CIESC Journal ›› 2023, Vol. 74 ›› Issue (8): 3171-3192.DOI: 10.11949/0438-1157.20230338
• Reviews and monographs • Previous Articles Next Articles
Yuyuan ZHENG1,2( ), Zhiwei GE1,2,3(
), Zhiwei GE1,2,3( ), Xiangyu HAN1,2, Liang WANG1,2,3, Haisheng CHEN1,2(
), Xiangyu HAN1,2, Liang WANG1,2,3, Haisheng CHEN1,2( )
)
Received:2023-04-06
Revised:2023-08-15
Online:2023-10-18
Published:2023-08-25
Contact:
Zhiwei GE, Haisheng CHEN
郑玉圆1,2( ), 葛志伟1,2,3(
), 葛志伟1,2,3( ), 韩翔宇1,2, 王亮1,2,3, 陈海生1,2(
), 韩翔宇1,2, 王亮1,2,3, 陈海生1,2( )
)
通讯作者:
葛志伟,陈海生
作者简介:郑玉圆(1999—),女,硕士研究生,zhengyuyuan@iet.cn
基金资助:CLC Number:
Yuyuan ZHENG, Zhiwei GE, Xiangyu HAN, Liang WANG, Haisheng CHEN. Progress and prospect of medium and high temperature thermochemical energy storage of calcium-based materials[J]. CIESC Journal, 2023, 74(8): 3171-3192.
郑玉圆, 葛志伟, 韩翔宇, 王亮, 陈海生. 中高温钙基材料热化学储热的研究进展与展望[J]. 化工学报, 2023, 74(8): 3171-3192.
Add to citation manager EndNote|Ris|BibTeX
| 反应类型 | 反应式 | ΔH/(kJ/mol) | T/℃ |
|---|---|---|---|
| 金属氢化物反应[ | 75 | 450 | |
| 88 | 947 | ||
| 氨类反应[ | 66 | 200 | |
| 335 | 467 | ||
| 氢氧化物反应[ | 84 | 330 | |
| 104 | 515 | ||
| 碳酸盐反应[ | 178 | 900 | |
| 125 | 400 | ||
| 氧化还原反应[ | 205 | 914 | |
| 910 | 948 |
Table 1 The classification and reaction conditions for thermochemical energy storage
| 反应类型 | 反应式 | ΔH/(kJ/mol) | T/℃ |
|---|---|---|---|
| 金属氢化物反应[ | 75 | 450 | |
| 88 | 947 | ||
| 氨类反应[ | 66 | 200 | |
| 335 | 467 | ||
| 氢氧化物反应[ | 84 | 330 | |
| 104 | 515 | ||
| 碳酸盐反应[ | 178 | 900 | |
| 125 | 400 | ||
| 氧化还原反应[ | 205 | 914 | |
| 910 | 948 |
| 分类 | 掺杂材料 | 特点 | 文献 |
|---|---|---|---|
| 活性掺杂 | Al2O3, ZrO2 | 在50次煅烧/碳酸化循环中保持80%以上吸附性;允许离子在整个晶体结构中迁移,对碳酸化反应起到催化作用;有效增强循环稳定性和反应活性 | [ |
| CeO2, Mn3O4 | 分散CaO颗粒,缓解烧结;Ce和Mn之间的电子转移促进了CO2扩散和O2-迁移;在40次循环中保持0.61 g/g的CO2吸附量 | [ | |
| Fe2O3, Mn3O4 | 薄片状多孔结构;促进氧空位产生并降低反应活化能;在20次循环中保持95%的转化率 | [ | |
| Al2O3, CeO2 | 掺杂量为5%时表现出最高的储热能力;30次循环有效转化率和能量密度仅下降7%;具有更大的比表面积和孔隙率,促进CO2吸附 | [ | |
| 惰性掺杂 | Al2O3 | 提高反应速率,缩短反应时间(减少42%);提高循环稳定性 | [ |
| SiO2 | 提高材料机械强度,在100次循环内保持28 N以上 | [ | |
| SiO2 | 提高材料导热性能,比热容提高20%;掺杂量为5%时材料反应动力学表现最佳;循环稳定性增强28% | [ | |
| TiO2 | 掺杂量为2.5% 储热密度达1256.68 kJ/kg,30次循环后仍为纯CaCO3的2.26倍;反应活化能从1117.39 kJ/mol降低到997.6 kJ/mol,脱碳温度从903.56℃降低到876.13℃;总转化率降低 | [ |
Table 2 Classification and characteristics of oxide doping modification
| 分类 | 掺杂材料 | 特点 | 文献 |
|---|---|---|---|
| 活性掺杂 | Al2O3, ZrO2 | 在50次煅烧/碳酸化循环中保持80%以上吸附性;允许离子在整个晶体结构中迁移,对碳酸化反应起到催化作用;有效增强循环稳定性和反应活性 | [ |
| CeO2, Mn3O4 | 分散CaO颗粒,缓解烧结;Ce和Mn之间的电子转移促进了CO2扩散和O2-迁移;在40次循环中保持0.61 g/g的CO2吸附量 | [ | |
| Fe2O3, Mn3O4 | 薄片状多孔结构;促进氧空位产生并降低反应活化能;在20次循环中保持95%的转化率 | [ | |
| Al2O3, CeO2 | 掺杂量为5%时表现出最高的储热能力;30次循环有效转化率和能量密度仅下降7%;具有更大的比表面积和孔隙率,促进CO2吸附 | [ | |
| 惰性掺杂 | Al2O3 | 提高反应速率,缩短反应时间(减少42%);提高循环稳定性 | [ |
| SiO2 | 提高材料机械强度,在100次循环内保持28 N以上 | [ | |
| SiO2 | 提高材料导热性能,比热容提高20%;掺杂量为5%时材料反应动力学表现最佳;循环稳定性增强28% | [ | |
| TiO2 | 掺杂量为2.5% 储热密度达1256.68 kJ/kg,30次循环后仍为纯CaCO3的2.26倍;反应活化能从1117.39 kJ/mol降低到997.6 kJ/mol,脱碳温度从903.56℃降低到876.13℃;总转化率降低 | [ |

Fig.7 Principle of hydration/ dehydration reaction of CaO/Ca(OH)2 system encapsulated in semi-permeable ceramic material (shell: brown part; core: white and gray parts)[58]
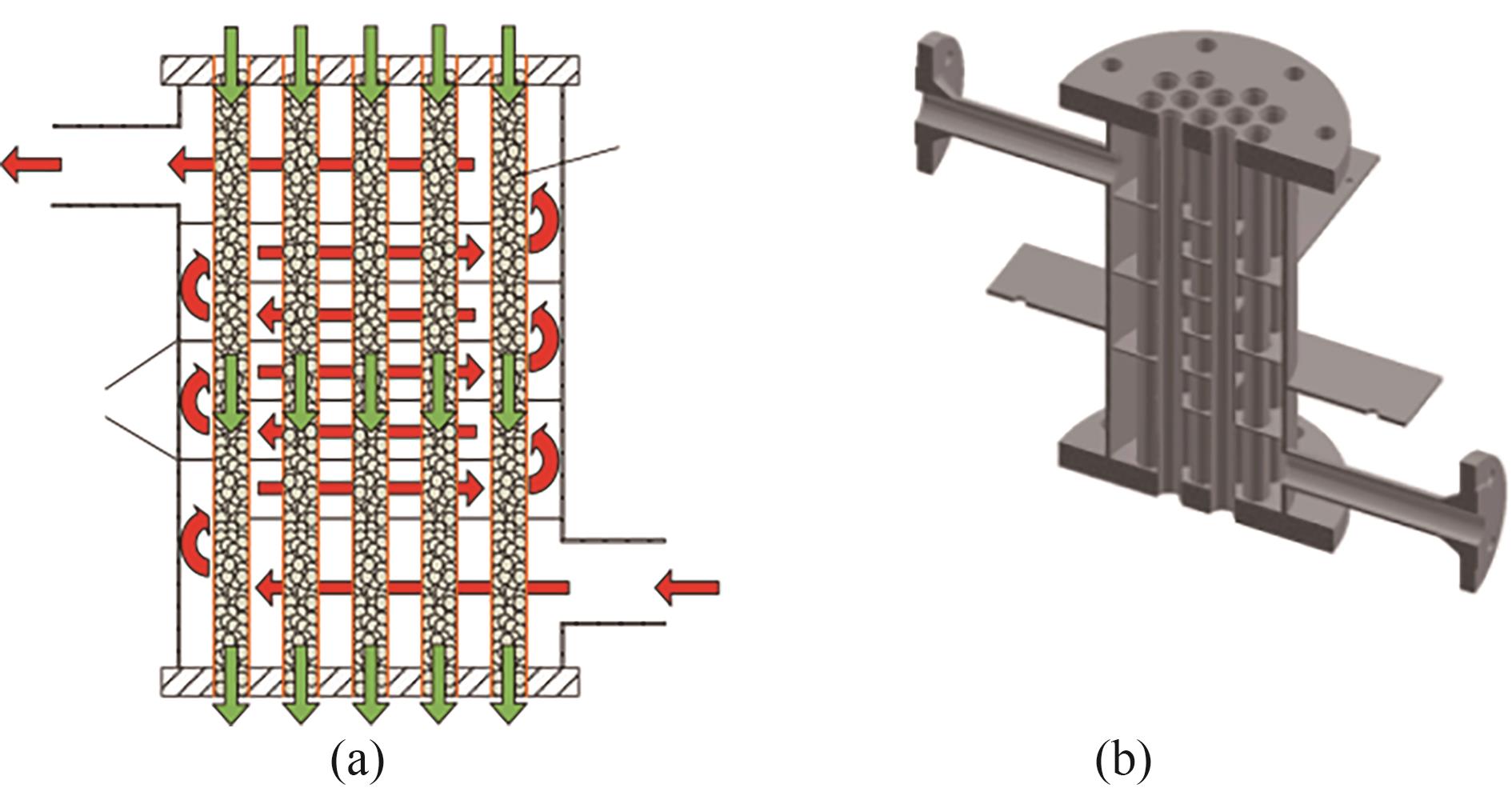
Fig.20 The flow routes of the heat transfer fluid in the shell (red arrows) and the heat storage material in the reactor tube (green arrows) (a) and 3D image of the reactor (b)[60]
| 1 | 陈海生, 刘畅, 徐玉杰, 等. 储能在碳达峰碳中和目标下的战略地位和作用[J]. 储能科学与技术, 2021, 10(5): 1477-1485. |
| Chen H S, Liu C, Xu Y J, et al. The strategic position and role of energy storage under the goal of carbon peak and carbon neutrality[J]. Energy Storage Science and Technology, 2021, 10(5): 1477-1485. | |
| 2 | Alvarez Rivero M, Rodrigues D, Pinheiro C I C, et al. Solid-gas reactors driven by concentrated solar energy with potential application to calcium looping: a comparative review[J]. Renewable and Sustainable Energy Reviews, 2022, 158: 112048 |
| 3 | 王泽众, 黄平瑞, 魏高升, 等. 太阳能热发电固-气两相化学储热技术研究进展[J]. 发电技术, 2021, 42(2): 238-246. |
