CIESC Journal ›› 2020, Vol. 71 ›› Issue (11): 5256-5264.DOI: 10.11949/0438-1157.20200342
• Energy and environmental engineering • Previous Articles Next Articles
Junjie REN1,2,3,4,6( ),Zhen LONG1,2,3,4,5(
),Zhen LONG1,2,3,4,5( ),Deqing LIANG1,2,3,4,5
),Deqing LIANG1,2,3,4,5
Received:2020-03-31
Revised:2020-06-02
Online:2020-11-05
Published:2020-11-05
Contact:
Zhen LONG
任俊杰1,2,3,4,6( ),龙臻1,2,3,4,5(
),龙臻1,2,3,4,5( ),梁德青1,2,3,4,5
),梁德青1,2,3,4,5
通讯作者:
龙臻
作者简介:任俊杰(1992—),男,硕士研究生,基金资助:CLC Number:
Junjie REN,Zhen LONG,Deqing LIANG. Effect of complex inhibitors containing ionic liquids and PVP K90 on formation of methane hydrate[J]. CIESC Journal, 2020, 71(11): 5256-5264.
任俊杰,龙臻,梁德青. 离子液体与PVP K90复合抑制剂对甲烷水合物的生成影响[J]. 化工学报, 2020, 71(11): 5256-5264.
Add to citation manager EndNote|Ris|BibTeX
| 试剂 | 缩写 | 摩尔质量/(g·mol-1) | 纯度/% | 供应商 |
|---|---|---|---|---|
| N-丁基-N-甲基吡咯烷四氟硼酸盐 | [BMP][BF4] | 229.07 | ≥ 99 | 中科院兰州化学物理研究所 |
| 聚乙烯基吡咯烷酮 K90 | PVP K90 | 360000 | 梯希爱(上海)化成工业发展有限公司 | |
| 甲烷气体 | CH4 | 16 | ≥ 99.99 | 佛山市科的气体有限公司 |
| 去离子水 | H2O | 18 | 实验室自制 |
Table 1 List of the materials used for the experiments
| 试剂 | 缩写 | 摩尔质量/(g·mol-1) | 纯度/% | 供应商 |
|---|---|---|---|---|
| N-丁基-N-甲基吡咯烷四氟硼酸盐 | [BMP][BF4] | 229.07 | ≥ 99 | 中科院兰州化学物理研究所 |
| 聚乙烯基吡咯烷酮 K90 | PVP K90 | 360000 | 梯希爱(上海)化成工业发展有限公司 | |
| 甲烷气体 | CH4 | 16 | ≥ 99.99 | 佛山市科的气体有限公司 |
| 去离子水 | H2O | 18 | 实验室自制 |
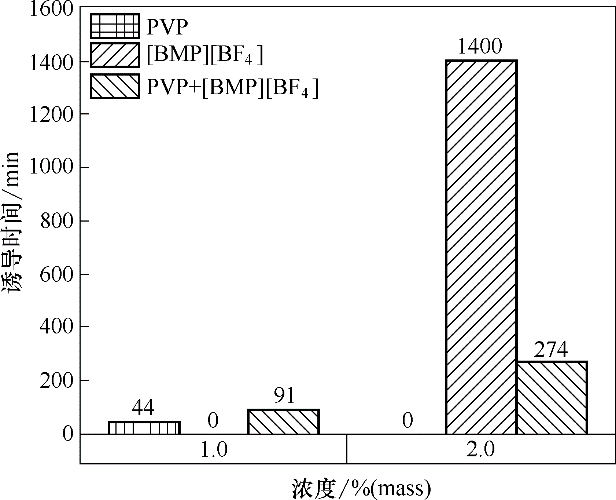
Fig.4 Induction time of methane hydrate formation in the presence of 1.0%(mass) and 2.0%(mass) [BMP][BF4] and the mixture of [BMP][BF4] with PVP (ratio of 1∶1)
| 反应体系 | 小笼占有率θS | 大笼占有率θL | 水合数n |
|---|---|---|---|
| 无 (纯水) | 0.936±0.080 | 0.946±0.041 | 6.095±0.124 |
| IL | 0.832±0.011 | 0.977±0.004 | 6.125±0.015 |
| PVP | 0.896±0.051 | 0.971±0.007 | 6.036±0.048 |
| [PVP+IL](1∶4) | 0.947±0.043 | 0.963±0.011 | 5.994±0.015 |
| [PVP+IL](1∶2) | 0.924±0.035 | 0.969±0.005 | 6.003±0.033 |
| [PVP+IL](1∶1) | 0.819±0.159 | 0.944±0.063 | 6.300±0.072 |
| [PVP+IL](2∶1) | 0.905±0.086 | 0.948±0.048 | 6.136±0.111 |
| [PVP+IL](4∶1) | 0.930±0.098 | 0.925±0.072 | 6.211±0.196 |
Table 2 Hydrate numbers and cage occupancies of methane hydrate formed in the presence of various inhibitors
| 反应体系 | 小笼占有率θS | 大笼占有率θL | 水合数n |
|---|---|---|---|
| 无 (纯水) | 0.936±0.080 | 0.946±0.041 | 6.095±0.124 |
| IL | 0.832±0.011 | 0.977±0.004 | 6.125±0.015 |
| PVP | 0.896±0.051 | 0.971±0.007 | 6.036±0.048 |
| [PVP+IL](1∶4) | 0.947±0.043 | 0.963±0.011 | 5.994±0.015 |
| [PVP+IL](1∶2) | 0.924±0.035 | 0.969±0.005 | 6.003±0.033 |
| [PVP+IL](1∶1) | 0.819±0.159 | 0.944±0.063 | 6.300±0.072 |
| [PVP+IL](2∶1) | 0.905±0.086 | 0.948±0.048 | 6.136±0.111 |
