CIESC Journal ›› 2022, Vol. 73 ›› Issue (11): 4838-4849.DOI: 10.11949/0438-1157.20221032
• Thermodynamics • Previous Articles Next Articles
Received:2022-07-26
Revised:2022-10-13
Online:2022-12-06
Published:2022-11-05
Contact:
Shuqian XIA
通讯作者:
夏淑倩
作者简介:高亚慧(1995—),女,博士研究生,yahuigao@tju.edu.cn
基金资助:CLC Number:
Yahui GAO, Shuqian XIA. Experiment and model for isochoric heat capacity of CO2-hydrocarbon liquid phase mixtures[J]. CIESC Journal, 2022, 73(11): 4838-4849.
高亚慧, 夏淑倩. CO2-烃类液相混合物比定容热容的实验与模型研究[J]. 化工学报, 2022, 73(11): 4838-4849.
Add to citation manager EndNote|Ris|BibTeX
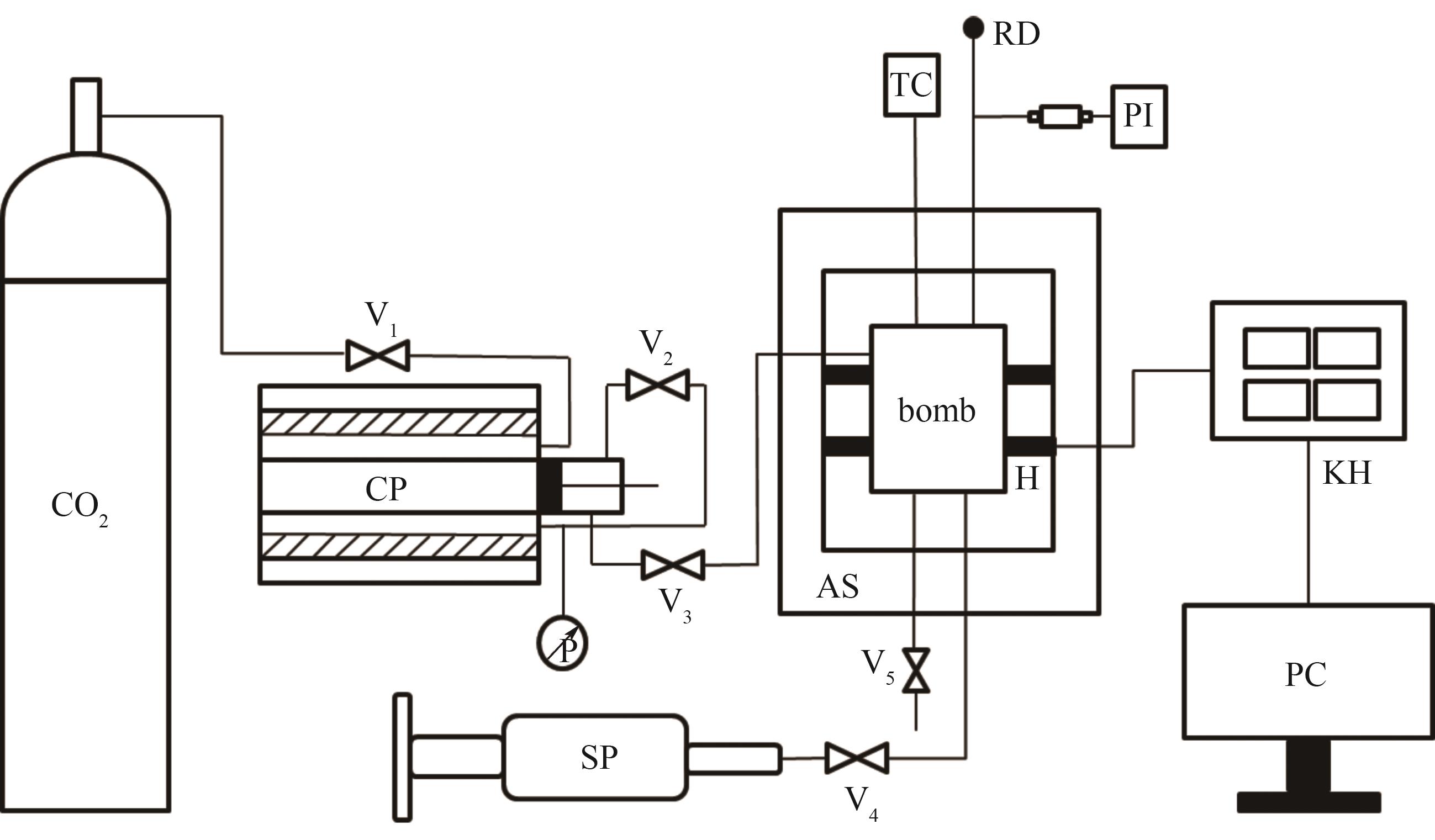
Fig.1 Schematic diagram of the apparatusCP—CO2 syringe pump; SP—liquid syringe pump; TC—temperature controller; PI—pressure indicator; bomb—internal bomb; H—heaters; AS—adiabatic shield; RD—rupture disk; KH—paperless recorder; PC—computer
| 参数 | 回归值 |
|---|---|
| a×10-2 | 7.5665 |
| b×10-2 | -3.0678 |
| c×10-2 | 2.3821 |
Table 1 Coefficients of Eq. (5)
| 参数 | 回归值 |
|---|---|
| a×10-2 | 7.5665 |
| b×10-2 | -3.0678 |
| c×10-2 | 2.3821 |
| T/K | p/MPa | Cv-exp/(J∙g-1∙K-1) | T/K | p/MPa | Cv-exp/(J∙g-1∙K-1) |
|---|---|---|---|---|---|
| 317.15 | 4.69 | 4.108 | 337.15 | 9.82 | 3.921 |
| 319.15 | 5.15 | 4.079 | 339.15 | 10.46 | 3.912 |
| 321.15 | 5.60 | 4.039 | 341.15 | 11.16 | 3.904 |
| 323.15 | 6.07 | 4.017 | 343.15 | 11.86 | 3.892 |
| 325.15 | 6.54 | 4.010 | 345.15 | 12.58 | 3.885 |
| 327.15 | 7.03 | 3.987 | 347.15 | 13.40 | 3.883 |
| 329.15 | 7.58 | 3.958 | 349.15 | 14.70 | 3.881 |
| 331.15 | 9.09 | 3.939 | 351.15 | 15.00 | 3.877 |
| 333.15 | 8.60 | 3.928 | 353.15 | 15.87 | 3.866 |
| 335.15 | 9.20 | 3.919 |
Table 2 Experimental isochoric heat capacity data (Cv ) of deionized water
| T/K | p/MPa | Cv-exp/(J∙g-1∙K-1) | T/K | p/MPa | Cv-exp/(J∙g-1∙K-1) |
|---|---|---|---|---|---|
| 317.15 | 4.69 | 4.108 | 337.15 | 9.82 | 3.921 |
| 319.15 | 5.15 | 4.079 | 339.15 | 10.46 | 3.912 |
| 321.15 | 5.60 | 4.039 | 341.15 | 11.16 | 3.904 |
| 323.15 | 6.07 | 4.017 | 343.15 | 11.86 | 3.892 |
| 325.15 | 6.54 | 4.010 | 345.15 | 12.58 | 3.885 |
| 327.15 | 7.03 | 3.987 | 347.15 | 13.40 | 3.883 |
| 329.15 | 7.58 | 3.958 | 349.15 | 14.70 | 3.881 |
| 331.15 | 9.09 | 3.939 | 351.15 | 15.00 | 3.877 |
| 333.15 | 8.60 | 3.928 | 353.15 | 15.87 | 3.866 |
| 335.15 | 9.20 | 3.919 |
| T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) |
|---|---|---|---|---|---|---|---|---|
| ρ/(g∙cm-3)=0.5678 x1=0.1834 | 343.15 | 8.26 | 1.792 | 329.15 | 10.68 | 1.633 | ||
| 317.15 | 3.22 | 1.749 | 345.15 | 9.00 | 1.812 | 331.15 | 11.27 | 1.642 |
| 319.65 | 3.74 | 1.759 | 347.15 | 9.78 | 1.815 | 333.15 | 11.94 | 1.644 |
| 321.15 | 4.07 | 1.765 | 349.15 | 10.70 | 1.819 | 335.15 | 12.74 | 1.649 |
| 325.15 | 5.15 | 1.774 | 351.15 | 11.30 | 1.836 | 337.15 | 13.54 | 1.651 |
| 327.65 | 6.38 | 1.779 | 353.15 | 12.03 | 1.865 | 339.15 | 14.35 | 1.658 |
| 329.65 | 6.87 | 1.787 | ρ/(g∙cm-3)=0.6045 x1=0.3351 | 341.15 | 15.17 | 1.661 | ||
| 331.15 | 7.40 | 1.794 | 317.15 | 9.98 | 1.687 | 343.15 | 16.00 | 1.663 |
| 333.15 | 8.14 | 1.803 | 319.15 | 10.78 | 1.688 | 345.15 | 16.78 | 1.665 |
| 335.15 | 8.87 | 1.816 | 321.15 | 11.63 | 1.689 | 347.15 | 17.72 | 1.669 |
| 337.15 | 9.67 | 1.831 | 323.15 | 12.49 | 1.694 | 349.15 | 18.57 | 1.670 |
| 339.15 | 10.38 | 1.835 | 325.15 | 13.29 | 1.700 | 351.15 | 19.42 | 1.673 |
| 341.15 | 11.21 | 1.842 | 327.15 | 14.07 | 1.705 | 353.15 | 19.97 | 1.681 |
| 343.15 | 11.92 | 1.856 | 329.15 | 14.73 | 1.707 | ρ/(g∙cm-3)=0.6276 x1=0.5020 | ||
| 345.15 | 12.73 | 1.865 | 331.15 | 15.45 | 1.714 | 317.15 | 5.43 | 1.531 |
| 347.15 | 13.52 | 1.871 | 333.15 | 16.25 | 1.721 | 319.65 | 5.92 | 1.540 |
| 349.15 | 14.28 | 1.884 | 335.15 | 17.09 | 1.723 | 321.15 | 6.39 | 1.557 |
| 351.15 | 15.06 | 1.892 | 337.15 | 18.02 | 1.725 | 323.15 | 7.15 | 1.569 |
| 353.15 | 15.85 | 1.908 | 339.15 | 18.89 | 1.734 | 325.15 | 7.94 | 1.588 |
| ρ/(g∙cm-3)=0.5807 x1=0.2417 | 341.15 | 19.76 | 1.736 | 327.15 | 8.68 | 1.601 | ||
| 317.15 | 3.66 | 1.697 | 343.15 | 20.68 | 1.738 | 329.15 | 9.37 | 1.617 |
| 319.15 | 3.71 | 1.709 | 345.65 | 21.87 | 1.748 | 331.15 | 9.96 | 1.626 |