| Wang Z Z, Huang P R, Wei G S, et al. Research progress of solid-gas two-phase chemical heat storage technology for solar thermal power generation[J]. Power Generation Technology, 2021, 42(2): 238-246. | |
| 4 | Vecchi A, Sciacovelli A. Long-duration thermo-mechanical energy storage—present and future techno-economic competitiveness[J]. Applied Energy, 2023, 334: 120628. |
| 5 | Borri E, Zsembinszki G, Cabeza L F. Recent developments of thermal energy storage applications in the built environment: a bibliometric analysis and systematic review[J]. Applied Thermal Engineering, 2021, 189: 116666. |
| 6 | Aydin D, Casey S P, Riffat S. The latest advancements on thermochemical heat storage systems[J]. Renewable and Sustainable Energy Reviews, 2015, 41: 356-367. |
| 7 | Salgado-Pizarro R, Calderón A, Svobodova-Sedlackova A, et al. The relevance of thermochemical energy storage in the last two decades: the analysis of research evolution[J]. Journal of Energy Storage, 2022, 51: 104377. |
| 8 | Desai F, Sunku Prasad J, Muthukumar P, et al. Thermochemical energy storage system for cooling and process heating applications: a review[J]. Energy Conversion and Management, 2021, 229: 113617. |
| 9 | Han X Y, Wang L, Ling H S, et al. Critical review of thermochemical energy storage systems based on cobalt, manganese, and copper oxides[J]. Renewable and Sustainable Energy Reviews, 2022, 158: 112076. |
| 10 | Pardo P, Deydier A, Anxionnaz-Minvielle Z, et al. A review on high temperature thermochemical heat energy storage[J]. Renewable and Sustainable Energy Reviews, 2014, 32: 591-610. |
| 11 | Carrillo A J, González-Aguilar J, Romero M, et al. Solar energy on demand: a review on high temperature thermochemical heat storage systems and materials[J]. Chemical Reviews, 2019, 119(7): 4777-4816. |
| 12 | Bhatnagar A, Shaz M A, Srivastava O N. Synthesis of MgH2 using autocatalytic effect of MgH2 [J]. International Journal of Hydrogen Energy, 2019, 44(13): 6738-6747. |
| 13 | Leng H Y, Pan Y B, Li Q, et al. Effect of LiH on hydrogen storage property of MgH2 [J]. International Journal of Hydrogen Energy, 2014, 39(25): 13622-13627. |
| 14 | Yan J, Pan Z H, Zhao C Y. Experimental study of MgO/Mg(OH)2 thermochemical heat storage with direct heat transfer mode[J]. Applied Energy, 2020, 275: 115356. |
| 15 | Bian Z G, Li Y J, Ren Y, et al. Thermochemical heat storage performance of CaO particles under fluidization in coupled CaO/Ca(OH)2 cycles and CaO/CaCO3 cycles[J]. Journal of Energy Storage, 2022, 56: 106045. |
| 16 | Shkatulov A I, Kim S T, Miura H, et al. Adapting the MgO-CO2 working pair for thermochemical energy storage by doping with salts[J]. Energy Conversion and Management, 2019, 185: 473-481. |
| 17 | Chen X, Dong Z, Zhu L, et al. Mass transfer performance inside Ca-based thermochemical energy storage materials under different operating conditions[J]. Renewable Energy, 2023, 205: 340-348. |
| 18 | Carrillo A J, Pizarro P, Coronado J M. Assessing Cr incorporation in Mn2O3/Mn3O4 redox materials for thermochemical heat storage applications[J]. Journal of Energy Storage, 2021, 33: 102028. |
| 19 | Fellet M, Buckley C E, Paskevicius M, et al. Research on metal hydrides revived for next-generation solutions to renewable energy storage[J]. MRS Bulletin, 2013, 38(12): 1012-1013. |
| 20 | Lovegrove K, Luzzi A, Soldiani I, et al. Developing ammonia based thermochemical energy storage for dish power plants[J]. Solar Energy, 2004, 76(1/2/3): 331-337. |
| 21 | Shkatulov A, Ryu J, Kato Y, et al. Composite material "Mg(OH)2/vermiculite": a promising new candidate for storage of middle temperature heat[J]. Energy, 2012, 44(1): 1028-1034. |
| 22 | Bian Z G, Li Y J, Zhang C X, et al. CaO/Ca(OH)2 heat storage performance of hollow nanostructured CaO-based material from Ca-looping cycles for CO2 capture[J]. Fuel Processing Technology, 2021, 217: 106834. |
| 23 | Criado Y A, Alonso M, Abanades J C. Enhancement of a CaO/Ca(OH)2 based material for thermochemical energy storage[J]. Solar Energy, 2016, 135: 800-809. |
