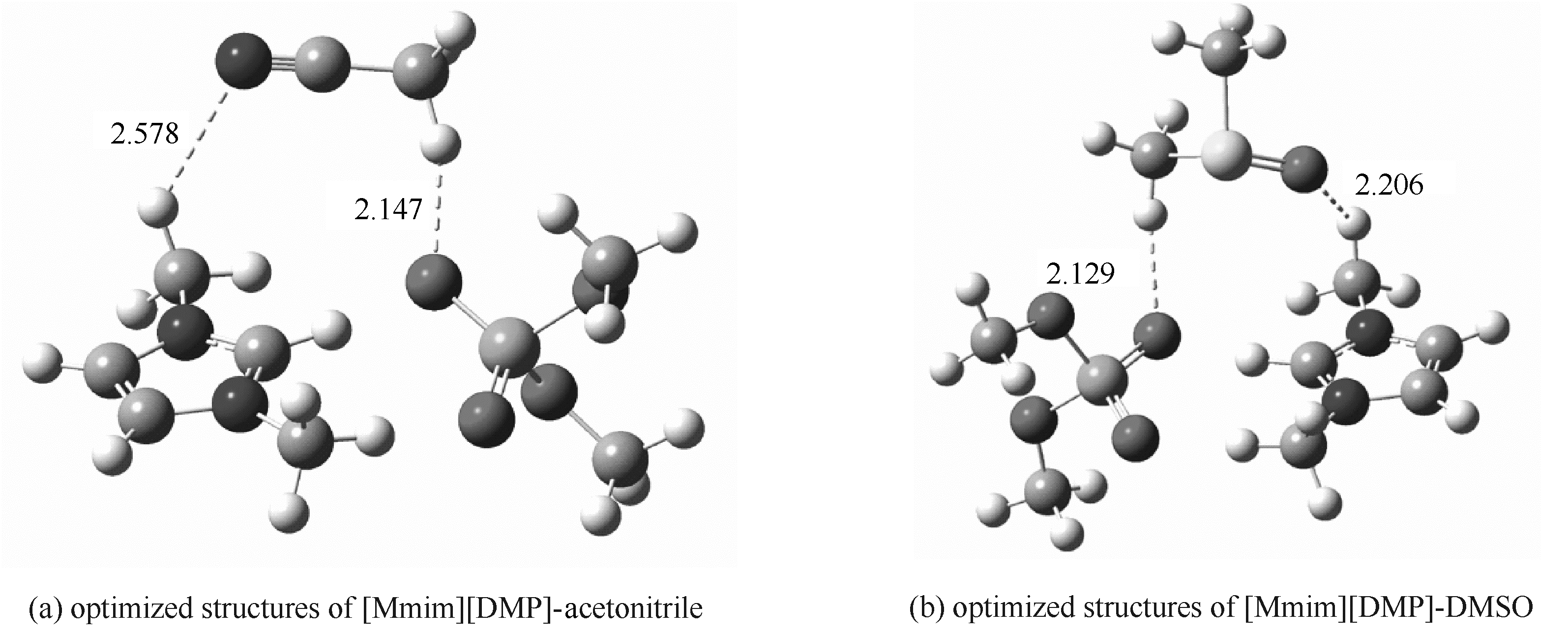
Fig.9 Optimized structures of [Mmim][DMP]-acetonitrile and [Mmim][DMP]-DMSO obtained at the B3LYP/6-311++G**level(Hydrogen bonds are indicated by dotted lines, and distances are in angstroms)
| T / K | V E/(cm3·mol-1) | Δη/(mPa·s) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A 0 | A 1 | A 2 | A 3 | σ(Y) | A 0 | A 1 | A 2 | A 3 | σ(Y) | |
| [Mmim][DMP] + DMSO | ||||||||||
| 293.15 | -2.171 | 1.142 | -0.532 | 1.704 | 0.005 | -605.0 | -214.1 | -193.0 | -277.3 | 6.098 |
| 298.15 | -2.236 | 1.111 | -0.601 | 1.793 | 0.007 | -412.8 | -157.9 | -56.80 | -61.20 | 3.020 |
| 303.15 | -2.262 | 1.098 | -0.807 | 1.752 | 0.011 | -287.2 | -107.1 | -24.24 | -17.72 | 1.937 |
| 308.15 | -2.293 | 1.155 | -0.860 | 1.792 | 0.011 | -203.3 | -69.46 | -10.52 | -6.481 | 1.499 |
| 313.15 | -2.336 | 1.191 | -0.889 | 1.840 | 0.011 | -149.2 | -46.55 | -6.010 | -4.752 | 1.255 |
| 318.15 | -2.381 | 1.229 | -0.947 | 1.894 | 0.012 | -110.8 | -30.66 | 0.984 | 1.179 | 1.116 |
| 323.15 | -2.436 | 1.289 | -0.988 | 1.904 | 0.013 | -85.25 | -22.46 | 4.856 | 7.009 | 0.897 |
| [Mmim][DMP] + 乙腈 | ||||||||||
| 293.15 | -5.157 | 3.292 | -2.771 | 3.492 | 0.032 | -720.3 | -514.3 | -291.2 | -72.87 | 3.540 |
| 298.15 | -5.400 | 3.269 | -2.798 | 4.162 | 0.030 | -499.1 | -371.5 | -114.9 | 93.96 | 4.418 |
| 303.15 | -5.630 | 3.372 | -2.961 | 4.368 | 0.030 | -350.8 | -246.0 | -58.80 | 78.62 | 2.564 |
| 308.15 | -5.837 | 3.527 | -3.051 | 4.620 | 0.029 | -253.2 | -165.1 | -17.75 | 74.31 | 1.552 |
| 313.15 | -6.059 | 3.673 | -3.169 | 4.931 | 0.028 | -189.2 | -121.2 | -7.246 | 64.44 | 1.295 |
| 318.15 | -6.279 | 3.712 | -3.261 | 5.477 | 0.034 | -143.9 | -93.14 | 4.74 | 67.30 | 1.258 |
| 323.15 | -6.529 | 3.778 | -3.255 | 6.099 | 0.040 | -112.4 | -72.45 | 6.374 | 58.34 | 1.098 |
Table 6 Coefficients of standard deviation and Redlich-Kister equation for V E and Δη for two mixtures of [Mmim][DMP] with DMSO and acetonitrile
| T / K | V E/(cm3·mol-1) | Δη/(mPa·s) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A 0 | A 1 | A 2 | A 3 | σ(Y) | A 0 | A 1 | A 2 | A 3 | σ(Y) | |
| [Mmim][DMP] + DMSO | ||||||||||
| 293.15 | -2.171 | 1.142 | -0.532 | 1.704 | 0.005 | -605.0 | -214.1 | -193.0 | -277.3 | 6.098 |
| 298.15 | -2.236 | 1.111 | -0.601 | 1.793 | 0.007 | -412.8 | -157.9 | -56.80 | -61.20 | 3.020 |
| 303.15 | -2.262 | 1.098 | -0.807 | 1.752 | 0.011 | -287.2 | -107.1 | -24.24 | -17.72 | 1.937 |
| 308.15 | -2.293 | 1.155 | -0.860 | 1.792 | 0.011 | -203.3 | -69.46 | -10.52 | -6.481 | 1.499 |
| 313.15 | -2.336 | 1.191 | -0.889 | 1.840 | 0.011 | -149.2 | -46.55 | -6.010 | -4.752 | 1.255 |
| 318.15 | -2.381 | 1.229 | -0.947 | 1.894 | 0.012 | -110.8 | -30.66 | 0.984 | 1.179 | 1.116 |