| 321.15 | 3.86 | 1.713 | 347.15 | 22.37 | 1.751 | 333.15 | 10.61 | 1.631 |
| 324.15 | 4.19 | 1.721 | 349.15 | 23.16 | 1.764 | 335.15 | 11.48 | 1.636 |
| 326.15 | 4.38 | 1.727 | 351.15 | 23.99 | 1.772 | 337.15 | 12.27 | 1.640 |
| 327.15 | 4.46 | 1.728 | 353.15 | 24.83 | 1.776 | 339.15 | 12.97 | 1.642 |
| 329.15 | 4.72 | 1.739 | ρ/(g∙cm-3)=0.6174 x1=0.4476 | 341.15 | 13.78 | 1.654 | ||
| 331.15 | 5.01 | 1.749 | 317.15 | 5.93 | 1.557 | 343.15 | 14.52 | 1.655 |
| 333.15 | 5.33 | 1.758 | 319.15 | 6.68 | 1.567 | 345.15 | 15.34 | 1.659 |
| 335.15 | 5.65 | 1.763 | 321.15 | 7.53 | 1.583 | 347.15 | 16.08 | 1.660 |
| 337.15 | 6.11 | 1.769 | 323.15 | 8.32 | 1.596 | 350.15 | 17.31 | 1.665 |
| 339.15 | 6.73 | 1.771 | 325.15 | 9.12 | 1.618 | 351.15 | 17.70 | 1.668 |
| 341.15 | 7.58 | 1.787 | 327.15 | 9.84 | 1.622 | 353.15 | 18.44 | 1.670 |
Table 3 Experimental isochoric heat capacity data (Cv ) of CO2-n-hexane liquid phase mixtures
| T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) |
|---|---|---|---|---|---|---|---|---|
| ρ/(g∙cm-3)=0.5678 x1=0.1834 | 343.15 | 8.26 | 1.792 | 329.15 | 10.68 | 1.633 | ||
| 317.15 | 3.22 | 1.749 | 345.15 | 9.00 | 1.812 | 331.15 | 11.27 | 1.642 |
| 319.65 | 3.74 | 1.759 | 347.15 | 9.78 | 1.815 | 333.15 | 11.94 | 1.644 |
| 321.15 | 4.07 | 1.765 | 349.15 | 10.70 | 1.819 | 335.15 | 12.74 | 1.649 |
| 325.15 | 5.15 | 1.774 | 351.15 | 11.30 | 1.836 | 337.15 | 13.54 | 1.651 |
| 327.65 | 6.38 | 1.779 | 353.15 | 12.03 | 1.865 | 339.15 | 14.35 | 1.658 |
| 329.65 | 6.87 | 1.787 | ρ/(g∙cm-3)=0.6045 x1=0.3351 | 341.15 | 15.17 | 1.661 | ||
| 331.15 | 7.40 | 1.794 | 317.15 | 9.98 | 1.687 | 343.15 | 16.00 | 1.663 |
| 333.15 | 8.14 | 1.803 | 319.15 | 10.78 | 1.688 | 345.15 | 16.78 | 1.665 |
| 335.15 | 8.87 | 1.816 | 321.15 | 11.63 | 1.689 | 347.15 | 17.72 | 1.669 |
| 337.15 | 9.67 | 1.831 | 323.15 | 12.49 | 1.694 | 349.15 | 18.57 | 1.670 |
| 339.15 | 10.38 | 1.835 | 325.15 | 13.29 | 1.700 | 351.15 | 19.42 | 1.673 |
| 341.15 | 11.21 | 1.842 | 327.15 | 14.07 | 1.705 | 353.15 | 19.97 | 1.681 |
| 343.15 | 11.92 | 1.856 | 329.15 | 14.73 | 1.707 | ρ/(g∙cm-3)=0.6276 x1=0.5020 | ||
| 345.15 | 12.73 | 1.865 | 331.15 | 15.45 | 1.714 | 317.15 | 5.43 | 1.531 |
| 347.15 | 13.52 | 1.871 | 333.15 | 16.25 | 1.721 | 319.65 | 5.92 | 1.540 |
| 349.15 | 14.28 | 1.884 | 335.15 | 17.09 | 1.723 | 321.15 | 6.39 | 1.557 |
| 351.15 | 15.06 | 1.892 | 337.15 | 18.02 | 1.725 | 323.15 | 7.15 | 1.569 |
| 353.15 | 15.85 | 1.908 | 339.15 | 18.89 | 1.734 | 325.15 | 7.94 | 1.588 |
| ρ/(g∙cm-3)=0.5807 x1=0.2417 | 341.15 | 19.76 | 1.736 | 327.15 | 8.68 | 1.601 | ||
| 317.15 | 3.66 | 1.697 | 343.15 | 20.68 | 1.738 | 329.15 | 9.37 | 1.617 |
| 319.15 | 3.71 | 1.709 | 345.65 | 21.87 | 1.748 | 331.15 | 9.96 | 1.626 |
| 321.15 | 3.86 | 1.713 | 347.15 | 22.37 | 1.751 | 333.15 | 10.61 | 1.631 |
| 324.15 | 4.19 | 1.721 | 349.15 | 23.16 | 1.764 | 335.15 | 11.48 | 1.636 |
| 326.15 | 4.38 | 1.727 | 351.15 | 23.99 | 1.772 | 337.15 | 12.27 | 1.640 |
| 327.15 | 4.46 | 1.728 | 353.15 | 24.83 | 1.776 | 339.15 | 12.97 | 1.642 |
| 329.15 | 4.72 | 1.739 | ρ/(g∙cm-3)=0.6174 x1=0.4476 | 341.15 | 13.78 | 1.654 | ||
| 331.15 | 5.01 | 1.749 | 317.15 | 5.93 | 1.557 | 343.15 | 14.52 | 1.655 |
| 333.15 | 5.33 | 1.758 | 319.15 | 6.68 | 1.567 | 345.15 | 15.34 | 1.659 |
| 335.15 | 5.65 | 1.763 | 321.15 | 7.53 | 1.583 | 347.15 | 16.08 | 1.660 |
| 337.15 | 6.11 | 1.769 | 323.15 | 8.32 | 1.596 | 350.15 | 17.31 | 1.665 |
| 339.15 | 6.73 | 1.771 | 325.15 | 9.12 | 1.618 | 351.15 | 17.70 | 1.668 |
| 341.15 | 7.58 | 1.787 | 327.15 | 9.84 | 1.622 | 353.15 | 18.44 | 1.670 |
| T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) |
|---|---|---|---|---|---|---|---|---|
| ρ/(g∙cm-3)=0.6153 x1=0.1434 | 345.15 | 16.99 | 1.928 | 343.15 | 18.86 | 1.801 | ||
| 331.15 | 5.07 | 1.913 | 347.15 | 17.81 | 1.933 | 345.15 | 19.75 | 1.809 |
| 333.15 | 5.87 | 1.920 | 349.15 | 18.65 | 1.945 | 347.15 | 20.55 | 1.813 |
| 335.15 | 6.64 | 1.925 | 351.15 | 19.48 | 1.957 | 349.15 | 21.34 | 1.817 |
| 337.15 | 7.40 | 1.939 | 353.15 | 20.27 | 1.962 | 351.15 | 22.20 | 1.825 |
| 339.15 | 8.18 | 1.946 | ρ/(g∙cm-3)=0.6410 x1=0.3228 | 353.15 | 22.98 | 1.829 | ||