| 24 | Wu S K, Zhou C, Doroodchi E, et al. A review on high-temperature thermochemical energy storage based on metal oxides redox cycle[J]. Energy Conversion and Management, 2018, 168: 421-453. |
| 25 | Chen X Y, Zhang Z, Qi C G, et al. State of the art on the high-temperature thermochemical energy storage systems[J]. Energy Conversion and Management, 2018, 177: 792-815. |
| 26 | Ortiz C, Valverde J M, Chacartegui R, et al. The calcium-looping (CaCO3/CaO) process for thermochemical energy storage in concentrating solar power plants[J]. Renewable and Sustainable Energy Reviews, 2019, 113: 109252. |
| 27 | Wu S K, Zhou C, Tremain P, et al. A phase change calcium looping thermochemical energy storage system based on CaCO3/CaO-CaCl2 [J]. Energy Conversion and Management, 2021, 227: 113503. |
| 28 | Wang K, Yan T, Li R K, et al. A review for Ca(OH)2/CaO thermochemical energy storage systems[J]. Journal of Energy Storage, 2022, 50: 104612. |
| 29 | Sunku Prasad J, Muthukumar P, Desai F, et al. A critical review of high-temperature reversible thermochemical energy storage systems[J]. Applied Energy, 2019, 254: 113733. |
| 30 | Hu M, Hu H Y, Ye Z H, et al. A review on turning sewage sludge to value-added energy and materials via thermochemical conversion towards carbon neutrality[J]. Journal of Cleaner Production, 2022, 379: 134657. |
| 31 | Li B, Magoua Mbeugang C F, Huang Y, et al. A review of CaO based catalysts for tar removal during biomass gasification[J]. Energy, 2022, 244: 123172. |
| 32 | Zhang X Y, Liu W Q, Zhou S M, et al. A review on granulation of CaO-based sorbent for carbon dioxide capture[J]. Chemical Engineering Journal, 2022, 446: 136880. |
| 33 | Yuan Y, Li Y J, Zhao J L. Development on thermochemical energy storage based on CaO-based materials: a review[J]. Sustainability, 2018, 10(8): 2660. |
| 34 | Yuan Y, Li Y J, Duan L B, et al. CaO/Ca(OH)2 thermochemical heat storage of carbide slag from calcium looping cycles for CO2 capture[J]. Energy Conversion and Management, 2018, 174: 8-19. |
| 35 | Hu Y C, Liu W Q, Chen H Q, et al. Screening of inert solid supports for CaO-based sorbents for high temperature CO2 capture[J]. Fuel, 2016, 181: 199-206. |
| 36 | Schaube F, Koch L, Wörner A, et al. A thermodynamic and kinetic study of the de- and rehydration of Ca(OH)2 at high H2O partial pressures for thermo-chemical heat storage[J]. Thermochimica Acta, 2012, 538: 9-20. |
| 37 | Dunstan M T, Donat F, Bork A H, et al. CO2 capture at medium to high temperature using solid oxide-based sorbents: fundamental aspects, mechanistic insights, and recent advances[J]. Chemical Reviews, 2021, 121(20): 12681-12745. |
| 38 | Fujii I, Ishino M, Akiyama S, et al. Behavior of Ca(OH)2/CaO pellet under dehydration and hydration[J]. Solar Energy, 1994, 53(4): 329-341. |
| 39 | Geng Y Q, Guo Y X, Fan B, et al. Research progress of calcium-based adsorbents for CO2 capture and anti-sintering modification[J]. Journal of Fuel Chemistry and Technology, 2021, 49(7): 998-1013. |
| 40 | Ridha F N, Manovic V, Wu Y H, et al. Post-combustion CO2 capture by formic acid-modified CaO-based sorbents[J]. International Journal of Greenhouse Gas Control, 2013, 16: 21-28. |
| 41 | Guo H X, Kou X C, Zhao Y J, et al. Effect of synergistic interaction between Ce and Mn on the CO2 capture of calcium-based sorbent: textural properties, electron donation, and oxygen vacancy[J]. Chemical Engineering Journal, 2018, 334: 237-246. |
| 42 | Møller K T, Berger A, Paskevicius M, et al. Synergetic effect of multicomponent additives on limestone when assessed as a thermochemical energy storage material[J]. Journal of Alloys and Compounds, 2022, 891: 161954. |
| 43 | Guo H, Kou X, Zhao Y, et al. Role of microstructure, electron transfer, and coordination state in the CO2 capture of calcium-based sorbent by doping (Zr-Mn) [J]. Chemical Engineering Journal, 2018, 336: 376-385. |