| [PVP+IL](4∶1) | 0.930±0.098 | 0.925±0.072 | 6.211±0.196 |
| 1 | Sloan E D, Koh C A. Clathrate Hydrates of Natural Gases[M]. 3rd ed. Boca Raton: CRC Press, 2007: 1-17. |
| 2 | Wang Y H, Fan S S, Lang X M. Reviews of gas hydrate inhibitors in gas-dominant pipelines and application of kinetic hydrate inhibitors in China[J]. Chinese Journal of Chemical Engineering, 2019, 27(9): 2118-2132. |
| 3 | 樊栓狮, 郭凯, 王燕鸿, 等. 天然气水合物动力学抑制剂性能评价方法的现状与展望[J]. 天然气工业, 2018, 38(9): 103-113. |
| Fan S S, Guo K, Wang Y H, et al. Present situation and prospect of performance evaluation methods for kinetic hydrate inhibitors (KHIs)[J]. Natural Gas Industry, 2018, 38(9): 103-113. | |
| 4 | 唐翠萍, 戴兴学, 梁德青, 等. 低剂量抑制剂Inhibex501存在下的甲烷水合物相平衡研究[J]. 新能源进展, 2018, 6(1): 42-46. |
| Tang C P, Dai X X, Liang D Q, et al. Equilibrium conditions for methane hydrate in the presence of low-dosage hydrate inhibitors aqueous solutions[J]. Advances in New and Renewable Enengy, 2018, 6(1): 42-46. | |
| 5 | Bavoh C B, Partoon B, Lal B, et al. Inhibition effect of amino acids on carbon dioxide hydrate[J]. Chemical Engineering Science, 2017, 171: 331-339. |
| 6 | Ke W, Svartaas T M, Abay H K. An experimental study on sⅠ hydrate formation in presence of methanol, PVP and PVCap in an isochoric cell[C]//Icgh 2011. ICGH, 2011: 17-21. |
| 7 | Ke W, Kelland M A. Kinetic hydrate inhibitor studies for gas hydrate systems: a review of experimental equipment and test methods[J]. Energy and Fuels, 2016, 30(12): 10015-10028. |
| 8 | Talaghat M R. Experimental investigation of induction time for double gas hydrate formation in the simultaneous presence of the PVP and L-tyrosine as kinetic inhibitors in a mini flow loop apparatus[J]. Journal of Natural Gas Science and Engineering, 2014, 19: 215-220. |
| 9 | Schicks J M, Ripmesster J A. The coexistence of two different methane hydrate phases under moderate pressure and temperature conditions: kinetic versus thermodynamic products[J]. Angewandte Chemie - International Edition, 2004, 43(25): 3310-3313. |
| 10 | Anderson B J, Tester J W, Borghi G P, et al. Properties of inhibitors of methane hydrate formation via molecular dynamics simulations[J]. Journal of the American Chemical Society, 2005, 127(50): 17852-17862. |
| 11 | Kelland M A. History of the development of low dosage hydrate inhibitors[J]. Energy and Fuels, 2006, 20(3): 825-847. |
| 12 | Kim J, Shin K, Seo Y, et al. Synergistic hydrate inhibition of monoethylene glycol with poly(vinylcaprolactam) in thermodynamically underinhibited system[J]. Journal of Physical Chemistry B, 2014, 118(30): 9065-9075. |
