CIESC Journal ›› 2023, Vol. 74 ›› Issue (3): 1033-1041.DOI: 10.11949/0438-1157.20221438
• Thermodynamics • Previous Articles Next Articles
Yuanjing MAO1( ), Zhi YANG1(
), Zhi YANG1( ), Songping MO1, Hao GUO2, Ying CHEN1, Xianglong LUO1, Jianyong CHEN1, Yingzong LIANG1
), Songping MO1, Hao GUO2, Ying CHEN1, Xianglong LUO1, Jianyong CHEN1, Yingzong LIANG1
Received:2022-11-03
Revised:2022-12-26
Online:2023-04-19
Published:2023-03-05
Contact:
Zhi YANG
毛元敬1( ), 杨智1(
), 杨智1( ), 莫松平1, 郭浩2, 陈颖1, 罗向龙1, 陈健勇1, 梁颖宗1
), 莫松平1, 郭浩2, 陈颖1, 罗向龙1, 陈健勇1, 梁颖宗1
通讯作者:
杨智
作者简介:毛元敬(2000—),男,硕士研究生,2112002077@mail2.gdut.edu.cn
基金资助:CLC Number:
Yuanjing MAO, Zhi YANG, Songping MO, Hao GUO, Ying CHEN, Xianglong LUO, Jianyong CHEN, Yingzong LIANG. Estimation of SAFT-VR Mie equation of state parameters and thermodynamic properties of C6—C10 alcohols[J]. CIESC Journal, 2023, 74(3): 1033-1041.
毛元敬, 杨智, 莫松平, 郭浩, 陈颖, 罗向龙, 陈健勇, 梁颖宗. C6~C10烷醇的SAFT-VR Mie状态方程参数回归及其热物性研究[J]. 化工学报, 2023, 74(3): 1033-1041.
Add to citation manager EndNote|Ris|BibTeX
| Substances | m | σ | (ε/k) | r | (εAB/k)/K | λr | AARD/% | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ps | ρs | ΔHvap | ρl | cP | u | |||||||
| 1-hexanol (C6) | 2.4364 | 4.2407 | 315.79 | 0.28852 | 3102.4 | 11.9366 | 0.81 | 1.06 | 1.64 | 0.23 | 1.60 | 2.09 |
| 1-heptanol (C7) | 2.4415 | 4.4413 | 336.05 | 0.26707 | 3404.3 | 12.2372 | 0.81 | 0.73 | 3.11 | 0.57 | 2.00 | 2.12 |
| 1-octanol (C8) | 2.7639 | 4.4240 | 342.37 | 0.29096 | 3180.6 | 12.9708 | 0.67 | 0.61 | 3.21 | 0.64 | 3.17 | 3.61 |
| 1-nonanol (C9) | 2.9172 | 4.4871 | 358.14 | 0.26918 | 3398.7 | 13.3660 | 0.57 | 1.05 | 3.84 | 0.48 | 2.12 | 1.77 |
| 1-decanol (C10) | 2.4778 | 4.9442 | 409.06 | 0.28053 | 3457.4 | 14.3970 | 0.83 | 0.66 | 3.29 | 0.77 | 5.51 | 1.98 |
| average | — | — | — | — | — | — | 0.74 | 0.82 | 3.02 | 0.54 | 2.88 | 2.31 |
Table 1 Results of SAFT-VR Mie EoS parameter regression and thermodynamic properties prediction for C6—C10 alcohols
| Substances | m | σ | (ε/k) | r | (εAB/k)/K | λr | AARD/% | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ps | ρs | ΔHvap | ρl | cP | u | |||||||
| 1-hexanol (C6) | 2.4364 | 4.2407 | 315.79 | 0.28852 | 3102.4 | 11.9366 | 0.81 | 1.06 | 1.64 | 0.23 | 1.60 | 2.09 |
| 1-heptanol (C7) | 2.4415 | 4.4413 | 336.05 | 0.26707 | 3404.3 | 12.2372 | 0.81 | 0.73 | 3.11 | 0.57 | 2.00 | 2.12 |
| 1-octanol (C8) | 2.7639 | 4.4240 | 342.37 | 0.29096 | 3180.6 | 12.9708 | 0.67 | 0.61 | 3.21 | 0.64 | 3.17 | 3.61 |
| 1-nonanol (C9) | 2.9172 | 4.4871 | 358.14 | 0.26918 | 3398.7 | 13.3660 | 0.57 | 1.05 | 3.84 | 0.48 | 2.12 | 1.77 |
| 1-decanol (C10) | 2.4778 | 4.9442 | 409.06 | 0.28053 | 3457.4 | 14.3970 | 0.83 | 0.66 | 3.29 | 0.77 | 5.51 | 1.98 |
| average | — | — | — | — | — | — | 0.74 | 0.82 | 3.02 | 0.54 | 2.88 | 2.31 |