| 44 | Guo H X, Wang X, Wang H, et al. Double-exchange-induced effective increased CO2 capture of CaO by doping bimetallic oxides with variable valence state[J]. Chemical Engineering Journal, 2022, 433: 134490. |
| 45 | Sun H, Li Y J, Yan X Y, et al. Thermochemical energy storage performance of Al2O3/CeO2 co-doped CaO-based material under high carbonation pressure[J]. Applied Energy, 2020, 263: 114650. |
| 46 | Mathew A, Nadim N, Chandratilleke T T, et al. Kinetic investigation and numerical modelling of CaCO3/Al2O3 reactor for high-temperature thermal energy storage application[J]. Solar Energy, 2022, 241: 262-274. |
| 47 | Chen X Y, Jin X G, Liu Z M, et al. Experimental investigation on the CaO/CaCO3 thermochemical energy storage with SiO2 doping[J]. Energy, 2018, 155: 128-138. |
| 48 | Xu T X, Tian X K, Khosa A A, et al. Reaction performance of CaCO3/CaO thermochemical energy storage with TiO2 dopant and experimental study in a fixed-bed reactor[J]. Energy, 2021, 236: 121451. |
| 49 | Guo H X, Feng J Q, Zhao Y J, et al. Effect of micro-structure and oxygen vacancy on the stability of (Zr-Ce)-additive CaO-based sorbent in CO2 adsorption[J]. Journal of CO2 Utilization, 2017, 19: 165-176. |
| 50 | Sakellariou K G, Karagiannakis G, Criado Y A, et al. Calcium oxide based materials for thermochemical heat storage in concentrated solar power plants[J]. Solar Energy, 2015, 122: 215-230. |
| 51 | Salvador C, Lu D, Anthony E J, et al. Enhancement of CaO for CO2 capture in an FBC environment[J]. Chemical Engineering Journal, 2003, 96(1/2/3): 187-195. |
| 52 | Shkatulov A, Aristov Y. Modification of magnesium and calcium hydroxides with salts: an efficient way to advanced materials for storage of middle-temperature heat[J]. Energy, 2015, 85: 667-676. |
| 53 | Shkatulov A, Aristov Y. Calcium hydroxide doped by KNO3 as a promising candidate for thermochemical storage of solar heat[J]. RSC Advances, 2017, 7(68): 42929-42939. |
| 54 | Lee C H, Choi S W, Yoon H J, et al. Na2CO3-doped CaO-based high-temperature CO2 sorbent and its sorption kinetics[J]. Chemical Engineering Journal, 2018, 352: 103-109. |
| 55 | Xu Y Q, Lu B W, Luo C, et al. Na2CO3 promoted CaO-based heat carrier for thermochemical energy storage in concentrated solar power plants[J]. Chemical Engineering Journal, 2022, 435: 134852. |
| 56 | Wang T, Zhao C Y, Yan J. Investigation on the Ca(OH)2/CaO thermochemical energy storage system with potassium nitrate addition[J]. Solar Energy Materials and Solar Cells, 2020, 215: 110646. |
| 57 | Xu Y Q, Ding H R, Luo C, et al. Potential synergy of chlorine and potassium and sodium elements in carbonation enhancement of CaO-based sorbents[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(9): 11677-11684. |
| 58 | Afflerbach S, Afflerbach K, Trettin R, et al. Improvement of a semipermeable shell for encapsulation of calcium hydroxide for thermochemical heat storage solutions[J]. Solar Energy, 2021, 217: 208-222. |
| 59 | Afflerbach S, Kappes M, Gipperich A, et al. Semipermeable encapsulation of calcium hydroxide for thermochemical heat storage solutions[J]. Solar Energy, 2017, 148: 1-11. |
| 60 | Mejia A C, Afflerbach S, Linder M, et al. Experimental analysis of encapsulated CaO/Ca(OH)2 granules as thermochemical storage in a novel moving bed reactor[J]. Applied Thermal Engineering, 2020, 169: 114961. |
| 61 | Chen Y N, Long Y, Sun J A, et al. Core-in-shell, cellulose-templated CaO-based sorbent pellets for CO2 capture at elevated temperatures[J]. Energy & Fuels, 2021, 35(16): 13215-13223. |
| 62 | Liu F J, Chou K S, Huang Y K. A novel method to make regenerable core-shell calcium-based sorbents[J]. Journal of Environmental Management, 2006, 79(1): 51-56. |
| 63 | Sun J A, Liu W Q, Hu Y C, et al. Structurally improved, core-in-shell, CaO-based sorbent pellets for CO2 capture[J]. Energy & Fuels, 2015, 29(10): 6636-6644. |