| 13 | Chua P C, Kelland M A. Tetra(iso-hexyl)ammonium bromide - the most powerful quaternary ammonium-based tetrahydrofuran crystal growth inhibitor and synergist with polyvinylcaprolactam kinetic gas hydrate inhibitor[J]. Energy and Fuels, 2012, 26(2): 1160-1168. |
| 14 | Xu S R, Fan S S, Fang S T, et al. Excellent synergy effect on preventing CH4 hydrate formation when glycine meets polyvinylcaprolactam[J]. Fuel, 2017, 206: 19-26. |
| 15 | Young W D, Cohen J M, Wolf P F. Enhanced hydrate inhibitors: powerful synergism with glycol ethers[J]. ACS Division of Fuel Chemistry, Preprints, 1997, 42(2): 503-506. |
| 16 | Xiao C W, Adidharma H. Dual function inhibitors for methane hydrate[J]. Chemical Engineering Science, 2009, 64(7): 1522-1527. |
| 17 | Xiao C W, Wibisono N, Adidharma H. Dialkylimidazolium halide ionic liquids as dual function inhibitors for methane hydrate[J]. Chemical Engineering Science, 2010, 65(10): 3080-3087. |
| 18 | Villano L D, Kelland M A. An investigation into the kinetic hydrate inhibitor properties of two imidazolium-based ionic liquids on structure II gas hydrate[J]. Chemical Engineering Science, 2010, 65(19): 5366-5372. |
| 19 | Kim K S, Kang J W, Kang S P. Tuning ionic liquids for hydrate inhibition[J]. Chemical Communications, 2011, 47(22): 6341-6343. |
| 20 | Lee W, Shin J Y, Kin K S, et al. Synergetic effect of ionic liquids on the kinetic inhibition performance of poly(N-vinylcaprolactam) for natural gas hydrate formation[J]. Energy and Fuels, 2016, 30(11): 9162-9169. |
| 21 | Lee W, Shin J Y, Kim K S, et al. Kinetic promotion and inhibition of methane hydrate formation by morpholinium ionic liquids with chloride and tetrafluoroborate anions[J]. Energy and Fuels, 2016, 30(5): 3879-3885. |
| 22 | Shen X D, Zhou X B, Liang D Q. Kinetic effects of ionic liquids on methane hydrate[J]. Energy and Fuels, 2019, 33(2): 1422-1432. |
| 23 | Coquelet C, Chapoy A, Richon D. Development of a new alpha function for the Peng-Robinson equation of state: comparative study of alpha function models for pure gases (natural gas components) and water-gas systems[J]. International Journal of Thermophysics, 2004, 25(1): 133-158. |
| 24 | Peng D Y, Robinson D B. A new two-constant equation of state[J]. Industrial and Engineering Chemistry Fundamentals, 1976, 15(1): 59-64. |
| 25 | Sangwai J S, Oellrich L. Phase equilibrium of semiclathrate hydrates of methane in aqueous solutions of tetra-n-butyl ammonium bromide (TBAB) and TBAB-NaCl[J]. Fluid Phase Equilibria, 2014, 367: 95-102. |
| 26 | Gayet P, Dicharry C, Marion G, et al. Experimental determination of methane hydrate dissociation curve up to 55 MPa by using a small amount of surfactant as hydrate promoter[J]. Chemical Engineering Science, 2005, 60(21): 5751-5758. |