| Substances | Vapor-liquid equilibrium | Compressed liquid phase | |||||||
|---|---|---|---|---|---|---|---|---|---|
| T range/K | T range/K | P range/MPa | |||||||
| Ps | ρs | ΔHvap | ρl | cP | u | ρl | cP | u | |
| 1-hexanol | 340—600 | 340—600 | 280—560 | 278—358 | 325—570 | 303—393 | 0.1—60 | 2—30 | 0.1—810 |
| 1-heptanol | 320—620 | 320—620 | 280—580 | 278—358 | 325—570 | 303—393 | 0.1—60 | 2—30 | 0.1—810 |
| 1-octanol | 320—640 | 320—640 | 280—630 | 278—358 | 325—570 | 291—433 | 0.1—60 | 2—30 | 0.1—811 |
| 1-nonanol | 340—640 | 340—640 | 300—640 | 278—358 | 298—318 | 303—393 | 0.1—60 | 0.1—100 | 0.1—506 |
| 1-decanol | 360—660 | 360—660 | 300—660 | 288—358 | 325—570 | 303—393 | 0.1—60 | 2—30 | 0.1—506 |
Table 2 The temperature and pressure range selected for the thermodynamic properties experimental data[7,31-34]
| Substances | Vapor-liquid equilibrium | Compressed liquid phase | |||||||
|---|---|---|---|---|---|---|---|---|---|
| T range/K | T range/K | P range/MPa | |||||||
| Ps | ρs | ΔHvap | ρl | cP | u | ρl | cP | u | |
| 1-hexanol | 340—600 | 340—600 | 280—560 | 278—358 | 325—570 | 303—393 | 0.1—60 | 2—30 | 0.1—810 |
| 1-heptanol | 320—620 | 320—620 | 280—580 | 278—358 | 325—570 | 303—393 | 0.1—60 | 2—30 | 0.1—810 |
| 1-octanol | 320—640 | 320—640 | 280—630 | 278—358 | 325—570 | 291—433 | 0.1—60 | 2—30 | 0.1—811 |
| 1-nonanol | 340—640 | 340—640 | 300—640 | 278—358 | 298—318 | 303—393 | 0.1—60 | 0.1—100 | 0.1—506 |
| 1-decanol | 360—660 | 360—660 | 300—660 | 288—358 | 325—570 | 303—393 | 0.1—60 | 2—30 | 0.1—506 |
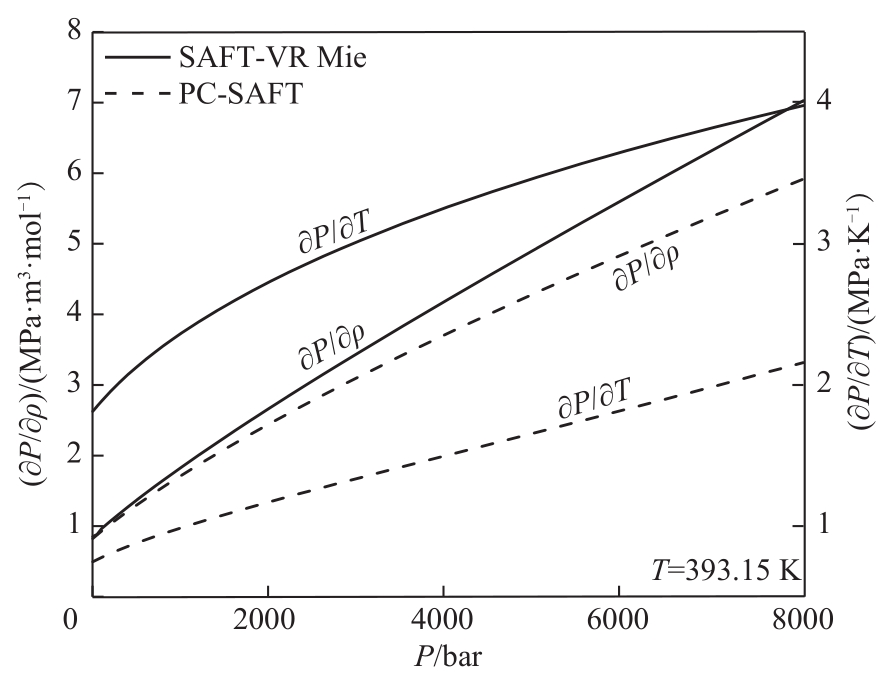
Fig.6 Comparison of the results of SAFT-VR Mie and PC-SAFT EoS for predicting the pressure-density and pressure-temperature derivatives of 1-hexanol (C6)
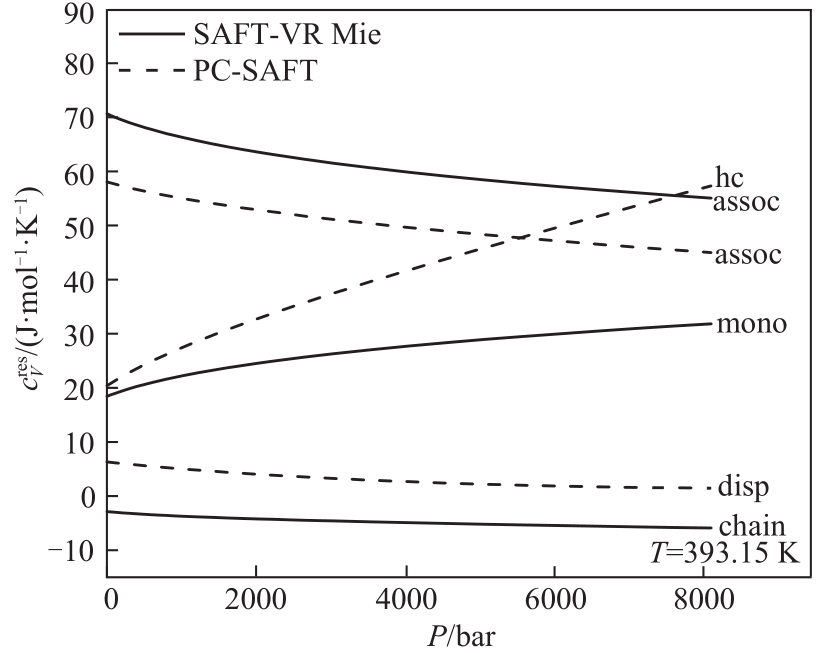
Fig.7 Comparison of different microscopic contributions of SAFT-VR Mie and PC-SAFT EoS for predicting residual isochoric specific heat capacity of 1-hexanol (C6)

Fig.8 Comparison of different microscopic contributions of SAFT-VR Mie and PC-SAFT EoS for predicting second-order temperature derivative of 1-hexanol (C6)
| 1 | Hayer H, Haghbakhsh R, Keshtkari S, et al. Support vector machine and CPA EoS for the prediction of high-pressure liquid densities of normal alkanols[J]. Journal of the Taiwan Institute of Chemical Engineers, 2014, 45(6): 2888-2898. |
| 2 | Menegazzo T A S, Soares Junior A M, Mota B T, et al. Application of an equation of state incorporating association to alcohols up to decanol[J]. Fluid Phase Equilibria, 2019, 482: 24-37. |
| 3 | Pokorný V, Štejfa V, Klajmon M, et al. Vapor pressures and thermophysical properties of 1-heptanol, 1-octanol, 1-nonanol, and 1-decanol: data reconciliation and PC-SAFT modeling[J]. Journal of Chemical & Engineering Data, 2021, 66(1): 805-821. |
