CIESC Journal ›› 2019, Vol. 70 ›› Issue (3): 830-839.DOI: 10.11949/j.issn.0438-1157.20181154
• Thermodynamics • Previous Articles Next Articles
Xudong YU1,2,3( ),Qin HUANG1,Lin WANG1,Maolan LI1,Hong ZHENG1,Ying ZENG1,3
),Qin HUANG1,Lin WANG1,Maolan LI1,Hong ZHENG1,Ying ZENG1,3
Received:2018-10-08
Revised:2018-12-18
Online:2019-03-05
Published:2019-03-05
Contact:
Xudong YU
于旭东1,2,3( ),黄琴1,王林1,李茂兰1,郑洪1,曾英1,3
),黄琴1,王林1,李茂兰1,郑洪1,曾英1,3
通讯作者:
于旭东
作者简介:于旭东(1985—),男,博士,副教授,<email>xwdlyxd@126.com</email>
基金资助:CLC Number:
Xudong YU, Qin HUANG, Lin WANG, Maolan LI, Hong ZHENG, Ying ZENG. Measurements and simulation for ternary system KCl-PEG4000-H2O at 288, 298 and 308 K[J]. CIESC Journal, 2019, 70(3): 830-839.
于旭东, 黄琴, 王林, 李茂兰, 郑洪, 曾英. KCl-PEG4000-H2O三元体系288、298、308 K相平衡测定及计算[J]. 化工学报, 2019, 70(3): 830-839.
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgxb.cip.com.cn/EN/10.11949/j.issn.0438-1157.20181154
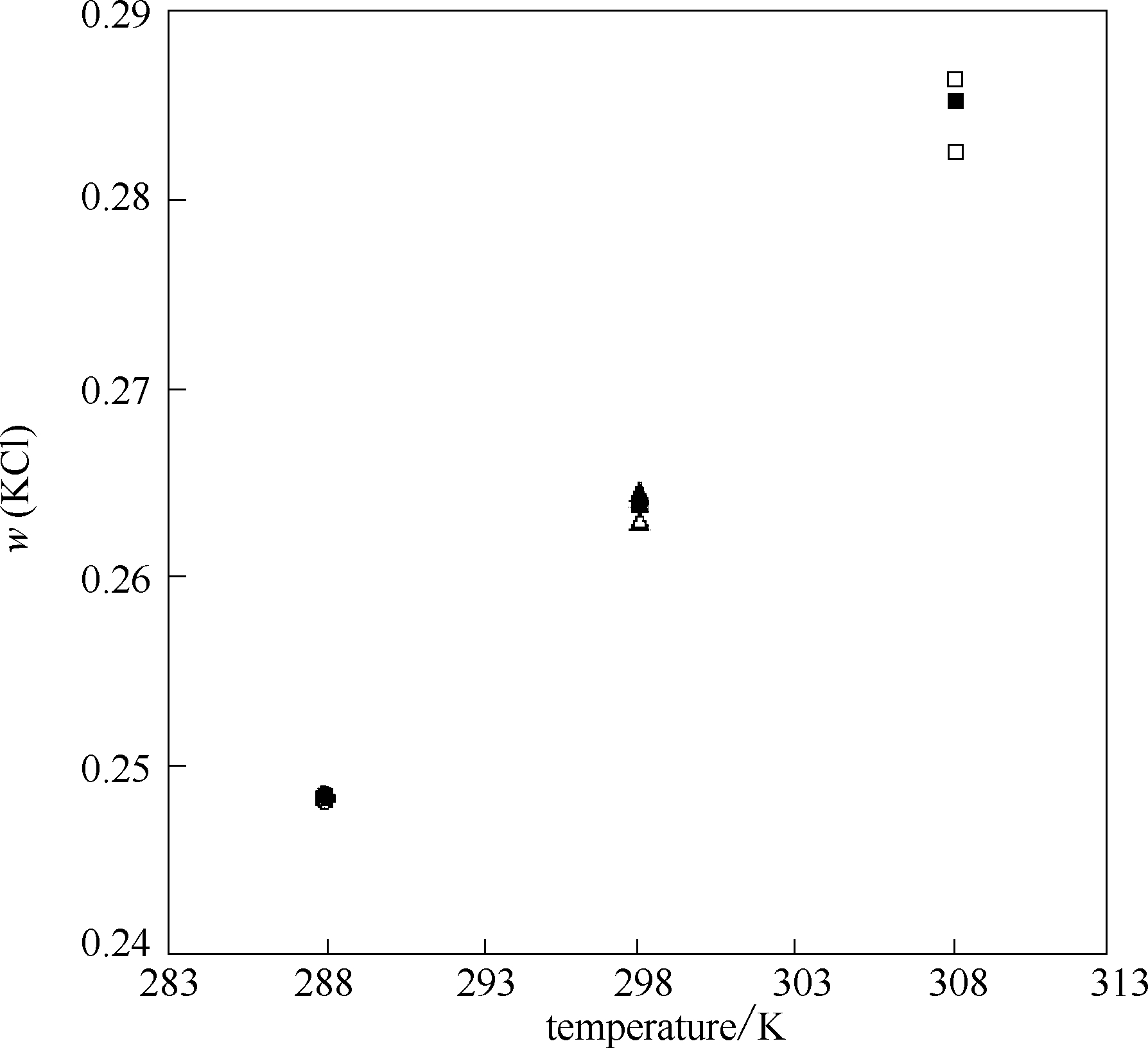
Fig.1 Solubility for KCl in pure water at 288,298 and 308 K: ●,▲,■ solubility for KCl at 288,298 and 308 K by this work,○,Δ,□ solubility for KCl at 288,298 and 308 K from literatures[23,24,25,26,27,28]
| T/K | Solubility/(g/g) | Relative deviation(RD)① | |
|---|---|---|---|
| This work | Ref. | ||
288 | 0.2485 | 0.2482[ | –0.0012 |
| 0.2484[ | –0.0004 | ||
298 | 0.2639 | 0.2642[ | 0.0011 |
| 0.2645[ | 0.0023 | ||
| 0.2630[ | –0.0034 | ||
308 | 0.2851 | 0.2824[ | –0.0095 |
| 0.2863[ | 0.0042 | ||
Table 1 Solubility of KCl in pure water at 288,298 and 308 K
| T/K | Solubility/(g/g) | Relative deviation(RD)① | |
|---|---|---|---|
| This work | Ref. | ||
288 | 0.2485 | 0.2482[ | –0.0012 |
| 0.2484[ | –0.0004 | ||
298 | 0.2639 | 0.2642[ | 0.0011 |
| 0.2645[ | 0.0023 | ||
| 0.2630[ | –0.0034 | ||
308 | 0.2851 | 0.2824[ | –0.0095 |
| 0.2863[ | 0.0042 | ||
| No. | Density,ρ/ (g?cm–3) | Refractive index,nD | Composition of equilibrated solution | R | Composition of wet solid phase | Equilibrated solid phase | |||
|---|---|---|---|---|---|---|---|---|---|
| w(KCl) | w(PEG4000) | w(KCl) | w(PEG4000) | ||||||
| T=288 K | |||||||||
| 1A | 1.1671 | 1.3680 | 0.2485 | 0.0000 | 0.0000 | - | - | KCl | |
| 2 | 1.1649 | 1.3723 | 0.2387 | 0.0381 | 0.0396 | 0.8875 | 0.0056 | KCl | |
| 3 | 1.1622 | 1.3764 | 0.2219 | 0.0778 | 0.1070 | 0.8694 | 0.0131 | KCl | |
| 4 | 1.1592 | 1.3807 | 0.2073 | 0.1188 | 0.1656 | 0.8615 | 0.0208 | KCl | |
| 5 | 1.1569 | 1.3852 | 0.1899 | 0.1619 | 0.2357 | 0.7081 | 0.0583 | KCl | |
| 6 | 1.1548 | 1.3898 | 0.1758 | 0.2061 | 0.2924 | 0.7530 | 0.0618 | KCl | |
| 7 | 1.1520 | 1.3950 | 0.1613 | 0.2517 | 0.3507 | 0.6919 | 0.0925 | KCl | |
| 8 | 1.1510 | 1.4003 | 0.1448 | 0.2993 | 0.4174 | 0.6924 | 0.1077 | KCl | |
