CIESC Journal ›› 2020, Vol. 71 ›› Issue (1): 245-253.DOI: 10.11949/0438-1157.20191215
• Thermodynamics • Previous Articles Next Articles
Tao LI1( ),Jiao SHA1,Rui ZHAO1,2,Pengshuai ZHANG1,Shiqi LIU1,Yu LI1,Baozeng REN1(
),Jiao SHA1,Rui ZHAO1,2,Pengshuai ZHANG1,Shiqi LIU1,Yu LI1,Baozeng REN1( )
)
Received:2019-10-23
Revised:2019-10-30
Online:2020-01-05
Published:2020-01-05
Contact:
Baozeng REN
李涛1( ),沙娇1,赵瑞1,2,张鹏帅1,刘士琪1,李玉1,任保增1(
),沙娇1,赵瑞1,2,张鹏帅1,刘士琪1,李玉1,任保增1( )
)
通讯作者:
任保增
作者简介:李涛(1982—),男,博士,副教授,基金资助:CLC Number:
Tao LI, Jiao SHA, Rui ZHAO, Pengshuai ZHANG, Shiqi LIU, Yu LI, Baozeng REN. Solid-liquid phase equilibrium of dipentaerythritol in three mixed solvents[J]. CIESC Journal, 2020, 71(1): 245-253.
李涛, 沙娇, 赵瑞, 张鹏帅, 刘士琪, 李玉, 任保增. 双季戊四醇在3种混合溶剂中的固-液相平衡[J]. 化工学报, 2020, 71(1): 245-253.
Add to citation manager EndNote|Ris|BibTeX
| Reagent name | Molecular formula | Specifications and grades | Provenance |
|---|---|---|---|
| DPE | C10H22O7 | ≥0.98 | Aladdin reagent co. LTD |
| methanol | CH3OH | analytical purity,0.995 | Kemiou chemical reagent co. LTD |
| ethanol | CH3CH2OH | analytical purity,0.995 | Kemiou chemical reagent co. LTD |
| isopropanol | (CH3)2CHOH | analytical purity,0.995 | Kemiou chemical reagent co. LTD |
| water | H2O | double-distilled water | our laboratory |
Table 1 List of solvents for solubility measurement
| Reagent name | Molecular formula | Specifications and grades | Provenance |
|---|---|---|---|
| DPE | C10H22O7 | ≥0.98 | Aladdin reagent co. LTD |
| methanol | CH3OH | analytical purity,0.995 | Kemiou chemical reagent co. LTD |
| ethanol | CH3CH2OH | analytical purity,0.995 | Kemiou chemical reagent co. LTD |
| isopropanol | (CH3)2CHOH | analytical purity,0.995 | Kemiou chemical reagent co. LTD |
| water | H2O | double-distilled water | our laboratory |
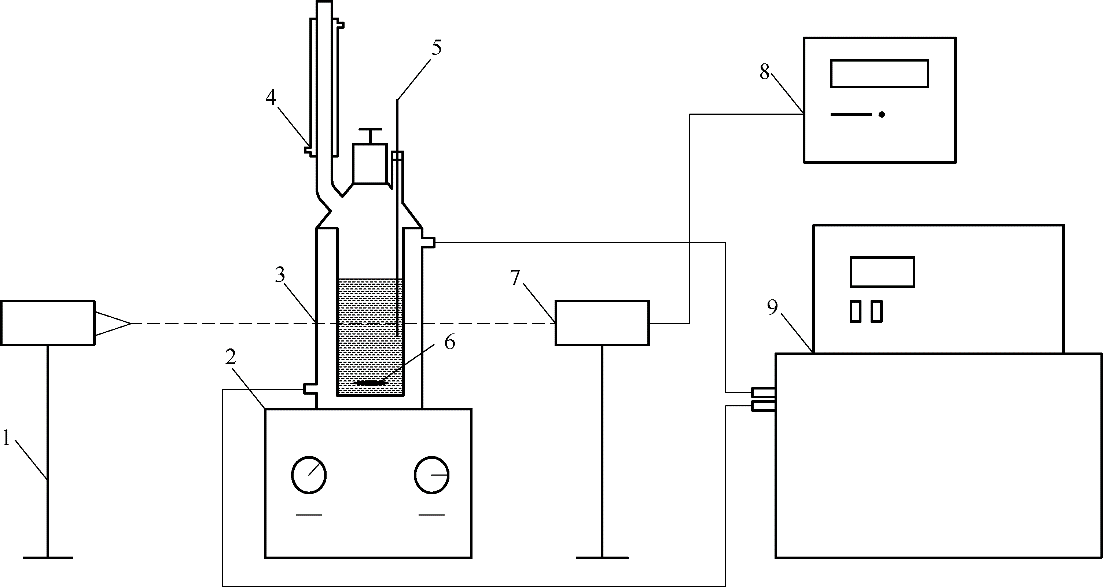
Fig.2 Schematic device for determination of solubility of DPE by dynamic method 1—laser generating device;2—magnetic stirrer;3—double-glazing crystallizer;4—condenser;5—precision thermometer;6—magnetic rotor;7—photoelectric conversion device;8—digital display;9—super constant temperature water bath
| T/K | x×104 | T/K | x×104 | T/K | x×104 |
|---|---|---|---|---|---|
| w=0.1 | 315.50 | 4.440 | 304.80 | 2.452 | |
| 295.85 | 1.187 | 317.65 | 5.346 | 308.20 | 3.002 |
| 298.50 | 1.406 | 320.45 | 6.391 | 311.45 | 3.626 |
| 301.30 | 1.675 | w=0.4 | 314.55 | 4.345 | |
| 303.90 | 1.971 | 295.75 | 1.121 | 317.55 | 5.091 |
| 307.45 | 2.327 | 298.65 | 1.393 | 320.45 | 5.916 |
| 310.20 | 2.721 | 300.65 | 1.679 | w=0.7 | |
| 313.15 | 3.239 | 303.30 | 2.051 | 294.70 | 0.7760 |
| 315.70 | 3.939 | 306.00 | 2.529 | 298.75 | 1.072 |
| 318.30 | 4.765 | 308.30 | 3.161 | 302.05 | 1.320 |
| 321.20 | 5.638 | 311.25 | 3.898 | 304.75 | 1.613 |
| w=0.2 | 314.45 | 4.693 | 307.50 | 1.970 | |
| 294.50 | 1.182 | 317.40 | 5.670 | 310.20 | 2.426 |
| 297.20 | 1.448 | 320.30 | 6.728 | 313.15 | 3.018 |
| 300.50 | 1.784 | w=0.5 | 316.40 | 3.698 | |
| 303.15 | 2.128 | 296.35 | 1.199 | 319.50 | 4.453 |
| 306.40 | 2.508 | 298.80 | 1.495 | 322.50 | 5.303 |
| 309.95 | 2.922 | 301.55 | 1.843 | w=0.8 | |
| 312.70 | 3.426 | 304.30 | 2.263 | 294.05 | 0.4178 |
| 314.90 | 4.072 | 307.20 | 2.758 | 297.30 | 0.6285 |
| 317.35 | 4.932 | 309.85 | 3.365 | 300.69 | 0.8973 |
| 320.55 | 5.880 | 312.60 | 4.114 | 304.00 | 1.188 |
| w=0.3 | 315.65 | 5.019 | 307.15 | 1.493 | |
| 295.25 | 1.119 | 318.50 | 6.046 | 310.15 | 1.827 |
| 297.85 | 1.364 | 321.20 | 7.102 | 313.45 | 2.259 |
| 300.75 | 1.676 | w=0.6 | 315.60 | 2.641 | |
| 303.75 | 2.037 | 293.50 | 0.9315 | 318.55 | 3.167 |
| 307.10 | 2.515 | 296.25 | 1.239 | 321.15 | 3.734 |
| 310.30 | 3.072 | 299.15 | 1.565 | ||
| 312.90 | 3.707 | 301.85 | 1.972 | ||
Table 2 Solubility of DPE in methanol(w)-water(1-w)
| T/K | x×104 | T/K | x×104 | T/K | x×104 |
|---|---|---|---|---|---|
| w=0.1 | 315.50 | 4.440 | 304.80 | 2.452 | |
| 295.85 | 1.187 | 317.65 | 5.346 | 308.20 | 3.002 |
| 298.50 | 1.406 | 320.45 | 6.391 | 311.45 | 3.626 |
| 301.30 | 1.675 | w=0.4 | 314.55 | 4.345 | |
| 303.90 | 1.971 | 295.75 | 1.121 | 317.55 | 5.091 |
| 307.45 | 2.327 | 298.65 | 1.393 | 320.45 | 5.916 |
| 310.20 | 2.721 | 300.65 | 1.679 | w=0.7 | |
| 313.15 | 3.239 | 303.30 | 2.051 | 294.70 | 0.7760 |
| 315.70 | 3.939 | 306.00 | 2.529 | 298.75 | 1.072 |