| 4 | Schwarz C E. High pressure phase behavior of the homologous series CO2+1-alcohols[J]. Journal of Chemical & Engineering Data, 2018, 63(7): 2451-2466. |
| 5 | Gunasekara S N, Martin V, Chiu J N. Phase equilibrium in the design of phase change materials for thermal energy storage: state-of-the-art[J]. Renewable and Sustainable Energy Reviews, 2017, 73: 558-581. |
| 6 | Pakravesh A, Zarei F, Zarei H. PρT parameterization of SAFT equation of state: developing a new parameterization method for equations of state[J]. Fluid Phase Equilibria, 2021, 538: 113024. |
| 7 | Dávila M J, Alcalde R, Atilhan M, et al. PρT measurements and derived properties of liquid 1-alkanols[J]. The Journal of Chemical Thermodynamics, 2012, 47: 241-259. |
| 8 | Schubert T. Production routes of advanced renewable C1 to C4 alcohols as biofuel components—a review[J]. Biofuels, Bioproducts and Biorefining, 2020, 14(4): 845-878. |
| 9 | Yang Z, Gong M Q, Zhou Y, et al. Vapor-liquid equilibria of CH4, CO2 and their binary system CH4+CO2: a comparison between the molecular simulation and equation of state[J]. Science China Technological Sciences, 2015, 58(4): 650-658. |
| 10 | Diamantonis N I, Boulougouris G C, Mansoor E, et al. Evaluation of cubic, SAFT, and PC-SAFT equations of state for the vapor-liquid equilibrium modeling of CO2 mixtures with other gases[J]. Industrial & Engineering Chemistry Research, 2013, 52(10): 3933-3942. |
| 11 | Redlich O, Kwong J N S. On the thermodynamics of solutions (Ⅴ): An equation of state. Fugacities of gaseous solutions[J]. Chemical Reviews, 1949, 44(1): 233-244. |
| 12 | Soave G. Equilibrium constants from a modified Redlich-Kwong equation of state[J]. Chemical Engineering Science, 1972, 27(6): 1197-1203. |
| 13 | Peng D Y, Robinson D B. A new two-constant equation of state[J]. Industrial & Engineering Chemistry Fundamentals, 1976, 15(1): 59-64. |
| 14 | Kontogeorgis G M, Folas G K. Thermodynamic Models for Industrial Applications[M]. United Kingdom: John Wiley & Sons Ltd., 2009. |
| 15 | 吴瑕, 贾文龙, 李长俊, 等. 基于CPA状态方程计算天然气-甲醇-水气液相平衡[J]. 化学工程, 2018, 46(6): 37-41. |
| Wu X, Jia W L, Li C J, et al. Phase equilibrium of natural gas/methanol/water mixtures by use of CPA EoS[J]. Chemical Engineering (China), 2018, 46(6): 37-41. | |
| 16 | De Villiers A J. Evaluation and improvement of the sPC-SAFT equation of state for complex mixtures[D]. Stellenbosch: Stellenbosch University, 2011. |
| 17 | Al-Saifi N M, Hamad E Z, Englezos P. Prediction of vapor-liquid equilibrium in water-alcohol-hydrocarbon systems with the dipolar perturbed-chain SAFT equation of state[J]. Fluid Phase Equilibria, 2008, 271(1/2): 82-93. |
| 18 | Chapman W G, Gubbins K E, Jackson G, et al. New reference equation of state for associating liquids[J]. Industrial & Engineering Chemistry Research, 1990, 29(8): 1709-1721. |