| 341.65 | 9.15 | 1.949 | 340.15 | 7.04 | 1.821 | ρ/(g∙cm-3)=0.6639 x1=0.5337 | ||
| 343.15 | 9.73 | 1.960 | 341.65 | 7.39 | 1.829 | 317.65 | 6.64 | 1.600 |
| 345.15 | 10.42 | 1.964 | 344.15 | 8.36 | 1.838 | 319.15 | 7.17 | 1.614 |
| 347.15 | 11.16 | 1.971 | 345.15 | 8.65 | 1.842 | 321.15 | 7.90 | 1.626 |
| 349.15 | 11.97 | 1.986 | 347.15 | 9.39 | 1.854 | 323.15 | 8.61 | 1.639 |
| 351.15 | 12.72 | 1.993 | 349.15 | 10.15 | 1.868 | 325.15 | 9.39 | 1.656 |
| 353.15 | 13.51 | 2.001 | 351.15 | 10.89 | 1.880 | 327.15 | 10.08 | 1.666 |
| ρ/(g∙cm-3)=0.6240 x1=0.2209 | 353.15 | 11.68 | 1.889 | 329.15 | 10.84 | 1.671 | ||
| 317.15 | 6.09 | 1.814 | ρ/(g∙cm-3)=0.6584 x1=0.4160 | 331.15 | 11.62 | 1.674 | ||
| 319.15 | 6.72 | 1.830 | 317.15 | 8.57 | 1.739 | 333.15 | 12.39 | 1.679 |
| 321.15 | 7.50 | 1.837 | 319.15 | 9.39 | 1.742 | 335.15 | 13.04 | 1.686 |
| 323.15 | 8.25 | 1.846 | 321.15 | 10.22 | 1.741 | 337.15 | 13.66 | 1.688 |
| 325.15 | 9.05 | 1.852 | 323.15 | 10.99 | 1.747 | 339.15 | 14.35 | 1.694 |
| 327.15 | 9.86 | 1.865 | 325.15 | 11.82 | 1.751 | 341.15 | 15.10 | 1.698 |
| 329.15 | 10.61 | 1.871 | 327.15 | 12.66 | 1.754 | 343.15 | 15.68 | 1.700 |
| 331.15 | 11.31 | 1.876 | 329.15 | 13.58 | 1.762 | 345.15 | 16.71 | 1.702 |
| 333.65 | 12.34 | 1.886 | 331.15 | 14.37 | 1.766 | 347.15 | 17.61 | 1.706 |
| 335.15 | 12.95 | 1.889 | 333.15 | 15.03 | 1.773 | 349.15 | 18.39 | 1.712 |
| 337.15 | 13.75 | 1.895 | 335.15 | 15.84 | 1.776 | 351.15 | 19.18 | 1.718 |
| 339.15 | 14.59 | 1.902 | 337.15 | 16.54 | 1.782 | 353.15 | 19.99 | 1.723 |
| 341.15 | 15.42 | 1.915 | 339.15 | 17.25 | 1.786 | |||
| 343.15 | 15.98 | 1.924 | 341.15 | 18.02 | 1.792 | |||
Table 4 Experimental isochoric heat capacity data (Cv ) of CO2-n-octane liquid phase mixtures
| T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) |
|---|---|---|---|---|---|---|---|---|
| ρ/(g∙cm-3)=0.6153 x1=0.1434 | 345.15 | 16.99 | 1.928 | 343.15 | 18.86 | 1.801 | ||
| 331.15 | 5.07 | 1.913 | 347.15 | 17.81 | 1.933 | 345.15 | 19.75 | 1.809 |
| 333.15 | 5.87 | 1.920 | 349.15 | 18.65 | 1.945 | 347.15 | 20.55 | 1.813 |
| 335.15 | 6.64 | 1.925 | 351.15 | 19.48 | 1.957 | 349.15 | 21.34 | 1.817 |
| 337.15 | 7.40 | 1.939 | 353.15 | 20.27 | 1.962 | 351.15 | 22.20 | 1.825 |
| 339.15 | 8.18 | 1.946 | ρ/(g∙cm-3)=0.6410 x1=0.3228 | 353.15 | 22.98 | 1.829 | ||
| 341.65 | 9.15 | 1.949 | 340.15 | 7.04 | 1.821 | ρ/(g∙cm-3)=0.6639 x1=0.5337 | ||
| 343.15 | 9.73 | 1.960 | 341.65 | 7.39 | 1.829 | 317.65 | 6.64 | 1.600 |
| 345.15 | 10.42 | 1.964 | 344.15 | 8.36 | 1.838 | 319.15 | 7.17 | 1.614 |
| 347.15 | 11.16 | 1.971 | 345.15 | 8.65 | 1.842 | 321.15 | 7.90 | 1.626 |
| 349.15 | 11.97 | 1.986 | 347.15 | 9.39 | 1.854 | 323.15 | 8.61 | 1.639 |
| 351.15 | 12.72 | 1.993 | 349.15 | 10.15 | 1.868 | 325.15 | 9.39 | 1.656 |
| 353.15 | 13.51 | 2.001 | 351.15 | 10.89 | 1.880 | 327.15 | 10.08 | 1.666 |
| ρ/(g∙cm-3)=0.6240 x1=0.2209 | 353.15 | 11.68 | 1.889 | 329.15 | 10.84 | 1.671 | ||
| 317.15 | 6.09 | 1.814 | ρ/(g∙cm-3)=0.6584 x1=0.4160 | 331.15 | 11.62 | 1.674 | ||
| 319.15 | 6.72 | 1.830 | 317.15 | 8.57 | 1.739 | 333.15 | 12.39 | 1.679 |
| 321.15 | 7.50 | 1.837 | 319.15 | 9.39 | 1.742 | 335.15 | 13.04 | 1.686 |
| 323.15 | 8.25 | 1.846 | 321.15 | 10.22 | 1.741 | 337.15 | 13.66 | 1.688 |
| 325.15 | 9.05 | 1.852 | 323.15 | 10.99 | 1.747 | 339.15 | 14.35 | 1.694 |
| 327.15 | 9.86 | 1.865 | 325.15 | 11.82 | 1.751 | 341.15 | 15.10 | 1.698 |
| 329.15 | 10.61 | 1.871 | 327.15 | 12.66 | 1.754 | 343.15 | 15.68 | 1.700 |
| 331.15 | 11.31 | 1.876 | 329.15 | 13.58 | 1.762 | 345.15 | 16.71 | 1.702 |
| 333.65 | 12.34 | 1.886 | 331.15 | 14.37 | 1.766 | 347.15 | 17.61 | 1.706 |
| 335.15 | 12.95 | 1.889 | 333.15 | 15.03 | 1.773 | 349.15 | 18.39 | 1.712 |
| 337.15 | 13.75 | 1.895 | 335.15 | 15.84 | 1.776 | 351.15 | 19.18 | 1.718 |
| 339.15 | 14.59 | 1.902 | 337.15 | 16.54 | 1.782 | 353.15 | 19.99 | 1.723 |
| 341.15 | 15.42 | 1.915 | 339.15 | 17.25 | 1.786 | |||
| 343.15 | 15.98 | 1.924 | 341.15 | 18.02 | 1.792 | |||
| T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) |
|---|---|---|---|---|---|---|---|---|
| ρ/(g∙cm-3)=0.7285 x1=0.1609 | ρ/(g∙cm-3)=0.7292 x1=0.2602 | ρ/(g∙cm-3)=0.7363 x1=0.4350 | ||||||