| 27 | Nixdorf J, Oellrich L R. Experimental determination of hydrate equilibrium conditions for pure gases, binary and ternary mixtures and natural gases[J]. Fluid Phase Equilibria, 1997, 139(1/2): 325-333. |
| 28 | Zhou X B, Long Z, Liang S, et al. In situ Raman analysis on the dissociation behavior of mixed CH4-CO2 Hydrates[J]. Energy and Fuels, 2016, 30(2): 1279-1286. |
| 29 | Wang J W, Lu H L, Ripmeester J A. Raman spectroscopy and cage occupancy of hydrogen clathrate hydrate from first-principle calculations[J]. Journal of the American Chemical Society, 2009, 131(40): 14132-14133. |
| 30 | Schober H, Itoh H, Klapproth A, et al. Guest-host coupling and anharmonicity in clathrate hydrates[J]. European Physical Journal E, 2003, 12(1): 41-49. |
| 31 | 周雪冰, 刘婵娟, 罗金琼, 等. 甲烷水合物分解过程的微尺度测量[J]. 化工学报, 2019, 70(3): 1042-1047. |
| Zhou X B, Liu C J, Luo J Q, et al. Microscopic measurements on methane hydrate dissociation[J]. CIESC Journal, 2019, 70(3): 1042-1047. | |
| 32 | Lu H L, Moudrakovski I, Riedel M, et al. Occurrence and structural characterization of gas hydrates associated with a cold vent field, offshore Vancouver Island[J]. Journal of Geophysical Research: Solid Earth, 2005, 110(10): 1-9. |
| 33 | Uchida T, Hirano T, Ebinuma T, et al. Raman spectroscopic determination of hydration number of methane hydrates[J]. AIChE Journal, 1999, 45(12): 2641-2645. |
| 34 | Li Z, Jiang F, Qin H B, et al. Molecular dynamics method to simulate the process of hydrate growth in the presence/absence of KHIs[J]. Chemical Engineering Science, 2017, 164: 307-312. |
| 35 | Cheng L W, Liao K, Li Z, et al. The invalidation mechanism of kinetic hydrate inhibitors under high subcooling conditions[J]. Chemical Engineering Science, 2019, 207: 305-316. |
| 36 | Kvamme B, Huseby G, Forrisdahl O K. Molecular dynamics simulations of PVP kinetic inhibitor in liquid water and hydrate/liquid water systems[J]. Molecular Physics, 1997, 90(6): 979-992. |
| [1] | Cheng CHENG, Zhongdi DUAN, Haoran SUN, Haitao HU, Hongxiang XUE. Lattice Boltzmann simulation of surface microstructure effect on crystallization fouling [J]. CIESC Journal, 2023, 74(S1): 74-86. |
| [2] | Qi WANG, Bin ZHANG, Xiaoxin ZHANG, Hujian WU, Haitao ZHAN, Tao WANG. Synthesis of isoxepac and 2-ethylanthraquinone catalyzed by chloroaluminate-triethylamine ionic liquid/P2O5 [J]. CIESC Journal, 2023, 74(S1): 245-249. |
| [3] | Ruimin CHE, Wenqiu ZHENG, Xiaoyu WANG, Xin LI, Feng XU. Research progress on homogeneous processing of cellulose in ionic liquids [J]. CIESC Journal, 2023, 74(9): 3615-3627. |