| 323.15 | -2.436 | 1.289 | -0.988 | 1.904 | 0.013 | -85.25 | -22.46 | 4.856 | 7.009 | 0.897 |
| [Mmim][DMP] + 乙腈 | ||||||||||
| 293.15 | -5.157 | 3.292 | -2.771 | 3.492 | 0.032 | -720.3 | -514.3 | -291.2 | -72.87 | 3.540 |
| 298.15 | -5.400 | 3.269 | -2.798 | 4.162 | 0.030 | -499.1 | -371.5 | -114.9 | 93.96 | 4.418 |
| 303.15 | -5.630 | 3.372 | -2.961 | 4.368 | 0.030 | -350.8 | -246.0 | -58.80 | 78.62 | 2.564 |
| 308.15 | -5.837 | 3.527 | -3.051 | 4.620 | 0.029 | -253.2 | -165.1 | -17.75 | 74.31 | 1.552 |
| 313.15 | -6.059 | 3.673 | -3.169 | 4.931 | 0.028 | -189.2 | -121.2 | -7.246 | 64.44 | 1.295 |
| 318.15 | -6.279 | 3.712 | -3.261 | 5.477 | 0.034 | -143.9 | -93.14 | 4.74 | 67.30 | 1.258 |
| 323.15 | -6.529 | 3.778 | -3.255 | 6.099 | 0.040 | -112.4 | -72.45 | 6.374 | 58.34 | 1.098 |

Fig.10 Excess molar volumes for mixtures of [Mmim][DMP] binary system as a function of mole fractions of IL at different temperatures(The solid curves are calculated with Redlich-Kister equation, and symbols represent experimental values) ■ 293.15 K; □ 298.15 K; ▲ 303.15 K; △ 308.15 K; ▼ 313.15 K; ▽ 318.15 K; ◆ 323.15 K
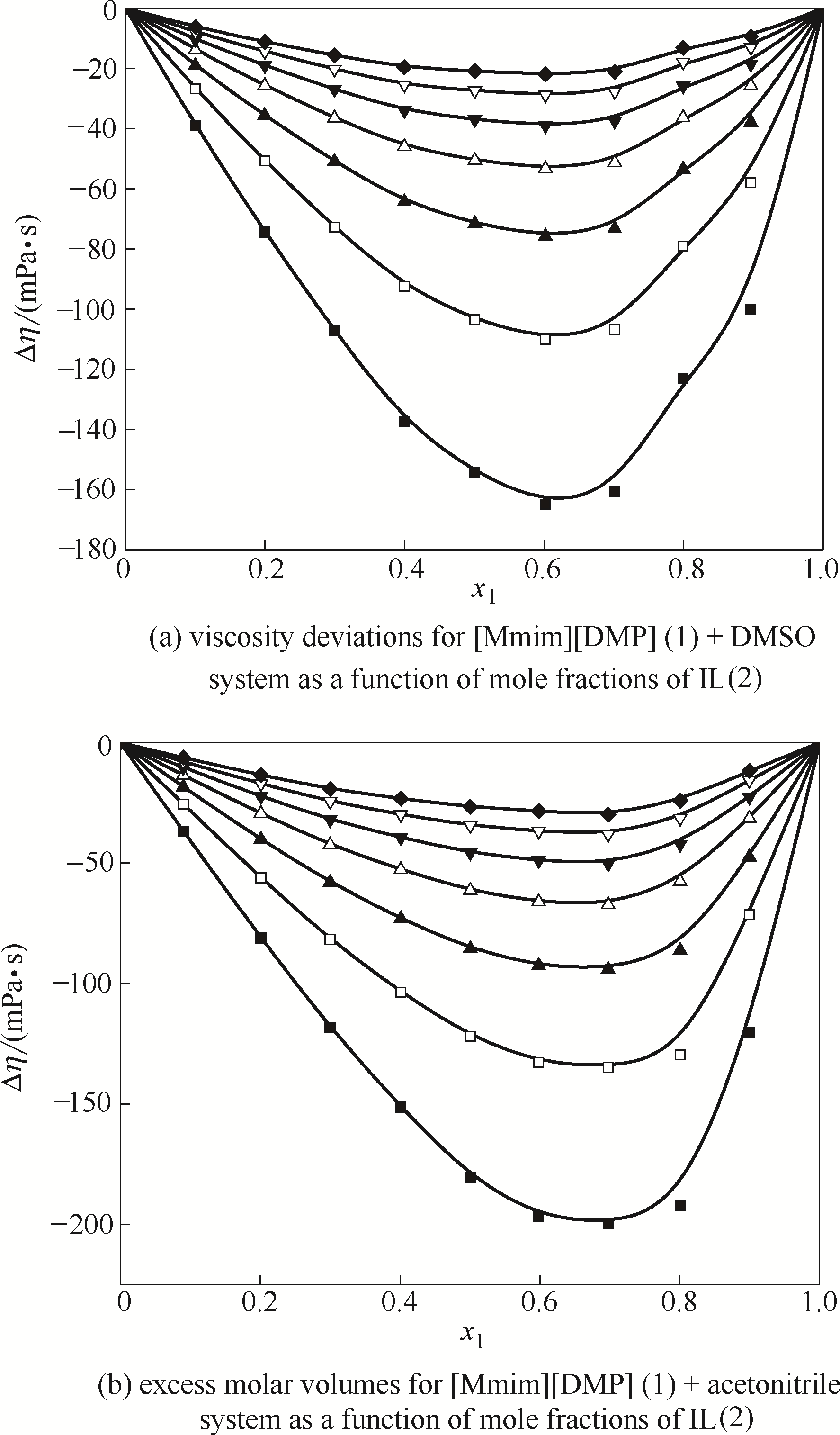
Fig.11 Viscosity deviations for mixtures of [Mmim][DMP]binary system at different temperatures(The solid curves are calculated with Redlich-Kister equation, and symbols represent experimental values) ■ 293.15 K; □ 298.15 K; ▲ 303.15 K; △ 308.15 K; ▼ 313.15 K; ▽ 318.15 K; ◆ 323.15 K
| 1 | Wang H , Gurau G , Rogers R D . Ionic liquid processing of cellulose[J]. Chem. Soc. Rev., 2012, 41(4): 1519-1537. |
| 2 | Zhao Y , Liu X , Wang J , et al . Insight into the cosolvent effect of cellulose dissolution in imidazolium-based ionic liquid systems[J]. J. Phys. Chem. B, 2013, 117(30): 9042-9049. |
| 3 | Xu J , Yao X , Zhou Q , et al . Enhanced delignification of cornstalk by employing superbase TBD in ionic liquids[J]. RSC Adv., 2014, 4(52): 27430-27438. |
| 4 | Xu J , Yao X , Xin J , et al . An effective two-step ionic liquids method for cornstalk pretreatment[J]. J. Chem. Technol. Biotechnol., 2015, 90: 2057-2065. |
| 5 | Chen Z , Zeng J , Di J , et al . Facile microwave-assisted ionic liquid synthesis of sphere-like BiOBr hollow and porous nanostructures with enhanced photocatalytic performance[J]. Green Energy Environ., 2017, 2(2): 124-133. |
| 6 | Vitz J , Erdmenger T , Haensch C , et al . Extended dissolution studies of cellulose in imidazolium based ionic liquids[J]. Green Chem., 2009, 11(3): 417-424. |
| 7 | Abe M , Fukaya Y , Ohno H . Extraction of polysaccharides from bran with phosphonate or phosphinate-derived ionic liquids under short mixing time and low temperature[J]. Green Chem., 2010, 12: 1274-1280. |