| 317.15 | 13.39 | 1.896 | 317.15 | 9.52 | 1.896 | 323.15 | 7.10 | 1.761 |
| 319.15 | 14.21 | 1.906 | 319.65 | 10.52 | 1.902 | 325.15 | 7.84 | 1.772 |
| 321.15 | 14.99 | 1.913 | 321.15 | 11.06 | 1.903 | 327.15 | 8.64 | 1.783 |
| 323.15 | 15.78 | 1.926 | 323.15 | 11.80 | 1.912 | 329.15 | 9.33 | 1.793 |
| 325.15 | 16.64 | 1.931 | 325.15 | 12.60 | 1.918 | 331.15 | 10.07 | 1.803 |
| 327.15 | 17.42 | 1.931 | 327.15 | 13.39 | 1.921 | 333.15 | 10.81 | 1.811 |
| 329.15 | 18.22 | 1.944 | 329.15 | 14.20 | 1.928 | 335.35 | 11.69 | 1.819 |
| 331.15 | 19.10 | 1.946 | 331.15 | 15.03 | 1.936 | 337.15 | 12.33 | 1.824 |
| 333.15 | 19.97 | 1.956 | 333.15 | 15.86 | 1.941 | 339.15 | 13.00 | 1.830 |
| 335.15 | 20.80 | 1.967 | 335.15 | 16.72 | 1.951 | 341.15 | 13.74 | 1.836 |
| 337.15 | 21.72 | 1.971 | 337.15 | 17.57 | 1.958 | 343.15 | 14.58 | 1.840 |
| 339.15 | 22.61 | 1.978 | 339.15 | 18.37 | 1.963 | 345.15 | 15.27 | 1.846 |
| 341.15 | 23.47 | 1.986 | 341.15 | 19.24 | 1.972 | 347.15 | 16.08 | 1.852 |
| 343.15 | 24.31 | 1.994 | 343.15 | 20.12 | 1.979 | 349.15 | 16.88 | 1.856 |
| 345.15 | 25.22 | 1.997 | 345.15 | 20.96 | 1.991 | 351.15 | 17.60 | 1.861 |
| 347.15 | 26.03 | 2.000 | 347.15 | 21.75 | 1.995 | 353.15 | 18.32 | 1.869 |
| 349.15 | 26.96 | 2.019 | 349.15 | 22.63 | 2.008 | |||
| 351.15 | 23.48 | 2.018 | ||||||
| 353.15 | 24.28 | 2.021 | ||||||
Table 5 Experimental isochoric heat capacity data (Cv ) of CO2-undecane liquid phase mixtures
| T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) |
|---|---|---|---|---|---|---|---|---|
| ρ/(g∙cm-3)=0.7285 x1=0.1609 | ρ/(g∙cm-3)=0.7292 x1=0.2602 | ρ/(g∙cm-3)=0.7363 x1=0.4350 | ||||||
| 317.15 | 13.39 | 1.896 | 317.15 | 9.52 | 1.896 | 323.15 | 7.10 | 1.761 |
| 319.15 | 14.21 | 1.906 | 319.65 | 10.52 | 1.902 | 325.15 | 7.84 | 1.772 |
| 321.15 | 14.99 | 1.913 | 321.15 | 11.06 | 1.903 | 327.15 | 8.64 | 1.783 |
| 323.15 | 15.78 | 1.926 | 323.15 | 11.80 | 1.912 | 329.15 | 9.33 | 1.793 |
| 325.15 | 16.64 | 1.931 | 325.15 | 12.60 | 1.918 | 331.15 | 10.07 | 1.803 |
| 327.15 | 17.42 | 1.931 | 327.15 | 13.39 | 1.921 | 333.15 | 10.81 | 1.811 |
| 329.15 | 18.22 | 1.944 | 329.15 | 14.20 | 1.928 | 335.35 | 11.69 | 1.819 |
| 331.15 | 19.10 | 1.946 | 331.15 | 15.03 | 1.936 | 337.15 | 12.33 | 1.824 |
| 333.15 | 19.97 | 1.956 | 333.15 | 15.86 | 1.941 | 339.15 | 13.00 | 1.830 |
| 335.15 | 20.80 | 1.967 | 335.15 | 16.72 | 1.951 | 341.15 | 13.74 | 1.836 |
| 337.15 | 21.72 | 1.971 | 337.15 | 17.57 | 1.958 | 343.15 | 14.58 | 1.840 |
| 339.15 | 22.61 | 1.978 | 339.15 | 18.37 | 1.963 | 345.15 | 15.27 | 1.846 |
| 341.15 | 23.47 | 1.986 | 341.15 | 19.24 | 1.972 | 347.15 | 16.08 | 1.852 |
| 343.15 | 24.31 | 1.994 | 343.15 | 20.12 | 1.979 | 349.15 | 16.88 | 1.856 |
| 345.15 | 25.22 | 1.997 | 345.15 | 20.96 | 1.991 | 351.15 | 17.60 | 1.861 |
| 347.15 | 26.03 | 2.000 | 347.15 | 21.75 | 1.995 | 353.15 | 18.32 | 1.869 |
| 349.15 | 26.96 | 2.019 | 349.15 | 22.63 | 2.008 | |||
| 351.15 | 23.48 | 2.018 | ||||||
| 353.15 | 24.28 | 2.021 | ||||||
| T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) |
|---|---|---|---|---|---|---|---|---|
| ρ/(g∙cm-3)=0.7771 x1=0.0966 | ρ/(g∙cm-3)=0.7783 x1=0.1516 | 335.15 | 8.07 | 1.394 | ||||
| 317.15 | 5.46 | 1.414 | 343.15 | 6.39 | 1.516 | 337.15 | 8.74 | 1.398 |
| 319.15 | 5.92 | 1.421 | 345.15 | 6.96 | 1.524 | 339.15 | 9.45 | 1.411 |
| 321.15 | 6.53 | 1.435 | 347.15 | 7.85 | 1.542 | 341.15 | 10.22 | 1.421 |
| 323.15 | 7.41 | 1.441 | 349.15 | 8.68 | 1.561 | 343.15 | 10.98 | 1.432 |
| 325.15 | 8.27 | 1.459 | 351.15 | 9.52 | 1.563 | 345.15 | 11.66 | 1.440 |
| 327.15 | 9.12 | 1.472 | 353.15 | 10.41 | 1.573 | 347.15 | 12.44 | 1.446 |
| 329.15 | 10.00 | 1.482 | ρ/(g∙cm-3)=0.7870 x1=0.2928 | 349.15 | 13.20 | 1.454 | ||
| 331.15 | 10.92 | 1.491 | 337.15 | 6.66 | 1.434 | 351.15 | 13.97 | 1.460 |
| 333.15 | 11.68 | 1.511 | 339.15 | 7.22 | 1.439 | 353.15 | 14.77 | 1.472 |
| 335.15 | 12.62 | 1.521 | 341.15 | 7.99 | 1.446 | ρ/(g∙cm-3)=0.7923 x1=0.4150 | ||
| 337.15 | 13.53 | 1.537 | 343.15 | 8.77 | 1.457 | 337.15 | 6.60 | 1.352 |
| 339.15 | 14.45 | 1.546 | 345.15 | 9.46 | 1.460 | 339.15 | 7.12 | 1.357 |