| 318.30 | 4.765 | 308.30 | 3.161 | 302.05 | 1.320 |
| 321.20 | 5.638 | 311.25 | 3.898 | 304.75 | 1.613 |
| w=0.2 | 314.45 | 4.693 | 307.50 | 1.970 | |
| 294.50 | 1.182 | 317.40 | 5.670 | 310.20 | 2.426 |
| 297.20 | 1.448 | 320.30 | 6.728 | 313.15 | 3.018 |
| 300.50 | 1.784 | w=0.5 | 316.40 | 3.698 | |
| 303.15 | 2.128 | 296.35 | 1.199 | 319.50 | 4.453 |
| 306.40 | 2.508 | 298.80 | 1.495 | 322.50 | 5.303 |
| 309.95 | 2.922 | 301.55 | 1.843 | w=0.8 | |
| 312.70 | 3.426 | 304.30 | 2.263 | 294.05 | 0.4178 |
| 314.90 | 4.072 | 307.20 | 2.758 | 297.30 | 0.6285 |
| 317.35 | 4.932 | 309.85 | 3.365 | 300.69 | 0.8973 |
| 320.55 | 5.880 | 312.60 | 4.114 | 304.00 | 1.188 |
| w=0.3 | 315.65 | 5.019 | 307.15 | 1.493 | |
| 295.25 | 1.119 | 318.50 | 6.046 | 310.15 | 1.827 |
| 297.85 | 1.364 | 321.20 | 7.102 | 313.45 | 2.259 |
| 300.75 | 1.676 | w=0.6 | 315.60 | 2.641 | |
| 303.75 | 2.037 | 293.50 | 0.9315 | 318.55 | 3.167 |
| 307.10 | 2.515 | 296.25 | 1.239 | 321.15 | 3.734 |
| 310.30 | 3.072 | 299.15 | 1.565 | ||
| 312.90 | 3.707 | 301.85 | 1.972 | ||
| T/K | x×104 | T/K | x×104 | T/K | x×104 |
|---|---|---|---|---|---|
| w=0.1 | 318.75 | 7.556 | 305.25 | 3.279 | |
| 296.15 | 1.658 | 320.90 | 8.882 | 307.45 | 3.904 |
| 298.95 | 1.877 | 324.00 | 10.26 | 310.60 | 4.755 |
| 302.15 | 2.105 | 327.15 | 11.79 | 314.65 | 5.724 |
| 305.45 | 2.389 | 329.75 | 13.37 | 317.65 | 6.727 |
| 307.95 | 2.761 | 332.15 | 15.09 | 320.25 | 7.901 |
| 310.95 | 3.213 | w=0.4 | 322.65 | 9.290 | |
| 313.30 | 3.722 | 294.95 | 2.137 | 326.00 | 10.79 |
| 315.70 | 4.320 | 298.25 | 2.408 | 329.05 | 12.29 |
| 318.25 | 5.048 | 300.65 | 2.795 | 331.75 | 13.84 |
| 320.40 | 5.854 | 303.55 | 3.247 | w=0.7 | |
| 323.75 | 6.868 | 306.05 | 3.880 | 296.50 | 1.386 |
| 326.55 | 7.889 | 308.05 | 4.678 | 299.25 | 1.654 |
| 329.30 | 9.043 | 311.75 | 5.716 | 301.85 | 2.016 |
| 332.00 | 10.32 | 314.95 | 6.844 | 304.40 | 2.501 |
| w=0.2 | 317.55 | 7.987 | 307.70 | 3.081 | |
| 296.15 | 1.800 | 320.15 | 9.339 | 310.75 | 3.591 |
| 298.40 | 2.061 | 322.95 | 10.81 | 313.85 | 4.294 |
| 302.05 | 2.458 | 325.90 | 12.50 | 316.35 | 5.062 |
| 304.85 | 2.877 | 328.45 | 14.36 | 318.25 | 5.847 |
| 307.20 | 3.360 | 330.95 | 16.36 | 320.75 | 6.735 |
| 310.25 | 3.989 | w=0.5 | 324.05 | 7.680 | |
| 312.40 | 4.636 | 296.40 | 2.402 | 326.85 | 8.748 |
| 315.10 | 5.328 | 299.80 | 2.795 | 329.95 | 10.08 |
| 317.60 | 6.155 | 303.15 | 3.240 | 332.55 | 11.54 |
| 320.15 | 7.124 | 305.30 | 3.735 | w=0.8 | |
| 322.55 | 8.259 | 307.90 | 4.521 | 294.90 | 0.5524 |
| 325.05 | 9.530 | 310.70 | 5.545 | 297.50 | 0.8101 |
| 327.75 | 10.86 | 313.90 | 6.735 | 300.40 | 1.123 |
| 330.55 | 12.34 | 317.70 | 7.944 | 304.00 | 1.514 |
| w=0.3 | 320.85 | 9.211 | 307.90 | 1.965 | |
| 296.25 | 2.044 | 323.65 | 10.64 | 311.85 | 2.448 |
| 299.30 | 2.422 | 326.55 | 12.19 | 315.70 | 3.037 |
| 301.50 | 2.791 | 329.30 | 13.93 | 319.10 | 3.741 |
| 304.40 | 3.244 | 332.20 | 15.73 | 322.50 | 4.528 |
| 306.95 | 3.741 | w=0.6 | 325.90 | 5.411 | |
| 309.15 | 4.243 | 295.10 | 2.175 | 329.05 | 6.317 |
| 311.70 | 4.808 | 297.70 | 2.380 | 332.55 | 7.291 |
| 313.95 | 5.518 | 300.00 | 2.603 | ||
| 316.10 | 6.409 | 302.75 | 2.844 | ||
Table 3 Solubility of DPE in ethanol(w)-water(1-w)
| T/K | x×104 | T/K | x×104 | T/K | x×104 |
|---|---|---|---|---|---|
| w=0.1 | 318.75 | 7.556 | 305.25 | 3.279 | |
| 296.15 | 1.658 | 320.90 | 8.882 | 307.45 | 3.904 |
| 298.95 | 1.877 | 324.00 | 10.26 | 310.60 | 4.755 |
| 302.15 | 2.105 | 327.15 | 11.79 | 314.65 | 5.724 |
| 305.45 | 2.389 | 329.75 | 13.37 | 317.65 | 6.727 |
| 307.95 | 2.761 | 332.15 | 15.09 | 320.25 | 7.901 |
| 310.95 | 3.213 | w=0.4 | 322.65 | 9.290 | |
| 313.30 | 3.722 | 294.95 | 2.137 | 326.00 | 10.79 |
| 315.70 | 4.320 | 298.25 | 2.408 | 329.05 | 12.29 |
| 318.25 | 5.048 | 300.65 | 2.795 | 331.75 | 13.84 |
| 320.40 | 5.854 | 303.55 | 3.247 | w=0.7 | |
| 323.75 | 6.868 | 306.05 | 3.880 | 296.50 | 1.386 |
| 326.55 | 7.889 | 308.05 | 4.678 | 299.25 | 1.654 |
| 329.30 | 9.043 | 311.75 | 5.716 | 301.85 | 2.016 |
| 332.00 | 10.32 | 314.95 | 6.844 | 304.40 | 2.501 |
| w=0.2 | 317.55 | 7.987 | 307.70 | 3.081 | |
| 296.15 | 1.800 | 320.15 | 9.339 | 310.75 | 3.591 |
| 298.40 | 2.061 | 322.95 | 10.81 | 313.85 | 4.294 |
| 302.05 | 2.458 | 325.90 | 12.50 | 316.35 | 5.062 |
| 304.85 | 2.877 | 328.45 | 14.36 | 318.25 | 5.847 |