| 9 | 1.1507 | 1.4061 | 0.1300 | 0.3478 | 0.4768 | 0.6974 | 0.1210 | KCl | |
| 10 | 1.1509 | 1.4122 | 0.1188 | 0.3966 | 0.5221 | 0.7025 | 0.1339 | KCl | |
| 11 | 1.1516 | 1.4174 | 0.1056 | 0.4468 | 0.5751 | 0.6464 | 0.1766 | KCl | |
| 12 | 1.1496 | 1.4240 | 0.0893 | 0.5010 | 0.6406 | 0.6672 | 0.1831 | KCl | |
| 13 | 1.1364 | 1.4282 | 0.0389 | 0.5767 | 0.8434 | 0.7232 | 0.1661 | KCl | |
| 14B | 1.1071 | 1.4281 | 0.0000 | 0.6485 | 1.0000 | - | - | - | |
| T=298 K | |||||||||
| 15C | 1.1798 | 1.3704 | 0.2639 | 0.0000 | 0.0000 | - | - | KCl | |
| 16 | 1.1746 | 1.3735 | 0.2532 | 0.0374 | 0.0446 | 0.8688 | 0.0066 | KCl | |
| 17 | 1.1712 | 1.3775 | 0.2382 | 0.0762 | 0.1013 | 0.9442 | 0.0056 | KCl | |
| 18 | 1.1675 | 1.3820 | 0.2238 | 0.1164 | 0.1555 | 0.8957 | 0.0156 | KCl | |
| 19 | 1.1641 | 1.3861 | 0.2073 | 0.1584 | 0.2178 | 0.8541 | 0.0292 | KCl | |
| 20 | 1.1612 | 1.3906 | 0.1903 | 0.2025 | 0.2818 | 0.7349 | 0.0663 | KCl | |
| 21 | 1.1587 | 1.3956 | 0.1744 | 0.2478 | 0.3418 | 0.7347 | 0.0796 | KCl | |
| 22 | 1.1561 | 1.4002 | 0.1581 | 0.2947 | 0.4033 | 0.7318 | 0.0939 | KCl | |
| 23 | 1.1539 | 1.4059 | 0.1409 | 0.3435 | 0.4684 | 0.7328 | 0.1068 | KCl | |
| 24 | 1.1523 | 1.4114 | 0.1258 | 0.3934 | 0.5254 | 0.7340 | 0.1197 | KCl | |
| 25 | 1.1516 | 1.4173 | 0.1087 | 0.4453 | 0.5896 | 0.7189 | 0.1404 | KCl | |
| 26 | 1.1490 | 1.4229 | 0.0929 | 0.4990 | 0.6496 | 0.6976 | 0.1664 | KCl | |
| 27 | 1.1431 | 1.4282 | 0.0669 | 0.5600 | 0.7477 | 0.6789 | 0.1927 | KCl | |
| 28 | 1.1428 | 1.4340 | 0.0548 | 0.6130 | 0.7933 | 0.7376 | 0.1702 | KCl | |
| 29D | 1.1118 | 1.4314 | 0.0000 | 0.6990 | 1.0000 | - | - | - | |
| T=308 K | |||||||||
| 30E | 1.1847 | 1.3692 | 0.2851 | 0.0000 | 0.0000 | - | - | KCl | |
| 31 | 1.1794 | 1.3744 | 0.2680 | 0.0367 | 0.0598 | 0.9781 | 0.0011 | KCl | |
| 32 | 1.1748 | 1.3780 | 0.2510 | 0.0749 | 0.1196 | 0.9224 | 0.0078 | KCl | |
| 33 | 1.1707 | 1.3829 | 0.2371 | 0.1144 | 0.1685 | 0.9498 | 0.0075 | KCl | |
| 34 | 1.1658 | 1.3861 | 0.2185 | 0.1562 | 0.2336 | 0.9496 | 0.0101 | KCl | |
| 35 | 1.1629 | 1.3905 | 0.2030 | 0.1993 | 0.2880 | 0.8288 | 0.0428 | KCl | |
| 36 | 1.1589 | 1.3945 | 0.1841 | 0.2449 | 0.3544 | 0.8756 | 0.0373 | KCl | |
| 37 | 1.1559 | 1.4000 | 0.1680 | 0.2912 | 0.4109 | 0.7442 | 0.0895 | KCl | |
| 38 | 1.1522 | 1.4050 | 0.1490 | 0.3402 | 0.4772 | 0.7634 | 0.0946 | KCl | |
| 39 | 1.1497 | 1.4101 | 0.1323 | 0.3905 | 0.5360 | 0.7283 | 0.1222 | KCl | |
| 40 | 1.1464 | 1.4159 | 0.1153 | 0.4420 | 0.5957 | 0.7034 | 0.1482 | KCl | |
| 41 | 1.1441 | 1.4215 | 0.0973 | 0.4966 | 0.6586 | 0.7191 | 0.1545 | KCl | |
| 42 | 1.1421 | 1.4272 | 0.0796 | 0.5523 | 0.7208 | 0.7530 | 0.1482 | KCl | |