| [4] | Zehao MI, Er HUA. DFT and COSMO-RS theoretical analysis of SO2 absorption by polyamines type ionic liquids [J]. CIESC Journal, 2023, 74(9): 3681-3696. |
| [5] | Meisi CHEN, Weida CHEN, Xinyao LI, Shangyu LI, Youting WU, Feng ZHANG, Zhibing ZHANG. Advances in silicon-based ionic liquid microparticle enhanced gas capture and conversion [J]. CIESC Journal, 2023, 74(9): 3628-3639. |
| [6] | Lizhi WANG, Qiancheng HANG, Yeling ZHENG, Yan DING, Jiaji CHEN, Qing YE, Jinlong LI. Separation of methyl propionate + methanol azeotrope using ionic liquid entrainers [J]. CIESC Journal, 2023, 74(9): 3731-3741. |
| [7] | Jie CHEN, Yongsheng LIN, Kai XIAO, Chen YANG, Ting QIU. Study on catalytic synthesis of sec-butanol by tunable choline-based basic ionic liquids [J]. CIESC Journal, 2023, 74(9): 3716-3730. |
| [8] | Minghao SONG, Fei ZHAO, Shuqing LIU, Guoxuan LI, Sheng YANG, Zhigang LEI. Multi-scale simulation and study of volatile phenols removal from simulated oil by ionic liquids [J]. CIESC Journal, 2023, 74(9): 3654-3664. |
| [9] | Shaoqi YANG, Shuheng ZHAO, Lungang CHEN, Chenguang WANG, Jianjun HU, Qing ZHOU, Longlong MA. Hydrodeoxygenation of lignin-derived compounds to alkanes in Raney Ni-protic ionic liquid system [J]. CIESC Journal, 2023, 74(9): 3697-3707. |
| [10] | Xudong YU, Qi LI, Niancu CHEN, Li DU, Siying REN, Ying ZENG. Phase equilibria and calculation of aqueous ternary system KCl + CaCl2 + H2O at 298.2, 323.2, and 348.2 K [J]. CIESC Journal, 2023, 74(8): 3256-3265. |
| [11] | Linzheng WANG, Yubing LU, Ruizhi ZHANG, Yonghao LUO. Analysis on thermal oxidation characteristics of VOCs based on molecular dynamics simulation [J]. CIESC Journal, 2023, 74(8): 3242-3255. |
| [12] | Mengmeng ZHANG, Dong YAN, Yongfeng SHEN, Wencui LI. Effect of electrolyte types on the storage behaviors of anions and cations for dual-ion batteries [J]. CIESC Journal, 2023, 74(7): 3116-3126. |
| [13] | Yuanliang ZHANG, Xinqi LUAN, Weige SU, Changhao LI, Zhongxing ZHAO, Liqin ZHOU, Jianmin CHEN, Yan HUANG, Zhenxia ZHAO. Study on selective extraction of nicotine by ionic liquids composite extractant and DFT calculation [J]. CIESC Journal, 2023, 74(7): 2947-2956. |
| [14] | Jipeng ZHOU, Wenjun HE, Tao LI. Reaction engineering calculation of deactivation kinetics for ethylene catalytic oxidation over irregular-shaped catalysts [J]. CIESC Journal, 2023, 74(6): 2416-2426. |
| [15] | Guangyu WANG, Kai ZHANG, Kaihua ZHANG, Dongke ZHANG. Heat and mass transfer and energy consumption for microwave drying of coal slime [J]. CIESC Journal, 2023, 74(6): 2382-2390. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||