| 342.15 | 15.88 | 1.556 | 347.15 | 10.23 | 1.472 | 341.15 | 7.75 | 1.369 |
| 343.15 | 16.20 | 1.561 | 349.15 | 11.02 | 1.483 | 343.15 | 8.34 | 1.384 |
| 345.15 | 17.19 | 1.569 | 351.15 | 11.86 | 1.496 | 345.15 | 8.99 | 1.395 |
| 347.15 | 18.08 | 1.578 | 353.15 | 12.60 | 1.505 | 347.15 | 9.65 | 1.399 |
| 349.15 | 19.02 | 1.587 | ρ/(g∙cm-3)=0.7891 x1=0.3646 | 349.15 | 10.30 | 1.416 | ||
| 351.15 | 19.93 | 1.601 | 331.15 | 6.69 | 1.377 | 351.15 | 11.02 | 1.425 |
| 353.15 | 20.88 | 1.613 | 333.15 | 7.30 | 1.385 | 353.15 | 11.98 | 1.432 |
Table 6 Experimental isochoric heat capacity data (Cv ) of CO2-cyclohexane liquid phase mixtures
| T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) |
|---|---|---|---|---|---|---|---|---|
| ρ/(g∙cm-3)=0.7771 x1=0.0966 | ρ/(g∙cm-3)=0.7783 x1=0.1516 | 335.15 | 8.07 | 1.394 | ||||
| 317.15 | 5.46 | 1.414 | 343.15 | 6.39 | 1.516 | 337.15 | 8.74 | 1.398 |
| 319.15 | 5.92 | 1.421 | 345.15 | 6.96 | 1.524 | 339.15 | 9.45 | 1.411 |
| 321.15 | 6.53 | 1.435 | 347.15 | 7.85 | 1.542 | 341.15 | 10.22 | 1.421 |
| 323.15 | 7.41 | 1.441 | 349.15 | 8.68 | 1.561 | 343.15 | 10.98 | 1.432 |
| 325.15 | 8.27 | 1.459 | 351.15 | 9.52 | 1.563 | 345.15 | 11.66 | 1.440 |
| 327.15 | 9.12 | 1.472 | 353.15 | 10.41 | 1.573 | 347.15 | 12.44 | 1.446 |
| 329.15 | 10.00 | 1.482 | ρ/(g∙cm-3)=0.7870 x1=0.2928 | 349.15 | 13.20 | 1.454 | ||
| 331.15 | 10.92 | 1.491 | 337.15 | 6.66 | 1.434 | 351.15 | 13.97 | 1.460 |
| 333.15 | 11.68 | 1.511 | 339.15 | 7.22 | 1.439 | 353.15 | 14.77 | 1.472 |
| 335.15 | 12.62 | 1.521 | 341.15 | 7.99 | 1.446 | ρ/(g∙cm-3)=0.7923 x1=0.4150 | ||
| 337.15 | 13.53 | 1.537 | 343.15 | 8.77 | 1.457 | 337.15 | 6.60 | 1.352 |
| 339.15 | 14.45 | 1.546 | 345.15 | 9.46 | 1.460 | 339.15 | 7.12 | 1.357 |
| 342.15 | 15.88 | 1.556 | 347.15 | 10.23 | 1.472 | 341.15 | 7.75 | 1.369 |
| 343.15 | 16.20 | 1.561 | 349.15 | 11.02 | 1.483 | 343.15 | 8.34 | 1.384 |
| 345.15 | 17.19 | 1.569 | 351.15 | 11.86 | 1.496 | 345.15 | 8.99 | 1.395 |
| 347.15 | 18.08 | 1.578 | 353.15 | 12.60 | 1.505 | 347.15 | 9.65 | 1.399 |
| 349.15 | 19.02 | 1.587 | ρ/(g∙cm-3)=0.7891 x1=0.3646 | 349.15 | 10.30 | 1.416 | ||
| 351.15 | 19.93 | 1.601 | 331.15 | 6.69 | 1.377 | 351.15 | 11.02 | 1.425 |
| 353.15 | 20.88 | 1.613 | 333.15 | 7.30 | 1.385 | 353.15 | 11.98 | 1.432 |
| T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) |
|---|---|---|---|---|---|---|---|---|
| ρ/(g∙cm-3)=0.8215 x1=0.1088 | ρ/(g∙cm-3)=0.8295 x1=0.2122 | ρ/(g∙cm-3)=0.8326 x1=0.3466 | ||||||
| 317.65 | 12.07 | 1.390 | 317.15 | 9.64 | 1.372 | 317.15 | 7.58 | 1.350 |
| 319.15 | 12.96 | 1.394 | 319.65 | 10.91 | 1.378 | 319.15 | 8.51 | 1.355 |
| 321.15 | 13.85 | 1.401 | 321.15 | 11.66 | 1.380 | 321.15 | 9.43 | 1.358 |
| 323.15 | 14.64 | 1.407 | 323.15 | 12.63 | 1.386 | 323.15 | 10.32 | 1.362 |
| 325.15 | 15.88 | 1.413 | 325.15 | 13.62 | 1.390 | 325.15 | 11.27 | 1.368 |
| 327.15 | 16.96 | 1.419 | 327.15 | 14.67 | 1.396 | 327.15 | 12.13 | 1.370 |
| 329.15 | 17.91 | 1.426 | 329.15 | 15.69 | 1.400 | 329.15 | 13.06 | 1.373 |
| 331.15 | 18.94 | 1.432 | 331.15 | 16.70 | 1.406 | 331.15 | 13.97 | 1.377 |
| 333.15 | 19.93 | 1.438 | 333.15 | 17.68 | 1.411 | 333.15 | 14.89 | 1.381 |
| 335.15 | 20.90 | 1.444 | 335.15 | 18.66 | 1.417 | 335.65 | 15.99 | 1.386 |
| 337.15 | 21.91 | 1.451 | 337.15 | 19.70 | 1.423 | 337.15 | 16.65 | 1.389 |
| 339.15 | 22.87 | 1.457 | 339.15 | 20.66 | 1.429 | 339.15 | 17.60 | 1.394 |
| 341.15 | 23.89 | 1.463 | 341.15 | 21.84 | 1.434 | 341.15 | 18.52 | 1.398 |
| 343.15 | 24.81 | 1.470 | 343.15 | 22.67 | 1.440 | 343.15 | 19.39 | 1.404 |
| 345.15 | 25.82 | 1.476 | 345.15 | 23.81 | 1.445 | 345.15 | 20.32 | 1.408 |
| 347.65 | 27.04 | 1.485 | 347.65 | 24.80 | 1.454 | 347.15 | 21.25 | 1.413 |
| 349.15 | 27.72 | 1.490 | 349.15 | 25.50 | 1.457 | 349.15 | 22.13 | 1.419 |
| 351.15 | 28.64 | 1.496 | 351.15 | 26.59 | 1.464 | 351.15 | 23.00 | 1.425 |
| 353.15 | 29.49 | 1.501 | 353.15 | 27.39 | 1.471 | 353.15 | 23.88 | 1.430 |
Table 7 Experimental isochoric heat capacity data (Cv ) of CO2-ethylbenzene liquid phase mixtures
| T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) |
|---|---|---|---|---|---|---|---|---|