| 307.20 | 3.360 | 330.95 | 16.36 | 320.75 | 6.735 |
| 310.25 | 3.989 | w=0.5 | 324.05 | 7.680 | |
| 312.40 | 4.636 | 296.40 | 2.402 | 326.85 | 8.748 |
| 315.10 | 5.328 | 299.80 | 2.795 | 329.95 | 10.08 |
| 317.60 | 6.155 | 303.15 | 3.240 | 332.55 | 11.54 |
| 320.15 | 7.124 | 305.30 | 3.735 | w=0.8 | |
| 322.55 | 8.259 | 307.90 | 4.521 | 294.90 | 0.5524 |
| 325.05 | 9.530 | 310.70 | 5.545 | 297.50 | 0.8101 |
| 327.75 | 10.86 | 313.90 | 6.735 | 300.40 | 1.123 |
| 330.55 | 12.34 | 317.70 | 7.944 | 304.00 | 1.514 |
| w=0.3 | 320.85 | 9.211 | 307.90 | 1.965 | |
| 296.25 | 2.044 | 323.65 | 10.64 | 311.85 | 2.448 |
| 299.30 | 2.422 | 326.55 | 12.19 | 315.70 | 3.037 |
| 301.50 | 2.791 | 329.30 | 13.93 | 319.10 | 3.741 |
| 304.40 | 3.244 | 332.20 | 15.73 | 322.50 | 4.528 |
| 306.95 | 3.741 | w=0.6 | 325.90 | 5.411 | |
| 309.15 | 4.243 | 295.10 | 2.175 | 329.05 | 6.317 |
| 311.70 | 4.808 | 297.70 | 2.380 | 332.55 | 7.291 |
| 313.95 | 5.518 | 300.00 | 2.603 | ||
| 316.10 | 6.409 | 302.75 | 2.844 | ||
| T/K | x×104 | T/K | x×104 | T/K | x×104 |
|---|---|---|---|---|---|
| w=0.1 | 319.35 | 8.181 | 297.45 | 2.486 | |
| 294.95 | 1.471 | 321.95 | 9.341 | 301.05 | 2.901 |
| 298.85 | 1.758 | 324.50 | 10.71 | 304.45 | 3.486 |
| 302.75 | 2.040 | 327.15 | 12.25 | 307.70 | 4.153 |
| 305.95 | 2.395 | 329.75 | 14.05 | 310.65 | 4.865 |
| 308.35 | 2.831 | 332.30 | 16.07 | 313.65 | 5.633 |
| 311.25 | 3.422 | w=0.4 | 317.60 | 6.609 | |
| 314.15 | 4.182 | 293.70 | 1.838 | 319.65 | 7.783 |
| 317.00 | 5.087 | 296.50 | 2.327 | 323.15 | 9.205 |
| 320.25 | 6.096 | 299.30 | 2.733 | 326.35 | 10.73 |
| 323.35 | 7.132 | 302.50 | 3.224 | 329.10 | 12.46 |
| 326.25 | 8.174 | 305.25 | 3.683 | 332.05 | 14.28 |
| 329.20 | 9.237 | 307.20 | 4.322 | w=0.7 | |
| 331.85 | 10.37 | 309.85 | 5.225 | 294.10 | 1.735 |
| w=0.2 | 313.35 | 6.401 | 298.25 | 2.291 | |
| 295.40 | 1.931 | 316.75 | 7.612 | 302.65 | 2.762 |
| 298.40 | 2.192 | 320.20 | 9.071 | 306.95 | 3.253 |
| 301.65 | 2.568 | 323.25 | 10.56 | 310.50 | 3.854 |
| 304.40 | 3.020 | 326.35 | 12.20 | 313.45 | 4.562 |
| 306.55 | 3.511 | 329.25 | 13.98 | 317.15 | 5.486 |
| 309.75 | 4.177 | 332.20 | 15.94 | 320.85 | 6.480 |
| 312.65 | 4.942 | w=0.5 | 324.10 | 7.627 | |
| 315.55 | 5.759 | 293.85 | 1.957 | 326.90 | 8.962 |
| 318.15 | 6.598 | 297.15 | 2.352 | 330.00 | 10.52 |
| 320.65 | 7.581 | 300.80 | 2.853 | 332.80 | 12.14 |
| 323.05 | 8.685 | 304.15 | 3.263 | w=0.8 | |
| 325.90 | 9.961 | 306.75 | 3.757 | 296.60 | 0.8477 |
| 328.35 | 11.39 | 308.85 | 4.363 | 300.55 | 1.218 |
| 330.70 | 12.93 | 311.80 | 5.266 | 303.90 | 1.540 |
| w=0.3 | 314.75 | 6.372 | 307.25 | 1.915 | |
| 294.95 | 2.100 | 317.65 | 7.543 | 311.10 | 2.366 |
| 297.75 | 2.392 | 320.40 | 8.895 | 313.55 | 2.875 |
| 300.25 | 2.843 | 323.05 | 10.35 | 317.25 | 3.621 |
| 303.20 | 3.425 | 326.10 | 12.09 | 320.75 | 4.371 |
| 306.05 | 4.201 | 328.85 | 14.01 | 324.15 | 5.207 |
| 309.95 | 5.183 | 331.95 | 16.02 | 327.85 | 6.263 |
| 312.90 | 6.132 | w=0.6 | 331.25 | 7.388 | |
| 316.35 | 7.107 | 293.75 | 2.047 | ||
Table 4 Solubility of DPE in isopropanol (w)-water(1-w)
| T/K | x×104 | T/K | x×104 | T/K | x×104 |
|---|---|---|---|---|---|
| w=0.1 | 319.35 | 8.181 | 297.45 | 2.486 | |
| 294.95 | 1.471 | 321.95 | 9.341 | 301.05 | 2.901 |
| 298.85 | 1.758 | 324.50 | 10.71 | 304.45 | 3.486 |
| 302.75 | 2.040 | 327.15 | 12.25 | 307.70 | 4.153 |
| 305.95 | 2.395 | 329.75 | 14.05 | 310.65 | 4.865 |
| 308.35 | 2.831 | 332.30 | 16.07 | 313.65 | 5.633 |
| 311.25 | 3.422 | w=0.4 | 317.60 | 6.609 | |
| 314.15 | 4.182 | 293.70 | 1.838 | 319.65 | 7.783 |
| 317.00 | 5.087 | 296.50 | 2.327 | 323.15 | 9.205 |
| 320.25 | 6.096 | 299.30 | 2.733 | 326.35 | 10.73 |
| 323.35 | 7.132 | 302.50 | 3.224 | 329.10 | 12.46 |
| 326.25 | 8.174 | 305.25 | 3.683 | 332.05 | 14.28 |
| 329.20 | 9.237 | 307.20 | 4.322 | w=0.7 | |
| 331.85 | 10.37 | 309.85 | 5.225 | 294.10 | 1.735 |
| w=0.2 | 313.35 | 6.401 | 298.25 | 2.291 | |
| 295.40 | 1.931 | 316.75 | 7.612 | 302.65 | 2.762 |
| 298.40 | 2.192 | 320.20 | 9.071 | 306.95 | 3.253 |
| 301.65 | 2.568 | 323.25 | 10.56 | 310.50 | 3.854 |
| 304.40 | 3.020 | 326.35 | 12.20 | 313.45 | 4.562 |
| 306.55 | 3.511 | 329.25 | 13.98 | 317.15 | 5.486 |
| 309.75 | 4.177 | 332.20 | 15.94 | 320.85 | 6.480 |
| 312.65 | 4.942 | w=0.5 | 324.10 | 7.627 | |