| 8 | Fukaya Y , Hayashi K , Wada M , et al . Cellulose dissolution with polar ionic liquids under mild conditions: required factors for anions[J]. Green Chem., 2008, 10(1): 44-46. |
| 9 | Xu A , Wang J , Wang H . Effects of anionic structure and lithium salts addition on the dissolution of cellulose in 1-butyl-3-methylimidazolium-based ionic liquid solvent systems[J]. Green Chem., 2010, 12(2): 268-275. |
| 10 | Swatloski R P , Spear S K , Holbrey J D , et al . Dissolution of cellose with ionic liquids[J]. J. Am. Chem. Soc., 2002, 124(18): 4974-4975. |
| 11 | Zhao Y , Liu X , Wang J , et al . Effects of anionic structure on the dissolution of cellulose in ionic liquids revealed by molecular simulation[J]. Carbohydr Polym., 2013, 94(2): 723-730. |
| 12 | Zhao Y , Liu X , Wang J , et al . Effects of cationic structure on cellulose dissolution in ionic liquids: a molecular dynamics study[J]. ChemPhysChem, 2012, 13(13): 3126-3133. |
| 13 | Rooney D , Jacquemin J , Gardas R . Thermophysical properties of ionic liquids[J]. Top Curr. Chem., 2009, 290: 185-212. |
| 14 | Seddon K R , Stark A , Torres M J . Influence of chloride, water, and organic solvents on the physical properties of ionic liquids[J]. Pure Appl. Chem., 2000, 72(12): 2275-2287. |
| 15 | Wahlström R , Rahikainen J , Kruus K , et al . Cellulose hydrolysis and binding with trihoderma reesei Cel5A and Cel7A and their core domains in ionic liquid solutions[J]. Biotechnol. Bioeng., 2014, 111(4): 726-733. |
| 16 | Qing Q , Hu R , He Y , et al . Investigation of a novel acid-catalyzed ionic liquid pretreatment method to improve biomass enzymatic hydrolysis conversion[J]. Appl. Microbiol. Biotechnol., 2014, 98(11): 5275-5286. |
| 17 | Wahlstrom R , Rovio S , Suurnakki A . Analysis of mono- and oligosaccharides in ionic liquid containing matrices[J]. Carbohydr. Res., 2013, 373: 42-51. |
| 18 | Engel P , Krull S , Seiferheld B , et al . Rational approach to optimize cellulase mixtures for hydrolysis of regenerated cellulose containing residual ionic liquid[J]. Bioresour. Technology., 2012, 115: 27-34. |
| 19 | Abels C , Redepenning C , Moll A , et al . Simple purification of ionic liquid solvents by nanofiltration in biorefining of lignocellulosic substrates[J]. J. Membr. Sci., 2012, 405/406: 1-10. |
| 20 | Engel P , Mladenov R , Wulfhorst H , et al . Point by point analysis: how ionic liquid affects the enzymatic hydrolysis of native and modified cellulose[J]. Green Chem., 2010, 12(11): 1959-1966. |
| 21 | Mazza M , Catana D A , Vaca-Garcia C , et al . Influence of water on the dissolution of cellulose in selected ionic liquids[J]. Cellulose, 2008, 16(2): 207-215. |
| 22 | Zhu S , Wu Y , Chen Q , et al . Dissolution of cellulose with ionic liquids and its application: a mini-review[J]. Green Chem., 2006, 8(4): 325-327. |