| ρ/(g∙cm-3)=0.8215 x1=0.1088 | ρ/(g∙cm-3)=0.8295 x1=0.2122 | ρ/(g∙cm-3)=0.8326 x1=0.3466 | ||||||
| 317.65 | 12.07 | 1.390 | 317.15 | 9.64 | 1.372 | 317.15 | 7.58 | 1.350 |
| 319.15 | 12.96 | 1.394 | 319.65 | 10.91 | 1.378 | 319.15 | 8.51 | 1.355 |
| 321.15 | 13.85 | 1.401 | 321.15 | 11.66 | 1.380 | 321.15 | 9.43 | 1.358 |
| 323.15 | 14.64 | 1.407 | 323.15 | 12.63 | 1.386 | 323.15 | 10.32 | 1.362 |
| 325.15 | 15.88 | 1.413 | 325.15 | 13.62 | 1.390 | 325.15 | 11.27 | 1.368 |
| 327.15 | 16.96 | 1.419 | 327.15 | 14.67 | 1.396 | 327.15 | 12.13 | 1.370 |
| 329.15 | 17.91 | 1.426 | 329.15 | 15.69 | 1.400 | 329.15 | 13.06 | 1.373 |
| 331.15 | 18.94 | 1.432 | 331.15 | 16.70 | 1.406 | 331.15 | 13.97 | 1.377 |
| 333.15 | 19.93 | 1.438 | 333.15 | 17.68 | 1.411 | 333.15 | 14.89 | 1.381 |
| 335.15 | 20.90 | 1.444 | 335.15 | 18.66 | 1.417 | 335.65 | 15.99 | 1.386 |
| 337.15 | 21.91 | 1.451 | 337.15 | 19.70 | 1.423 | 337.15 | 16.65 | 1.389 |
| 339.15 | 22.87 | 1.457 | 339.15 | 20.66 | 1.429 | 339.15 | 17.60 | 1.394 |
| 341.15 | 23.89 | 1.463 | 341.15 | 21.84 | 1.434 | 341.15 | 18.52 | 1.398 |
| 343.15 | 24.81 | 1.470 | 343.15 | 22.67 | 1.440 | 343.15 | 19.39 | 1.404 |
| 345.15 | 25.82 | 1.476 | 345.15 | 23.81 | 1.445 | 345.15 | 20.32 | 1.408 |
| 347.65 | 27.04 | 1.485 | 347.65 | 24.80 | 1.454 | 347.15 | 21.25 | 1.413 |
| 349.15 | 27.72 | 1.490 | 349.15 | 25.50 | 1.457 | 349.15 | 22.13 | 1.419 |
| 351.15 | 28.64 | 1.496 | 351.15 | 26.59 | 1.464 | 351.15 | 23.00 | 1.425 |
| 353.15 | 29.49 | 1.501 | 353.15 | 27.39 | 1.471 | 353.15 | 23.88 | 1.430 |
| 组分 | a0 | a1×103 | a2×105 | a3×108 | a4×1011 |
|---|---|---|---|---|---|
| CO2 | 3.259 | 1.356 | 1.502 | -2.374 | 1.056 |
| 正己烷 | 8.831 | -0.166 | 14.302 | -18.314 | 7.124 |
| 正辛烷 | 10.824 | 4.983 | 17.751 | -23.137 | 8.980 |
| 十一烷 | 15.285 | 5.485 | 24.990 | -35.821 | 14.200 |
| 环己烷 | 4.035 | -4.443 | 16.834 | -20.775 | 7.746 |
| 乙苯 | 4.544 | 10.578 | 13.644 | -19.276 | 7.885 |
Table 8 Ideal gas heat capacity coefficients of pure compound[29]
| 组分 | a0 | a1×103 | a2×105 | a3×108 | a4×1011 |
|---|---|---|---|---|---|
| CO2 | 3.259 | 1.356 | 1.502 | -2.374 | 1.056 |
| 正己烷 | 8.831 | -0.166 | 14.302 | -18.314 | 7.124 |
| 正辛烷 | 10.824 | 4.983 | 17.751 | -23.137 | 8.980 |
| 十一烷 | 15.285 | 5.485 | 24.990 | -35.821 | 14.200 |
| 环己烷 | 4.035 | -4.443 | 16.834 | -20.775 | 7.746 |
| 乙苯 | 4.544 | 10.578 | 13.644 | -19.276 | 7.885 |
| PR模型 | 经验模型 | ||||
|---|---|---|---|---|---|
| 体系 | AARD/% | 参数 | 数值 | 训练集 | 测试集 |
| CO2-正己烷 | 3.25 | a | 4.4095 | ||
| CO2-正辛烷 | 1.06 | b | 0.3246 | ||
| CO2-十一烷 | 0.81 | c | 0.1544 | ||
| CO2-环己烷 | 2.71 | d | -2.3119 | ||
| CO2-乙苯 | 3.66 | e | 0.0718 | ||
| f | -2.2543×10-4 | ||||
| g | 2.0609 | ||||
| R2 | 0.9438 | ||||
| RMSE/% | 4.58 | 5.47 | |||
| AARD/% | 2.45 | 2.56 | |||
Table 9 The calculated results of the two models
| PR模型 | 经验模型 | ||||
|---|---|---|---|---|---|
| 体系 | AARD/% | 参数 | 数值 | 训练集 | 测试集 |
| CO2-正己烷 | 3.25 | a | 4.4095 | ||
| CO2-正辛烷 | 1.06 | b | 0.3246 | ||
| CO2-十一烷 | 0.81 | c | 0.1544 | ||
| CO2-环己烷 | 2.71 | d | -2.3119 | ||
| CO2-乙苯 | 3.66 | e | 0.0718 | ||
| f | -2.2543×10-4 | ||||
| g | 2.0609 | ||||
| R2 | 0.9438 | ||||
| RMSE/% | 4.58 | 5.47 | |||
| AARD/% | 2.45 | 2.56 | |||
| 1 | Sedaghat M, Rouhibakhsh K. Investigation of carbon dioxide capture and storage by a novel LSSVM-GA method[J]. Pet. Sci. Technol., 2020, 38(5): 421-427. |
| 2 | Marocco Stuardi F, MacPherson F, Leclaire J. Integrated CO2 capture and utilization: a priority research direction[J]. Curr. Opin. Green Sustainable Chem., 2019, 16: 71-76. |
| 3 | Rudyk S, Hussain S, Spirov P. Supercritical extraction of crude oil by methanol- and ethanol-modified carbon dioxide[J]. J. Supercrit. Fluids, 2013, 78: 63-69. |
| 4 | Jia B, Tsau J S, Barati R. A review of the current progress of CO2 injection EOR and carbon storage in shale oil reservoirs[J]. Fuel, 2019, 236: 404-427. |
| 5 | Gui X, Wang W, Gao Q, et al. Measurement and correlation of high pressure phase equilibria for CO2+ alkanes and CO2+ crude oil systems[J]. J. Chem. Eng. Data, 2017, 62(11): 3807-3822. |