| 19 | Huang S H, Radosz M. Equation of state for small, large, polydisperse, and associating molecules[J]. Industrial & Engineering Chemistry Research, 1990, 29(11): 2284-2294. |
| 20 | Gross J, Sadowski G. Perturbed-chain SAFT: an equation of state based on a perturbation theory for chain molecules[J]. Industrial & Engineering Chemistry Research, 2001, 40(4): 1244-1260. |
| 21 | Lafitte T, Apostolakou A, Avendaño C, et al. Accurate statistical associating fluid theory for chain molecules formed from Mie segments[J]. The Journal of Chemical Physics, 2013, 139(15): 154504. |
| 22 | Jamali A, Behnejad H. Observations regarding the first and second order thermodynamic derivative properties of non-polar and light polar fluids by perturbed chain-SAFT equations of state[J]. Cryogenics, 2019, 99: 78-86. |
| 23 | 杨智, 公茂琼, 李会亚, 等. 基于不同的SAFT类状态方程研究甲烷的热物性[J]. 低温工程, 2015(3): 6-12. |
| Yang Z, Gong M Q, Li H Y, et al. Thermophysical properties of methane studied by different SAFT-type equation of state[J]. Cryogenics, 2015(3): 6-12. | |
| 24 | 屈绍广, 王昶昊, 施云海, 等. 基团贡献状态方程的开发与热力学模型参数的理论预测[J]. 化工学报, 2020, 71(1): 200-208. |
| Qu S G, Wang C H, Shi Y H, et al. Development of group-contribution equation of state and theoretical prediction of thermodynamic model parameters[J]. CIESC Journal, 2020, 71(1): 200-208. | |
| 25 | Hurter R M. Comparing the group-contribution SAFT-γ Mie equation of state with SAFT-VR Mie[D]. Stellenbosch: Stellenbosch University, 2019. |
| 26 | Ramírez-Vélez N, Piña-Martinez A, Jaubert J N, et al. Parameterization of SAFT models: analysis of different parameter estimation strategies and application to the development of a comprehensive database of PC-SAFT molecular parameters[J]. Journal of Chemical & Engineering Data, 2020, 65(12): 5920-5932. |
| 27 | Anoune I, Mimoune Z, Madani H, et al. New modified PC-SAFT pure component parameters for accurate VLE and critical phenomena description[J]. Fluid Phase Equilibria, 2021, 532: 112916. |
| 28 | Rehner P, Gross J. Multiobjective optimization of PCP-SAFT parameters for water and alcohols using surface tension data[J]. Journal of Chemical & Engineering Data, 2020, 65(12): 5698-5707. |
| 29 | Dufal S, Lafitte T, Galindo A, et al. Developing intermolecular-potential models for use with the SAFT-VR Mie equation of state[J]. AIChE Journal, 2015, 61(9): 2891-2912. |
| 30 | Cripwell J, Smith S A M, Schwarz C E, et al. SAFT-VR Mie: application to phase equilibria of alcohols in mixtures with n-alkanes and water[J]. Industrial & Engineering Chemistry Research, 2018, 57(29): 9693-9706. |
| 31 | Cibulka I. Saturated liquid densities of 1-alkanols from C1 to C10 and n-alkanes from C5 to C16: a critical evaluation of experimental data[J]. Fluid Phase Equilibria, 1993, 89(1): 1-18. |