| 315.55 | 5.759 | 293.85 | 1.957 | 326.90 | 8.962 |
| 318.15 | 6.598 | 297.15 | 2.352 | 330.00 | 10.52 |
| 320.65 | 7.581 | 300.80 | 2.853 | 332.80 | 12.14 |
| 323.05 | 8.685 | 304.15 | 3.263 | w=0.8 | |
| 325.90 | 9.961 | 306.75 | 3.757 | 296.60 | 0.8477 |
| 328.35 | 11.39 | 308.85 | 4.363 | 300.55 | 1.218 |
| 330.70 | 12.93 | 311.80 | 5.266 | 303.90 | 1.540 |
| w=0.3 | 314.75 | 6.372 | 307.25 | 1.915 | |
| 294.95 | 2.100 | 317.65 | 7.543 | 311.10 | 2.366 |
| 297.75 | 2.392 | 320.40 | 8.895 | 313.55 | 2.875 |
| 300.25 | 2.843 | 323.05 | 10.35 | 317.25 | 3.621 |
| 303.20 | 3.425 | 326.10 | 12.09 | 320.75 | 4.371 |
| 306.05 | 4.201 | 328.85 | 14.01 | 324.15 | 5.207 |
| 309.95 | 5.183 | 331.95 | 16.02 | 327.85 | 6.263 |
| 312.90 | 6.132 | w=0.6 | 331.25 | 7.388 | |
| 316.35 | 7.107 | 293.75 | 2.047 | ||
| Solvent | λ | h | R 2 | AAD×102 | RMSD×105 |
|---|---|---|---|---|---|
| methanol(w)-water(1-w) | |||||
| w=0.1 | 0.39780 | 15280 | 0.995 | 4.54 | 1.25 |
| w=0.2 | 0.34884 | 16873 | 0.993 | 4.81 | 1.43 |
| w=0.3 | 0.96175 | 6997.7 | 0.997 | 4.03 | 1.28 |
| w=0.4 | 1.0226 | 6556.6 | 0.997 | 3.35 | 1.41 |
| w=0.5 | 0.96584 | 6898.9 | 0.999 | 2.58 | 1.16 |
| w=0.6 | 0.36007 | 16309 | 0.996 | 5.23 | 1.27 |
| w=0.7 | 0.56586 | 11509 | 0.999 | 2.14 | 0.902 |
| w=0.8 | 0.57280 | 11819 | 0.997 | 6.42 | 0.786 |
| ethanol(w)-water(1-w) | |||||
| w=0.1 | 0.21642 | 25207 | 0.998 | 4.23 | 1.76 |
| w=0.2 | 0.34266 | 16499 | 0.999 | 2.52 | 1.77 |
| w=0.3 | 0.36008 | 15513 | 0.998 | 2.62 | 2.50 |
| w=0.4 | 0.45927 | 12400 | 0.999 | 3.04 | 2.29 |
| w=0.5 | 0.25455 | 20388 | 0.998 | 3.46 | 2.60 |
| w=0.6 | 0.25387 | 20858 | 0.998 | 3.71 | 2.37 |
| w=0.7 | 0.23835 | 22905 | 0.997 | 4.02 | 2.18 |
| w=0.8 | 0.18837 | 30098 | 0.996 | 8.98 | 1.63 |
| isopropanol(w)-water(1-w) | |||||
| w=0.1 | 0.22014 | 24706 | 0.996 | 4.46 | 2.18 |
| w=0.2 | 0.29333 | 18677 | 0.999 | 2.81 | 1.70 |
| w=0.3 | 0.27984 | 18919 | 0.999 | 2.45 | 2.26 |
| w=0.4 | 0.27913 | 18880 | 0.999 | 3.12 | 2.40 |
| w=0.5 | 0.42246 | 13422 | 0.999 | 3.70 | 2.46 |
| w=0.6 | 0.22352 | 23123 | 0.999 | 3.72 | 2.11 |
| w=0.7 | 0.17280 | 29636 | 0.997 | 4.25 | 2.17 |
| w=0.8 | 0.21077 | 27115 | 0.998 | 3.78 | 1.28 |
Table 5 Parameters, average absolute deviation AAD, root-mean square deviation RMSDfor DPE in Solvents by λh equation
| Solvent | λ | h | R 2 | AAD×102 | RMSD×105 |
|---|---|---|---|---|---|
| methanol(w)-water(1-w) | |||||
| w=0.1 | 0.39780 | 15280 | 0.995 | 4.54 | 1.25 |
| w=0.2 | 0.34884 | 16873 | 0.993 | 4.81 | 1.43 |
| w=0.3 | 0.96175 | 6997.7 | 0.997 | 4.03 | 1.28 |
| w=0.4 | 1.0226 | 6556.6 | 0.997 | 3.35 | 1.41 |
| w=0.5 | 0.96584 | 6898.9 | 0.999 | 2.58 | 1.16 |
| w=0.6 | 0.36007 | 16309 | 0.996 | 5.23 | 1.27 |
| w=0.7 | 0.56586 | 11509 | 0.999 | 2.14 | 0.902 |
| w=0.8 | 0.57280 | 11819 | 0.997 | 6.42 | 0.786 |
| ethanol(w)-water(1-w) | |||||
| w=0.1 | 0.21642 | 25207 | 0.998 | 4.23 | 1.76 |
| w=0.2 | 0.34266 | 16499 | 0.999 | 2.52 | 1.77 |
| w=0.3 | 0.36008 | 15513 | 0.998 | 2.62 | 2.50 |
| w=0.4 | 0.45927 | 12400 | 0.999 | 3.04 | 2.29 |
| w=0.5 | 0.25455 | 20388 | 0.998 | 3.46 | 2.60 |
| w=0.6 | 0.25387 | 20858 | 0.998 | 3.71 | 2.37 |
| w=0.7 | 0.23835 | 22905 | 0.997 | 4.02 | 2.18 |
| w=0.8 | 0.18837 | 30098 | 0.996 | 8.98 | 1.63 |
| isopropanol(w)-water(1-w) | |||||
| w=0.1 | 0.22014 | 24706 | 0.996 | 4.46 | 2.18 |
| w=0.2 | 0.29333 | 18677 | 0.999 | 2.81 | 1.70 |
| w=0.3 | 0.27984 | 18919 | 0.999 | 2.45 | 2.26 |
| w=0.4 | 0.27913 | 18880 | 0.999 | 3.12 | 2.40 |
| w=0.5 | 0.42246 | 13422 | 0.999 | 3.70 | 2.46 |
| w=0.6 | 0.22352 | 23123 | 0.999 | 3.72 | 2.11 |
| w=0.7 | 0.17280 | 29636 | 0.997 | 4.25 | 2.17 |
| w=0.8 | 0.21077 | 27115 | 0.998 | 3.78 | 1.28 |
| Solvent | A | B | C | R 2 | AAD×102 | RMSD×105 |
|---|---|---|---|---|---|---|
| methanol(w)-water(1-w) | ||||||
| w=0.1 | -568.53 | 20743 | 86.013 | 0.998 | 1.69 | 0.567 |
| w=0.2 | -308.77 | 8922.3 | 47.397 | 0.995 | 2.81 | 0.978 |
| w=0.3 | -409.87 | 12859 | 62.808 | 0.999 | 1.38 | 0.515 |
| w=0.4 | 549.70 | -31494 | -79.502 | 0.999 | 1.55 | 0.530 |
| w=0.5 | 248.62 | -17543 | -34.865 | 0.999 | 0.638 | 0.211 |
| w=0.6 | 952.00 | -49158 | -139.70 | 0.999 | 1.27 | 0.467 |
| w=0.7 | 86.161 | -9982.2 | -10.861 | 0.999 | 1.37 | 0.424 |
| w=0.8 | 1164.7 | -59865 | -170.86 | 0.998 | 2.49 | 0.536 |
| ethanol(w)-water(1-w) | ||||||