| 43 | 1.1356 | 1.4321 | 0.0592 | 0.6101 | 0.7923 | 0.7134 | 0.1859 | KCl | |
| 44 | 1.1308 | 1.4337 | 0.0441 | 0.6689 | 0.8453 | 0.1845 | 0.5707 | KCl | |
| 45 | 1.1274 | 1.4376 | 0.0400 | 0.7191 | 0.8599 | 0.1704 | 0.6214 | KCl | |
| 46F | 1.1089 | 1.4445 | 0.0000 | 0.7800 | 1.0000 | - | - | - | |
| No. | Density,ρ/ (g?cm–3) | Refractive index,nD | Composition of equilibrated solution | R | Composition of wet solid phase | Equilibrated solid phase | |||
|---|---|---|---|---|---|---|---|---|---|
| w(KCl) | w(PEG4000) | w(KCl) | w(PEG4000) | ||||||
| T=288 K | |||||||||
| 1A | 1.1671 | 1.3680 | 0.2485 | 0.0000 | 0.0000 | - | - | KCl | |
| 2 | 1.1649 | 1.3723 | 0.2387 | 0.0381 | 0.0396 | 0.8875 | 0.0056 | KCl | |
| 3 | 1.1622 | 1.3764 | 0.2219 | 0.0778 | 0.1070 | 0.8694 | 0.0131 | KCl | |
| 4 | 1.1592 | 1.3807 | 0.2073 | 0.1188 | 0.1656 | 0.8615 | 0.0208 | KCl | |
| 5 | 1.1569 | 1.3852 | 0.1899 | 0.1619 | 0.2357 | 0.7081 | 0.0583 | KCl | |
| 6 | 1.1548 | 1.3898 | 0.1758 | 0.2061 | 0.2924 | 0.7530 | 0.0618 | KCl | |
| 7 | 1.1520 | 1.3950 | 0.1613 | 0.2517 | 0.3507 | 0.6919 | 0.0925 | KCl | |
| 8 | 1.1510 | 1.4003 | 0.1448 | 0.2993 | 0.4174 | 0.6924 | 0.1077 | KCl | |
| 9 | 1.1507 | 1.4061 | 0.1300 | 0.3478 | 0.4768 | 0.6974 | 0.1210 | KCl | |
| 10 | 1.1509 | 1.4122 | 0.1188 | 0.3966 | 0.5221 | 0.7025 | 0.1339 | KCl | |
| 11 | 1.1516 | 1.4174 | 0.1056 | 0.4468 | 0.5751 | 0.6464 | 0.1766 | KCl | |
| 12 | 1.1496 | 1.4240 | 0.0893 | 0.5010 | 0.6406 | 0.6672 | 0.1831 | KCl | |
| 13 | 1.1364 | 1.4282 | 0.0389 | 0.5767 | 0.8434 | 0.7232 | 0.1661 | KCl | |
| 14B | 1.1071 | 1.4281 | 0.0000 | 0.6485 | 1.0000 | - | - | - | |
| T=298 K | |||||||||
| 15C | 1.1798 | 1.3704 | 0.2639 | 0.0000 | 0.0000 | - | - | KCl | |
| 16 | 1.1746 | 1.3735 | 0.2532 | 0.0374 | 0.0446 | 0.8688 | 0.0066 | KCl | |
| 17 | 1.1712 | 1.3775 | 0.2382 | 0.0762 | 0.1013 | 0.9442 | 0.0056 | KCl | |
| 18 | 1.1675 | 1.3820 | 0.2238 | 0.1164 | 0.1555 | 0.8957 | 0.0156 | KCl | |
| 19 | 1.1641 | 1.3861 | 0.2073 | 0.1584 | 0.2178 | 0.8541 | 0.0292 | KCl | |
| 20 | 1.1612 | 1.3906 | 0.1903 | 0.2025 | 0.2818 | 0.7349 | 0.0663 | KCl | |
| 21 | 1.1587 | 1.3956 | 0.1744 | 0.2478 | 0.3418 | 0.7347 | 0.0796 | KCl | |
| 22 | 1.1561 | 1.4002 | 0.1581 | 0.2947 | 0.4033 | 0.7318 | 0.0939 | KCl | |
| 23 | 1.1539 | 1.4059 | 0.1409 | 0.3435 | 0.4684 | 0.7328 | 0.1068 | KCl | |
| 24 | 1.1523 | 1.4114 | 0.1258 | 0.3934 | 0.5254 | 0.7340 | 0.1197 | KCl | |
| 25 | 1.1516 | 1.4173 | 0.1087 | 0.4453 | 0.5896 | 0.7189 | 0.1404 | KCl | |
| 26 | 1.1490 | 1.4229 | 0.0929 | 0.4990 | 0.6496 | 0.6976 | 0.1664 | KCl | |
| 27 | 1.1431 | 1.4282 | 0.0669 | 0.5600 | 0.7477 | 0.6789 | 0.1927 | KCl | |