| 23 | Cao Y , Zhang R , Cheng T , et al . Imidazolium-based ionic liquids for cellulose pretreatment: recent progresses and future perspectives[J]. Appl. Microbiol. Biotechnol., 2017, 101(2): 521-532. |
| 24 | Zhao D , Li H , Zhang J , et al . Dissolution of cellulose in phosphate-based ionic liquids[J]. Carbohydr. Polym., 2012, 87(2): 1490-1494. |
| 25 | Cai F , Zhu W , Wang Y , et al . Dialkylphosphate-based ionic liquids as solvents to extract toluene from heptane[J]. J. Chem. Eng. Data, 2015, 60(6): 1776-1780. |
| 26 | Cai F , Zhao M , Wang Y , et al . Phosphoric-based ionic liquids as solvents to separate the azeotropic mixture of ethanol and hexane[J]. J. Chem. Thermodyn., 2015, 81: 177-183. |
| 27 | Cai F , Xiao G . Liquid-liquid equilibria for ternary systems ethanol + heptane + phosphoric-based ionic liquids[J]. Fluid Phase Equilib., 2015, 386: 155-161. |
| 28 | Cai F , Xiao G . (Liquid + liquid) extraction of methanol from alkanes using dialkylphosphate-based ionic liquids as solvents[J]. J. Chem. Thermodyn., 2015, 87: 110-116. |
| 29 | Cai F , Ibrahim J J , Niu L , et al . Liquid-liquid equilibrium for ternary system methanol + methyl acetate + 1,3-dimethylimidazolium dimethylphosphate at several temperatures and atmospheric pressure[J]. J. Chem. Eng. Data, 2015, 60(1): 57-64. |
| 30 | Sakal S A , Shen C , Li C X . (Liquid + liquid) equilibria of {benzene + cyclohexane + two ionic liquids} at different temperature and atmospheric pressure[J]. J. Chem. Thermodyn., 2012, 49: 81-86. |
| 31 | Cao J , Yu G , Chen X , et al . Determination of vapor-liquid equilibrium of methyl acetate + methanol + 1-alkyl-3-methylimidazolium dialkylphosphates at 101.3 kPa[J]. J. Chem. Eng. Data, 2017, 62(2): 816-824. |
| 32 | Chen X , Yang B , Abdeltawab A A , et al . Isobaric vapor-liquid equilibrium for acetone + methanol + phosphate ionic liquids[J]. J. Chem. Eng. Data, 2015, 60(3): 612-620. |
| 33 | Li Q , Cao L , Zhang Y , et al . Isobaric vapor-liquid equilibrium for chloroform + methanol + 1,3-dimethylimidazolium dimethylphosphate at 101.3 kPa[J]. J. Chem. Eng. Data, 2014, 59(2): 234-239. |
| 34 | Wang J , Li Z . Measurement and modeling of vapor-liquid equilibria for systems containing alcohols, water, and imidazolium-based phosphate ionic liquids[J]. J. Chem. Eng. Data, 2013, 58(6): 1641-1649. |
| 35 | Dong L , Zheng D , Li J , et al . Suitability prediction and affinity regularity assessment of H2O + imidazolium ionic liquid working pairs of absorption cycle by excess property criteria and UNIFAC model[J]. Fluid Phase Equilib., 2013, 348: 1-8. |
| 36 | Li Q , Zhu W , Wang H , et al . Isobaric vapor-liquid equilibrium for the ethanol + water + 1,3-dimethylimidazolium dimethylphosphate system at 101.3 kPa[J]. J. Chem. Eng. Data, 2012, 57(3): 696-700. |