| w=0.1 | -466.90 | 16886 | 70.490 | 0.997 | 2.73 | 1.75 |
| w=0.2 | -160.80 | 2331.1 | 25.355 | 0.999 | 1.34 | 1.20 |
| w=0.3 | -111.03 | 53.025 | 17.983 | 0.998 | 1.86 | 2.06 |
| w=0.4 | -59.656 | -2459.6 | 10.460 | 0.998 | 2.49 | 1.41 |
| w=0.5 | 119.54 | -10462 | -16.275 | 0.996 | 2.44 | 1.58 |
| w=0.6 | -290.86 | 8696.0 | 44.469 | 0.996 | 2.90 | 2.50 |
| w=0.7 | 385.80 | -23244 | -55.568 | 0.999 | 1.78 | 1.03 |
| w=0.8 | 898.76 | -47482 | -131.44 | 0.995 | 4.38 | 1.21 |
| isopropanol(w)-water(1-w) | ||||||
| w=0.1 | -200.50 | 4262.9 | 31.153 | 0.994 | 4.17 | 2.45 |
| w=0.2 | -180.83 | 3455.2 | 28.225 | 0.999 | 1.17 | 0.562 |
| w=0.3 | 97.955 | -9417.8 | -13.102 | 0.999 | 1.83 | 1.56 |
| w=0.4 | 171.33 | -12936 | -23.911 | 0.999 | 1.77 | 1.03 |
| w=0.5 | -269.57 | 7401.8 | 41.496 | 0.998 | 2.37 | 2.48 |
| w=0.6 | -228.92 | 6009.1 | 35.189 | 0.999 | 1.08 | 0.988 |
| w=0.7 | -298.52 | 9386.8 | 45.388 | 0.998 | 1.66 | 0.701 |
| w=0.8 | 410.09 | -24579 | -59.126 | 0.999 | 1.28 | 0.372 |
Table 7 Parameters, average absolute deviation AAD, root-mean square deviation RMSDfor DPE in Solvents by Apelblat equation
| Solvent | A | B | C | R 2 | AAD×102 | RMSD×105 |
|---|---|---|---|---|---|---|
| methanol(w)-water(1-w) | ||||||
| w=0.1 | -568.53 | 20743 | 86.013 | 0.998 | 1.69 | 0.567 |
| w=0.2 | -308.77 | 8922.3 | 47.397 | 0.995 | 2.81 | 0.978 |
| w=0.3 | -409.87 | 12859 | 62.808 | 0.999 | 1.38 | 0.515 |
| w=0.4 | 549.70 | -31494 | -79.502 | 0.999 | 1.55 | 0.530 |
| w=0.5 | 248.62 | -17543 | -34.865 | 0.999 | 0.638 | 0.211 |
| w=0.6 | 952.00 | -49158 | -139.70 | 0.999 | 1.27 | 0.467 |
| w=0.7 | 86.161 | -9982.2 | -10.861 | 0.999 | 1.37 | 0.424 |
| w=0.8 | 1164.7 | -59865 | -170.86 | 0.998 | 2.49 | 0.536 |
| ethanol(w)-water(1-w) | ||||||
| w=0.1 | -466.90 | 16886 | 70.490 | 0.997 | 2.73 | 1.75 |
| w=0.2 | -160.80 | 2331.1 | 25.355 | 0.999 | 1.34 | 1.20 |
| w=0.3 | -111.03 | 53.025 | 17.983 | 0.998 | 1.86 | 2.06 |
| w=0.4 | -59.656 | -2459.6 | 10.460 | 0.998 | 2.49 | 1.41 |
| w=0.5 | 119.54 | -10462 | -16.275 | 0.996 | 2.44 | 1.58 |
| w=0.6 | -290.86 | 8696.0 | 44.469 | 0.996 | 2.90 | 2.50 |
| w=0.7 | 385.80 | -23244 | -55.568 | 0.999 | 1.78 | 1.03 |
| w=0.8 | 898.76 | -47482 | -131.44 | 0.995 | 4.38 | 1.21 |
| isopropanol(w)-water(1-w) | ||||||
| w=0.1 | -200.50 | 4262.9 | 31.153 | 0.994 | 4.17 | 2.45 |
| w=0.2 | -180.83 | 3455.2 | 28.225 | 0.999 | 1.17 | 0.562 |
| w=0.3 | 97.955 | -9417.8 | -13.102 | 0.999 | 1.83 | 1.56 |
| w=0.4 | 171.33 | -12936 | -23.911 | 0.999 | 1.77 | 1.03 |
| w=0.5 | -269.57 | 7401.8 | 41.496 | 0.998 | 2.37 | 2.48 |
| w=0.6 | -228.92 | 6009.1 | 35.189 | 0.999 | 1.08 | 0.988 |
| w=0.7 | -298.52 | 9386.8 | 45.388 | 0.998 | 1.66 | 0.701 |
| w=0.8 | 410.09 | -24579 | -59.126 | 0.999 | 1.28 | 0.372 |
| Solvent | a | b | R 2 | AAD×102 | RMSD×105 |
|---|---|---|---|---|---|
| methanol(w)-water(1-w) | |||||
| w=0.1 | 11.437 | -6080.7 | 0.995 | 3.92 | 0.959 |
| w=0.2 | 10.917 | -5889.1 | 0.993 | 3.91 | 1.18 |
| w=0.3 | 13.634 | -6729.4 | 0.997 | 3.11 | 0.818 |
| w=0.4 | 13.647 | -6705.2 | 0.997 | 3.58 | 0.969 |
| w=0.5 | 13.505 | -6663.6 | 0.999 | 1.21 | 0.402 |
| w=0.6 | 10.925 | -5876.5 | 0.996 | 4.76 | 0.971 |
| w=0.7 | 12.669 | -6514.3 | 0.999 | 1.76 | 0.454 |
| w=0.8 | 13.204 | -6771.5 | 0.997 | 5.23 | 0.529 |
| ethanol(w)-water(1-w) | |||||
| w=0.1 | 9.5915 | -5466.5 | 0.998 | 3.81 | 1.27 |
| w=0.2 | 10.441 | -5661.1 | 0.999 | 1.73 | 0.891 |
| w=0.3 | 10.356 | -5594.3 | 0.998 | 2.13 | 1.72 |
| w=0.4 | 10.813 | -5700.7 | 0.999 | 2.24 | 1.09 |
| w=0.5 | 9.2214 | -5203.1 | 0.998 | 2.90 | 1.81 |
| w=0.6 | 9.4279 | -5307.0 | 0.998 | 3.27 | 1.74 |
| w=0.7 | 9.6958 | -5470.5 | 0.997 | 4.06 | 1.69 |
| w=0.8 | 9.8839 | -5679.7 | 0.996 | 8.37 | 1.35 |
| isopropanol(w)-water(1-w) | |||||
| w=0.1 | 9.5751 | -5450.1 | 0.996 | 4.23 | 1.76 |
| w=0.2 | 9.9367 | -5487.9 | 0.999 | 1.37 | 0.663 |
| w=0.3 | 9.5237 | -5306.2 | 0.999 | 1.68 | 1.28 |
| w=0.4 | 9.4716 | -5281.9 | 0.999 | 3.06 | 1.57 |
| w=0.5 | 10.683 | -5677.2 | 0.999 | 3.06 | 1.54 |
| w=0.6 | 9.0514 | -5182.8 | 0.999 | 2.31 | 1.36 |
| w=0.7 | 8.7062 | -5138.0 | 0.997 | 3.69 | 1.68 |
| w=0.8 | 10.085 | -5724.1 | 0.998 | 3.74 | 0.865 |
Table 6 Parameters, average absolute deviation AAD, root-mean square deviation RMSDfor DPE in Solvents by equation 7
| Solvent | a | b | R 2 | AAD×102 | RMSD×105 |