| 28 | 1.1428 | 1.4340 | 0.0548 | 0.6130 | 0.7933 | 0.7376 | 0.1702 | KCl | |
| 29D | 1.1118 | 1.4314 | 0.0000 | 0.6990 | 1.0000 | - | - | - | |
| T=308 K | |||||||||
| 30E | 1.1847 | 1.3692 | 0.2851 | 0.0000 | 0.0000 | - | - | KCl | |
| 31 | 1.1794 | 1.3744 | 0.2680 | 0.0367 | 0.0598 | 0.9781 | 0.0011 | KCl | |
| 32 | 1.1748 | 1.3780 | 0.2510 | 0.0749 | 0.1196 | 0.9224 | 0.0078 | KCl | |
| 33 | 1.1707 | 1.3829 | 0.2371 | 0.1144 | 0.1685 | 0.9498 | 0.0075 | KCl | |
| 34 | 1.1658 | 1.3861 | 0.2185 | 0.1562 | 0.2336 | 0.9496 | 0.0101 | KCl | |
| 35 | 1.1629 | 1.3905 | 0.2030 | 0.1993 | 0.2880 | 0.8288 | 0.0428 | KCl | |
| 36 | 1.1589 | 1.3945 | 0.1841 | 0.2449 | 0.3544 | 0.8756 | 0.0373 | KCl | |
| 37 | 1.1559 | 1.4000 | 0.1680 | 0.2912 | 0.4109 | 0.7442 | 0.0895 | KCl | |
| 38 | 1.1522 | 1.4050 | 0.1490 | 0.3402 | 0.4772 | 0.7634 | 0.0946 | KCl | |
| 39 | 1.1497 | 1.4101 | 0.1323 | 0.3905 | 0.5360 | 0.7283 | 0.1222 | KCl | |
| 40 | 1.1464 | 1.4159 | 0.1153 | 0.4420 | 0.5957 | 0.7034 | 0.1482 | KCl | |
| 41 | 1.1441 | 1.4215 | 0.0973 | 0.4966 | 0.6586 | 0.7191 | 0.1545 | KCl | |
| 42 | 1.1421 | 1.4272 | 0.0796 | 0.5523 | 0.7208 | 0.7530 | 0.1482 | KCl | |
| 43 | 1.1356 | 1.4321 | 0.0592 | 0.6101 | 0.7923 | 0.7134 | 0.1859 | KCl | |
| 44 | 1.1308 | 1.4337 | 0.0441 | 0.6689 | 0.8453 | 0.1845 | 0.5707 | KCl | |
| 45 | 1.1274 | 1.4376 | 0.0400 | 0.7191 | 0.8599 | 0.1704 | 0.6214 | KCl | |
| 46F | 1.1089 | 1.4445 | 0.0000 | 0.7800 | 1.0000 | - | - | - | |
| 二元参数 | |||||||
|---|---|---|---|---|---|---|---|
| T/K | B11×102 | C111×105 | Np | σ×102 | |||
| 288 | 0.7088[ | 1.296[ | –0.4398 | –1.095 | 0.7345 | 11 | 0.3129 |
| 298 | 0.7088[ | 1.296[ | 0.7523 | –0.8338 | 0.9664 | 16 | 0.4356 |
| 308 | 0.7088[ | 1.296[ | 0.9950 | –1.053 | 1.981 | 14 | 0.2434 |
| 交互作用参数 | |||||||
| T/K | C112 | C122 | Np | σ | |||
| 288 | 0.101683 | –0.09897 | 0.000586 | –0.00325 | 13 | 0.26 | |
| 298 | 0.114476 | –0.10325 | –0.00015 | –0.00294 | 13 | 0.09 | |
| 308 | 0.110781 | 0.056002 | –0.0007 | –0.00233 | 15 | 0.20 | |
Table 3 Binary parameters and cross parameters for ternary system KCl-PEG4000-H2O at 288,298 and 308 K
| 二元参数 | |||||||
|---|---|---|---|---|---|---|---|
| T/K | B11×102 | C111×105 | Np | σ×102 | |||
| 288 | 0.7088[ | 1.296[ | –0.4398 | –1.095 | 0.7345 | 11 | 0.3129 |
| 298 | 0.7088[ | 1.296[ | 0.7523 | –0.8338 | 0.9664 | 16 | 0.4356 |
| 308 | 0.7088[ | 1.296[ | 0.9950 | –1.053 | 1.981 | 14 | 0.2434 |
| 交互作用参数 | |||||||
| T/K | C112 | C122 | Np | σ | |||
| 288 | 0.101683 | –0.09897 | 0.000586 | –0.00325 | 13 | 0.26 | |
| 298 | 0.114476 | –0.10325 | –0.00015 | –0.00294 | 13 | 0.09 | |
| 308 | 0.110781 | 0.056002 | –0.0007 | –0.00233 | 15 | 0.20 | |