| 64 | Jin D L, Yu X J, Yue L H, et al. Decomposition kinetics study of AlOOH coated calcium carbonate[J]. Materials Chemistry and Physics, 2009, 115(1): 418-422. |
| 65 | Sun Z K, Sedghkerdar M H, Saayman J, et al. A facile fabrication of mesoporous core-shell CaO-based pellets with enhanced reactive stability and resistance to attrition in cyclic CO2 capture[J]. Journal of Materials Chemistry A, 2014, 2(39): 16577-16588. |
| 66 | Ogura H, Miyazaki M, Matsuda H, et al. Experimental study on heat transfer enhancement of the solid reactant particle bed in a chemical heat pump using Ca(OH)2/CaO reaction[J]. Kagaku Kogaku Ronbunshu, 1991, 17(5): 916-923. |
| 67 | Wokon M, Block T, Nicolai S, et al. Thermodynamic and kinetic investigation of a technical grade manganese-iron binary oxide for thermochemical energy storage[J]. Solar Energy, 2017, 153: 471-485. |
| 68 | Schaube F, Utz I, Wörner A, et al. De- and rehydration of Ca(OH)2 in a reactor with direct heat transfer for thermo-chemical heat storage(Part B): Validation of model[J]. Chemical Engineering Research and Design, 2013, 91(5): 865-873. |
| 69 | Yan J, Zhao C Y. Experimental study of CaO/Ca(OH)2 in a fixed-bed reactor for thermochemical heat storage[J]. Applied Energy, 2016, 175: 277-284. |
| 70 | Schmidt M, Gutierrez A, Linder M. Thermochemical energy storage with CaO/Ca(OH)2—experimental investigation of the thermal capability at low vapor pressures in a lab scale reactor[J]. Applied Energy, 2017, 188: 672-681. |
| 71 | Wang B Y, Wang Z Y, Ma Y, et al. Heat transfer enhancement of indirect heat transfer reactors for Ca(OH)2/CaO thermochemical energy storage system[J]. Processes, 2021, 9(7): 1136. |
| 72 | Wang M Y, Chen L, He P, et al. Numerical study and enhancement of Ca(OH)2/CaO dehydration process with porous channels embedded in reactors[J]. Energy, 2019, 181: 417-428. |
| 73 | Han X C, Xu H J, Zhao C Y. Design and performance evaluation of multi-layered reactor for calcium-based thermochemical heat storage with multi-physics coupling[J]. Renewable Energy, 2022, 195: 1324-1340. |
| 74 | Funayama S, Takasu H, Kim S T, et al. Thermochemical storage performance of a packed bed of calcium hydroxide composite with a silicon-based ceramic honeycomb support[J]. Energy, 2020, 201: 117673. |
| 75 | Kato Y, Yamada M, Kanie T, et al. Calcium oxide/carbon dioxide reactivity in a packed bed reactor of a chemical heat pump for high-temperature gas reactors[J]. Nuclear Engineering and Design, 2001, 210(1/2/3): 1-8. |
| 76 | Scaltsoyiannes A, Lemonidou A. CaCO3 decomposition for calcium-looping applications: kinetic modeling in a fixed-bed reactor[J]. Chemical Engineering Science: X, 2020, 8: 100071. |
| 77 | Sun H, Li Y J, Bian Z G, et al. Thermochemical energy storage performances of Ca-based natural and waste materials under high pressure during CaO/CaCO3 cycles[J]. Energy Conversion and Management, 2019, 197: 111885. |
| 78 | Criado Y A, Alonso M, Abanades J C, et al. Conceptual process design of a CaO/Ca(OH)2 thermochemical energy storage system using fluidized bed reactors[J]. Applied Thermal Engineering, 2014, 73(1): 1087-1094. |
| 79 | Criado Y A, Huille A, Rougé S, et al. Experimental investigation and model validation of a CaO/Ca(OH)2 fluidized bed reactor for thermochemical energy storage applications[J]. Chemical Engineering Journal, 2017, 313: 1194-1205. |
| 80 | Pardo P, Anxionnaz-Minvielle Z, Rougé S, et al. Ca(OH)2/CaO reversible reaction in a fluidized bed reactor for thermochemical heat storage[J]. Solar Energy, 2014, 107: 605-616. |
| 81 | Kai R, Marc L, Matthias S. Experimental investigation of a novel mechanically fluidized bed reactor for thermochemical energy storage with calcium hydroxide/calcium oxide[J]. Applied Energy, 2022, 315:118976. |
| 82 | Uchino T, Fushimi C. Fluidized bed reactor for thermochemical heat storage using Ca(OH)2/CaO to absorb the fluctuations of electric power supplied by variable renewable energy sources: a dynamic model[J]. Chemical Engineering Journal, 2021, 419: 129571. |