| 32 | Fulem M, Růžička K, Růžička V. Heat capacities of alkanols. Part I. Selected 1-alkanols C2 to C10 at elevated temperatures and pressures[J]. Thermochimica Acta, 2002, 382(1): 119-128. |
| 33 | Postnikov E B, Goncharov A L, Cohen N, et al. Estimating the liquid properties of 1-alkanols from C5 to C12 by FT-EoS and CP-PC-SAFT: simplicity versus complexity[J]. The Journal of Supercritical Fluids, 2015, 104: 193-203. |
| 34 | Green D W, Southard M Z. Perry’s Chemical Engineers’ Handbook[M]. New York: McGraw-Hill Education, 2019. |
| 35 | Lee L L. Molecular Thermodynamics of Nonideal Fluids[M]. Amsterdam: Elsevier, 1988: 373-393. |
| 36 | Barker J A, Henderson D. Perturbation theory and equation of state for fluids (Ⅱ): A successful theory of liquids[J]. The Journal of Chemical Physics, 1967, 47(11): 4714-4721. |
| 37 | Barker J A, Henderson D. What is “liquid”? Understanding the states of matter[J]. Reviews of Modern Physics, 1976, 48(4): 587-671. |
| 38 | Wertheim M S. Fluids of dimerizing hard spheres, and fluid mixtures of hard spheres and dispheres[J]. The Journal of Chemical Physics, 1986, 85(5): 2929-2936. |
| 39 | Chapman W G, Gubbins K E, Jackson G, et al. SAFT: equation-of-state solution model for associating fluids[J]. Fluid Phase Equilibria, 1989, 52: 31-38. |
| 40 | Lafitte T, Piñeiro M M, Daridon J L, et al. A comprehensive description of chemical association effects on second derivative properties of alcohols through a SAFT-VR approach[J]. The Journal of Physical Chemistry. B, 2007, 111(13): 3447-3461. |
| 41 | Gross J, Sadowski G. Application of the perturbed-chain SAFT equation of state to associating systems[J]. Industrial & Engineering Chemistry Research, 2002, 41(22): 5510-5515. |
| 42 | Zheng K, Wu H S, Geng C Y, et al. A comparative study of the perturbed-chain statistical associating fluid theory equation of state and activity coefficient models in phase equilibria calculations for mixtures containing associating and polar components[J]. Industrial & Engineering Chemistry Research, 2018, 57(8): 3014-3030. |
| [1] | Xiaoyu YAO, Jun SHEN, Jian LI, Zhenxing LI, Huifang KANG, Bo TANG, Xueqiang DONG, Maoqiong GONG. Research progress in measurement methods in vapor-liquid critical properties of mixtures [J]. CIESC Journal, 2023, 74(5): 1847-1861. |
| [2] | Ke CHEN, Li DU, Ying ZENG, Siying REN, Xudong YU. Phase equilibria and calculation of quaternary system LiCl+MgCl2+CaCl2+H2O at 323.2 K [J]. CIESC Journal, 2023, 74(5): 1896-1903. |
| [3] | Jinfeng HE, Xiuzhen LI, Jianyao KOU, Tingjie TAO, Can YU, Huan LIU, Yongyuan CHEN, Haojian ZHAO, Dahao JIANG, Xiaonian LI. Ethanol upgrading to higher alcohols over ordered mesoporous alumina supported Cu-based catalysts [J]. CIESC Journal, 2023, 74(3): 1082-1091. |