| 1 | 侯献华, 樊馥, 郑绵平, 等. 青海盐湖钾盐资源开发利用及产业发展[J]. 科技导报, 2017, 35(12): 67-71. |
| HouX H, FanF, ZhengM P, et al. Development and utilization of potash resources of saline lakes in Qinghai province[J]. Sci. Technol. Rev. 2017, 35(12): 67-71. | |
| 2 | FareloF, FernandesC, AvelinoA. Solubilities for six ternary systems: NaCl + NH4Cl + H2O, KCl + NH4Cl + H2O, NaCl + LiCl + H2O, KCl + LiCl + H2O, NaCl + AlCl3 + H2O, NaCl + AlCl3 + H2O and KCl + AlCl3 + H2O at T = (298 to 333) K[J]. J. Chem. Eng. Data., 2005, 50(4): 1470-1477. |
| 3 | YangH T, LiangT Y, ZengD W, et al. Phase diagram of the quaternary system LiCl + MgCl2 + KCl +H2O at 323.15 K[J]. CALPHAD: Comput. Coupling Phase Diagrams Thermochem., 2017, 57: 126-133. |
| 4 | MengL Z, LiD, DengT L, et al. Solubility calculation for the brine system Na+, K+//Cl–, Br–-H2O using Pitzer thermodynamic model[J]. J. Chem. Eng. Jpn., 2018, 51(3): 185-189. |
| 5 | 桑世华, 张婷婷, 傅超, 等. 四元体系Li+, K+, Mg2+//B4O72–-H2O 273 K相平衡[J]. 化工学报, 2017, 68(9): 3343-3349. |
| SangS H, ZhangT T, FuC, et al. Phase equilibria in quaternary system Li+, K+, Mg2+//B4O72–-H2O at 273 K[J]. CIESC Journal, 2017, 68(9): 3343-3349. | |
| 6 | ZhangX, SangS H, ZhongS Y, et al. Equilibria in the ternary system SrCl2-KCl-H2O and the quaternary system SrCl2-KCl-NaCl-H2O at 323 K[J]. Russ. J. Phys. Chem. A, 2015, 89(12): 2322-2326. |
| 7 | 于旭东, 刘敏, 王林, 等. 三元体系硼酸钾+硼酸铷+水和硼酸铷+硼酸镁+水323 K相平衡[J]. 高校化学工程学报, 2018, 32(3): 514-521. |
| YuX D, LiuM, WangL, et al. Phase equilibria of potassium borate + rubidium borate + H2O and rubidium borate + magnesium borate + H2O aqueoue ternary systems at 323 K[J]. J. Chem. Eng. Chin. Univ., 2018, 32(3): 514-521. | |
| 8 | GuoS S, YuX D, ZengY. Phase equilibria for the aqueous reciprocal quaternary system K+, Mg2+//Cl-, Borate-H2O at 298 K[J]. J. Chem. Eng. Data, 2016, 61(4): 1566-1572. |
| 9 | YuX D, ZengY, GuoS S, et al. Stable phase equilibrium and phase diagram of the quinary system Li+, K+, Rb+, Mg2+//borate-H2O at T=348.15 K[J]. J.Chem. Eng. Data, 2016, 61(3): 1246-1253. |
| 10 | YuX D, ZengY, ChenP J, et al. Solid-liquid equilibrium of the quaternary system lithium, potassium, rubidium, and borate at T = 323 K[J]. J. Chem. Eng. Data, 2018, 63(8): 3125-3129. |
| 11 | YuX D, ZengY, MuP T, et al. Solid-liquid equilibria in the quinary system LiCl-KCl-RbCl-MgCl2-H2O at T = 323 K[J]. Fluid Phase Equilib., 2015, 387: 88-94. |
| 12 | LiZ Q, YuX D, YinQ H, et al. Thermodynamics metastable phase equilibria of aqueous quaternary system LiCl + KCl + RbCl+ H2O at 323.15 K[J]. Fluid Phase Equilib., 2013, 358: 131-136. |
| 13 | 任永胜, 何婷婷, 谢娟, 等. 333.15 K K+, NH4+//Cl–, SO42–-H2O和K+, NH4+//Cl–, SO42–-(CH2OH)2-H2O体系固液相平衡[J]. 化工学报, 2018, 69(7): 2838-2850. |