| 37 | Jia P , Zhao Z , Gao Q , et al . Isobaric vapor-liquid equilibrium of the acetonitrile + 1-propanol + ionic liquids at an atmospheric pressure[J]. J. Chem. Eng. Data, 2019, 64(7): 2963-2972. |
| 38 | Luo C , Wang Y , Li Y , et al . Thermodynamic properties and application of LiNO3-MMIM DMP /H2O ternary working pair[J]. Renewable Energy, 2019, 134: 147-160. |
| 39 | Dong L , Zheng D , Nie N , et al . Performance prediction of absorption refrigeration cycle based on the measurements of vapor pressure and heat capacity of H2O + [DMIM]DMP system[J]. Appl. Energy, 2012, 98: 326-332. |
| 40 | Ge M L , Lu C Y , Liu X Y , et al . Activity coefficients at infinite dilution of alkanes, alkenes, alkyl benzenes in dimethylphosphate based ionic liquids using gas-liquid chromatography[J]. J. Chem. Thermodyn., 2015, 91: 279-285. |
| 41 | Gaciño F M , Regueira T , Bolotov A V , et al . Volumetric behaviour of six ionic liquids from T = (278 to 398) K and up to 120 MPa[J]. J. Chem. Thermodyn., 2016, 93: 24-33. |
| 42 | Ghani N A , Sairi N A , Aroua M K , et al . Density, surface tension, and viscosity of ionic liquids (1-ethyl-3-methylimidazolium diethylphosphate and 1,3-dimethylimidazolium dimethylphosphate) aqueous ternary mixtures with MDEA[J]. J. Chem. Eng. Data, 2014, 59(6): 1737-1746. |
| 43 | He Z , Zhao Z , Zhang X , et al . Thermodynamic properties of new heat pump working pairs: 1,3-dimethylimidazolium dimethylphosphate and water, ethanol and methanol[J]. Fluid Phase Equilib., 2010, 298(1): 83-91. |
| 44 | Zhang Z , Zhou Q , Lu X , et al . Densities and viscosities of binary mixtures containing 1,3-dimethylimidazolium dimethylphosphate and alcohols[J]. J. Chem. Eng. Data, 2014, 59(8): 2377-2388. |
| 45 | Wang J , Zhang Z , Jin S , et al . Efficient conversion of carbohydrates into 5-hydroxylmethylfurfan and 5-ethoxymethylfurfural over sufonic acid-functionalized mesoporous carbon catalyst[J]. Fuel, 2017, 192: 102-107. |
| 46 | Sampath G , Srinivasan K . Remarkable catalytic synergism of alumina, metal salt and solvent for conversion of biomass sugars to furan compounds[J]. Appl. Catal., A, 2017, 533: 75-80. |
| 47 | Morais-de-Carvalho D , Martinez-Abad A , Evtuguin D V , et al . Isolation and characterization of acetylated glucuronoarabinoxylan from sugarcane bagasse and straw[J]. Carbohydr Polym., 2017, 156: 223-234. |
| 48 | Holding A J , Parviainen A , Kilpeläinen I , et al . Efficiency of hydrophobic phosphonium ionic liquids and DMSO as recyclable cellulose dissolution and regeneration media[J]. RSC Adv., 2017, 7(28): 17451-17461. |