| 6 | Mosavat N, Abedini A, Torabi F. Phase behaviour of CO2-brine and CO2-oil systems for CO2 storage and enhanced oil recovery: experimental studies[J]. Energy Procedia, 2014, 63: 5631-5645. |
| 7 | Zábranský M, Kolská Z, Růžička V, et al. Heat capacity of liquids: critical review and recommended values. Supplement Ⅱ[J]. J. Phys. Chem. Ref. Data, 2010, 39(1): 013103. |
| 8 | Yamaya K, Matsuguchi A, Kagawa N, et al. Isochoric specific heat capacity of trans-1,3,3,3-tetrafluoropropene (HFO-1234ze(E)) and the HFO-1234ze(E) + CO2 mixture in the liquid phase[J]. J. Chem. Eng. Data, 2011, 56(4): 1535-1539. |
| 9 | Xiao X, Al Ghafri S Z S, Rowland D, et al. Isobaric heat capacity measurements of natural gas model mixtures (methane + n-heptane) and (propane + n-heptane) by differential scanning calorimetry at temperatures from 313 K to 422 K and pressures up to 31 MPa[J]. Fuel, 2021, 296: 120668. |
| 10 | Xiao X, Oakley J, Al Ghafri S Z S, et al. Isobaric heat capacities of a methane (1) + propane (2) mixture by differential scanning calorimetry at near-critical and supercritical conditions[J]. Fuel, 2021, 289: 119840. |
| 11 | Dai Z X, Chen Y F, Liu C, et al. Prediction and verification of heat capacities for pure ionic liquids[J]. Chin. J. Chem. Eng., 2021, 31: 169-176. |
| 12 | Wang L, Song J, Sheng B W, et al. The isochoric specific heat capacity for R1234ze(E) at temperatures from (237 to 349) K and pressures up to 9.2 MPa[J]. J. Chem. Thermodyn., 2020, 141: 105936. |
| 13 | Li H P, Zhang X G, Han B X, et al. Effect of phase behavior and pressure on the constant-volume heat capacity and intermolecular interaction of CO2-ethanol and CO2-n-pentane mixtures in the critical region[J]. Chem. - Eur. J., 2002, 8(2): 451-456. |
| 14 | Zhang X F, Zhang X G, Han B X, et al. Determination of constant volume heat capacity of mixed supercritical fluids and study on the intermolecular interaction[J]. J. Supercrit. Fluids, 2002, 24(3): 193-201. |
| 15 | Polikhronidi N G, Batyrova R G, Abdulagatov I M, et al. Isochoric heat capacity measurements for a CO2 + n-decane mixture in the near-critical and supercritical regions[J]. J. Supercrit. Fluids, 2005, 33(3): 209-222. |
| 16 | Yousefi Seyf J. Evaluation of the PR, tc-PR, CPA, PC-SAFT and IAPWS-95 models in the predicting thermodynamic properties of pure water at the supercooled, compressed liquid, saturated liquid-vapor and superheat regions[J]. J. Mol. Liq., 2019, 288: 111088. |
| 17 | Saeed A, Ghader S. Calculation of density, vapor pressure and heat capacity near the critical point by incorporating cubic SRK EoS and crossover translation[J]. Fluid Phase Equilib., 2019, 493: 10-25. |
| 18 | Liu Y, He X, Zhao X M, et al. Modeling heat capacity of saturated hydrocarbon in liquid phase over a wide range of temperature and pressure[J]. J. Mol. Liq., 2020, 319: 114068. |
| 19 | Zhong Q, Dong X Q, Zhao Y X, et al. A simple generalized equation for compressed liquid isochoric heat capacity of pure and mixture refrigerants[J]. Fluid Phase Equilib., 2019, 490: 33-38. |
| 20 | Kuroki T, Kagawa N, Endo H, et al. Specific heat capacity at constant volume for water, methanol, and their mixtures at temperatures from 300 K to 400 K and pressures to 20 MPa[J]. J. Chem. Eng. Data, 2001, 46(5): 1101-1106. |
| 21 | Zhong Q, Dong X Q, Zhao Y X, et al. Adiabatic calorimeter for isochoric specific heat capacity measurements and experimental data of compressed liquid R1234yf[J]. J. Chem. Thermodyn., 2018, 125: 86-92. |
| 22 | Japan Society of Mechanical Engineers. JSME Data Book: Heat Transfer[M]. 4th ed. Tokyo: JSME, 1994. |
| 23 | Lindstrom P J. NIST Standard Reference Database Number 69[DB/OL]. [2020-06-28]. . |
| 24 | Wagner W, Pruß A. The IAPWS formulation 1995 for the thermodynamic properties of ordinary water substance for general and scientific use[J]. J. Phys. Chem. Ref. Data, 2002, 31(2): 387-535. |
| 25 | Shi Q Z, Jing L S, Qiao W H. Solubility of n-alkanes in supercritical CO2 at diverse temperature and pressure[J]. J. CO2 Util., 2015, 9: 29-38. |
| 26 | Mu T C, Zhang X G, Han B X, et al. Effect of phase behavior on the constant volume heat capacity of ethane + ethanol and ethane + acetone mixed fluids near the critical region and the intermolecular interaction[J]. Fluid Phase Equilib., 2003, 214(1): 53-65. |