| [4] | Wenting CHENG, Jie LI, Li XU, Fangqin CHENG, Guoji LIU. Experiment and prediction for the solubility of AlCl3·6H2O in FeCl3, CaCl2, KCl and KCl-FeCl3 solutions [J]. CIESC Journal, 2023, 74(2): 642-652. |
| [5] | Songtao YANG, Dongyang LI, Yuqing NIU, Xingang LI, Shaohui KANG, Hong LI, Kaikai YE, Zhiquan ZHOU, Xin GAO. Molecular simulation progress in studying thermodynamic properties and potential functions of fluorides [J]. CIESC Journal, 2022, 73(9): 3828-3840. |
| [6] | Hailin JIA, Bo CUI, Nan CHEN, Yongqin YANG, Qingyin WANG, Fumin ZHU. Foam performance analysis of fluorine-free foam modified by low carbon alcohol and experimental study on extinguishing oil pool fire [J]. CIESC Journal, 2022, 73(9): 4235-4244. |
| [7] | Jing YANG, Zhenkang LIN, Jun TANG, Cheng FAN, Kening SUN. A review of fault characteristics, fault diagnosis and identification for lithium-ion battery systems [J]. CIESC Journal, 2022, 73(8): 3394-3405. |
| [8] | Jiahui REN, Yu LIU, Chao LIU, Lang LIU, Ying LI. Critical temperature prediction of working fluids using molecular fingerprints and topological indices [J]. CIESC Journal, 2022, 73(4): 1493-1500. |
| [9] | Zirui WU, Rui SUN, Lingfeng SHI, Hua TIAN, Xuan WANG, Gequn SHU. A comparative and predictive study of the mixing rules for the vapor-liquid equilibria of CO2-based mixtures [J]. CIESC Journal, 2022, 73(4): 1483-1492. |
| [10] | Rongshan BI, Zhihui HAN, Shaohui TAO, Xiaoyan SUN, Shuguang XIANG. Recognizing historical operating conditions by determining the density peaks at kernel density estimation of heat diffusion [J]. CIESC Journal, 2022, 73(4): 1615-1622. |
| [11] | Biqiang LIU, Haishan CAO. Adsorption measurement method based on flow calibration and its error analysis [J]. CIESC Journal, 2022, 73(4): 1597-1605. |
| [12] | Mingze SUN, Ning MA, Haoran LI, Haifeng JIANG, Wenpeng HONG, Xiaojuan NIU. Thermodynamic analysis of Brayton cycle of medium and low temperature supercritical CO2 and its mixed working medium [J]. CIESC Journal, 2022, 73(3): 1379-1388. |
| [13] | Maolin YE, Fenghua TAN, Yuping LI, Yuhe LIAO, Chenguang WANG, Longlong MA. Life cycle environmental impact assessment of mixed alcohol via gasification of agricultural and forestry residues and catalytic synthesis [J]. CIESC Journal, 2022, 73(3): 1369-1378. |
| [14] | Cheng ZHANG, Lizhi PAN, Yuan LI. Fault detection and diagnosis method based on weighted statistical feature KICA [J]. CIESC Journal, 2022, 73(2): 827-837. |
| [15] | Huaixu LI, Xiaoyan SUN, Shaohui TAO, Li XIA, Shuguang XIANG. Lumping gasoline with molecular properties and density peak clustering [J]. CIESC Journal, 2022, 73(12): 5449-5460. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||