| 49 | Gajula S , Inthumathi K , Arumugam S R , et al . Strategic designing on selection of solvent systems for conversion of biomass sugars to furan derivatives and their separation[J]. ACS Sustainable Chem. Eng., 2017, 5(6): 5373-5381. |
| 50 | Chen T Y , Wang B , Shen X J , et al . Assessment of structural characteristics of regenerated cellulolytic enzyme lignin based on a mild DMSO/[Emim]OAc dissolution system from triploid of populus tomentosa carr[J]. RSC Adv., 2017, 7(6): 3376-3387. |
| 51 | Brzonova I , Asina F , Andrianova A A , et al . Fungal biotransformation of insoluble kraft lignin into a water soluble polymer[J]. Ind. Eng. Chem. Res., 2017, 56(21): 6103-6113. |
| 52 | Bhanja P , Modak A , Chatterjee S , et al . Bifunctionalized mesoporous SBA-15: a new heterogeneous catalyst for the facile synthesis of 5-hydroxymethylfurfural[J]. ACS Sustainable Chem. Eng., 2017, 5(3): 2763-2773. |
| 53 | Xue Z , Zhao X , Sun R C , et al . Biomass-derived γ-valerolactone-based solvent systems for highly efficient dissolution of various lignins: dissolution behavior and mechanism study[J]. ACS Sustainable Chem. Eng., 2016, 4(7): 3864-3870. |
| 54 | Zuo M , Le K , Li Z , et al . Green process for production of 5-hydroxymethylfurfural from carbohydrates with high purity in deep eutectic solvents[J]. Ind. Crops Prod., 2017, 99: 1-6. |
| 55 | Hattori K , Arai A . Preparation and hydrolysis of water-stable amorphous cellulose[J]. ACS Sustainable Chem. Eng., 2016, 4(3): 1180-1186. |
| 56 | Chen H , Zhou J , Mao J , et al . Enhancement of mass transfer through bubbling effect during extraction and reaction in biphasic systems containing ionic liquid[J]. RSC Adv., 2016, 6(103): 101485-101491. |
| 57 | Chidambaram M , Bell A T . A two-step approach for the catalytic conversion of glucose to 2,5-dimethylfuran in ionic liquids[J]. Green Chem., 2010, 12(7): 1253-1262. |
| 58 | Tian S , Ren S , Hou Y , et al . Densities, viscosities and excess properties of binary mixtures of 1,1,3,3-tetramethylguanidinium lactate + water at T = (303.15 to 328.15) K[J]. J. Chem. Eng. Data, 2013, 58(7): 1885-1892. |
| 59 | Kermanpour F , Niakan H Z , Sharifi T . Density and viscosity measurements of binary alkanol mixtures from (293.15 to 333.15) K at atmospheric pressure[J]. J. Chem. Eng. Data, 2013, 58(5): 1086-1091. |
| 60 | Ciocirlan O , Croitoru O , Iulian O . Densities and viscosities for binary mixtures of 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid with molecular solvents[J]. J. Chem. Eng. Data, 2011, 56(4): 1526-1534. |
| 61 | Roy M N , Sarkar B K , Chanda R . Viscosity, density, and speed of sound for the binary mixtures of formamide with 2-methoxyethanol, acetophenone, acetonitrile, 1,2-dimethoxyethane, and dimethylsulfoxide at different temperatures[J]. J. Chem. Eng. Data, 2007, 52: 1630-1637. |