| RenY S, HeT T, XieJ, et al. Phase equilibria in systems K+, NH4+//Cl–, SO42–-H2O and K+, NH4+//Cl–, SO42--(CH2OH)2-H2O at 333.15 K[J]. CIESC Journal, 2018, 69(7): 2838-2850. | |
| 14 | SampaioV S, BonomoR C F, Monteiro FilhoE S, et al. Phyical properties and liquid-liquid equilibrium of aqueous two-phase systems containing poly(ethylene glycol) + potassium chloride + sodium polyacrylate [J]. J. Chem. Eng. Data, 2012, 57(12): 3651-3657. |
| 15 | 雷红, 李淑妮, 翟全国, 等. 298.15和308.15 K时1,2-丙二醇 + MCl (M=Na, K, Rb, Cs) + H2O三元体系的溶解度、密度和折射率[J]. 物理化学学报, 2012, 28(7): 1599-1607. |
| LeiH, LiS N, ZhaiQ G, et al. Solibility, density and refractive index for the ternary systems of 1,2-propanediol, MCl (M=Na, K, Rb, Cs) and H2O at 298.15 and 308.15 K [J]. Acta Phys. -Chim. Sin., 2012, 28(7): 1599-1607. | |
| 16 | TaboadaM E, GalleguillosH R, GraberT A. Compositions, densities, conductivities, and refractive indices of potassium chloride or/and sodium chloride +PEG4000 + water at 298.15 and liquid-liquid equilibrium of potassium chloride or sodium chloride +PEG4000 + water at 333.15 K[J]. J. Chem. Eng. Data, 2005, 50 (1): 264-269. |
| 17 | Hernandes-LuisF, Rodriguez-RaposoR, GalleguillosH, et al. Acivity coefficients of KCl in PEG4000 + water mixtures at 288.15, 298.15 and 308.15 K[J]. Fluid Phase Equilib., 2010, 295: 163-171. |
| 18 | LoveraJ A, PadillaA P, GalleguillosH, et al. Correlation of the solubilities of alkali chlorides in mixed solvents: polyethylene glycol + H2O and ethanol + H2O[J]. CALPHAD: Comput. Coupling Phase Diagrams Thermochem., 2012, 38: 35-42. |
| 19 | WuY T, LinD Q, ZhuZ Q, et al. Prediction of liquid-liquid equilibria of polymer-salt aqueous two-phase systms by modified Pitzer’s virial equation[J]. Fluid Phase Equilib., 1996, 124: 67-79. |
| 20 | FosbolP L, ThomsenK, StenbyE H. Reverse schreinemakers method for experimental analysis of mixed-solvent electrolyte systems [J]. J. Solution Chem., 2009, 38(1): 1-14. |
| 21 | 中国科学院青海盐湖研究所. 卤水和盐的分析方法(第二版)[M]. 北京:科学出版社, 1988: 69-72. |
| Institute of Qinghai Salt-Lake of Chinese Academy of Sciences. Analytical Methods of Brines and Salts (2nd ed) [M]. Beijing: Chinese Science Press, 1988: 69-72. | |
| 22 | ChelugetE L, GelinasS, VeraJ H, et al. Liquid-liquid equilibrium of aqueous mixtures of poly(propylene glycol) with NaCl [J]. J. Chem. Eng. Data, 1994, 39(1): 127-130. |
| 23 | DengT L, LiD C, WangS Q. Metastable phase equilibrium in the aqueous ternary system (KCl-CaCl2-H2O) at (288.15 and 308.15) K[J]. J. Chem. Eng. Data, 2008, 53(4): 1007-1011. |
| 24 | LongJ, TangJ H, YouY K, et al. Phase equilibrium in the aqueous ternary system KH2PO4+KCl+H2O at (288.15 and 303.15) K[J]. J. Chem. Eng. Data, 2015, 60(6): 1906-1909. |