| 83 | Shimizu T, Hirama T, Hosoda H, et al. A twin fluid-bed reactor for removal of CO2 from combustion processes[J]. Chemical Engineering Research and Design, 1999, 77(1): 62-68. |
| 84 | Grasa G S, Abanades J C. CO2 capture capacity of CaO in long series of carbonation/calcination cycles[J]. Industrial & Engineering Chemistry Research, 2006, 45(26): 8846-8851. |
| 85 | Arias B, Diego M E, Abanades J C, et al. Demonstration of steady state CO2 capture in a 1.7 MWth calcium looping pilot[J]. International Journal of Greenhouse Gas Control, 2013, 18: 237-245. |
| 86 | Blamey J, Al-Jeboori M J, Manovic V, et al. CO2 capture by calcium aluminate pellets in a small fluidized bed[J]. Fuel Processing Technology, 2016, 142: 100-106. |
| 87 | Blamey J, Manovic V, Anthony E J, et al. On steam hydration of CaO-based sorbent cycled for CO2 capture[J]. Fuel, 2015, 150: 269-277. |
| 88 | Zheng H B, Liu X L, Xuan Y M, et al. Thermochemical heat storage performances of fluidized black CaCO3 pellets under direct concentrated solar irradiation[J]. Renewable Energy, 2021, 178: 1353-1369. |
| 89 | Nikulshina V, Gebald C, Steinfeld A. CO2 capture from atmospheric air via consecutive CaO-carbonation and CaCO3-calcination cycles in a fluidized-bed solar reactor[J]. Chemical Engineering Journal, 2009, 146(2): 244-248. |
| 90 | Tregambi C, Padula S, Galbusieri M, et al. Directly irradiated fluidized bed reactor for thermochemical energy storage and solar fuels production[J]. Powder Technology, 2020, 366: 460-469. |
| 91 | Farcot L, Le Pierrès N, Michel B, et al. Numerical investigations of a continuous thermochemical heat storage reactor[J]. Journal of Energy Storage, 2018, 20: 109-119. |
| 92 | Roßkopf C, Afflerbach S, Schmidt M, et al. Investigations of nano coated calcium hydroxide cycled in a thermochemical heat storage[J]. Energy Conversion and Management, 2015, 97: 94-102. |
| 93 | Schmidt M, Szczukowski C, Roßkopf C, et al. Experimental results of a 10 kW high temperature thermochemical storage reactor based on calcium hydroxide[J]. Applied Thermal Engineering, 2014, 62(2): 553-559. |
| 94 | Chen X Y, Jin X G, Zhang Z H, et al. Experimental investigation of CaCO3/CaO in a spiral coil reactor for thermochemical energy storage[J]. Chemical Engineering Journal, 2022, 428: 131971. |
| 95 | Barker R. The reactivity of calcium oxide towards carbon dioxide and its use for energy storage[J]. Journal of Applied Chemistry and Biotechnology, 1974, 24(4/5): 221-227. |
| 96 | Pascual S, Lisbona P, Bailera M, et al. Design and operational performance maps of calcium looping thermochemical energy storage for concentrating solar power plants[J]. Energy, 2021, 220: 119715. |
| 97 | Hughes R W, Lu D Y, Anthony E J, et al. Design, process simulation and construction of an atmospheric dual fluidized bed combustion system for in situ CO2 capture using high-temperature sorbents[J]. Fuel Processing Technology, 2005, 86(14/15): 1523-1531. |
| 98 | Lu D Y, Hughes R W, Anthony E J. Ca-based sorbent looping combustion for CO2 capture in pilot-scale dual fluidized beds[J]. Fuel Processing Technology, 2008, 89(12): 1386-1395. |
| 99 | Ströhle J, Hilz J, Epple B. Performance of the carbonator and calciner during long-term carbonate looping tests in a 1 MWth pilot plant[J]. Journal of Environmental Chemical Engineering, 2020, 8(1): 103578. |
| 100 | Ströhle J, Junk M, Kremer J, et al. Carbonate looping experiments in a 1 MWth pilot plant and model validation[J]. Fuel, 2014, 127: 13-22. |
| 101 | Reitz M, Junk M, Ströhle J, et al. Design and operation of a 300 kWth indirectly heated carbonate looping pilot plant[J]. International Journal of Greenhouse Gas Control, 2016, 54: 272-281. |
| 102 | Ogura H, Yamamoto T, Kage H, et al. Effects of heat exchange condition on hot air production by a chemical heat pump dryer using CaO/H2O/Ca(OH)2 reaction[J]. Chemical Engineering Journal, 2002, 86(1/2): 3-10. |