| 62 | Yasmeen S , Riyazuddeen, Anwar N . Interaction of 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)-imide with methanol/dimethyl sulfoxide at (298.15, 303.15, 308.15, 313.15, 318.15 and 323.15) K: measurements and correlations of thermophysical properties[J]. J. Mol. Liq., 2016, 221: 1207-1217. |
| 63 | Keshapolla D , Gardas R L . Apparent molar volumes and isentropic compressions of benzylalkylammonium ionic liquids in dimethylsulfoxide from 293.15 K to 328.15 K[J]. Fluid Phase Equilib., 2014, 383: 32-42. |
| 64 | Redlich O , Meyer D M . The molal volumes of electrolytes[J]. Chem Rev., 1964, 24(3): 221-227. |
| 65 | Carmen Grande M D , Juliá J A , García M , et al . On the density and viscosity of (water + dimethylsulphoxide) binary mixtures[J]. J. Chem. Thermodyn., 2007, 39(7): 1049-1056. |
| 66 | Tôrres R B , Marchiore A C M , Volpe P L O . Volumetric properties of binary mixtures of (water + organic solvents) at temperatures between T = 288.15 K and T = 303.15 K at p = 0.1 MPa[J]. J. Chem. Thermodyn., 2006, 38(5): 526-541. |
| 67 | González E J , Alonso L , Á Domínguez . Physical properties of binary mixtures of the ionic liquid 1-methyl-3-octylimidazolium chloride with methanol, ethanol, and 1-propanol at T = (298.15, 313.15, and 328.15) K and at P = 0.1 MPa[J]. J. Chem. Eng. Data, 2006, 51(4): 1446-1452. |
| 68 | González E J , González B , Macedo E A . Thermophysical properties of the pure ionic liquid 1-butyl-1-methylpyrrolidinium dicyanamide and its binary mixtures with alcohols[J]. J. Chem. Eng. Data, 2013, 58(6): 1440-1448. |
| 69 | Zheng Y , Dong K , Wang Q , et al . Density, viscosity, and conductivity of lewis acidic 1-butyl- and 1-hydrogen-3-methylimidazolium chloroaluminate ionic liquids[J]. J. Chem. Eng. Data, 2013, 58(1): 32-42. |
| 70 | Ciocirlan O , Iulian O . Properties of pure 1-butyl-2,3-dimethylimidazolium tetrafluoroborate ionic liquid and its binary mixtures with dimethyl sulfoxide and acetonitrile[J]. J. Chem. Eng. Data, 2012, 57(11): 3142-3148. |
| 71 | Iulian O , Ciocirlan O . Volumetric properties of binary mixtures of two 1-alkyl-3-methylimidazolium tetrafluoroborate ionic liquids with molecular solvents[J]. J. Chem. Eng. Data, 2012, 57(10): 2640-2646. |
| [1] | Jingwei CHAO, Jiaxing XU, Tingxian LI. Investigation on the heating performance of the tube-free-evaporation based sorption thermal battery [J]. CIESC Journal, 2023, 74(S1): 302-310. |
| [2] | Long ZHANG, Mengjie SONG, Keke SHAO, Xuan ZHANG, Jun SHEN, Runmiao GAO, Zekang ZHEN, Zhengyong JIANG. Simulation study on frosting at windward fin end of heat exchanger [J]. CIESC Journal, 2023, 74(S1): 179-182. |