| 27 | Kian K, Scurto A M. Heat transport properties of CO2-expanded liquids: n-hexane, n-decane, and n-tetradecane[J]. Ind. Eng. Chem. Res., 2017, 56(44): 12822-12832. |
| 28 | Yang Z H, Li M Y, Peng B, et al. Dispersion property of CO2 in oil (2): Volume expansion of CO2 + organic liquid at near-critical and supercritical conditions of CO2 [J]. J. Chem. Eng. Data, 2012, 57(4): 1305-1311. |
| 29 | Poling B E, Prausnitz J M, O’connell J P. The Properties of Gases and Liquids[M]. 5th ed. New York: McGraw-Hill Professional, 2001. |
| 30 | Peng D Y, Robinson D B. A new two-constant equation of state[J]. Ind. Eng. Chem. Fundam., 1976, 15(1): 59-64. |
| 31 | Hankinson R W, Thomson G H. A new correlation for saturated densities of liquids and their mixtures[J]. AIChE J., 1979, 25(4): 653-663. |
| [1] | Yifei ZHANG, Fangchen LIU, Shuangxing ZHANG, Wenjing DU. Performance analysis of printed circuit heat exchanger for supercritical carbon dioxide [J]. CIESC Journal, 2023, 74(S1): 183-190. |
| [2] | Ruitao SONG, Pai WANG, Yunpeng WANG, Minxia LI, Chaobin DANG, Zhenguo CHEN, Huan TONG, Jiaqi ZHOU. Numerical simulation of flow boiling heat transfer in pipe arrays of carbon dioxide direct evaporation ice field [J]. CIESC Journal, 2023, 74(S1): 96-103. |
| [3] | Yepin CHENG, Daqing HU, Yisha XU, Huayan LIU, Hanfeng LU, Guokai CUI. Application of ionic liquid-based deep eutectic solvents for CO2 conversion [J]. CIESC Journal, 2023, 74(9): 3640-3653. |
| [4] | Ruihang ZHANG, Pan CAO, Feng YANG, Kun LI, Peng XIAO, Chun DENG, Bei LIU, Changyu SUN, Guangjin CHEN. Analysis of key parameters affecting product purity of natural gas ethane recovery process via ZIF-8 nanofluid [J]. CIESC Journal, 2023, 74(8): 3386-3393. |
| [5] | Rui HONG, Baoqiang YUAN, Wenjing DU. Analysis on mechanism of heat transfer deterioration of supercritical carbon dioxide in vertical upward tube [J]. CIESC Journal, 2023, 74(8): 3309-3319. |
| [6] | Qiyu ZHANG, Lijun GAO, Yuhang SU, Xiaobo MA, Yicheng WANG, Yating ZHANG, Chao HU. Recent advances in carbon-based catalysts for electrochemical reduction of carbon dioxide [J]. CIESC Journal, 2023, 74(7): 2753-2772. |
| [7] | Caihong LIN, Li WANG, Yu WU, Peng LIU, Jiangfeng YANG, Jinping LI. Effect of alkali cations in zeolites on adsorption and separation of CO2/N2O [J]. CIESC Journal, 2023, 74(5): 2013-2021. |
| [8] | Chenxi LI, Yongfeng LIU, Lu ZHANG, Haifeng LIU, Jin’ou SONG, Xu HE. Quantum chemical analysis of n-heptane combustion mechanism under O2/CO2 atmosphere [J]. CIESC Journal, 2023, 74(5): 2157-2169. |
| [9] | Bingguo ZHU, Jixiang HE, Jinliang XU, Bin PENG. Heat transfer characteristics of supercritical pressure CO2 in diverging/converging tube under cooling conditions [J]. CIESC Journal, 2023, 74(3): 1062-1072. |
| [10] | Jingbo GAO, Qiang SUN, Qing LI, Yiwei WANG, Xuqiang GUO. Hydrate equilibrium model of hydrogen-containing gas considering hydrates structure transformation [J]. CIESC Journal, 2023, 74(2): 666-673. |
| [11] | Renchu HE, Zhaohui ZHANG, Minglei YANG, Cong WANG, Zhenhao XI. Online optimization of gasoline blending considering carbon emissions [J]. CIESC Journal, 2023, 74(2): 818-829. |
| [12] | Jiaqing ZHANG, Rongpei JIANG, Weikang SHI, Boxiang WU, Chao YANG, Zhaohui LIU. Study on viscosity-temperature characteristics and component characteristics of rocket kerosene [J]. CIESC Journal, 2023, 74(2): 653-665. |
| [13] | Feng WANG, Shunxin ZHANG, Fangbo YU, Ya LIU, Liejin GUO. Optimization strategy for producing carbon based fuels by photocatalytic CO2 reduction [J]. CIESC Journal, 2023, 74(1): 29-44. |
| [14] | Chenyang SHEN, Kaihang SUN, Yueping ZHANG, Changjun LIU. Research progresses on In2O3 and In2O3 supported metal catalysts for CO2 hydrogenation to methanol [J]. CIESC Journal, 2023, 74(1): 145-156. |
| [15] | Chen CHEN, Qian YANG, Yun CHEN, Rui ZHANG, Dong LIU. Chemical kinetic study on coal volatiles combustion for various oxygen concentrations [J]. CIESC Journal, 2022, 73(9): 4133-4146. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