| 103 | Ogura H, Yamamoto T, Kage H. Efficiencies of CaO/H2O/Ca(OH)2 chemical heat pump for heat storing and heating/cooling[J]. Energy, 2003, 28(14): 1479-1493. |
| 104 | Ogura H, Yasuda S, Otsubo Y, et al. Continuous operation of a chemical heat pump[J]. Asia-Pacific Journal of Chemical Engineering, 2007, 2(2): 118-123. |
| 105 | Ogura H, Shimojyo R, Kage H, et al. Simulation of hydration/dehydration of CaO/Ca(OH)2 chemical heat pump reactor for cold/hot heat generation[J]. Drying Technology, 1999, 17(7/8): 1579-1592. |
| 106 | Kato Y, Saku D, Harada N, et al. Utilization of high temperature heat from nuclear reactor using inorganic chemical heat pump[J]. Progress in Nuclear Energy, 1998, 32(3/4): 563-570. |
| 107 | Fujimoto S, Bilgen E, Ogura H. Dynamic simulation of CaO/Ca(OH)2 chemical heat pump systems[J]. Exergy, An International Journal, 2002, 2(1): 6-14. |
| 108 | Fujimoto S, Bilgen E, Ogura H. CaO/Ca(OH)2 chemical heat pump system[J]. Energy Conversion and Management, 2002, 43(7): 947-960. |
| 109 | Ogura H, Kubota M, Suzuki H, et al. Fundamental experimental study on chemical heat pump for storing low-temperature waste heat and releasing cold-heat[J]. Kagaku Kogaku Ronbunshu, 2009, 35(5): 506-510. |
| 110 | Zhang H, Ogura H, Umezu M, et al. Hydration reaction characteristics of CaO from various local limestone samples as chemical heat pump/storage materials[J]. Journal of Materials Science, 2017, 52(19): 11360-11369. |
| 111 | Arjmand M, Liu L C, Neretnieks I. Exergetic efficiency of high-temperature-lift chemical heat pump (CHP) based on CaO/CO2 and CaO/H2O working pairs[J]. International Journal of Energy Research, 2013, 37(9): 1122-1131. |
| 112 | Cerkvenik B, Kato Y, Storkenmaier F. Applicability of calcium oxide in a cascading sorption cooling system[J]. Journal of Chemical Engineering of Japan, 2002, 35(10): 969-976. |
| 113 | Alovisio A, Chacartegui R, Ortiz C, et al. Optimizing the CSP-calcium looping integration for thermochemical energy storage[J]. Energy Conversion and Management, 2017, 136: 85-98. |
| 114 | Tregambi C, Montagnaro F, Salatino P, et al. A model of integrated calcium looping for CO2 capture and concentrated solar power[J]. Solar Energy, 2015, 120: 208-220. |
| 115 | Edwards S E B, Materić V. Calcium looping in solar power generation plants[J]. Solar Energy, 2012, 86(9): 2494-2503. |
| 116 | Chacartegui R, Alovisio A, Ortiz C, et al. Thermochemical energy storage of concentrated solar power by integration of the calcium looping process and a CO2 power cycle[J]. Applied Energy, 2016, 173: 589-605. |
| 117 | Tregambi C, Bareschino P, Mancusi E, et al. Modelling of a concentrated solar power-photovoltaics hybrid plant for carbon dioxide capture and utilization via calcium looping and methanation[J]. Energy Conversion and Management, 2021, 230: 113792. |
| 118 | Binotti M, Astolfi M, Campanari S, et al. Preliminary assessment of sCO2 cycles for power generation in CSP solar tower plants[J]. Applied Energy, 2017, 204: 1007-1017. |
| 119 | Hanak D P, Manovic V. Calcium looping with supercritical CO2 cycle for decarbonisation of coal-fired power plant[J]. Energy, 2016, 102: 343-353. |
| 120 | Ortiz C, Romano M C, Valverde J M, et al. Process integration of calcium-looping thermochemical energy storage system in concentrating solar power plants[J]. Energy, 2018, 155: 535-551. |
| 121 | Fernández R, Ortiz C, Chacartegui R, et al. Dispatchability of solar photovoltaics from thermochemical energy storage[J]. Energy Conversion and Management, 2019, 191: 237-246. |
| 122 | Colelli G, Chacartegui R, Ortiz C, et al. Life cycle and environmental assessment of calcium looping (CaL) in solar thermochemical energy storage[J]. Energy Conversion and Management, 2022, 257: 115428. |
| 123 | Karasavvas E, Panopoulos K D, Papadopoulou S, et al. Energy and exergy analysis of the integration of concentrated solar power with calcium looping for power production and thermochemical energy storage[J]. Renewable Energy, 2020, 154: 743-753. |