| [3] | Qi WANG, Bin ZHANG, Xiaoxin ZHANG, Hujian WU, Haitao ZHAN, Tao WANG. Synthesis of isoxepac and 2-ethylanthraquinone catalyzed by chloroaluminate-triethylamine ionic liquid/P2O5 [J]. CIESC Journal, 2023, 74(S1): 245-249. |
| [4] | Ruimin CHE, Wenqiu ZHENG, Xiaoyu WANG, Xin LI, Feng XU. Research progress on homogeneous processing of cellulose in ionic liquids [J]. CIESC Journal, 2023, 74(9): 3615-3627. |
| [5] | Zehao MI, Er HUA. DFT and COSMO-RS theoretical analysis of SO2 absorption by polyamines type ionic liquids [J]. CIESC Journal, 2023, 74(9): 3681-3696. |
| [6] | Meisi CHEN, Weida CHEN, Xinyao LI, Shangyu LI, Youting WU, Feng ZHANG, Zhibing ZHANG. Advances in silicon-based ionic liquid microparticle enhanced gas capture and conversion [J]. CIESC Journal, 2023, 74(9): 3628-3639. |
| [7] | Lizhi WANG, Qiancheng HANG, Yeling ZHENG, Yan DING, Jiaji CHEN, Qing YE, Jinlong LI. Separation of methyl propionate + methanol azeotrope using ionic liquid entrainers [J]. CIESC Journal, 2023, 74(9): 3731-3741. |
| [8] | Jie CHEN, Yongsheng LIN, Kai XIAO, Chen YANG, Ting QIU. Study on catalytic synthesis of sec-butanol by tunable choline-based basic ionic liquids [J]. CIESC Journal, 2023, 74(9): 3716-3730. |
| [9] | Minghao SONG, Fei ZHAO, Shuqing LIU, Guoxuan LI, Sheng YANG, Zhigang LEI. Multi-scale simulation and study of volatile phenols removal from simulated oil by ionic liquids [J]. CIESC Journal, 2023, 74(9): 3654-3664. |
| [10] | Shaoqi YANG, Shuheng ZHAO, Lungang CHEN, Chenguang WANG, Jianjun HU, Qing ZHOU, Longlong MA. Hydrodeoxygenation of lignin-derived compounds to alkanes in Raney Ni-protic ionic liquid system [J]. CIESC Journal, 2023, 74(9): 3697-3707. |
| [11] | Shuang LIU, Linzhou ZHANG, Zhiming XU, Suoqi ZHAO. Study on molecular level composition correlation of viscosity of residual oil and its components [J]. CIESC Journal, 2023, 74(8): 3226-3241. |
| [12] | Ji CHEN, Ze HONG, Zhao LEI, Qiang LING, Zhigang ZHAO, Chenhui PENG, Ping CUI. Study on coke dissolution loss reaction and its mechanism based on molecular dynamics simulations [J]. CIESC Journal, 2023, 74(7): 2935-2946. |
| [13] | Yuanliang ZHANG, Xinqi LUAN, Weige SU, Changhao LI, Zhongxing ZHAO, Liqin ZHOU, Jianmin CHEN, Yan HUANG, Zhenxia ZHAO. Study on selective extraction of nicotine by ionic liquids composite extractant and DFT calculation [J]. CIESC Journal, 2023, 74(7): 2947-2956. |
| [14] | Bowen LEI, Jianhua WU, Qihang WU. Research on high injection superheat cycle for R290 low pressure ratio heat pump [J]. CIESC Journal, 2023, 74(5): 1875-1883. |
| [15] | Bimao ZHOU, Shisen XU, Xiaoxiao WANG, Gang LIU, Xiaoyu LI, Yongqiang REN, Houzhang TAN. Effect of burner bias angle on distribution characteristics of gasifier slag layer [J]. CIESC Journal, 2023, 74(5): 1939-1949. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||