|---|---|---|---|---|---|
| methanol(w)-water(1-w) | |||||
| w=0.1 | 11.437 | -6080.7 | 0.995 | 3.92 | 0.959 |
| w=0.2 | 10.917 | -5889.1 | 0.993 | 3.91 | 1.18 |
| w=0.3 | 13.634 | -6729.4 | 0.997 | 3.11 | 0.818 |
| w=0.4 | 13.647 | -6705.2 | 0.997 | 3.58 | 0.969 |
| w=0.5 | 13.505 | -6663.6 | 0.999 | 1.21 | 0.402 |
| w=0.6 | 10.925 | -5876.5 | 0.996 | 4.76 | 0.971 |
| w=0.7 | 12.669 | -6514.3 | 0.999 | 1.76 | 0.454 |
| w=0.8 | 13.204 | -6771.5 | 0.997 | 5.23 | 0.529 |
| ethanol(w)-water(1-w) | |||||
| w=0.1 | 9.5915 | -5466.5 | 0.998 | 3.81 | 1.27 |
| w=0.2 | 10.441 | -5661.1 | 0.999 | 1.73 | 0.891 |
| w=0.3 | 10.356 | -5594.3 | 0.998 | 2.13 | 1.72 |
| w=0.4 | 10.813 | -5700.7 | 0.999 | 2.24 | 1.09 |
| w=0.5 | 9.2214 | -5203.1 | 0.998 | 2.90 | 1.81 |
| w=0.6 | 9.4279 | -5307.0 | 0.998 | 3.27 | 1.74 |
| w=0.7 | 9.6958 | -5470.5 | 0.997 | 4.06 | 1.69 |
| w=0.8 | 9.8839 | -5679.7 | 0.996 | 8.37 | 1.35 |
| isopropanol(w)-water(1-w) | |||||
| w=0.1 | 9.5751 | -5450.1 | 0.996 | 4.23 | 1.76 |
| w=0.2 | 9.9367 | -5487.9 | 0.999 | 1.37 | 0.663 |
| w=0.3 | 9.5237 | -5306.2 | 0.999 | 1.68 | 1.28 |
| w=0.4 | 9.4716 | -5281.9 | 0.999 | 3.06 | 1.57 |
| w=0.5 | 10.683 | -5677.2 | 0.999 | 3.06 | 1.54 |
| w=0.6 | 9.0514 | -5182.8 | 0.999 | 2.31 | 1.36 |
| w=0.7 | 8.7062 | -5138.0 | 0.997 | 3.69 | 1.68 |
| w=0.8 | 10.085 | -5724.1 | 0.998 | 3.74 | 0.865 |
| Solvent | Δsol H 0/ (kJ·mol-1) | Δsol S 0/ (J·mol-1·K-1) | Δsol G 0/ (kJ·mol-1) |
|---|---|---|---|
| methanol(w)-water(1-w) | |||
| w=0.1 | 40.75 | 62.77 | 22.04 |
| w=0.2 | 43.31 | 72.13 | 21.80 |
| w=0.3 | 48.78 | 89.73 | 22.03 |
| w=0.4 | 64.77 | 143.23 | 22.07 |
| w=0.5 | 59.43 | 125.61 | 21.98 |
| w=0.6 | 62.41 | 135.90 | 21.89 |
| w=0.7 | 56.07 | 111.56 | 22.81 |
| w=0.8 | 74.19 | 169.18 | 23.75 |
| ethanol(w)-water(1-w) | |||
| w=0.1 | 34.34 | 43.35 | 21.42 |
| w=0.2 | 43.47 | 74.97 | 21.12 |
| w=0.3 | 44.14 | 78.26 | 20.80 |
| w=0.4 | 46.38 | 86.47 | 20.60 |
| w=0.5 | 46.64 | 87.60 | 20.52 |
| w=0.6 | 37.93 | 57.99 | 20.64 |
| w=0.7 | 55.51 | 113.30 | 21.73 |
| w=0.8 | 68.95 | 153.21 | 23.27 |
| isopropanol(w)-water(1-w) | |||
| w=0.1 | 41.78 | 67.76 | 21.58 |
| w=0.2 | 41.24 | 68.26 | 20.89 |
| w=0.3 | 45.82 | 84.83 | 20.53 |
| w=0.4 | 48.28 | 92.98 | 20.56 |
| w=0.5 | 41.32 | 69.45 | 20.62 |
| w=0.6 | 37.27 | 56.22 | 20.51 |
| w=0.7 | 34.47 | 45.48 | 20.91 |
| w=0.8 | 57.79 | 117.13 | 22.87 |
Table 8 Δsol H 0, Δsol S 0 and Δsol G 0 of DPE in different solvents
| Solvent | Δsol H 0/ (kJ·mol-1) | Δsol S 0/ (J·mol-1·K-1) | Δsol G 0/ (kJ·mol-1) |
|---|---|---|---|
| methanol(w)-water(1-w) | |||
| w=0.1 | 40.75 | 62.77 | 22.04 |
| w=0.2 | 43.31 | 72.13 | 21.80 |
| w=0.3 | 48.78 | 89.73 | 22.03 |
| w=0.4 | 64.77 | 143.23 | 22.07 |
| w=0.5 | 59.43 | 125.61 | 21.98 |
| w=0.6 | 62.41 | 135.90 | 21.89 |
| w=0.7 | 56.07 | 111.56 | 22.81 |
| w=0.8 | 74.19 | 169.18 | 23.75 |
| ethanol(w)-water(1-w) | |||
| w=0.1 | 34.34 | 43.35 | 21.42 |
| w=0.2 | 43.47 | 74.97 | 21.12 |
| w=0.3 | 44.14 | 78.26 | 20.80 |
| w=0.4 | 46.38 | 86.47 | 20.60 |
| w=0.5 | 46.64 | 87.60 | 20.52 |
| w=0.6 | 37.93 | 57.99 | 20.64 |
| w=0.7 | 55.51 | 113.30 | 21.73 |
| w=0.8 | 68.95 | 153.21 | 23.27 |
| isopropanol(w)-water(1-w) | |||
| w=0.1 | 41.78 | 67.76 | 21.58 |
| w=0.2 | 41.24 | 68.26 | 20.89 |
| w=0.3 | 45.82 | 84.83 | 20.53 |
| w=0.4 | 48.28 | 92.98 | 20.56 |
| w=0.5 | 41.32 | 69.45 | 20.62 |
| w=0.6 | 37.27 | 56.22 | 20.51 |
| w=0.7 | 34.47 | 45.48 | 20.91 |
| w=0.8 | 57.79 | 117.13 | 22.87 |
| 1 | Li L S , Kamiya Y , Okuhara T . Catalytic dehydration of pentaerythritol to dipentaerythritol over heteropoly compounds[J]. Applied Catalysis A: General, 2003, 253(1): 29-32. |
| 2 | 陈坤 . 双季戊四醇的应用[J]. 化工生产与技术, 2004, 11(6): 25-28 |
| Chen K . Application of dipentaerythritol [J]. Chemical Production and Technology, 2004, 11(6): 25-28. | |
| 3 | Nagendramma P , Kaul S . Study of synethesised ecofriendly and biodegradable esters: fire resistance and lubricating properties[J]. Lubrication Science, 2010, 22: 103-110. |
| 4 | Paredes X , Pensado A S , Comuñas M J P , et al . Experimental dynamic viscosities of dipentaerythritol ester lubricants at high pressure[J]. Journal of Chemical & Engineering Data, 2010, 55(9): 3216-3223. |