| 25 | ShenW, RenY S, ZhangX R, et al. Solid-liquid phase equilibrium for the ternary system (potassium chloride + potassium dihydrogen phosphate + water) at (298.15 and 313.15) K[J]. J. Chem. Eng. Data, 2015, 60(7): 2070-2078. |
| 26 | LinS Q, TangJ H, TengJ, et al. Phase equilibrium in the system KH2PO4 + KCl +H3PO4 at 298.15 K and 308.15 K[J]. J. Chem. Eng. Data, 2017, 62(12): 4169-4173. |
| 27 | JiaX Y, LiJ, JinY, et al. Solid-liquid equilibria in the quaternary system Na+, K+//HPO42–, Cl–-H2O and its subsystems Na+//HPO42–, Cl–-H2O, K+//HPO42–, Cl–-H2O, and Na+, K+//HPO42–-H2O at 298.2 K[J]. J. Chem. Eng. Data, 2017, 62(11): 3679-3686. |
| 28 | MengR, LiS N, ZhaiQ G, et al. Solubilities, densities, and refractive indices for the ternary systems glycerin + MCl + H2O (M=Na, K, Rb, Cs) at (298.15 and 308.15) K[J]. J. Chem. Eng. Data, 2011, 56(12): 4643-4650. |
| 29 | PitzerK S. Thermodynamics of electrolytes. I. Theoretical basis and general equations[J]. J. Phys. Chem., 1973, 77(2): 268-277. |
| 30 | HariveC E, EugsterH P, WeareJ H. Mineral equilibria in the six-component seawater system, Na-K-Mg-Ca-SO4-Cl-H2O at 25℃. II: Compositions of the saturated solutions[J]. Geochim. Cosmochim. Acta., 1982, 46(9): 1603-1618. |
| 31 | CleggS L, RardJ A, MillerD G. Isopiestic determination of the osmotic and activity coefficients of NaCl+SrCl2+H2O at 298.15K and representation with an extended ion-interaction model. J. Chem. Eng. Data, 2005, 50(4): 1162-1170. |
| 32 | KrevelenD W V, NijehuisK T. Properties of Polymers: Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contributions[M].(4th ed). Amsterdam: Elsevier, 2009: 319-321. |
| 33 | GuoL J, HanH J, DongO Y, et al. Thermodynamics and phase equilibrium of the high concentration solid solution-aqueous solution system KCl-RbCl-H2O from T = 298.15 K to T = 323.15 K[J]. J. Chem. Thermodyn., 2016, 106: 285-294. |
| [1] | Chao HU, Yuming DONG, Wei ZHANG, Hongling ZHANG, Peng ZHOU, Hongbin XU. Preparation of high-concentration positive electrolyte of vanadium redox flow battery by activating vanadium pentoxide with highly concentrated sulfuric acid [J]. CIESC Journal, 2023, 74(S1): 338-345. |
| [2] | Xudong YU, Qi LI, Niancu CHEN, Li DU, Siying REN, Ying ZENG. Phase equilibria and calculation of aqueous ternary system KCl + CaCl2 + H2O at 298.2, 323.2, and 348.2 K [J]. CIESC Journal, 2023, 74(8): 3256-3265. |
| [3] | Xiaoyu YAO, Jun SHEN, Jian LI, Zhenxing LI, Huifang KANG, Bo TANG, Xueqiang DONG, Maoqiong GONG. Research progress in measurement methods in vapor-liquid critical properties of mixtures [J]. CIESC Journal, 2023, 74(5): 1847-1861. |