| 124 | Tesio U, Guelpa E, Verda V. Integration of thermochemical energy storage in concentrated solar power (Part 1): Energy and economic analysis/optimization[J]. Energy Conversion and Management: X, 2020, 6: 100039. |
| 125 | Ortiz C, Tejada C, Chacartegui R, et al. Solar combined cycle with high-temperature thermochemical energy storage[J]. Energy Conversion and Management, 2021, 241: 114274. |
| 126 | Bravo R, Ortiz C, Chacartegui R, et al. Hybrid solar power plant with thermochemical energy storage: a multi-objective operational optimisation[J]. Energy Conversion and Management, 2020, 205: 112421. |
| 127 | Khosa A A, Xu T X, Xia B Q, et al. Technological challenges and industrial applications of CaCO3/CaO based thermal energy storage system—a review[J]. Solar Energy, 2019, 193: 618-636. |
| [1] | He JIANG, Junfei YUAN, Lin WANG, Guyu XING. Experimental study on the effect of flow sharing cavity structure on phase change flow characteristics in microchannels [J]. CIESC Journal, 2023, 74(S1): 235-244. |
| [2] | Zhewen CHEN, Junjie WEI, Yuming ZHANG. System integration and energy conversion mechanism of the power technology with integrated supercritical water gasification of coal and SOFC [J]. CIESC Journal, 2023, 74(9): 3888-3902. |
| [3] | Lingding MENG, Ruqing CHONG, Feixue SUN, Zihui MENG, Wenfang LIU. Immobilization of carbonic anhydrase on modified polyethylene membrane and silica [J]. CIESC Journal, 2023, 74(8): 3472-3484. |
| [4] | Guixian LI, Abo CAO, Wenliang MENG, Dongliang WANG, Yong YANG, Huairong ZHOU. Process design and evaluation of CO2 to methanol coupled with SOEC [J]. CIESC Journal, 2023, 74(7): 2999-3009. |
| [5] | Lei MAO, Guanzhang LIU, Hang YUAN, Guangya ZHANG. Efficient preparation of carbon anhydrase nanoparticles capable of capturing CO2 and their characteristics [J]. CIESC Journal, 2023, 74(6): 2589-2598. |
| [6] | Bin CAI, Xiaolin ZHANG, Qian LUO, Jiangtao DANG, Liyuan ZUO, Xinmei LIU. Research progress of conductive thin film materials [J]. CIESC Journal, 2023, 74(6): 2308-2321. |
| [7] | Wenchao XU, Zhigao SUN, Cuimin LI, Juan LI, Haifeng HUANG. Effect of surfactant E-1310 on the formation of HCFC-141b hydrate under static conditions [J]. CIESC Journal, 2023, 74(5): 2179-2185. |
| [8] | Cheng YUN, Qianlin WANG, Feng CHEN, Xin ZHANG, Zhan DOU, Tingjun YAN. Deep-mining risk evolution path of chemical processes based on community structure [J]. CIESC Journal, 2023, 74(4): 1639-1650. |
| [9] | Zijian WANG, Ming KE, Jiahan LI, Shuting LI, Jinru SUN, Yanbing TONG, Zhiping ZHAO, Jiaying LIU, Lu REN. Progress in preparation and application of short b-axis ZSM-5 molecular sieve [J]. CIESC Journal, 2023, 74(4): 1457-1473. |
| [10] | Xiangshang CHEN, Zhenjie MA, Xihua REN, Yue JIA, Xiaolong LYU, Huayan CHEN. Preparation and mass transfer efficiency of three-dimensional network extraction membrane [J]. CIESC Journal, 2023, 74(3): 1126-1133. |
| [11] | Runzhu LIU, Tiantian CHU, Xiaoa ZHANG, Chengzhong WANG, Junying ZHANG. Synthesis and properties of phenylene-containing α,ω-hydroxy-terminated fluorosilicone polymers [J]. CIESC Journal, 2023, 74(3): 1360-1369. |
| [12] | Yalin WANG, Yuqing PAN, Chenliang LIU. Intermittent process monitoring based on GSA-LSTM dynamic structure feature extraction [J]. CIESC Journal, 2022, 73(9): 3994-4002. |
| [13] | Hongxin YANG, Xingya LI, Liang GE, Tongwen XU. Preparation of mono-/divalent anion permselective membranes with piperidinium-type long side-chain [J]. CIESC Journal, 2022, 73(8): 3739-3748. |
| [14] | Kun WANG, Hongbo SHI, Shuai TAN, Bing SONG, Yang TAO. Local time difference constrained neighborhood preserving embedding algorithm for fault detection [J]. CIESC Journal, 2022, 73(7): 3109-3119. |
| [15] | Jianfei SONG, Liqiang SUN, Ming XIE, Yaodong WEI. Experimental study of instability of gas-phase swirling flow in cyclone [J]. CIESC Journal, 2022, 73(7): 2858-2864. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||