| 5 | Zhang L T , Cai G X , Wang Y L , et al . Synthesis and characterisation of antioxidant-modified esters of dipentaerythritol as lubricating base oil[J]. Lubrication Science, 2013, 25(5): 329-337. |
| 6 | García J , Abou Naccoul R , Fernández J , et al . Vapor-pressure measurements and modeling of dipentaerythritol ester lubricants[J]. Industrial & Engineering Chemistry Research, 2011, 50(8): 4231-4237. |
| 7 | Shaver M P , Cameron D J A . Tacticity control in the synthesis of poly (lactic acid) polymer stars with dipentaerythritol cores[J]. Biomacromolecules, 2010, 11(12): 3673-3679. |
| 8 | Ma J Q , Dai Q , Li X Y , et al . Dipentaerythritol penta-/hexa-acrylate based-highly cross-linked hybrid monolithic column: preparation and its applications for ultrahigh efficiency separation of proteins[J]. Analytica Chimica Acta, 2017, 963: 143-152. |
| 9 | Wang G J , Yang J Y . Thermal degradation study of fire resistive coating containing melamine polyphosphate and dipentaerythritol[J]. Progress in Organic Coatings, 2011, 72(4): 605-611. |
| 10 | Snejdrova E , Dittrich M , Drastik M . Plasticized branched aliphatic oligoesters as potential mucoadhesive drug carriers [J]. International Journal of Pharmaceutics, 2013, 458(2): 282-286. |
| 11 | Cho Y H , Shin C W , Kim N , et al . High-performance transmission holographic gratings via different polymerization rates of dipentaerythritol acrylates and siloxane-containing epoxides [J]. Chemistry of Materials, 2005, 17(25): 6263-6271. |
| 12 | Lewin M , Brozek J , Martens M M . The system polyamide/sulfamate/dipentaerythritol: flame retardancy and chemical reactions [J]. Polymers for Advanced Technologies, 2002, 13(10/11/12): 1091-1102. |
| 13 | Wang Z Z , Wu K , Hu Y . Study on flame retardance of co-microencapsulated ammonium polyphosphate and dipentaerythritol in polypropylene [J]. Polymer Engineering & Science, 2008, 48(12): 2426-2431. |
| 14 | Lu Y B , Zhang Y J , Xu W J . Flame retardancy and mechanical properties of ethylene-vinyl acetate rubber with expandable graphite/ammonium polyphosphate/dipentaerythritol system [J]. Journal of Macromolecular Science, Part B, 2011, 50(10): 1864-1872. |
| 15 | Zhang L , Cai G , Eli W . Synthesis and characterization of novel liquid ester-phenolic antioxidant based on dipentaerythritol [J]. Lubrication Science, 2013, 25(3): 209-216. |
| 16 | Vannini M , Marchese P , Celli A , et al . Synergistic effect of dipentaerythritol and montmorillonite in EVOH-based nanocomposites [J]. Journal of Applied Polymer Science, 2015, 132(28): 42265. |
| 17 | 沈国良, 徐铁军, 傅承碧, 等 . 季戊四醇水溶液结晶介稳区性质的研究[J]. 化学世界, 2003, (4): 181-184. |
| Shen G L , Xu T J , Fu C B , et al . Study on the properties of crystallization metastable zone of pentaerythritol aqueous solution [J]. Chemical World, 2003, (4): 181-184. | |
| 18 | Buchowski H , Ksiazczak A , Pietrzyk S . Solvent activity along a saturation line and solubility of hydrogen-bonding solids[J]. The Journal of Physical Chemistry, 1980, 84(9): 975-979. |
| 19 | Buchowski H , Khiat A . Solubility of solids in liquids: one-parameter solubility equation[J]. Fluid Phase Equilibria, 1986, 25(3): 273-278. |
| 20 | Zhang P S , Zhao R , Zhang C , et al . Thermodynamic analysis and correlation of cyromazine in three (acetic acid, propanoic acid or ethylene glycol+ water) binary solvents at different temperatures[J]. Journal of Molecular Liquids, 2018, 272: 158-169. |
| 21 | Prausnitz J M , Lichtenthaler R N , de Azevedo E G . Molecular Thermodynamics of Fluid-Phase Equilibria[M]. US: Pearson Education, 1998. |
| 22 | Li Y , Wang F A , Xu L , et al . Solubilities of cefodizime disodium in aqueous alcohol mixtures at atmospheric pressure and different temperatures[J]. Fluid Phase Equilibria, 2010, 298: 246-252. |
| 23 | [23] Li T , Jiang Z X , Chen F X , et al . Solubilities of d-xylose in water+(acetic acid or propionic acid) mixtures at atmospheric pressure and different temperatures[J]. Fluid Phase Equilibria, 2012, 333: 13-17. |
| 24 | Apelblat A , Manzurola E . Solubilities of L-glutamic acid, 3-nitrobenzoic acid, p-toluic acid, calcium-L-lactate, calcium gluconate, magnesium-DL-aspartate, and magnesium-L-lactate in water[J]. J. Chem. Thermodyn., 2002, 34: 1127-1136 |
| 25 | Apelblat A , Manzurola E . Solubilities of o-acetylsalicylic, 4-aminosalicylic, 3, 5-dinitrosalicylic, and p-toluic acid, and magnesium-DL-aspartate in water from T=(278 to 348) K [J]. J. Chem. Thermodyn., 1999, 31: 85-91. |