| [4] | Ke CHEN, Li DU, Ying ZENG, Siying REN, Xudong YU. Phase equilibria and calculation of quaternary system LiCl+MgCl2+CaCl2+H2O at 323.2 K [J]. CIESC Journal, 2023, 74(5): 1896-1903. |
| [5] | Yuanjing MAO, Zhi YANG, Songping MO, Hao GUO, Ying CHEN, Xianglong LUO, Jianyong CHEN, Yingzong LIANG. Estimation of SAFT-VR Mie equation of state parameters and thermodynamic properties of C6—C10 alcohols [J]. CIESC Journal, 2023, 74(3): 1033-1041. |
| [6] | Jingbo GAO, Qiang SUN, Qing LI, Yiwei WANG, Xuqiang GUO. Hydrate equilibrium model of hydrogen-containing gas considering hydrates structure transformation [J]. CIESC Journal, 2023, 74(2): 666-673. |
| [7] | Wenting CHENG, Jie LI, Li XU, Fangqin CHENG, Guoji LIU. Experiment and prediction for the solubility of AlCl3·6H2O in FeCl3, CaCl2, KCl and KCl-FeCl3 solutions [J]. CIESC Journal, 2023, 74(2): 642-652. |
| [8] | Jin CAI, Xiaohui WANG, Han TANG, Guangjin CHEN, Changyu SUN. Prediction of the phase equilibrium of semi-clathrate hydrate in TBAB aqueous solution [J]. CIESC Journal, 2023, 74(1): 408-415. |
| [9] | Huan ZHOU, Mengli ZHANG, Qing HAO, Si WU, Jie LI, Cunbing XU. Process mechanism and dynamic behaviors of magnesium sulfate type carnallite converting into kainite [J]. CIESC Journal, 2022, 73(9): 3841-3850. |
| [10] | Qian LIU, Xianglan ZHANG, Zhiping LI, Yulong LI, Mengxing HAN. Screening of deep eutectic solvents and study on extraction performance for oil-hydroxybenzene separation [J]. CIESC Journal, 2022, 73(9): 3915-3928. |
| [11] | Songtao YANG, Dongyang LI, Yuqing NIU, Xingang LI, Shaohui KANG, Hong LI, Kaikai YE, Zhiquan ZHOU, Xin GAO. Molecular simulation progress in studying thermodynamic properties and potential functions of fluorides [J]. CIESC Journal, 2022, 73(9): 3828-3840. |
| [12] | Yuxin REN, Runfeng XU, Wanying WANG, Pengzhong CHEN, Xiaojun PENG. Synthesis and stability study of anthraquinone dyes for color photoresist [J]. CIESC Journal, 2022, 73(5): 2251-2261. |
| [13] | Jiahui REN, Yu LIU, Chao LIU, Lang LIU, Ying LI. Critical temperature prediction of working fluids using molecular fingerprints and topological indices [J]. CIESC Journal, 2022, 73(4): 1493-1500. |
| [14] | Mingze SUN, Ning MA, Haoran LI, Haifeng JIANG, Wenpeng HONG, Xiaojuan NIU. Thermodynamic analysis of Brayton cycle of medium and low temperature supercritical CO2 and its mixed working medium [J]. CIESC Journal, 2022, 73(3): 1379-1388. |
| [15] | Huaixu LI, Xiaoyan SUN, Shaohui TAO, Li XIA, Shuguang XIANG. Lumping gasoline with molecular properties and density peak clustering [J]. CIESC Journal, 2022, 73(12): 5449-5460. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||