| 26 | Li T , Li Y , Li Y H , et al . Solubilities of {α-d-glucose in water+(acetic acid or propionic acid)} mixtures at atmospheric pressure and different temperatures[J]. The Journal of Chemical Thermodynamics, 2013, 65: 7-10. |
| 27 | 王福安, 蒋元力 . 分子热力学与色谱保留[M].北京: 气象出版社, 2001. |
| Wang F A , Jiang Y L . Molecular Thermodynamics and Chromatographic Retention[M]. Beijing: Meteorological Publishing House, 2001. | |
| 28 | Zhang C L , Wang F A , Wang Y . Solubilities of sulfadiazine, sulfamethazine, sulfadimethoxine, sulfamethoxydiazine, sulfamonomethoxine, sulfamethoxazole, and sulfachloropyrazine in water from (298.15 to 333.15) K[J]. Journal of Chemical & Engineering Data. 2007, 52(5): 1563-1566. |
| 29 | Zhang H , Wang J , Chen Y , et al . Solubiliy of sodium cefotaxime in aqueous 2-propanol mixtures [J]. Journal of Chemical & Engineering Data, 2006, 51(6): 2239-2241. |
| [1] | Chao HU, Yuming DONG, Wei ZHANG, Hongling ZHANG, Peng ZHOU, Hongbin XU. Preparation of high-concentration positive electrolyte of vanadium redox flow battery by activating vanadium pentoxide with highly concentrated sulfuric acid [J]. CIESC Journal, 2023, 74(S1): 338-345. |
| [2] | Xudong YU, Qi LI, Niancu CHEN, Li DU, Siying REN, Ying ZENG. Phase equilibria and calculation of aqueous ternary system KCl + CaCl2 + H2O at 298.2, 323.2, and 348.2 K [J]. CIESC Journal, 2023, 74(8): 3256-3265. |
| [3] | Xiaoyu YAO, Jun SHEN, Jian LI, Zhenxing LI, Huifang KANG, Bo TANG, Xueqiang DONG, Maoqiong GONG. Research progress in measurement methods in vapor-liquid critical properties of mixtures [J]. CIESC Journal, 2023, 74(5): 1847-1861. |
| [4] | Ke CHEN, Li DU, Ying ZENG, Siying REN, Xudong YU. Phase equilibria and calculation of quaternary system LiCl+MgCl2+CaCl2+H2O at 323.2 K [J]. CIESC Journal, 2023, 74(5): 1896-1903. |
| [5] | Yuanjing MAO, Zhi YANG, Songping MO, Hao GUO, Ying CHEN, Xianglong LUO, Jianyong CHEN, Yingzong LIANG. Estimation of SAFT-VR Mie equation of state parameters and thermodynamic properties of C6—C10 alcohols [J]. CIESC Journal, 2023, 74(3): 1033-1041. |
| [6] | Wenting CHENG, Jie LI, Li XU, Fangqin CHENG, Guoji LIU. Experiment and prediction for the solubility of AlCl3·6H2O in FeCl3, CaCl2, KCl and KCl-FeCl3 solutions [J]. CIESC Journal, 2023, 74(2): 642-652. |
| [7] | Songtao YANG, Dongyang LI, Yuqing NIU, Xingang LI, Shaohui KANG, Hong LI, Kaikai YE, Zhiquan ZHOU, Xin GAO. Molecular simulation progress in studying thermodynamic properties and potential functions of fluorides [J]. CIESC Journal, 2022, 73(9): 3828-3840. |
| [8] | Yuxin REN, Runfeng XU, Wanying WANG, Pengzhong CHEN, Xiaojun PENG. Synthesis and stability study of anthraquinone dyes for color photoresist [J]. CIESC Journal, 2022, 73(5): 2251-2261. |
| [9] | Jiahui REN, Yu LIU, Chao LIU, Lang LIU, Ying LI. Critical temperature prediction of working fluids using molecular fingerprints and topological indices [J]. CIESC Journal, 2022, 73(4): 1493-1500. |
| [10] | Mingze SUN, Ning MA, Haoran LI, Haifeng JIANG, Wenpeng HONG, Xiaojuan NIU. Thermodynamic analysis of Brayton cycle of medium and low temperature supercritical CO2 and its mixed working medium [J]. CIESC Journal, 2022, 73(3): 1379-1388. |
| [11] | Huaixu LI, Xiaoyan SUN, Shaohui TAO, Li XIA, Shuguang XIANG. Lumping gasoline with molecular properties and density peak clustering [J]. CIESC Journal, 2022, 73(12): 5449-5460. |
| [12] |
Siying REN, Xudong YU, Jun LUO, Xia FENG, Zhixing ZHAO, Zhihao YAO.
Phase equilibria of aqueous quaternary system Li+, K+, |
| [13] | Xueping ZHANG, Ruizhi CUI, Shihua SANG. Experiment and calculation of phase equilibrium in ternary systems NaBr-CaBr2-H2O and KBr-CaBr2-H2O at 273.15 K [J]. CIESC Journal, 2021, 72(9): 4479-4486. |
| [14] | Shengzheng GUO, Songgu WU, Xin SU, Wei GAO, Zhiping NIU, Junbo GONG. Determination of solubility and metastable zone width of rebaudioside A and study on its crystallization process [J]. CIESC Journal, 2021, 72(8): 3997-4008. |
| [15] | Xian WANG, Yanxing ZHAO, Xueqiang DONG, Xiaogang CHEN, Maoqiong GONG. Prediction of solid-liquid equilibrium of binary mixtures containing methane [J]. CIESC Journal, 2021, 72(8): 4009-4018. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||